Reduction of Thermal Conductivity for Icosahedral Al-Cu-Fe Quasicrystal through Heavy Element Substitution
Abstract
:1. Introduction
- (i)
- (ii)
- (iii)
- The constituent elements are nontoxic, readily available, and show favorable costs for industrial use [11].
2. Experimental Procedure and Sample Characterization
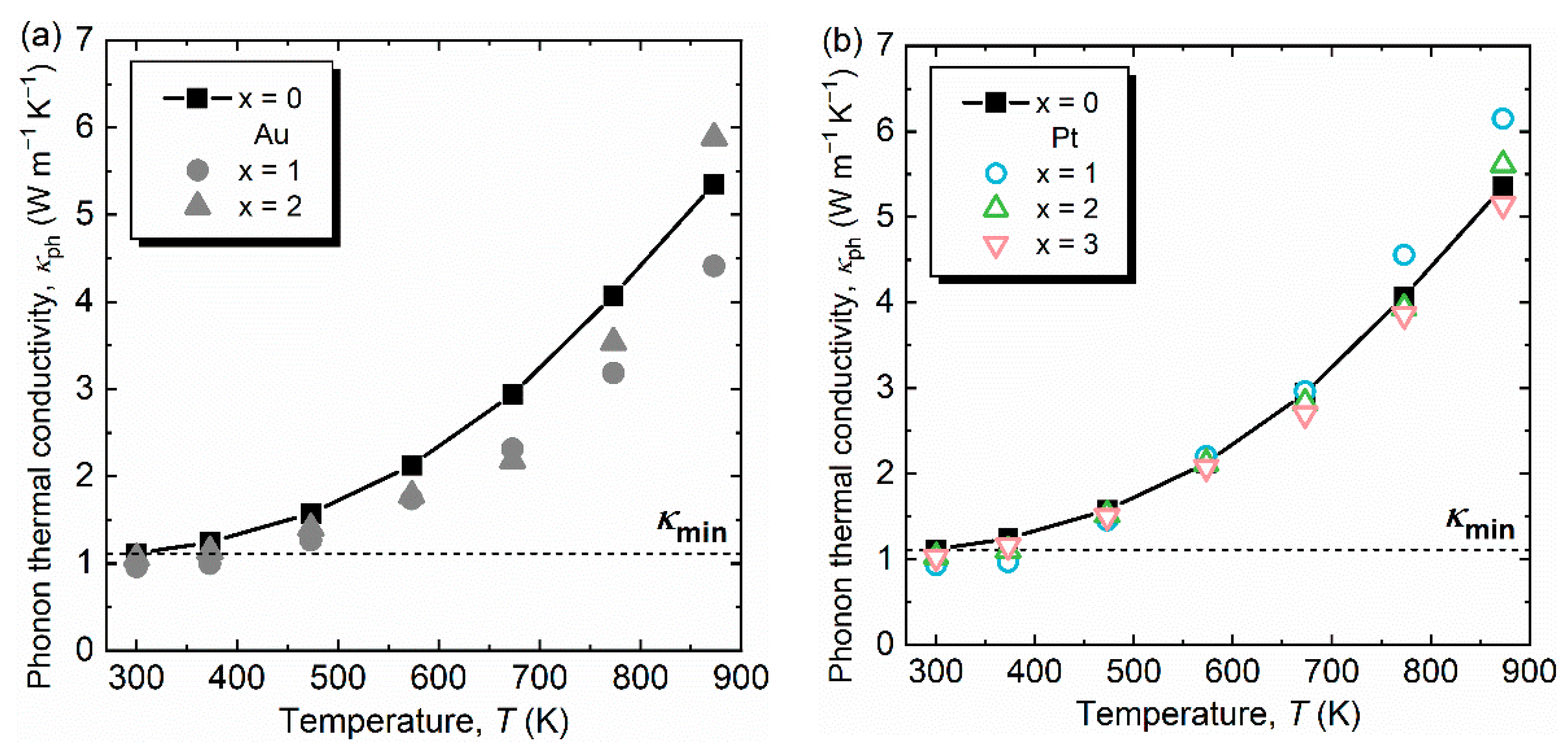
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Dresselhaus, M. Nanostructured thermoelectric materials. In Thermoelectrics and Its Energy Harvesting: Modules, Systems, and Applications in Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 1. [Google Scholar]
- Takagiwa, Y.; Kimura, K. Metallic-covalent bonding conversion and thermoelectric properties of Al-based icosahedral quasicrystals and approximants. Sci. Technol. Adv. Mater. 2014, 15, 044802. [Google Scholar] [CrossRef] [Green Version]
