The Influence of CoO/P2O5 Substitutions on the Structural, Mechanical, and Radiation Shielding of Boro-Phosphate Glasses
Abstract
:1. Introduction
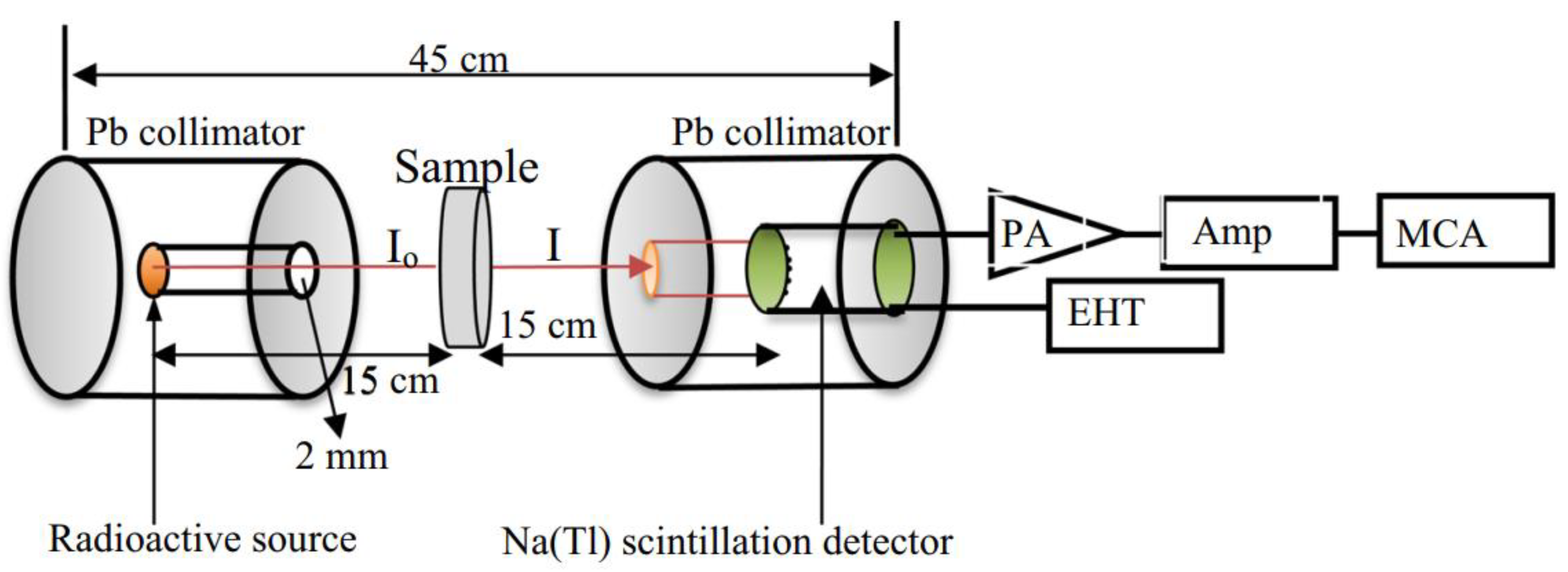
2. Materials and Methods
3. Results and Discussions
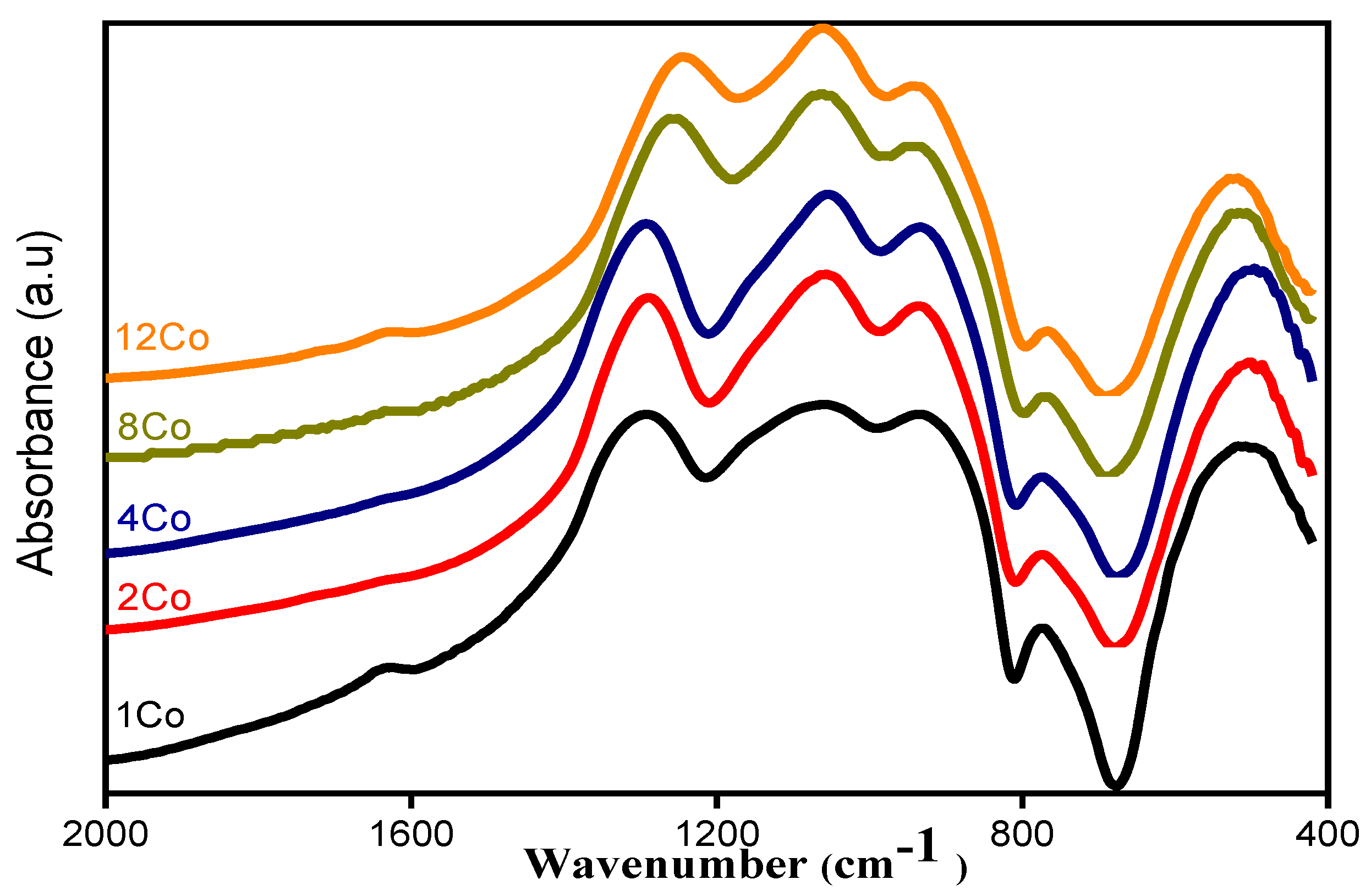
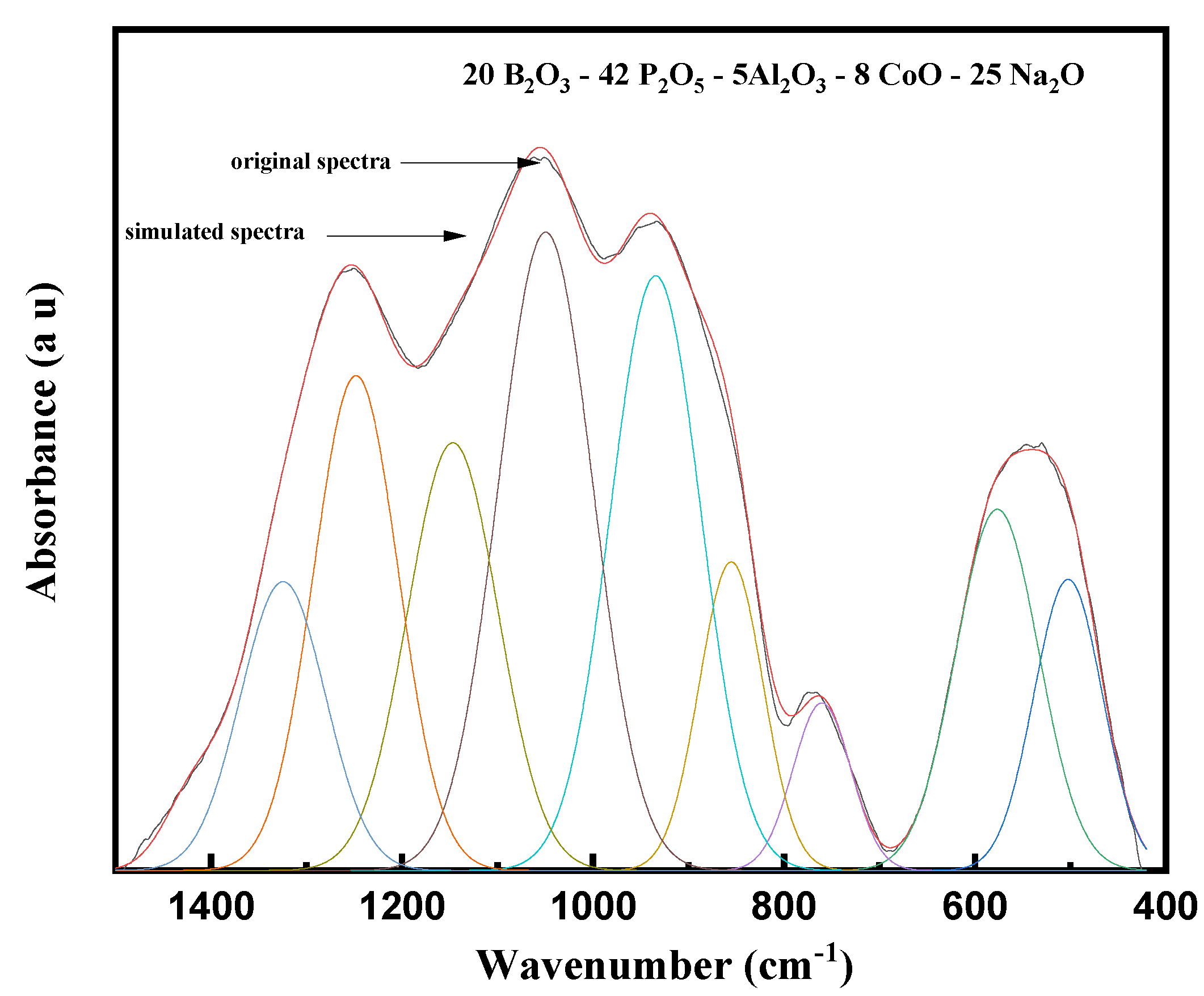
3.1. FTIR Analysis
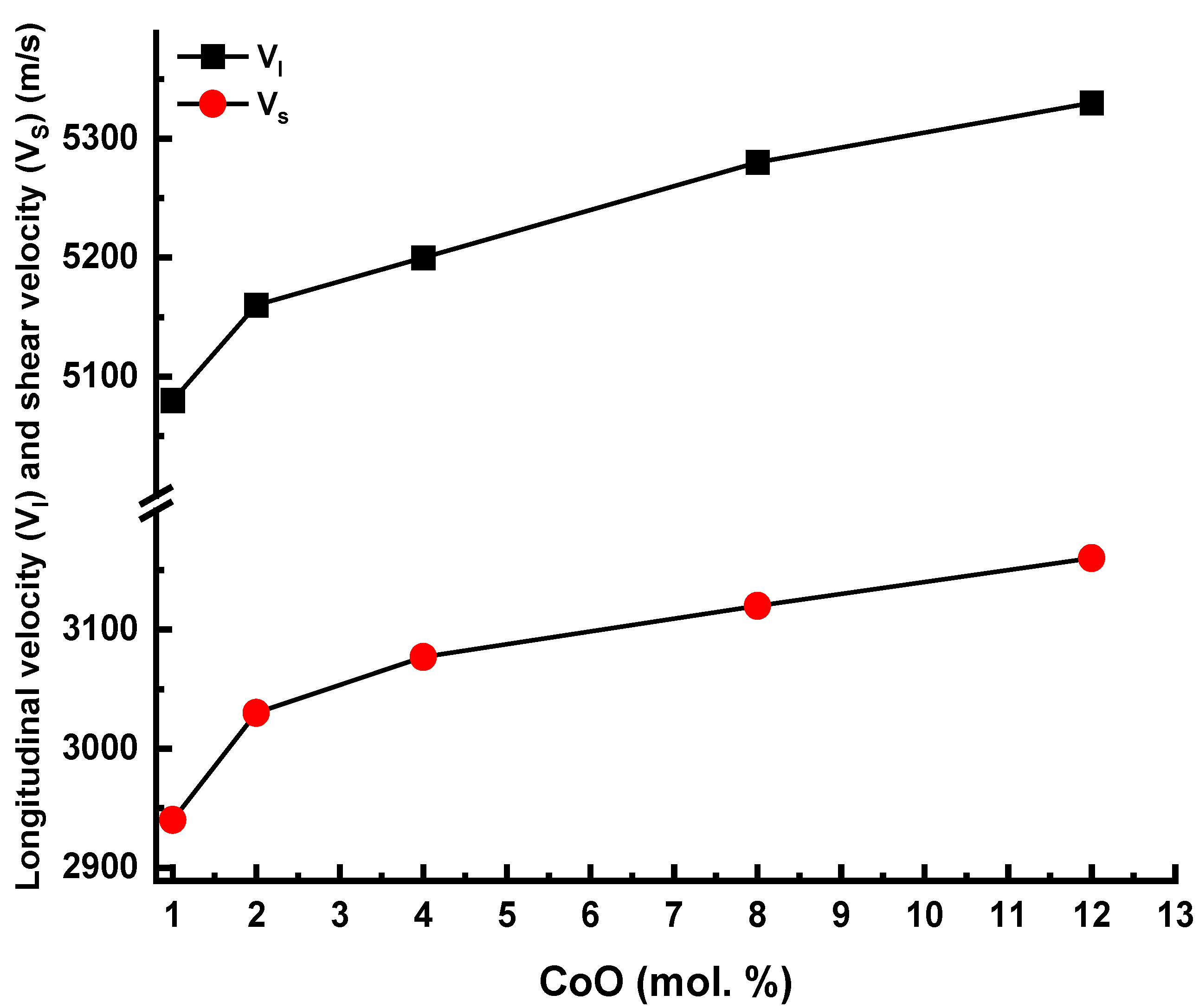
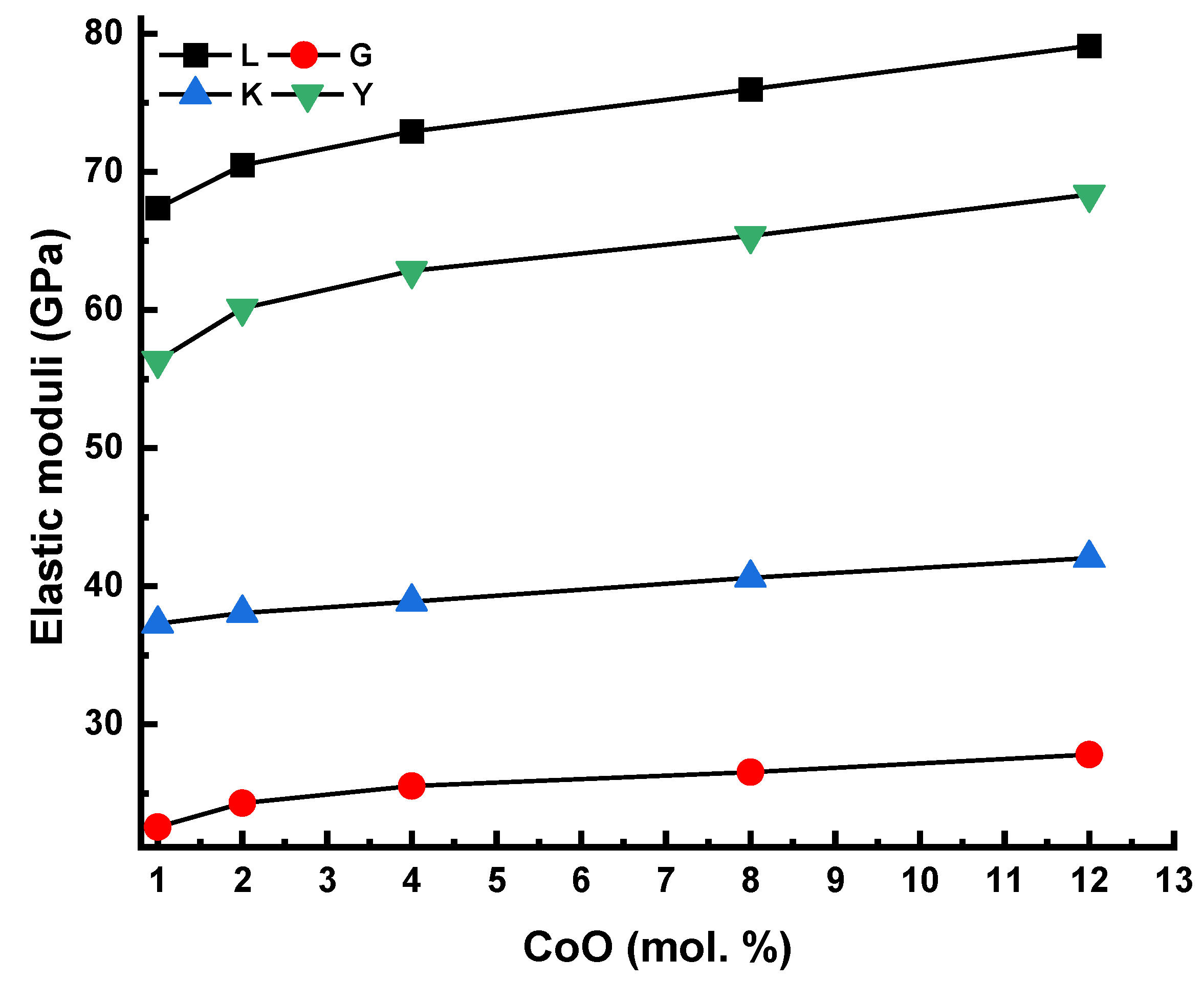
3.2. Analysis of Elastic Characteristics
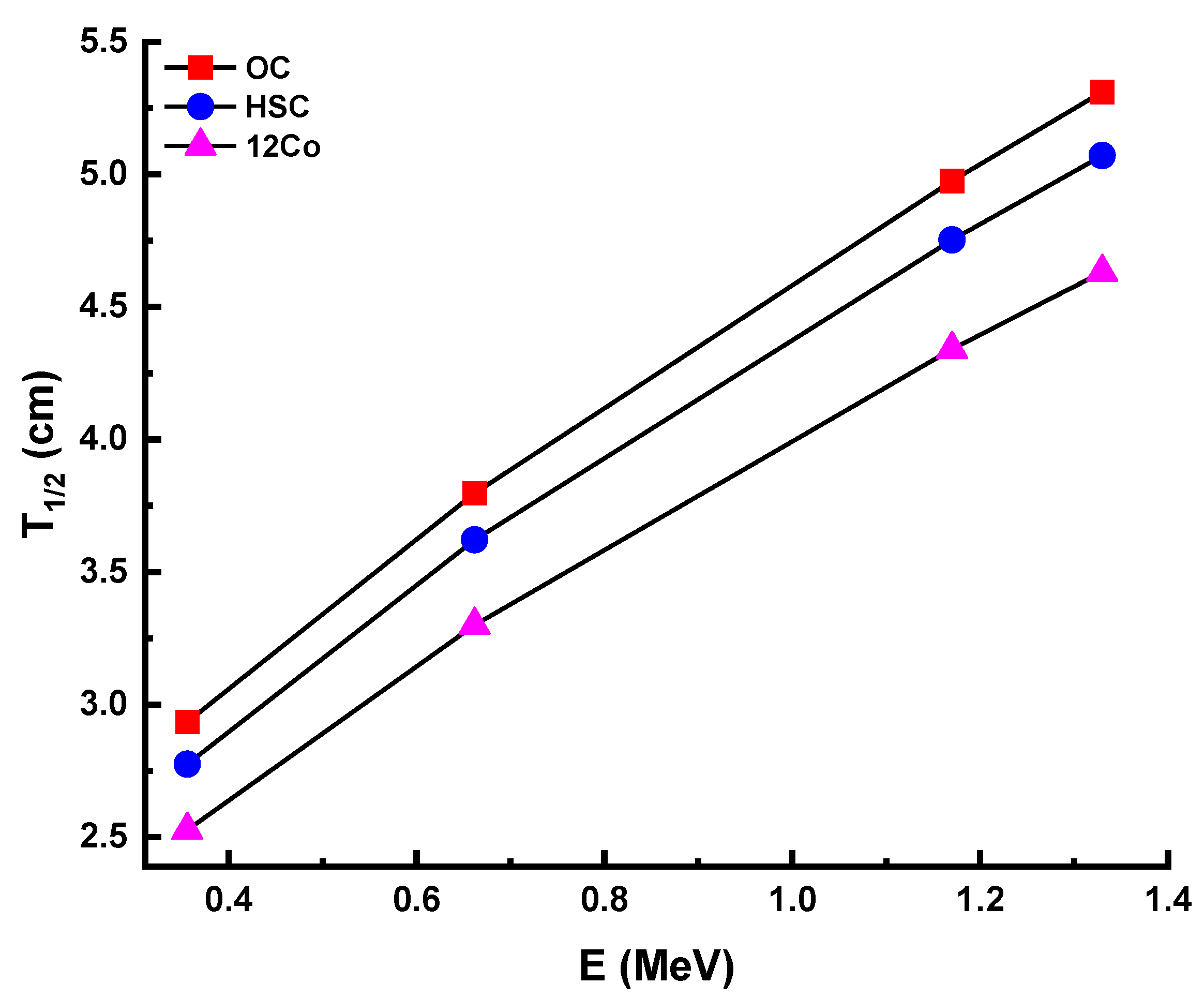
3.3. Thermal Properties
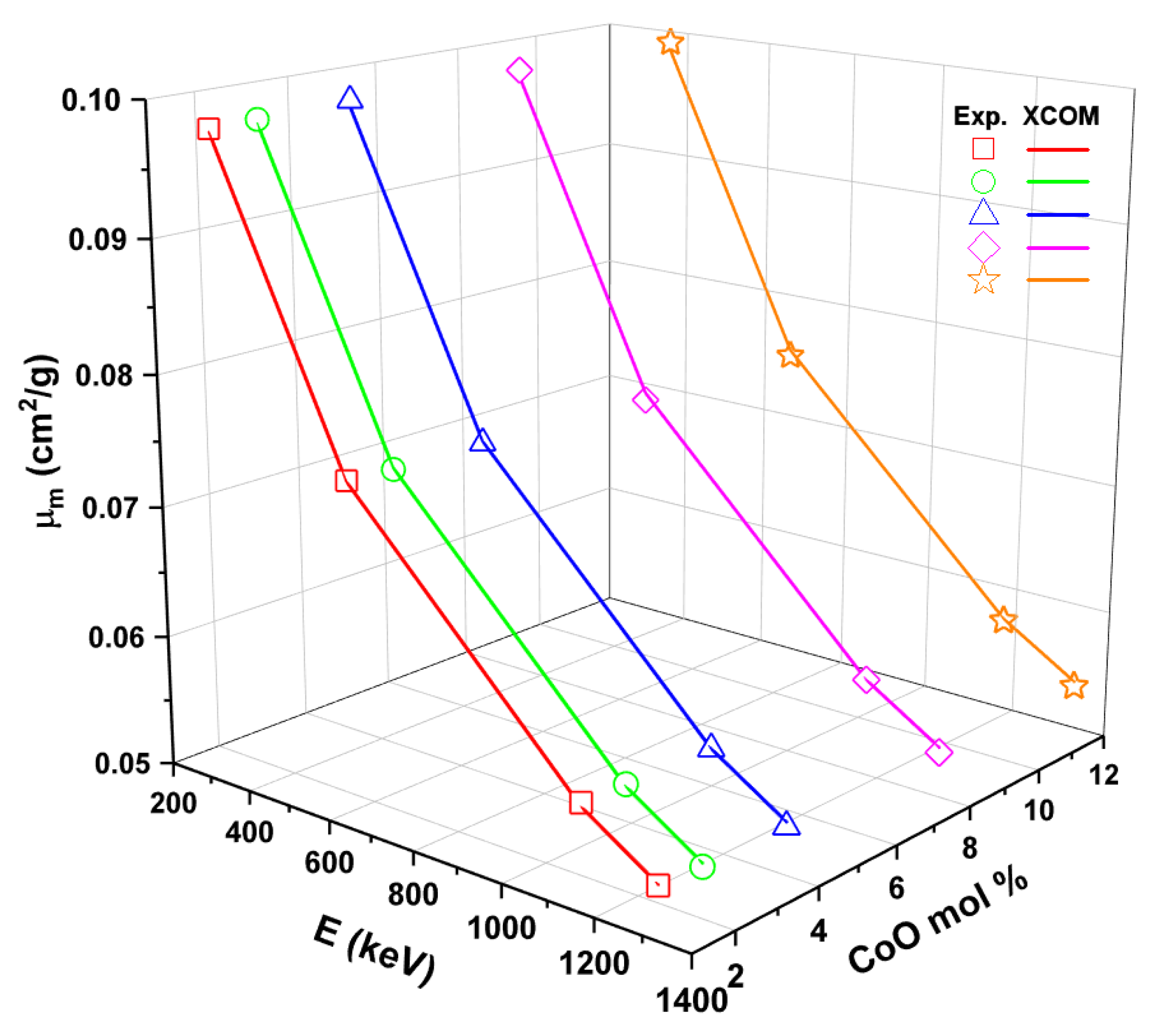
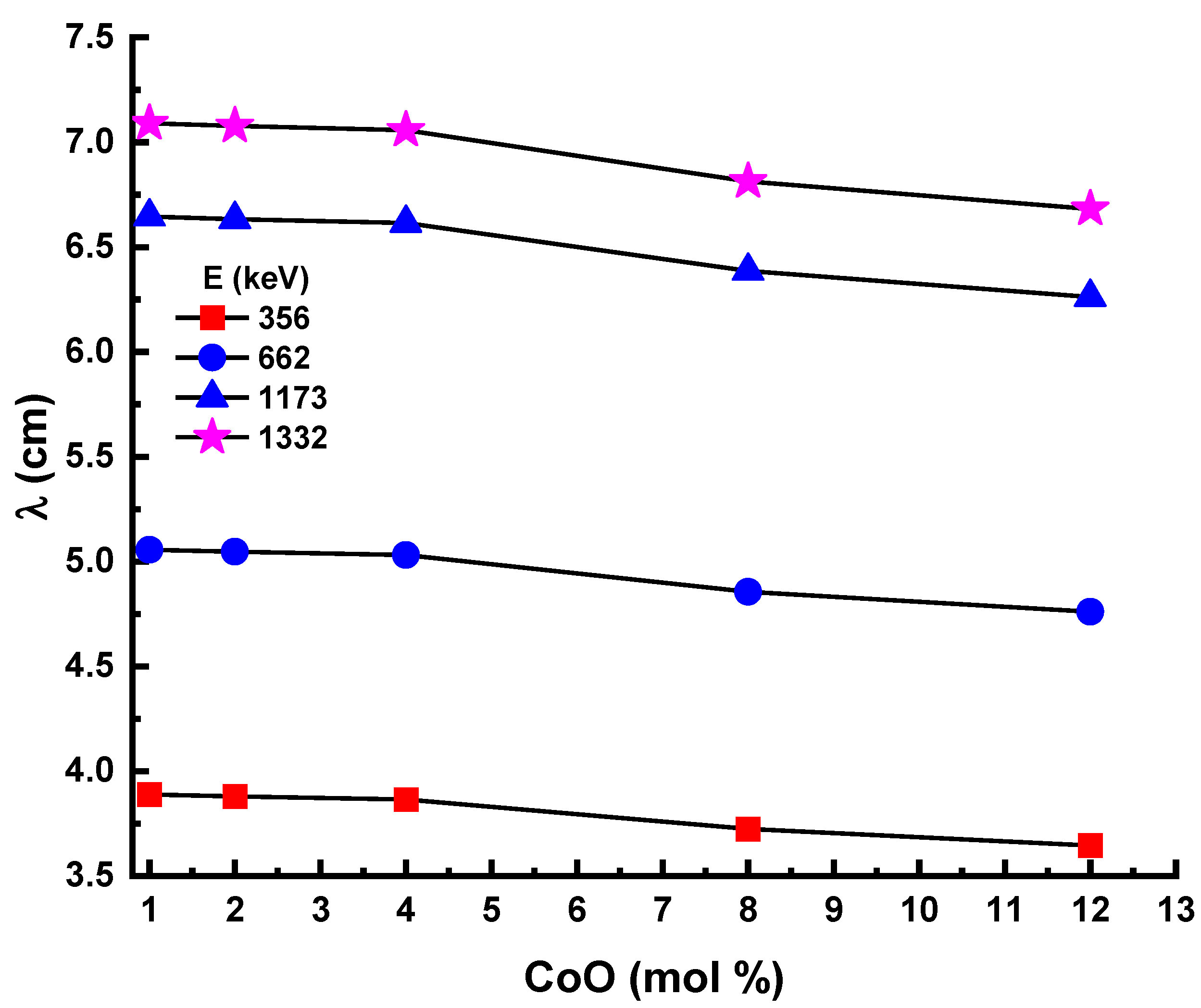
3.4. Radiation Shielding Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Issa, S.A.M.; Saddeek, Y.B.; Tekin, H.O.; Sayyed, M.I.; Shaaban, K.S. Investigations of radiation shielding using Monte Carlo method and elastic properties of PbO-SiO2-B2O3-Na2O glasses. Curr. Appl. Phys. 2018, 518, 184–194. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Mostafa, A.M.A. Effect of Bi2O3 in borate-tellurite-silicate glass system for development of gamma-rays shielding materials. J. Alloys Compd. 2017, 695, 302–310. [Google Scholar] [CrossRef]
- Villa, M.; Scagliotti, M.; Chiodelli, G. Short range order in the network of the borophosphate glasses: A 31P NMR-MAS (Magic Angle Spinning) study. J. Non-Cryst. Solids 1987, 94, 101–121. [Google Scholar] [CrossRef]
