Investigations of Structural and Electrical Properties of ALD Films Formed with the Ozone Precursor
Abstract
:1. Introduction
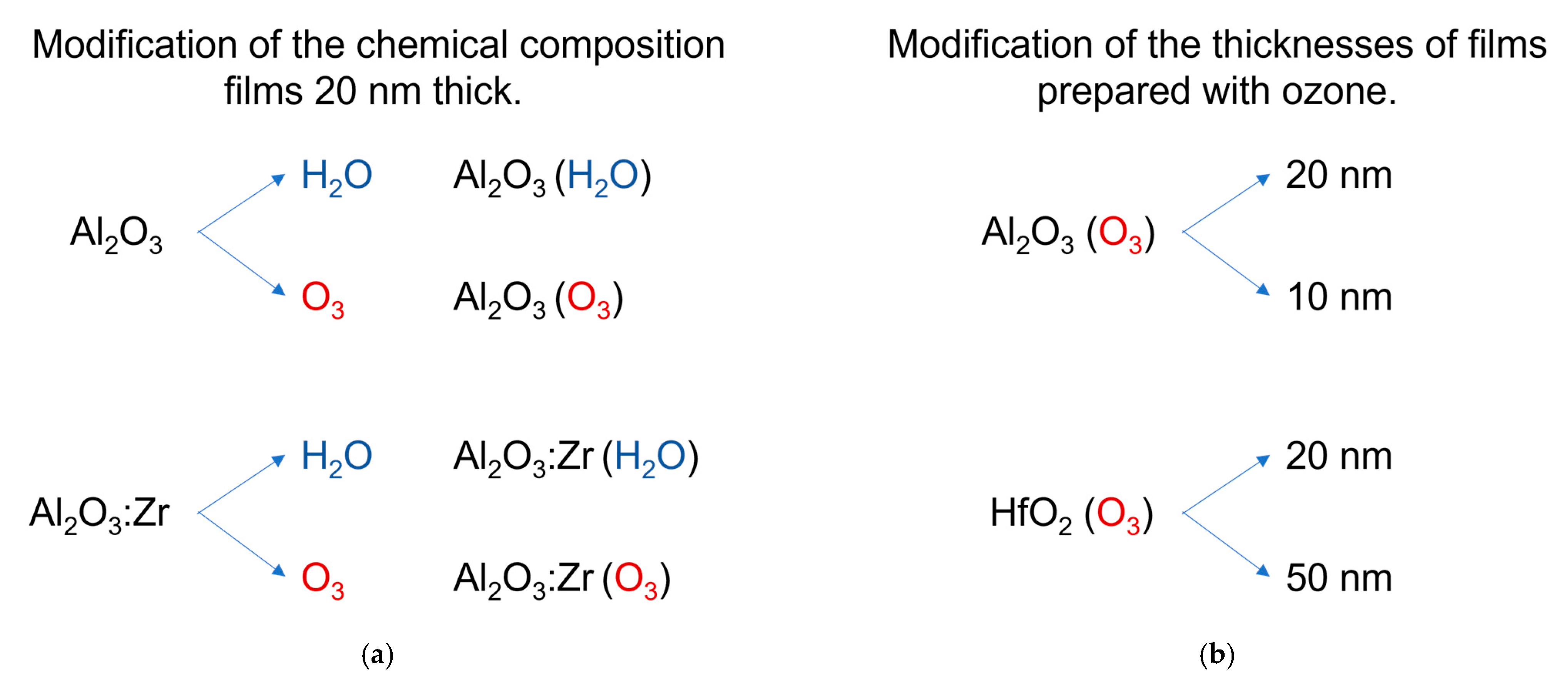
2. Materials and Methods
2.1. ALD of High-k Oxides
2.2. Structural Characterization of Dielectric Films
2.3. MIS Structures Fabrication and Electrical Characterization
3. Results and Discussion
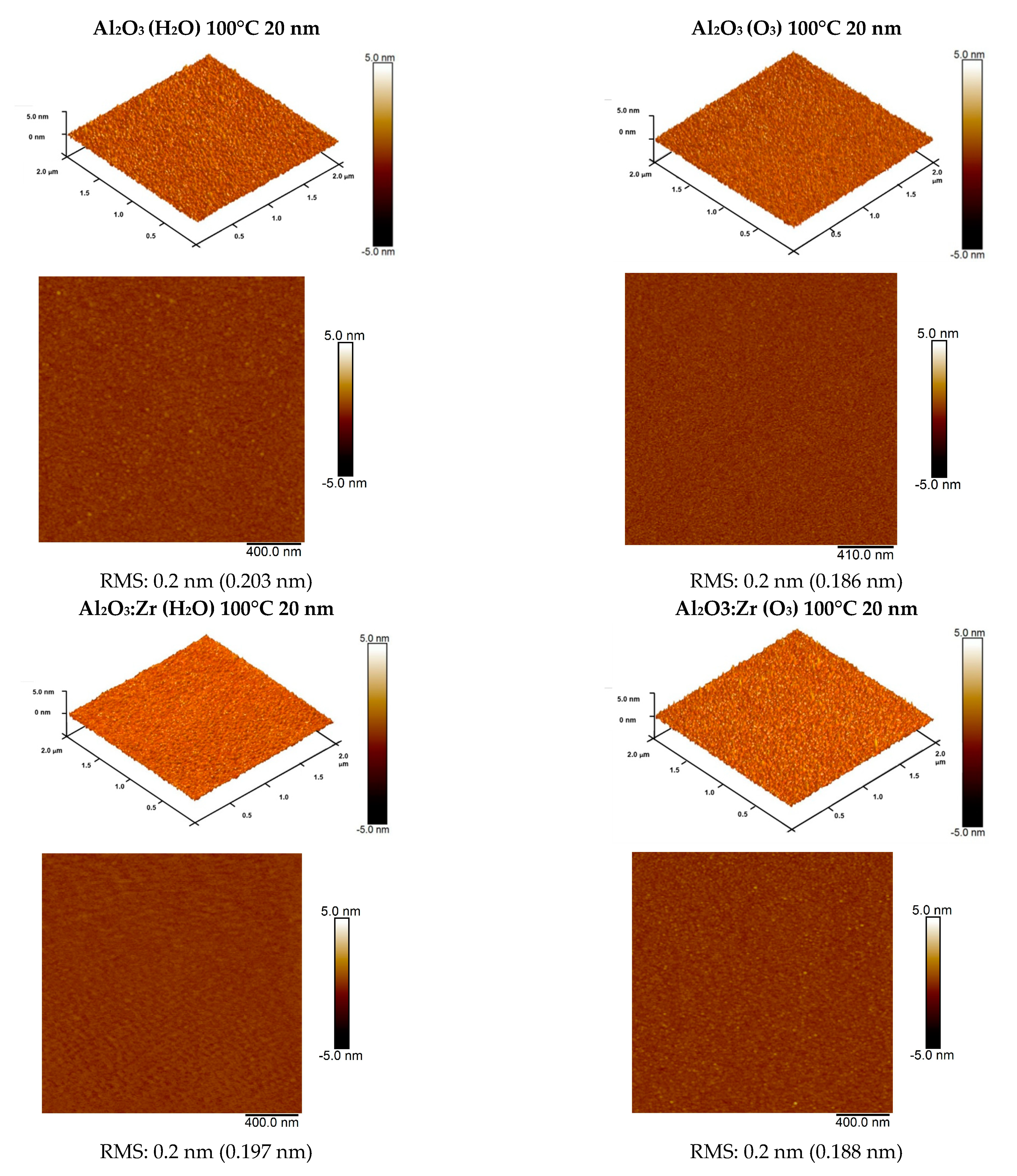
3.1. Structural Characterization of Investigated Samples
3.2. Electrical Characterization of MIS Structures with ALD Films as Gate-Dielectric Layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Crowell, J.E. Chemical methods of thin film deposition: Chemical vapor deposition, atomic layer deposition, and related technologies. J. Vac. Sci. Technol. A 2003, 21, S88–S95. [Google Scholar] [CrossRef]
- Mallick, B.C.; Hsieh, C.-T.; Yin, K.-M.; Gandomi, Y.A.; Huang, K.-T. Review—On Atomic Layer Deposition: Current Progress and Future Challenges. ECS J. Solid State Sci. Technol. 2019, 8, N55–N78. [Google Scholar] [CrossRef]
- Biercuk, M.; Monsma, D.J.; Marcus, C.M.; Becker, J.S.; Gordon, R.G. Low-temperature atomic-layer-deposition lift-off method for microelectronic and nanoelectronic applications. Appl. Phys. Lett. 2003, 83, 2405–2407. [Google Scholar] [CrossRef] [Green Version]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [Green Version]
