Fabrication of Low-Temperature Sintering Building Bricks Using Drilling Cutting and Geopolymeric Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Building Brick
2.3. Materials Characterization
3. Results and Discussions
3.1. Conventional DC Building Brick & DC Analysis
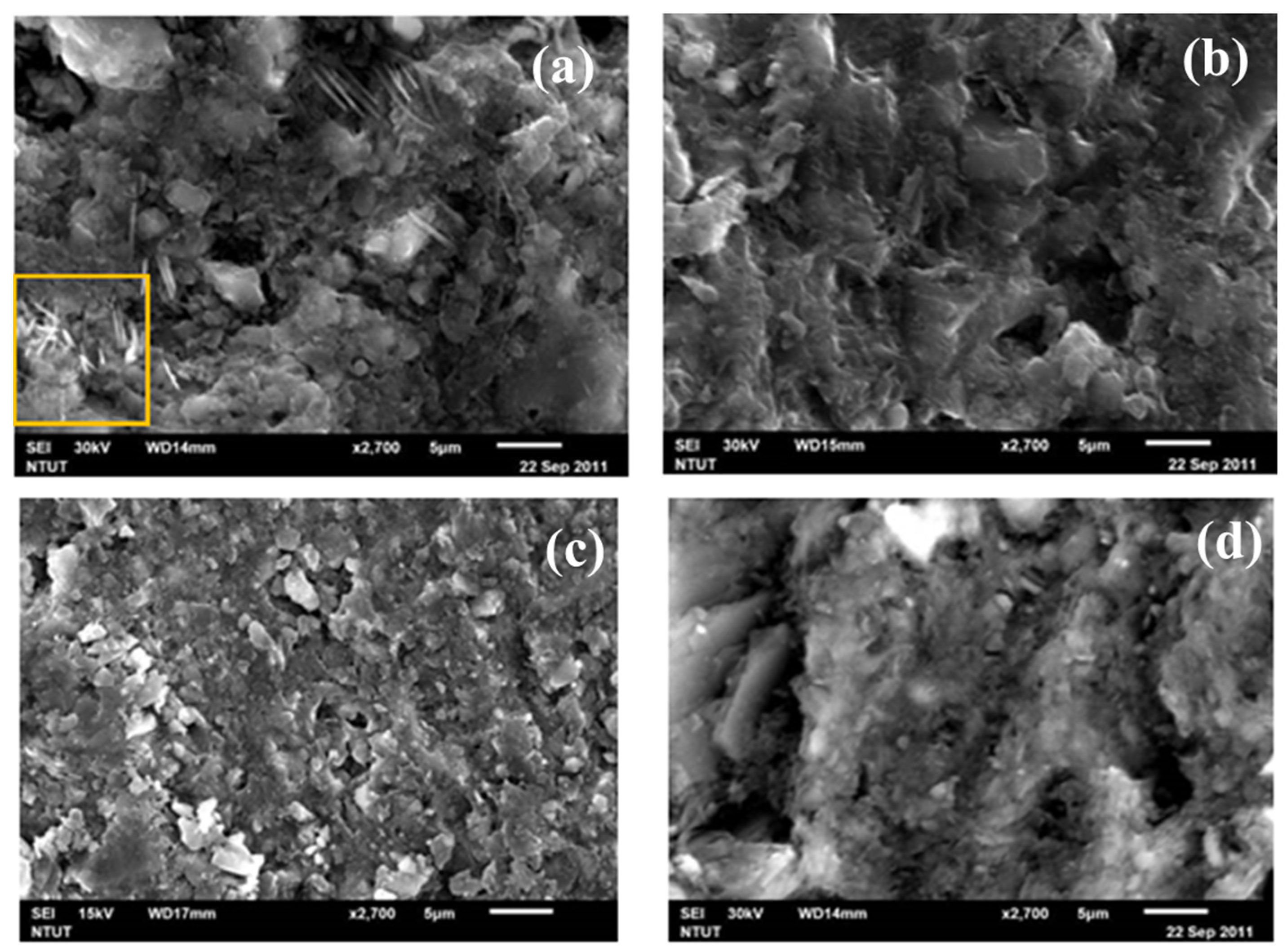
3.1.1. SEM Microstructure Analysis
3.1.2. Physical Properties
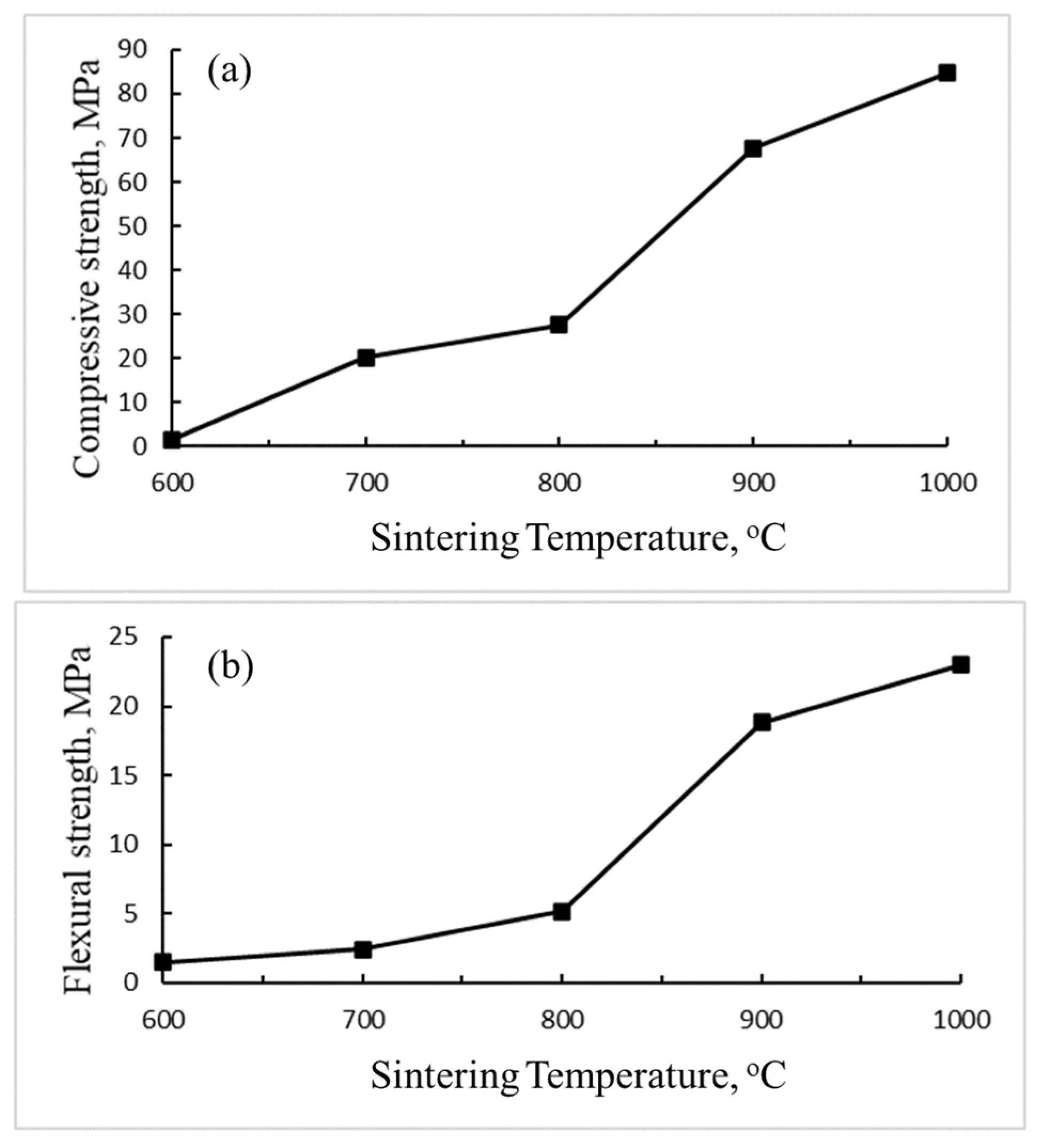
3.1.3. Mechanical Properties
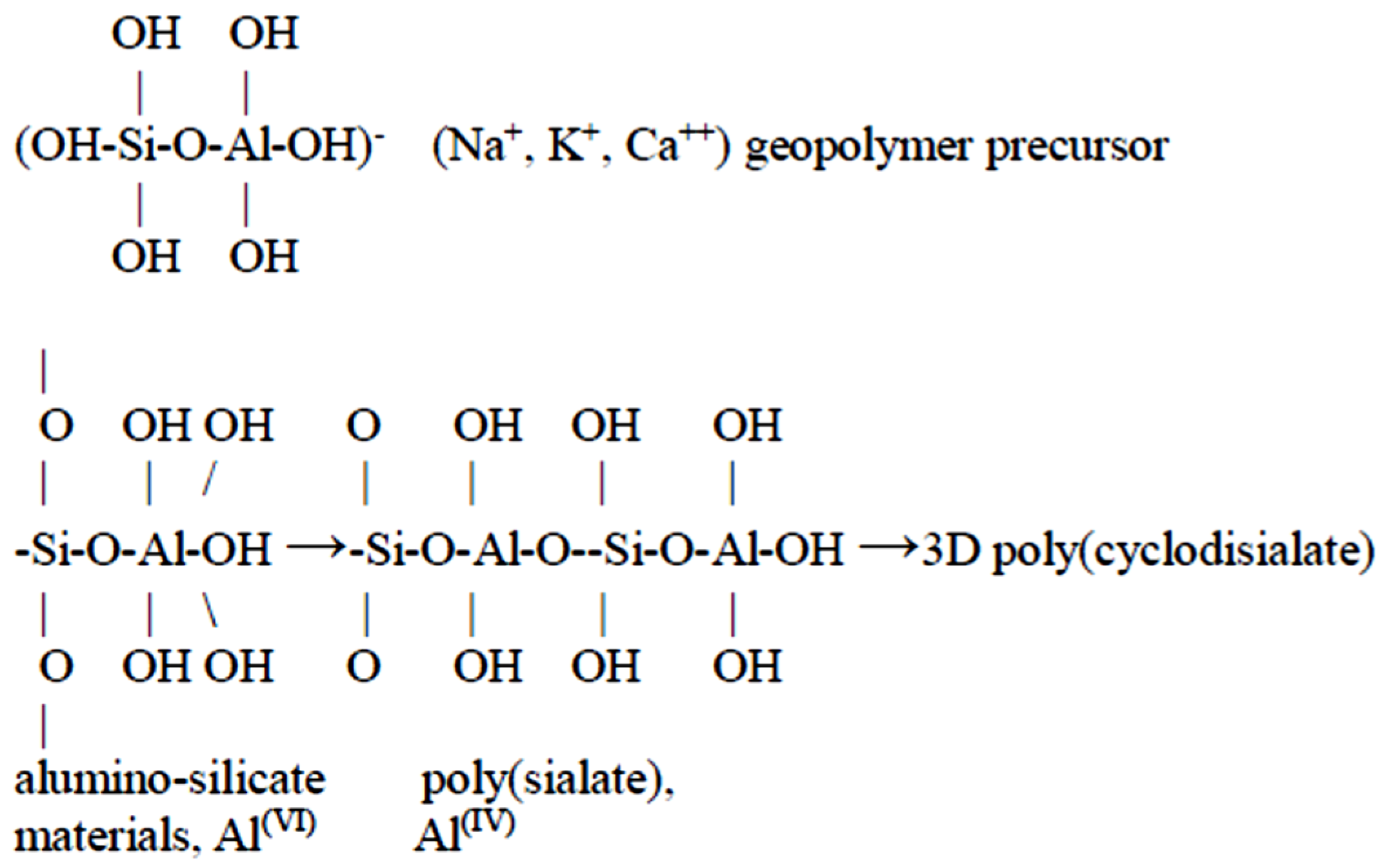
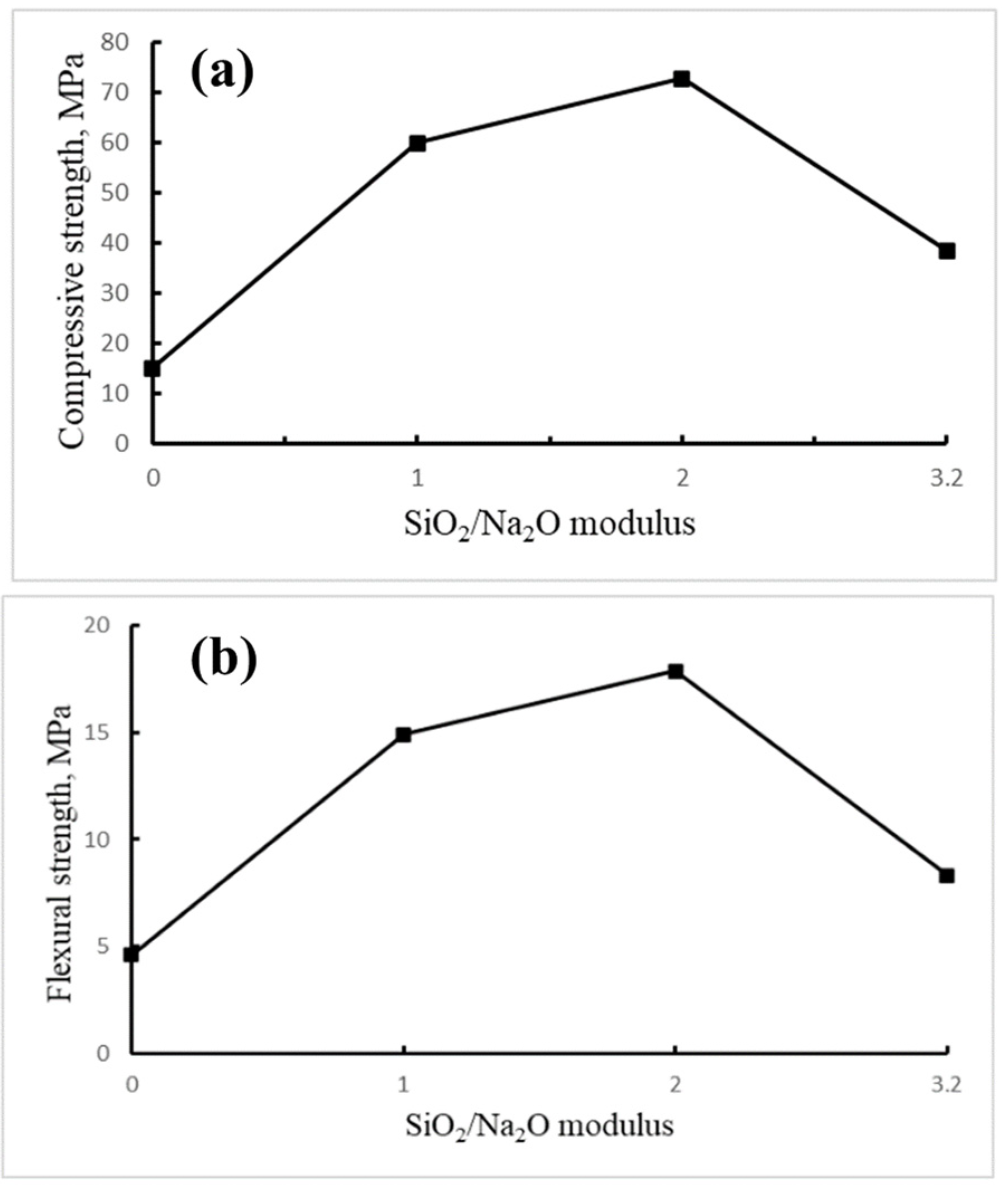