- Hong, S.K.; Jung, D.S.; Cho, J.S.; Kang, Y.C. Characteristics of ZnO–B2O3–CaO–Na2O–P2O5 glass powders prepared by spray pyrolysis. J. Non-Cryst. Solids 2008, 354, 3012–3018. [Google Scholar] [CrossRef]
- Seshadri, M.; Batesttin, C.; Silva, I.L.; Bell, M.J.V.; Anjos, V. Effect of compositional changes on the structural properties of borophosphate glasses: ATR-FTIR and Raman spectroscopy. Vib. Spectrosc. 2020, 110, 103137. [Google Scholar] [CrossRef]
- Kabi, S.; Ghosh, A. Correlation of Structure and Electrical Conductivity of CdI 2 Doped Silver Borophosphate Glass and Nanocomposite. J. Phys. Chem. C 2011, 115, 9760–9766. [Google Scholar] [CrossRef]
- Schuch, M.; Christensen, R.; Trott, C.; Maass, P.; Martin, S.W. Investigation of the Structures of Sodium Borophosphate Glasses by Reverse Monte Carlo Modeling to Examine the Origins of the Mixed Glass Former Effect. J. Phys. Chem. C 2012, 116, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Sadeq, M.S. Influence of cobalt oxide on the structure, optical transitions and ligand field parameters of lithium phosphate glasses. Ceram. Int. 2021, 47, 28536–28542. [Google Scholar] [CrossRef]