- Takagiwa, Y.; Kamimura, T.; Hosoi, S.; Okada, J.T.; Kimura, K. Thermoelectric properties of polygrained icosahedral Al71−xGaxPd20Mn9 (x = 0, 2, 3, 4) quasicrystals. J. Appl. Phys. 2008, 104, 073721. [Google Scholar] [CrossRef]
- Takagiwa, Y.; Kamimura, T.; Okada, J.T.; Kimura, K. Thermoelectric Properties of Icosahedral Al-Pd-(Mn or Re) Quasicrystals: Improvement of the ZT Value by Ga Substitution for Al Atoms. J. Electron. Mater. 2010, 39, 1885–1889. [Google Scholar] [CrossRef]
- Pierce, F.S.; Bancel, P.A.; Biggs, B.D.; Guo, Q.; Poon, S.J. Composition dependence of the electronic properties of Al-Cu-Fe and Al-Cu-Ru-Si semimetallic quasicrystals. Phys. Rev. B 1993, 47, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Bilušić, A.; Smontara, A.; Lasjaunias, J.C.; Ivkov, J.; Calvayrac, Y. Thermal and thermoelectric properties of icosahedral Al62Cu25.5Fe12.5 quasicrystal. Mater. Sci. Eng. A 2020, 294–296, 711–714. [Google Scholar] [CrossRef]
- Bilušić, A.; Pavuna, D.; Smontara, A. Figure of merit of quasicrystals: The case of Al-Cu-Fe. Vacuum 2001, 61, 345–348. [Google Scholar] [CrossRef]