3.2. Influence of SiO2/Na2O Modulus on the Properties of LTGS Bricks
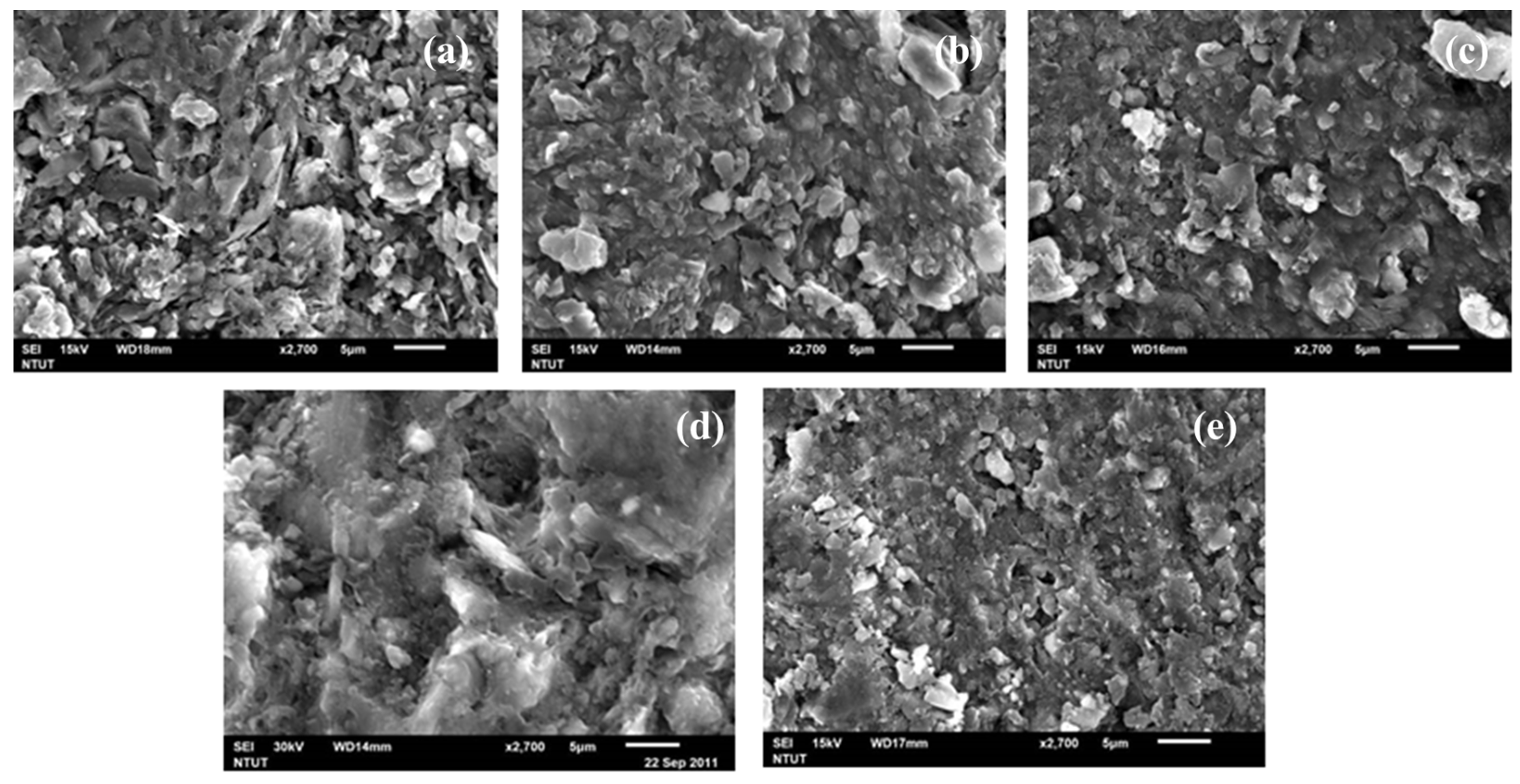
3.2.1. SEM Microstructure Analysis
3.2.2. Physical Properties
3.2.3. Mechanical Properties
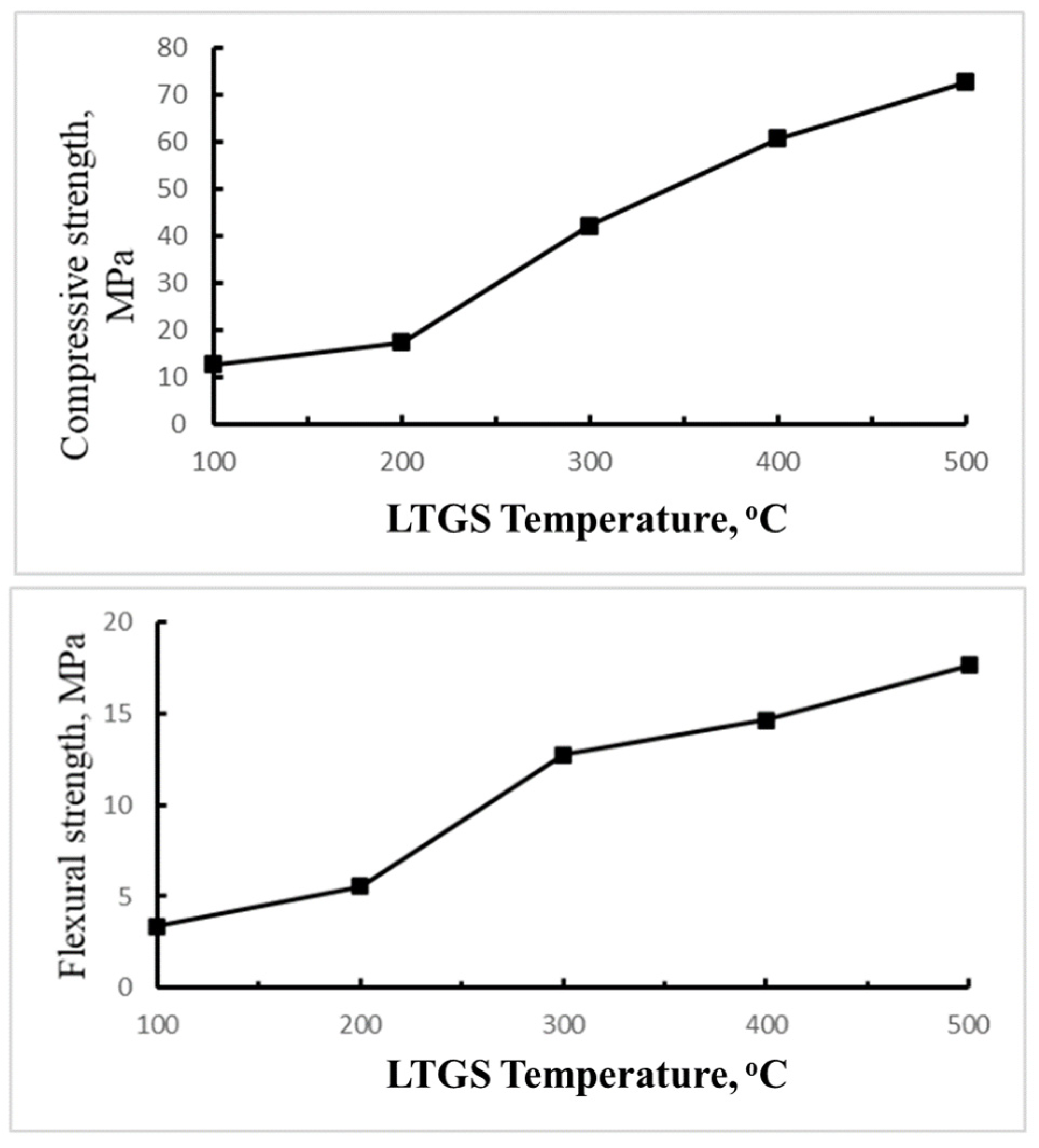
3.3. Effect of Temperature on the Properties of LTGS Bricks
3.3.1. SEM Microstructure Analysis
3.3.2. Physical Properties
3.3.3. Mechanical Properties
3.3.4. Ranking of Sintering Bricks and LTGS Bricks
4. Conclusions
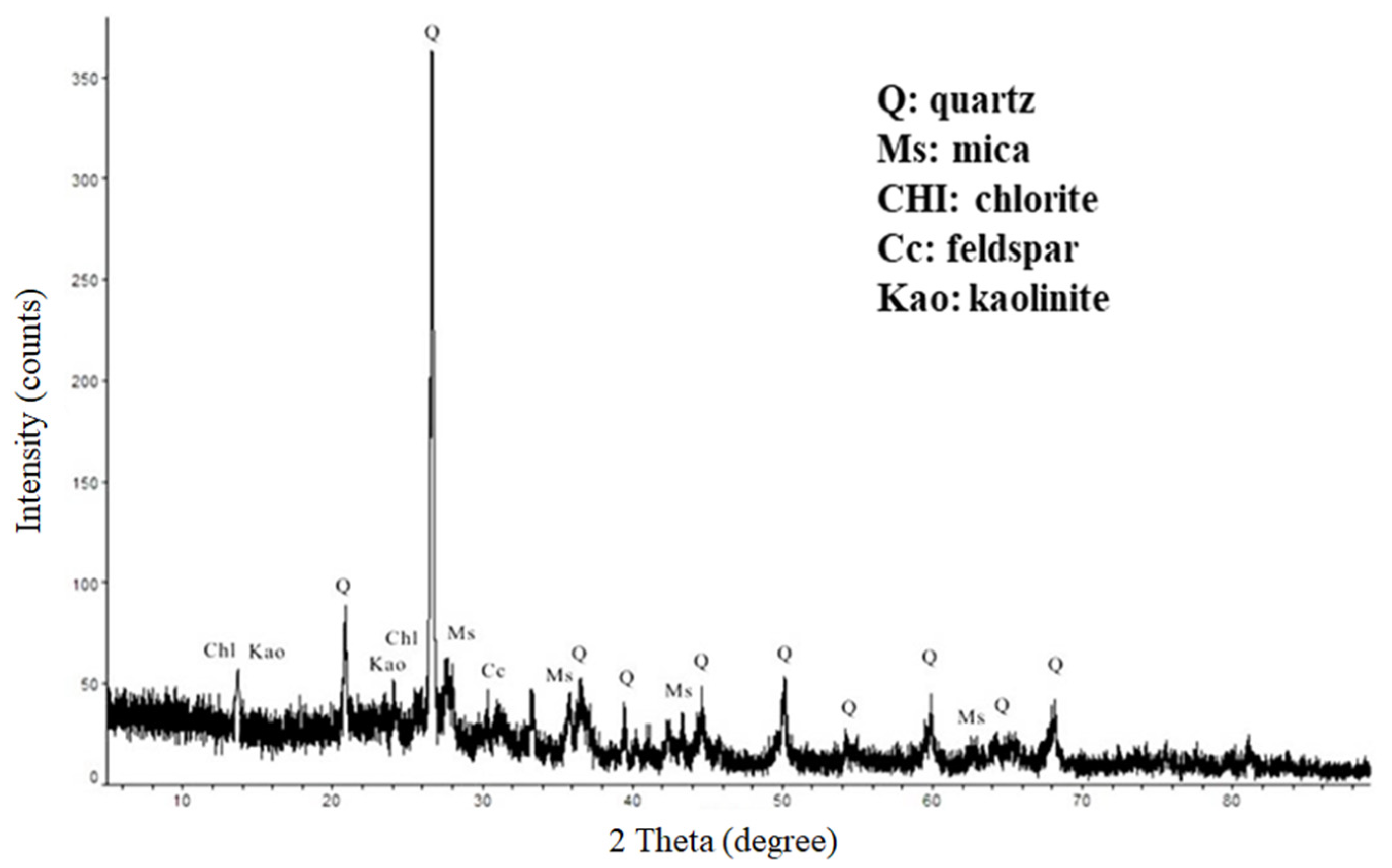