- El-Batal, A.M.; Saeed, A.; Hendawy, N.; El-Okr, M.M.; El-Mansy, M.K. Influence of Mo or/and Co ions on the structural and optical properties of phosphate zinc lithium glasses. J. Non-Cryst. Solids 2021, 559, 120678. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; El-Damrawi, G.; Oraby, A.H.; Madshal, M.A. Optical and FTIR structural studies on CoO-doped strontium phosphate glasses. J. Non-Cryst. Solids 2018, 499, 153–158. [Google Scholar] [CrossRef]
- Metwalli, E.; Karabulut, M.; Sidebottom, D.L.; Morsi, M.M.; Brow, R.K. Properties and structure of copper ultraphosphate glasses. J. Non-Cryst. Solids 2004, 344, 128–134. [Google Scholar] [CrossRef]
- ElBatal, F.H.; Ouis, M.A.; Morsi, R.M.M.; Marzouk, S.Y. Interaction of gamma rays with some sodium phosphate glasses containing cobalt. J. Non-Cryst. Solids 2010, 356, 46–55. [Google Scholar] [CrossRef]
- Ray, N.H. Composition—property relationships in inorganic oxide glasses. J. Non-Cryst. Solids 1974, 15, 423–434. [Google Scholar] [CrossRef]
- Sedmale, G.; Vaivads, J.; Sedmalis, U.; Kabanov, V.O.; Yanush, O.V. Formation of borophosphate glass structure within the system BaO–B2O3–P2O5. J. Non-Cryst. Solids 1991, 129, 284–291. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Saudi, H.A.; Issa, S.A.M.; Rashad, M.; Elazaka, A.I.; Tekin, H.O.; Saddeek, Y.B. Alteration of optical, structural, mechanical durability and nuclear radiation attenuation properties of barium borosilicate glasses through BaO reinforcement: Experimental and numerical analyses. Ceram. Int. 2021, 47, 5587–5596. [Google Scholar] [CrossRef]