- Nakayama, R.; Takeuchi, T. Thermal Rectification in Bulk Material Through Unusual Behavior of Electron Thermal Conductivity of Al-Cu-Fe Icosahedral Quasicrystal. J. Electron. Mater. 2015, 44, 356–361. [Google Scholar] [CrossRef]
- Dolinšek, J.; Vrtnik, S.; Klanjšek, M.; Jagličić, Z.; Smontara, A.; Smiljanić, I.; Bilušić, A.; Yokoyama, Y.; Inoue, A.; Landauro, C.V. Intrinsic electrical, magnetic, and thermal properties of single-crystalline Al64Cu23Fe13 icosahedral quasicrystal: Experiment and modeling. Phys. Rev. B 2007, 76, 054201. [Google Scholar] [CrossRef] [Green Version]
- Hurd, A.J.; Kelley, R.L.; Eggert, R.G.; Lee, M.-H. Energy-critical elements for sustainable development. MRS Bull. 2012, 37, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T. Very large thermal rectification in bulk composites consisting partly of icosahedral quasicrystals. Sci. Technol. Adv. Mater. 2014, 15, 064801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, T.; Kirihara, K.; Kimura, K. Effect of Ru substitution for Re on the thermoelectric properties of AlPdRe icosahedral quasicrystals. J. Appl. Phys. 2003, 94, 6560–6565. [Google Scholar] [CrossRef]
- Okada, J.T.; Hamamatsu, T.; Hosoi, S.; Nagata, T.; Kimura, K. Improvement of thermoelectric properties of icosahedral AlPdRe quasicrystals by Fe substitution for Re. J. Appl. Phys. 2007, 101, 103702. [Google Scholar] [CrossRef]
- Edagawa, K.; Kajiyama, K.; Tamura, R.; Takeuchi, S. High-temperature specific heat of quasicrystals and a crystal approximant. Mater. Sci. Eng. A 2001, 312, 293–298. [Google Scholar] [CrossRef]
- Prekul, A.F.; Kazantsev, V.A.; Shchegolikhina, N.I.; Gulyaeva, R.I.; Edagawa, K. High-Temperature Heat Capacity of the Al63Cu25Fe12 Quasicrystal. Phys. Solid State 2008, 50, 2013–2015. [Google Scholar] [CrossRef]