- According to the chemical composition and XRD analysis, drill cutting is mainly composed of SiO2, Al2O3, and Fe2O3 which are very similar to natural clay minerals, and can be used as raw material for building brick fabrication. In this study, building bricks were fabricated with drill cutting and met the CNS common brick standard using either high temperature sintering or LTGS process.
- For bricks prepared with the sintering process, the sintering temperature is the key factor affecting the physical and mechanical properties of the bricks. Low porosity and water absorption bricks can be produced at high sintering temperatures because the high temperature liquid phase can decrease in the pore volume, resulting in a denser surface. The compressive strength of the brick sintered at 1000 °C can reach 85.4 MPa.
- For building bricks fabricated with the LTGS process, it was found that the SiO2/Na2O modulus of alkali activator and temperature are two dominant factors affecting the properties of the brick. The LTGS brick made with 2.0 SiO2/Na2O alkali solution has the lowest porosity and highest mechanical strength due to complete geopolymerization of the drill cutting. The LTGS bricks fabricated at temperatures above 400 °C exhibit excellent physical and mechanical properties because of the strong geopolymeric cross-linking structure formed at higher temperatures.
- Building bricks fabricated with either the sintering process or LTGS process all meet CNS common brick rank #1 to rank #3 requirements. However, building bricks made with the LTGS process seem to be of a higher quality and are more cost effective than those made with the high temperature sintering process. The reutilization of drill cutting for building brick fabrication not only addresses some environmental issues but also elevate its application in a more cost effective and energy efficient manner.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CPC Corporation, Taiwan. Available online: http://www.cpc.com.tw/big5/home/index.asp (accessed on 20 January 2011).