- Mostafa, A.M.A.; Issa, S.A.M.; Zakaly, H.M.H.; Zaid, M.H.M.; Tekin, H.O.; Matori, K.A.; Sidek, H.A.A.; Elsaman, R. The influence of heavy elements on the ionizing radiation shielding efficiency and elastic properties of some tellurite glasses: Theoretical investigation. Results Phys. 2020, 19, 103496. [Google Scholar] [CrossRef]
- Mahmoud, I.S.; Issa, S.A.M.; Zakaly, H.M.H.; Saudi, H.A.; Ali, A.S.; Saddeek, Y.B.; Alharbi, T.; Tekin, H.O. Material characterization of WO3/Bi2O3 substituted calcium-borosilicate glasses: Structural, physical, mechanical properties and gamma-ray resistance competencies. J. Alloys Compd. 2021, 888, 161419. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; ElBatal, F.H.; ElBatal, H.A.; EzzElDin, F.M. Optical and FTIR structural studies of CoO-doped sodium borate, sodium silicate and sodium phosphate glasses and effects of gamma irradiation-a comparative study. J. Mol. Struct. 2014, 1074, 503–510. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Kityk, I.V.; Piasecki, M.; Bragiel, P.; Brik, M.G.; Gandhi, Y.; Veeraiah, N. Structural investigations on PbO–Sb2O3–B2O3:CoO glass ceramics by means of spectroscopic and dielectric studies. J. Phys. Condens. Matter 2009, 21, 245104. [Google Scholar] [CrossRef] [PubMed]