- Tamura, S.; Fukushima, K.; Tokumoto, Y.; Takagiwa, Y.; Edagawa, K. High-Temperature Specific Heat of Al-Cu-Ru Icosahedral Quasicrystals and 1/1 Crystal Approximants. Mater. Trans. 2021, 62, 356–359. [Google Scholar] [CrossRef]
- Fukushima, K.; Suyama, H.; Tokumoto, Y.; Kamimura, Y.; Takagiwa, Y.; Edagawa, K. Comparative study of high-temperature specific heat for Al-Pd-Mn icosahedral quasicrystals and crystal approximants. J. Phys. Commun. 2021, 5, 085002. [Google Scholar] [CrossRef]
- Takeuchi, T. Unusual Increase of Electron Thermal Conductivity Caused by a Pseudogap at the Fermi Level. J. Electron. Mater. 2009, 38, 1354–1359. [Google Scholar] [CrossRef]
- Kirihara, K.; Kimura, K. Composition dependence of thermoelectric properties of AlPdRe icosahedral quasicrystals. J. Appl. Phys. 2002, 92, 979–985. [Google Scholar] [CrossRef]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, U.; Sato, H.; Inukai, M.; Zijlstra, E.S. e/a determination for 4d- and 5d-transition metal elements and their intermetallic compounds with Mg, Al, Zn, Cd and In. Philos. Mag. 2013, 93, 3353–3390. [Google Scholar] [CrossRef]