- Chen, T.; Lin, S.; Lin, Z. An innovative utilization of drilling waste as building materials. In Proceedings of the SPE E&P Environmental and Safety Conference, Galveston, TX, USA, 5–7 March 2007. [Google Scholar]
- Willis, J.M.; Hester, M.W.; Shaffer, G.P. A mesocosm evaluation of processed drill cuttings for wetland restoration. Ecol. Eng. 2005, 25, 41–50. [Google Scholar] [CrossRef]
- Ji, G.D.; Yang, Y.S.; Zhou, Q.; Sun, T.; Ni, J.R. Phytodegradation of extra heavy oil-based drill cuttings using mature reed wetland: An in situ pilot study. Environ. Int. 2004, 30, 509–517. [Google Scholar] [CrossRef]
- Weiss, J.S.; Weiss, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Veil, J.A. Innovative Technologies For Managing Oil Field Waste. J. Energy Resour. Tech. 2003, 125, 238–248. [Google Scholar] [CrossRef]
- Bernardo, G.; Marroccoli, M.; Nobili, M.; Telesca, A.; Valenti, G. The use of oil well-derived drilling waste and electric arc furnace slag as alternative raw materials in clinker production. Resour. Conserv. Recycl. 2007, 52, 95–102. [Google Scholar] [CrossRef]
- Smith, M.; Manning, A.; Lang, M. Research on the Re-Use of Drill Cuttings Onshore; Report no. Cordah/COR012/1999; for Talisman Energy; Cordah Research Limited: Aberdeen, Scotland, 1999. [Google Scholar]
- Abbe, O.E.; Grimes, S.M.; Fowler, G.D.; Boccaccini, A.R. Novel sintered glass-ceramics from vitrified oil well drill cuttings. J. Mater. Sci. 2009, 44, 4296–4302. [Google Scholar] [CrossRef]
- Lin, D.F.; Luo, H.L.; Sheen. Y., N. Glazed tiles manufactured from incinerated sewage sludge ash and clay. J. Air Waste Manag. 2005, 55, 163–172. [Google Scholar] [CrossRef][Green Version]
- Lin, C.F.; Wu, C.H.; Ho, H.M. Recovery of municipal waste incineration bottom ash and water treatment sludge to water permeable pavement materials. Waste Manag. 2006, 26, 970–978. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Qi, J. Study on use of MSWI fly ash in ceramic tile. J. Hazard. Mater. 2007, 141, 106–114. [Google Scholar]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick materials. Adv. Environ. Res. 2003, 7, 679–685. [Google Scholar] [CrossRef]
- Chiang, K.Y.; Chien, K.L.; Hwang, S.J. Study on the characteristics of building bricks produced from reservoir sediment. J. Hazard. Mater. 2008, 159, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeoisa, M.; Maserib, F.; Rulmonta, A.; Clootsa, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; dl Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Komljenovi, M.; Bacarevi, Z.; Bradic, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Sindhunata, J.S.J.; Lukey, G.C.; Xu, H. Effect of curing temperature and silicate concentration on fly-ash-based geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; Riessen, A.V. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Buchwald, A.; Zellmann, H.D.; Kaps, C. Condensation of aluminosilicate gels—Model system for geopolymer binders. J. Non-Cryst. Solids 2011, 357, 1376–1382. [Google Scholar] [CrossRef]
- Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K.; Sato, T. Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mat. Sci. Eng. 2011, 528, 6616–6623. [Google Scholar] [CrossRef]
- Feng, D.; Provis, J.L.; van Deventer, J.S.J. Thermal activation of albite for the synthesis of one-part mix geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, A.; Palomo, G.C.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute France: Saint-Quentin, France, 2008. [Google Scholar]
- Xu, H.; van Deventer, J.S.J. Ab initio calculations on the five-membered alumino-silicateframe work rings model: Implications for dissolutionin alkaline solutions. Comput. Chem. Eng. 2000, 24, 391–404. [Google Scholar] [CrossRef]
- Tempest, B.; Sanusi, O.; Gergely, J.; Ogunro, V.; Weggel, D. Compressive strength and embodied energy optimization of fly ash based geopolymer concrete. In Proceedings of the World of Coal Ash Conference, Lexington, KY, USA, 5 May 2009; pp. 1–17. [Google Scholar]