- Sobhanachalam, P.; Ravi Kumar, V.; Raghavaiah, B.V.; Ravi Kumar, V.; Sahaya Baskaran, G.; Gandhi, Y.; Syam Prasad, P.; Veeraiah, N. In Vitro investigations on CoO doped CaF2CaO B2O3P2O5−MO bioactive glasses by means of spectroscopic studies. Opt. Mater. Amst. 2017, 73, 628–637. [Google Scholar] [CrossRef]
- Ivascu, C.; Timar Gabor, A.; Cozar, O.; Daraban, L.; Ardelean, I. FT-IR, Raman and thermoluminescence investigation of P2O5–BaO–Li2O glass system. J. Mol. Struct. 2011, 993, 249–253. [Google Scholar] [CrossRef]
- Vedeanu, N.S.; Cozar, I.B.; Stanescu, R.; Stefan, R.; Vodnar, D.; Cozar, O. Structural investigation of V2O5–P2O5–K2O glass system with antibacterial potential. Bull. Mater. Sci. 2016, 39, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.V.; Goswami, M.; Deo, M.N.; Sarkar, A.; Manikandan, S.; Shrikhande, V.K.; Kothiyal, G.P. Effect of B2O3 addition on microhardness and structural features of 40Na2O–10BaO–xB2O3–(50–x)P2O5 glass system. Bull. Mater. Sci. 2006, 29, 43–48. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Kaid, M.A.; Ebeid, M.R. FTIR and physical features of Al2O3–La2O3–P2O5–PbO glasses. J. Non-Cryst. Solids 2014, 387, 30–35. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Patsis, A.P.; Karakassides, M.A.; Chryssikos, G.D. Infrared reflectance spectra of lithium borate glasses. J. Non-Cryst. Solids 1990, 126, 52–67. [Google Scholar] [CrossRef]