- Gieraltowska, S.; Wachnicki, L.; Witkowski, B.S.; Mroczynski, R.; Dluzewski, P.; Godlewski, M. Characterization of dielectric layers grown at low temperature by atomic layer deposition. Thin Solid Film. 2015, 577, 97–102. [Google Scholar] [CrossRef]
- Choi, J.; Mao, Y.; Chang, J. Development of hafnium based high-k materials—A review. Mater. Sci. Eng. R Rep. 2011, 72, 97–136. [Google Scholar] [CrossRef]
- Bethge, O.; Abermann, S.; Henkel, C.; Bertagnolli, E. Low temperature atomic layer deposition of high-k dielectric stacks for scaled metal-oxide-semiconductor devices. Thin Solid Film. 2009, 517, 5543–5547. [Google Scholar] [CrossRef]
- Maikap, S.; Lee, H.Y.; Wang, T.-Y.; Tzeng, P.-J.; Wang, C.C.; Lee, L.S.; Liu, K.C.; Yang, J.-R.; Tsai, M.-J. Charge trapping characteristics of atomic-layer-deposited HfO2 films with Al2O3 as a blocking oxide for high-density non-volatile memory device applications. Semicond. Sci. Technol. 2007, 22, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Mroczyński, R.; Taube, A.; Gieraltowska, S.; Guziewicz, E.; Godlewski, M. Application of deposited by ALD HfO2 and Al2O3 layers in double-gate dielectric stacks for non-volatile semiconductor memory (NVSM) devices. Appl. Surf. Sci. 2012, 258, 8366–8370. [Google Scholar] [CrossRef]
- Leskelä, M.; Mattinen, M.; Ritala, M. Review Article: Atomic layer deposition of optoelectronic materials. J. Vac. Sci. Technol. B 2019, 37, 030801. [Google Scholar] [CrossRef] [Green Version]