- Ewelina, K.W.; Małolepszya, J.; Murzyn, P. Sintering Behavior of Kaolin with Calcite. Proc. Eng. 2013, 57, 572–582. [Google Scholar]
- Li, X.G.; Yang, L.V.; Ma, B.G.; Jian, S.W.; Tan, H.B. Influence of sintering temperature on the characteristics of shale brick containing oil well-derived drilling waste. Environ. Sci. Pollut. Res. 2011, 18, 1617–1622. [Google Scholar] [CrossRef]
- Lee, V.G.; Yeh, T.H. Sintering effects on the development of mechanical properties of fired clay ceramics. Mater. Sci. Eng. 2008, 485, 5–13. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Bakri, A.; Kamarudin, H.; Bnhussain, M.; Khairul Nizar, I.; Mastura, W.I.W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. J. Asian Sci. Res. 2011, 5, 247–253. [Google Scholar]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Gao, K.; Lin, K.L.; Wang, D.Y.; Hwang, C.L.; Shiu, H.S.; Chang, Y.M.; Cheng, T.W. Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr. Build. Mater. 2014, 52, 503–510. [Google Scholar] [CrossRef]
- Mbumbia, L.; Wilmars, A.M.; Tirlocq, J. Performance characteristics of lateritic soil bricks fired at low temperatures: A case study of Cameroon. Constr. Build. Mater. 2000, 14, 121–131. [Google Scholar] [CrossRef]

| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|
| wt.% | 47.3 | 20.1 | 14.4 | 3.7 | 2.8 | 1.7 | 1.5 |
| Sintering Temperature (℃) | Bulk Density (g/cm3) | Apparent (sp.gr.) | Porosity (%) | Water Absorption (%) | Shrinkage Rate (%) |
|---|---|---|---|---|---|
| 600 | - | - | - | - | 0.4 |
| 700 | 1.9 | 2.8 | 30.1 | 15.6 | 0.5 |
| 800 | 1.9 | 2.8 | 29.1 | 14.6 | 1.4 |
| 900 | 2.1 | 2.7 | 23.7 | 11.4 | 3.0 |
| 1000 | 2.2 | 2.7 | 17.7 | 8.1 | 5.2 |
| LTGS Bricks | SiO2/Na2O Modulus | Bulk Density (g/cm3) | Apparent (sp.gr.) | Porosity (%) | Water Absorption (%) | Shrinkage Rate (%) |
|---|---|---|---|---|---|---|
| DC0 | 0 | 1.9 | 2.8 | 33.2 | 17.9 | 0.3 |
| DC1 | 1 | 2.2 | 2.6 | 15.4 | 6.9 | 0.3 |
| DC2 | 2 | 2.3 | 2.7 | 13.9 | 6.0 | 0.3 |
| DC3.2 | 3.2 | 2.1 | 2.7 | 23.1 | 11.2 | 0.3 |
| LTGS Bricks | Sintering Temperature (℃) | Bulk Density (g/cm3) | Apparent (sp.gr.) | Porosity (%) | Water Absorption (%) | Shrinkage Rate (%) |
|---|---|---|---|---|---|---|
| DC100 | 100 | - | - | - | - | 0 |
| DC200 | 200 | - | - | - | - | 0.1 |
| DC300 | 300 | 2.2 | 2.8 | 22.4 | 10.5 | 0.2 |
| DC400 | 400 | 2.3 | 2.7 | 15.0 | 6.6 | 0.3 |
| DC500 | 5000 | 2.3 | 2.7 | 13.9 | 6.0 | 0.3 |
| Specifications | Rank #1 | Rank #2 | Rank #3 |
|---|---|---|---|
| Water absorption (%) | <10.0 | <13.0 | <15.0 |
| Compressive strength (MPa) | >30.0 | >20.0 | >15.0 |
| Sintering Bricks | CNS Rank | LTGS Bricks | CNS Rank | LTGS Bricks | CNS Rank |
|---|---|---|---|---|---|
| 800 °C | 3 | DC1 | 1 | DC300 | 2 |
| 900 °C | 2 | DC2 | 1 | DC400 | 1 |
| 1000 °C | 1 | DC3.2 | 2 | DC500 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Hsieh, Y.-C.; Wang, H.-W.; Ding, Y.-C.; Cheng, T.-W. Fabrication of Low-Temperature Sintering Building Bricks Using Drilling Cutting and Geopolymeric Technology. Materials 2021, 14, 5940. https://doi.org/10.3390/ma14205940
Lee W-H, Hsieh Y-C, Wang H-W, Ding Y-C, Cheng T-W. Fabrication of Low-Temperature Sintering Building Bricks Using Drilling Cutting and Geopolymeric Technology. Materials. 2021; 14(20):5940. https://doi.org/10.3390/ma14205940
Chicago/Turabian StyleLee, Wei-Hao, Yi-Che Hsieh, Hsin-Wen Wang, Yung-Chin Ding, and Ta-Wui Cheng. 2021. "Fabrication of Low-Temperature Sintering Building Bricks Using Drilling Cutting and Geopolymeric Technology" Materials 14, no. 20: 5940. https://doi.org/10.3390/ma14205940
APA StyleLee, W.-H., Hsieh, Y.-C., Wang, H.-W., Ding, Y.-C., & Cheng, T.-W. (2021). Fabrication of Low-Temperature Sintering Building Bricks Using Drilling Cutting and Geopolymeric Technology. Materials, 14(20), 5940. https://doi.org/10.3390/ma14205940