- Shih, P.; Ding, J.; Lee, S. MAS-NMR and FTIR analyses on the structure of CuO-containing sodium poly- and meta-phosphate glasses. Mater. Chem. Phys. 2003, 80, 391–396. [Google Scholar] [CrossRef]
- Jha, P.K.; Pandey, O.P.; Singh, K. Structure and crystallization kinetics of Li2O modified sodium-phosphate glasses. J. Mol. Struct. 2015, 1094, 174–182. [Google Scholar] [CrossRef]
- Ahmad, M.; Aly, K.; Saddeek, Y.B.; Dahshan, A. Glass transition and crystallization kinetics of Na2O–B2O3–Nb2O5–Bi2O3 ceramic glasses. J. Non-Cryst. Solids 2020, 546, 120260. [Google Scholar] [CrossRef]
- Aly, K.A.; Saddeek, Y.B.; Dahshan, A. Structure and crystallization kinetics of manganese lead tellurite glasses. J. Therm. Anal. Calorim. 2015, 119, 1215–1224. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Afifi, H.A.; Abd El-Aal, N.S. Interpretation of mechanical properties and structure of TeO2-Li2O-B2O3 glasses. Phys. B Condens. Matter 2007, 398, 1–7. [Google Scholar] [CrossRef]
- Bashter, I.I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]




| Sample Code | B2O3 | P2O5 | Al2O3 | CoO | Na2O | ρ (g/cm3) |
|---|---|---|---|---|---|---|
| 1Co | 20 | 49 | 5 | 1 | 25 | 2.611 |
| 2Co | 20 | 48 | 5 | 2 | 25 | 2.617 |
| 4Co | 20 | 46 | 5 | 4 | 25 | 2.627 |
| 8Co | 20 | 42 | 5 | 8 | 25 | 2.726 |
| 12Co | 20 | 38 | 5 | 12 | 25 | 2.785 |