- Mangote, B.; Gallais, L.; Zerrad, M.; Commandré, M.; Gao, L.H.; LeMarchand, F.; Lequime, M.; Melninkaitis, A.; Mirauskas, J.; Sirutkaitis, V.; et al. Study of the laser matter interaction in the femtosecond regime: Application to the analysis of the laser damage of optical thin films. SPIE Opt. Syst. Des. 2011, 8168, 816815. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Membranes: Basics, Challenges, and Opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Heikkinen, I.T.; Repo, P.; Vähänissi, V.; Pasanen, T.; Malinen, V.; Savin, H. Efficient surface passivation of black silicon using spatial atomic layer deposition. Energy Procedia 2017, 124, 282–287. [Google Scholar] [CrossRef]
- Caballero-Espitia, D.; Lizarraga, E.; Borbon-Nuñez, H.; Contreras-Lopez, O.; Tiznado, H.; Marquez, H. Study of Al2O3 thin films by ALD using H2O and O3 as oxygen source for waveguide applications. Opt. Mater. 2020, 109, 110370. [Google Scholar] [CrossRef]
- Dullweber, T.; Schmidt, J. Industrial Silicon Solar Cells Applying the Passivated Emitter and Rear Cell (PERC) Concept—A Review. IEEE J. Photovolt. 2016, 6, 1366–1381. [Google Scholar] [CrossRef]
- Pietruszka, R.; Witkowski, B.; Zielony, E.; Gwóźdź, K.; Placzek-Popko, E.; Godlewski, M. ZnO/Si heterojunction solar cell fabricated by atomic layer deposition and hydrothermal methods. Sol. Energy 2017, 155, 1282–1288. [Google Scholar] [CrossRef]
- von Gastrow, G.; Li, S.; Repo, P.; Bao, Y.; Putkonen, M.; Savin, H. Ozone-based Batch Atomic Layer Deposited Al2O3 for Effective Surface Passivation. Energy Procedia 2013, 38, 890–894. [Google Scholar] [CrossRef]