| 1Co | C | 479 | 542 | 608 | 725 | 772 | 862 | 938 | 1044 | 1155 | 1268 | 1344 |
| A | 4.8 | 10.4 | 5.49 | 2.09 | 3.7 | 8.4 | 14 | 17.5 | 13.9 | 12.1 | 7.66 | |
| 2Co | C | 487 | 550 | 604 | 735 | 767 | 865 | 940 | 1049 | 1153 | 1286 | 1376 |
| A | 6.9 | 8.81 | 3.58 | 1.05 | 2.8 | 8.2 | 15.8 | 18.5 | 13.7 | 15.8 | 4.85 | |
| 4Co | C | 474 | 540 | 597 | 724 | 768 | 859 | 933 | 1049 | 1157 | 1287 | 1383 |
| A | 6.7 | 10 | 5.85 | 1.95 | 3.52 | 8.54 | 15.6 | 17.9 | 12.2 | 13.9 | 3.79 | |
| 8Co | C | 502 | 577 | - | - | 760 | 855 | 934 | 1050 | 1147 | 1248 | 1324 |
| A | 6.59 | 9.9 | - | - | 3.2 | 6.51 | 17.8 | 19.9 | 12.8 | 15.2 | 8.11 | |
| 12Co | C | 511 | 591 | - | - | 753 | 852 | 935 | 1051 | 1137 | 1253 | 1388 |
| A | 8.55 | 7.65 | - | - | 3.04 | 8.93 | 17 | 19.2 | 15.3 | 17.3 | 2.98 |
| Code | ρ (g/cm3) | Vl (m/s) | Vs (m/s) | L (GPa) | G (GPa) | σ | K (GPa) | Y (GPa) | Θ | Tg |
|---|---|---|---|---|---|---|---|---|---|---|
| 1Co | 2.611 | 5080 | 2940 | 67.38 | 22.57 | 0.25 | 37.29 | 56.34 | 422 | 314 |
| 2Co | 2.647 | 5160 | 3030 | 70.48 | 24.30 | 0.24 | 38.08 | 60.12 | 436 | 319 |
| 4Co | 2.697 | 5200 | 3077 | 72.93 | 25.54 | 0.23 | 38.88 | 62.85 | 444 | 325 |
| 8Co | 2.726 | 5280 | 3120 | 75.99 | 26.54 | 0.23 | 40.62 | 65.37 | 451 | 331 |