- Lüdera, T.; Lauermann, T.; Zuschlag, A.; Hahn, G.; Terheiden, B. Al2O3/SiNx-Stacks at Increased Temperatures: Avoiding Blistering During Contact Firing. Energy Procedia 2012, 27, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Rojas, D.; Maindron, T.; Esteve, A.; Piallat, F.; Kools, J.; Decams, J.-M. Speeding up the unique assets of atomic layer deposition. Mater. Today Chem. 2019, 12, 96–120. [Google Scholar] [CrossRef]
- Mahapatra, R.; Maikap, S.; Ray, S.K. Electrical properties of ultrathin HfO2 gate dielectrics on partially strain compensated SiGeC/Si heterostructures. J. Electroceramics 2006, 16, 545–548. [Google Scholar] [CrossRef]
- Kim, J.B.; Kwon, D.R.; Chakrabarti, K.; Lee, C.; Oh, K.Y.; Lee, J.H. Improvement in Al2O3 dielectric behavior by using ozone as an oxidant for the atomic layer deposition technique. J. Appl. Phys. 2002, 92, 6739–6742. [Google Scholar] [CrossRef]
- Antson, J. United States Patent (19). Geothermics 1977, 14, 595–599. [Google Scholar]
- Puurunen, R.L. A Short History of Atomic Layer Deposition: Tuomo Suntola’s Atomic Layer Epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Seweryn, A.; Pietruszka, R.; Witkowski, B.S.; Wierzbicka, A.; Jakiela, R.; Sybilski, P.; Godlewski, M. Structural and electrical parameters of ZnO thin films grown by ALD with either water or ozone as oxygen precursors. Crystals 2019, 9, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Seweryn, A.; Pielok, A.; Lawniczak-Jablonska, K.; Pietruszka, R.; Marcinkowska, K.; Sikora, M.; Witkowski, B.S.; Godlewski, M.; Marycz, K.; Śmieszek, A. Zirconium Oxide Thin Films Obtained by Atomic Layer Deposition Technology Abolish the Anti-Osteogenic Effect Resulting from miR-21 Inhibition in the Pre-Osteoblastic MC3T3 Cell Line. Int. J. Nanomed. 2020, 15, 1595–1610. [Google Scholar] [CrossRef] [Green Version]
- Mroczyński, R.; Iwanicki, D.; Fetliński, B.; Ożga, M.; Świniarski, M.; Gertych, A.; Zdrojek, M.; Godlewski, M. Optimization of Ultra-Thin Pulsed-DC Magnetron Sputtered Aluminum Films for the Technology of Hyperbolic Metamaterials. Crystal 2020, 10, 384. [Google Scholar] [CrossRef]
- Belosludtsev, A.; Yakimov, Y.; Mroczyński, R.; Stanionytė, S.; Skapas, M.; Buinovskis, D.; Kyžas, N. Effect of Annealing on Optical, Mechanical, Electrical Properties and Structure of Scandium Oxide Films. Phys. Status Solidi A 2019, 216, 1–7. [Google Scholar] [CrossRef]
- Lawniczak-Jablonska, K.; Zytkiewicz, Z.R.; Gieraltowska, S.; Sobanska, M.; Kuzmiuk, P.; Klosek, K. Chemical bonding of nitrogen formed by nitridation of crystalline and amorphous aluminum oxide studied by X-ray photoelectron spectroscopy. RSC Adv. 2020, 10, 27932–27939. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wu, L.Q.; Li, Y.C.; Li, S.Q.; Li, Z.Z.; Tang, G.D.; Qi, W.H.; Xue, L.C.; Ge, X.S.; Ding, L.L. Method for estimating ionicities of oxides using O1s photoelectron spectra. AIP Adv. 2015, 5, 097210. [Google Scholar] [CrossRef]
- Crist, B.V. Handbooks of Monochromatic XPS Spectra; XPS International LLC.: Mountain View, CA, USA, 2005; Volume 2. [Google Scholar]
- Gieraltowska, S.; Sztenkiel, D.; Guziewicz, E.; Godlewski, M.; Łuka, G.; Witkowski, B.; Wachnicki, Ł.; Łusakowska, E.; Dietl, T.; Sawicki, M. Properties and Characterization of ALD Grown Dielectric Oxides for MIS Structures. Acta Phys. Pol. A 2011, 119, 692–695. [Google Scholar] [CrossRef]
- Mroczyński, R.; Beck, R.B. Reliability issues of double gate dielectric stacks based on hafnium dioxide (HfO2) layers for non-volatile semiconductor memory (NVSM) applications. Microelectron. Reliab. 2012, 52, 107–111. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.; Anthony, J.M. High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243–5275. [Google Scholar] [CrossRef]
- Reklaitis, I.; Radiunas, E.; Malinauskas, T.; Stanionytė, S.; Juška, G.; Ritasalo, R.; Pilvi, T.; Taeger, S.; Strassburg, M.; Tomašiūnas, R. A comparative study on atomic layer deposited oxide film morphology and their electrical breakdown. Surf. Coat. Technol. 2020, 399, 126123. [Google Scholar] [CrossRef]
- Groner, M.; Elam, J.; Fabreguette, F.; George, S. Electrical characterization of thin Al2O3 films grown by atomic layer deposition on silicon and various metal substrates. Thin Solid Film. 2002, 413, 186–197. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant gate oxides for metal oxide Si transistors. Rep. Prog. Phys. 2005, 69, 327–396. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant oxides. Eur. Phys. J. Appl. Phys. 2004, 28, 265–291. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.H.; Buchanan, D.A.; Taur, Y.; Wang, W. Quantum-mechanical modeling of electron tunneling current from the inversion layer of ultra-thin-oxide nMOSFET’s. IEEEE Electron Device Lett. 1997, 18, 209–211. [Google Scholar] [CrossRef]
- Houssa, M. High-k Gate Dielectrics; Institute of Physics Publishing: Bristol, UK, 2004. [Google Scholar]
- Hori, T. Gate Dielectrics and MOS ULSIs: Principles, Technologies, and Applications; Springer: Berlin, Germany, 1997. [Google Scholar]
- Natori, K.; Otani, D.; Sano, N. Thickness dependence of the effective dielectric constant in a thin film capacitor. Appl. Phys. Lett. 1998, 73, 632–634. [Google Scholar] [CrossRef] [Green Version]
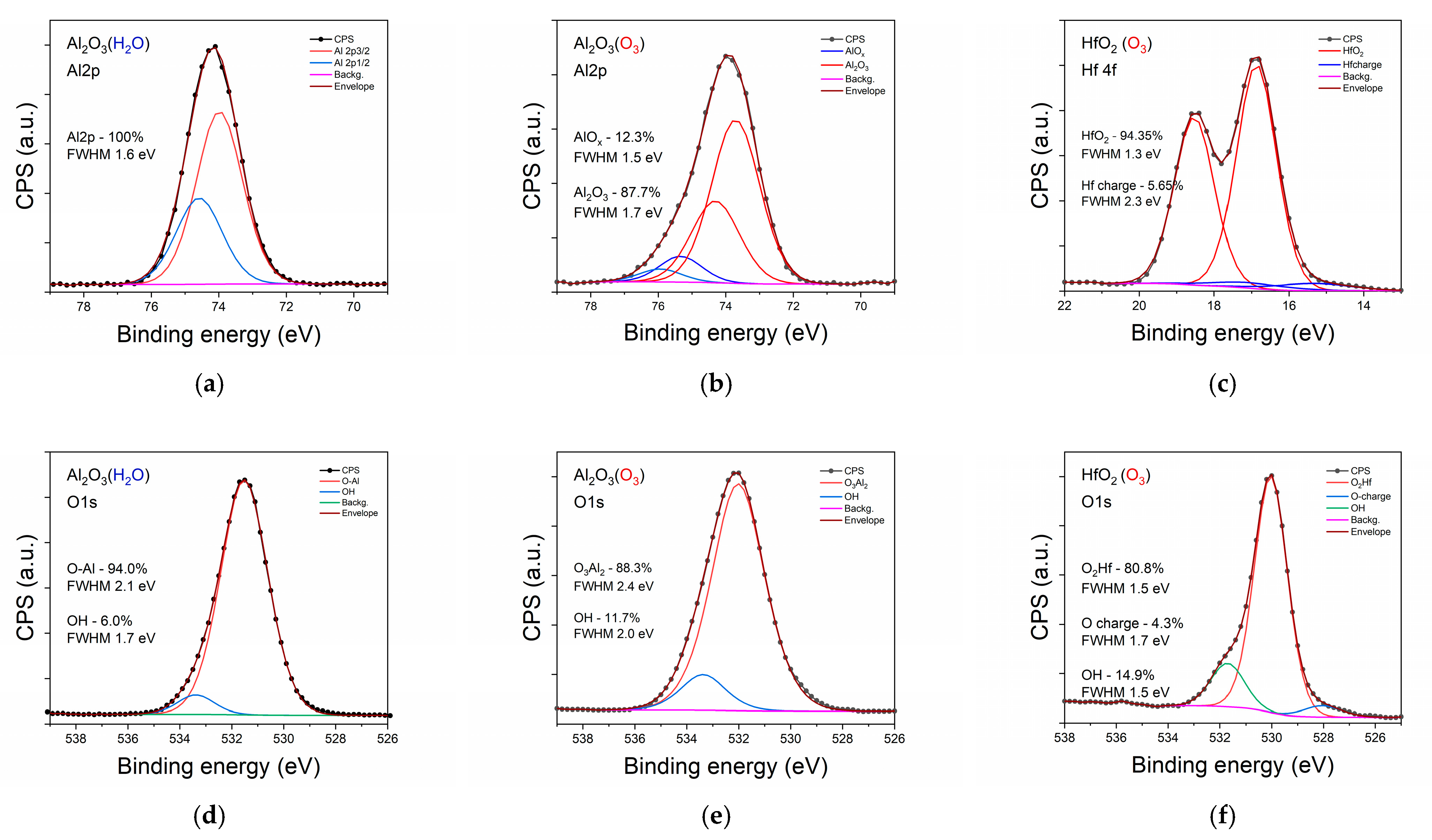
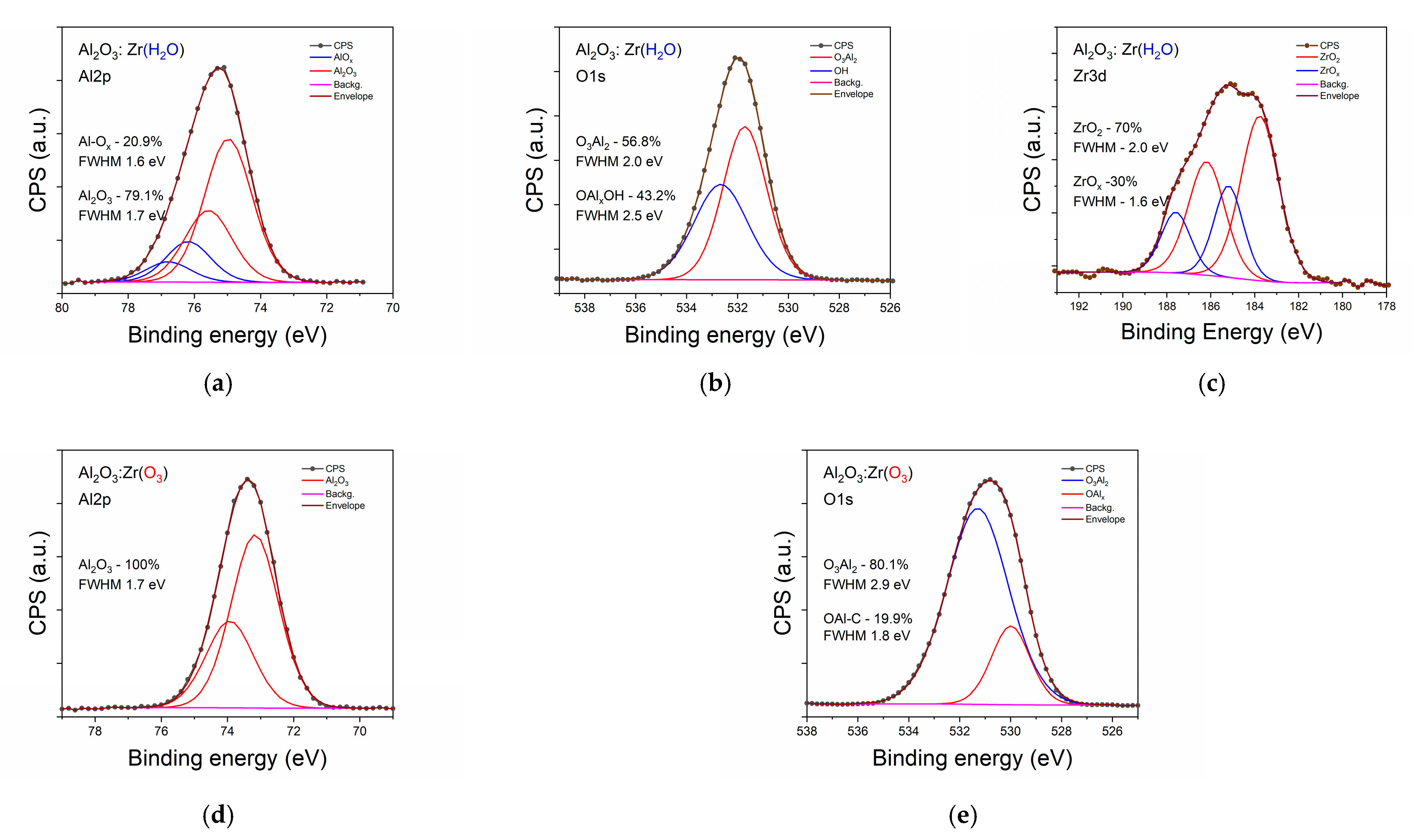

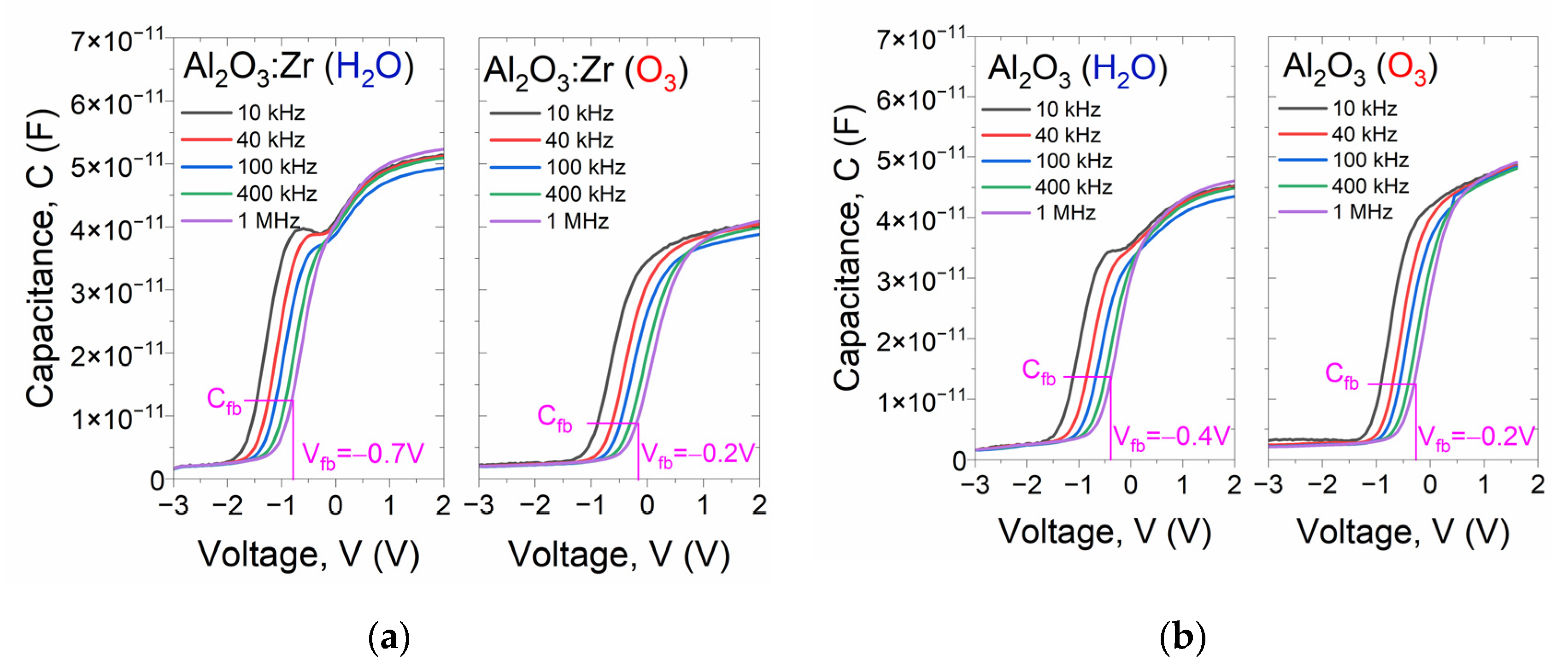
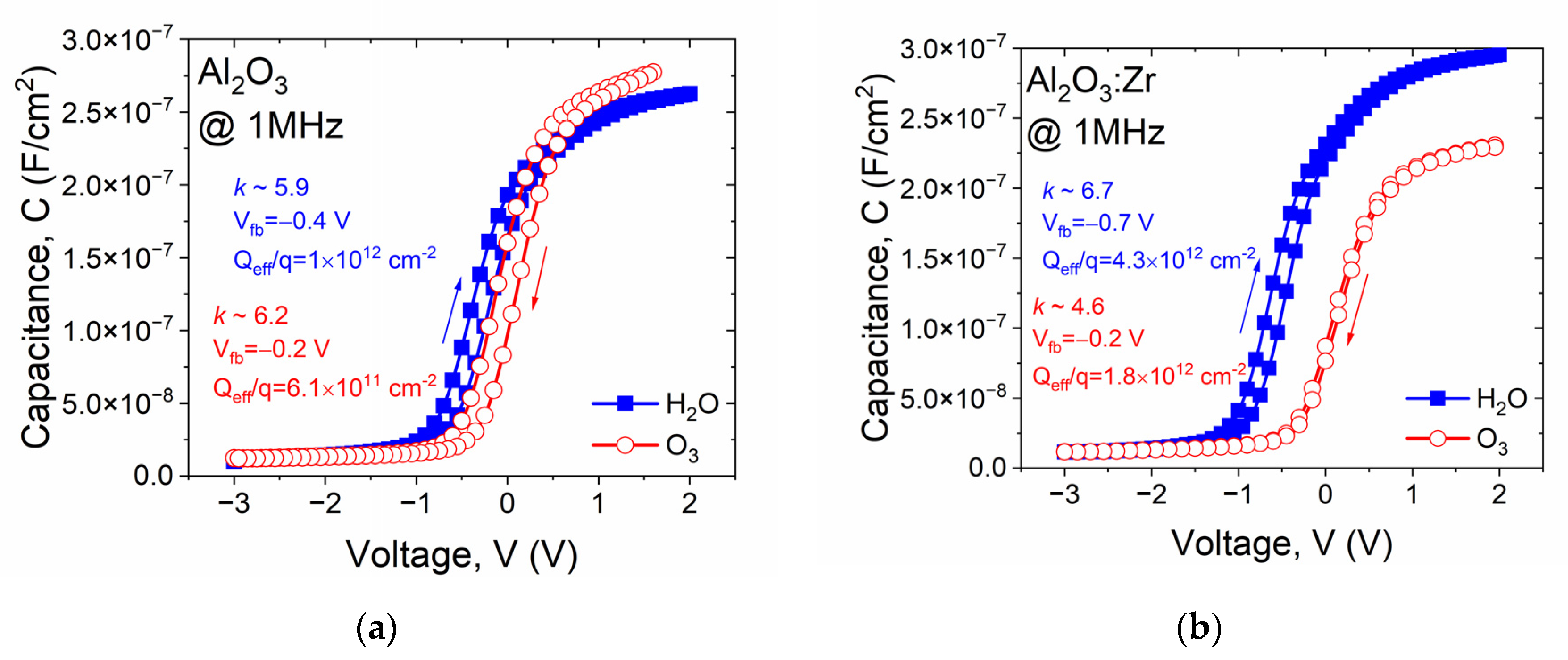
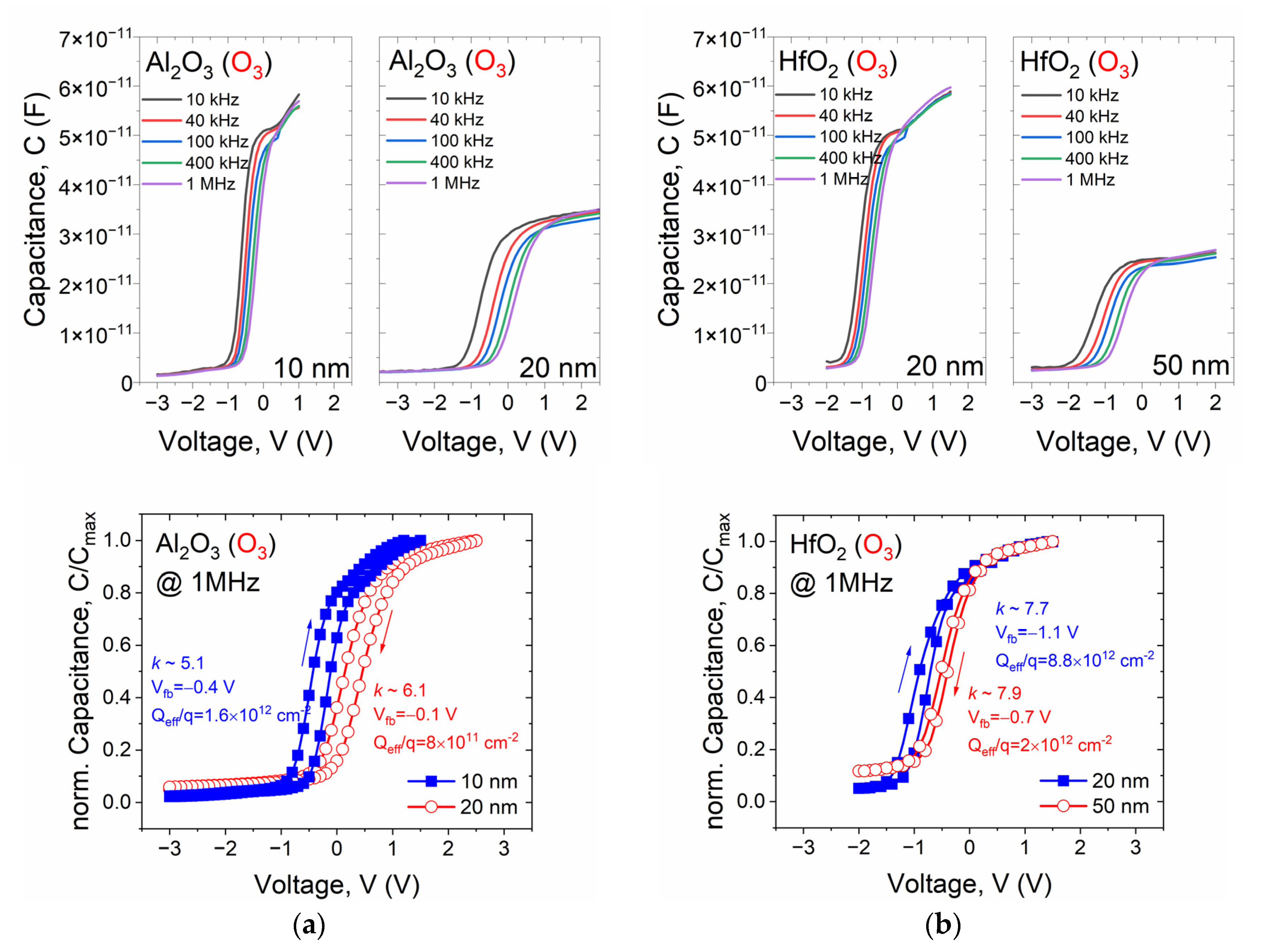
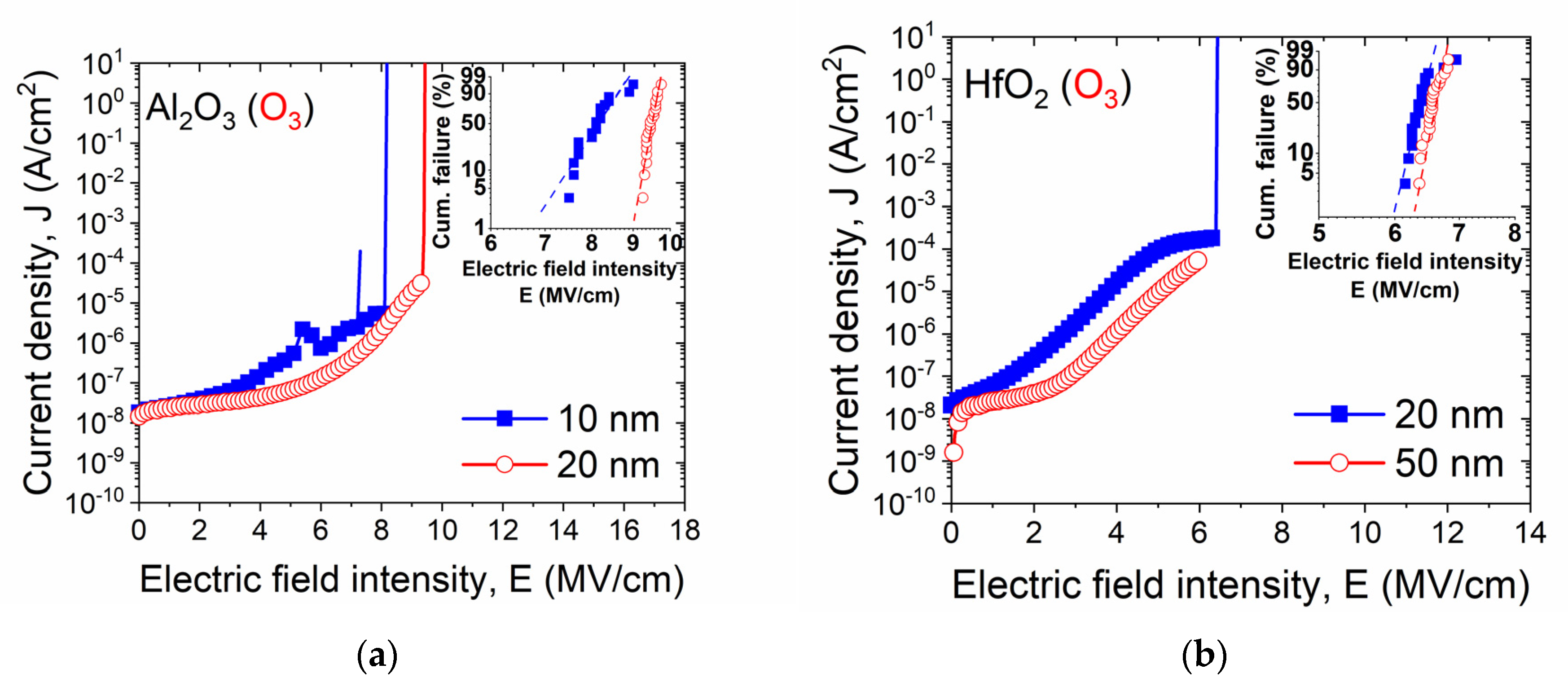
| Sample | Csurface | Cbulk | Osurface | Obulk | Al(Hf)surface | Al(Hf)bulk | Zrsurface | Zrbulk |
|---|---|---|---|---|---|---|---|---|
| Al2O3 (H2O) | 14.2 | 4.9 | 48.5 | 53.7 | 37.3 Al/O = 0.77 | 41.4 Al/O = 0.77 | ||
| Al2O3 (O3) | 20.5 | 4.8 | 46.8 | 54.9 | 32.7 Al/O = 0.70 | 40.3 Al/O = 0.73 | ||