| 12Co | 2.785 | 5330 | 3160 | 79.12 | 27.81 | 0.23 | 42.039 | 68.36 | 457 | 338 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.M.A.; Uosif, M.A.M.; Alrowaili, Z.A.; Elsaman, R.; Showahy, A.A.; Saddeek, Y.B.; Issa, S.A.M.; Ene, A.; Zakaly, H.M.H. The Influence of CoO/P2O5 Substitutions on the Structural, Mechanical, and Radiation Shielding of Boro-Phosphate Glasses. Materials 2021, 14, 6632. https://doi.org/10.3390/ma14216632
Mostafa AMA, Uosif MAM, Alrowaili ZA, Elsaman R, Showahy AA, Saddeek YB, Issa SAM, Ene A, Zakaly HMH. The Influence of CoO/P2O5 Substitutions on the Structural, Mechanical, and Radiation Shielding of Boro-Phosphate Glasses. Materials. 2021; 14(21):6632. https://doi.org/10.3390/ma14216632
Chicago/Turabian StyleMostafa, Ahmed M. A., Mohamed A. M. Uosif, Ziyad A. Alrowaili, Reda Elsaman, Ahmed A. Showahy, Yasser B. Saddeek, Shams A. M. Issa, Antoaneta Ene, and Hesham M. H. Zakaly. 2021. "The Influence of CoO/P2O5 Substitutions on the Structural, Mechanical, and Radiation Shielding of Boro-Phosphate Glasses" Materials 14, no. 21: 6632. https://doi.org/10.3390/ma14216632
APA StyleMostafa, A. M. A., Uosif, M. A. M., Alrowaili, Z. A., Elsaman, R., Showahy, A. A., Saddeek, Y. B., Issa, S. A. M., Ene, A., & Zakaly, H. M. H. (2021). The Influence of CoO/P2O5 Substitutions on the Structural, Mechanical, and Radiation Shielding of Boro-Phosphate Glasses. Materials, 14(21), 6632. https://doi.org/10.3390/ma14216632