| Al2O3:Zr (H2O) | 18.6 | 4.5 | 46.8 | 52.7 | 33.3 Al/O = 0.71 | 40.6 Al/O = 0.77 | 1.3 | 2.2 |
| Al2O3:Zr (O3) | 15.6 | 5.9 | 50.5 | 55.8 | 34.0 Al/O = 0.67 | 38.3 Al/O = 0.69 | 0 | |
| HfO2 (O3) | 33.9 | 4.5 | 46.0 | 63.7 | 20.1 Hf/O = 0.44 | 31.8 Hf/O = 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seweryn, A.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; Gieraltowska, S.; Godlewski, M.; Mroczynski, R. Investigations of Structural and Electrical Properties of ALD Films Formed with the Ozone Precursor. Materials 2021, 14, 5395. https://doi.org/10.3390/ma14185395
Seweryn A, Lawniczak-Jablonska K, Kuzmiuk P, Gieraltowska S, Godlewski M, Mroczynski R. Investigations of Structural and Electrical Properties of ALD Films Formed with the Ozone Precursor. Materials. 2021; 14(18):5395. https://doi.org/10.3390/ma14185395
Chicago/Turabian StyleSeweryn, Aleksandra, Krystyna Lawniczak-Jablonska, Piotr Kuzmiuk, Sylwia Gieraltowska, Marek Godlewski, and Robert Mroczynski. 2021. "Investigations of Structural and Electrical Properties of ALD Films Formed with the Ozone Precursor" Materials 14, no. 18: 5395. https://doi.org/10.3390/ma14185395
APA StyleSeweryn, A., Lawniczak-Jablonska, K., Kuzmiuk, P., Gieraltowska, S., Godlewski, M., & Mroczynski, R. (2021). Investigations of Structural and Electrical Properties of ALD Films Formed with the Ozone Precursor. Materials, 14(18), 5395. https://doi.org/10.3390/ma14185395