- Maciá, E.; Rodríguez-Oliveros, R. Theoretical assessment on the validity of the Wiedemann–Franz law for icosahedral quasicrystals. Phys. Rev. B 2007, 75, 104210. [Google Scholar] [CrossRef] [Green Version]
- Cahill, D.G.; Pohl, R.O. Heat flow and lattice vibrations in glasses. Solid State Commun. 1989, 70, 927–930. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131–6140. [Google Scholar] [CrossRef] [PubMed]
- Kanatzidis, M.G. Nanostructured Thermoelectrics: The New Paradigm? Chem. Mater. 2010, 22, 648–659. [Google Scholar] [CrossRef]
- Takagiwa, Y.; Kimura, K.; Sawama, K.; Hiroto, T.; Nishio, K.; Tamura, R. Thermoelectric properties of Tsai-type Au-Al-RE (RE: Yb, Tm, Gd) quasicrystals and approximants. J. Alloys Compd. 2015, 652, 139–144. [Google Scholar] [CrossRef]
- Ishikawa, A.; Takagiwa, Y.; Kimura, K.; Tamura, R. Probing the pseudogap via thermoelectric properties in the Au-Al-Gd quasicrystal approximant. Phys. Rev. B 2017, 95, 104201. [Google Scholar] [CrossRef]
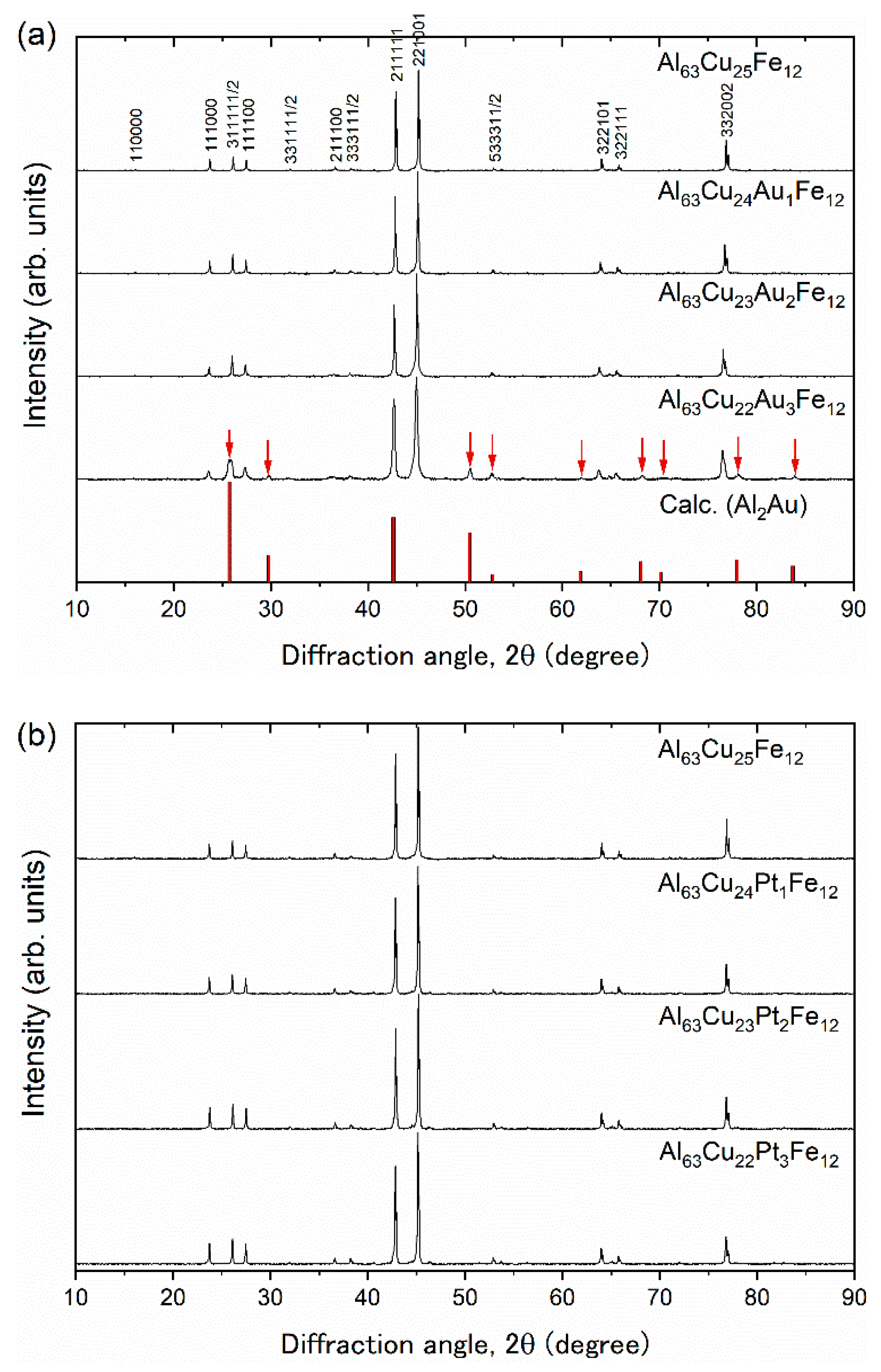
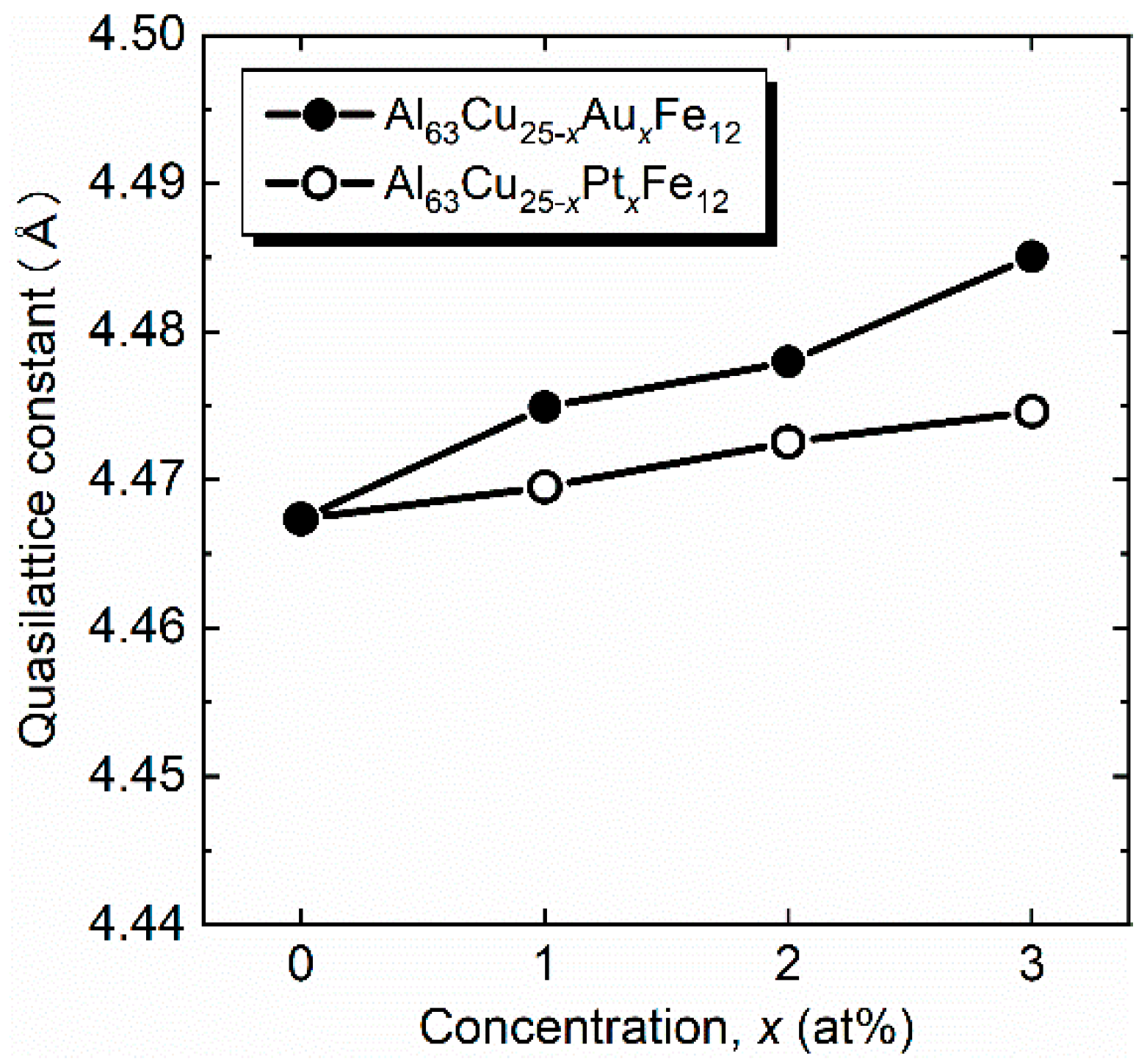
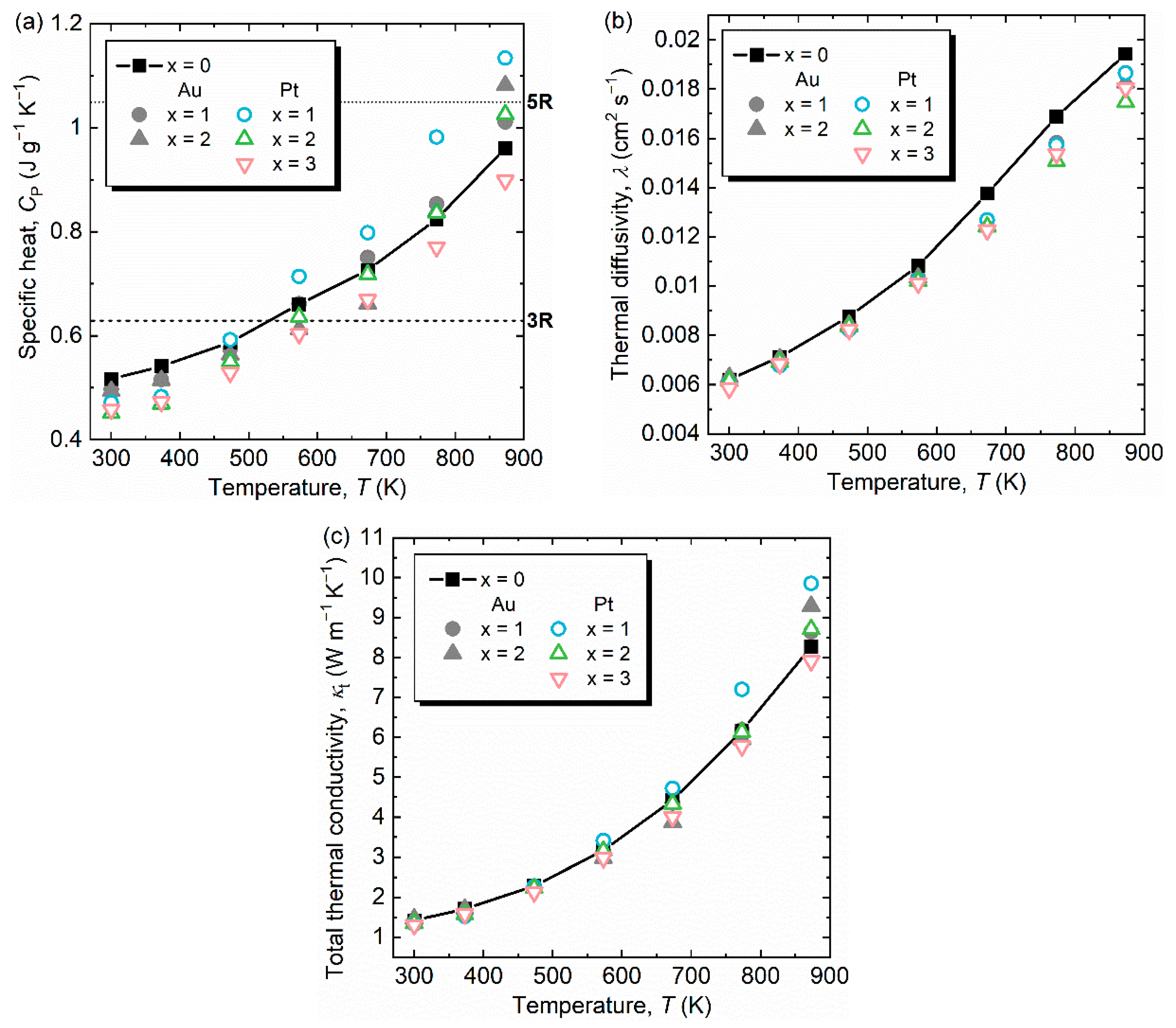
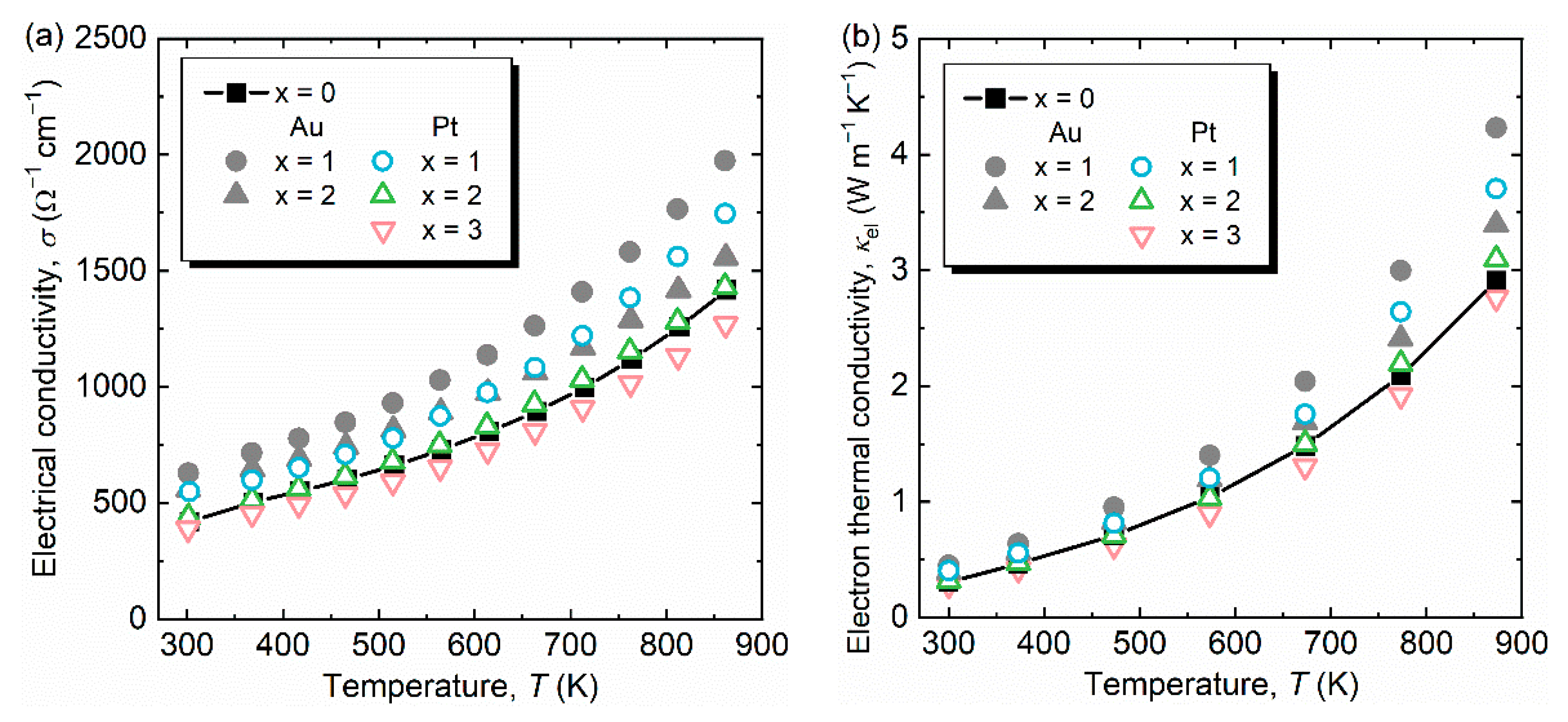

| Samples | Consodidating Temperature (K) | Bulk Density (g cm−3) | Crystalline Size (Å) |
|---|---|---|---|
| Al63Cu25Fe12 | 898 | 4.43 | 1256(62) |
| Al63Cu24Au1Fe12 | 1013 | 4.58 | 1090(58) |
| Al63Cu23Au2Fe12 | 1018 | 4.70 | 824(70) |
| Al63Cu22Au3Fe12 | 1033 | 4.80 | 379(13) |
| Al63Cu24Pt1Fe12 | 948 | 4.66 | 1062(16) |
| Al63Cu23Pt2Fe12 | 968 | 4.86 | 979(17) |
| Al63Cu22Pt3Fe12 | 1083 | 4.88 | 974(16) |
| Nominal Compositions | Phase | ICP Analysis of Chemical Composition |
|---|---|---|
| Al63Cu24Au1Fe12 | i | Al63.1Cu24.2Au0.9Fe11.8 |
| Al63Cu24Au2Fe12 | i | Al64Cu22.4Au1.9Fe11.7 |
| Al63Cu22Au3Fe12 | i + Al2Au | Al65.7Cu21.2Au1.7Fe11.4 |
| Al63Cu24Pt1Fe12 | i | Al63.2Cu24.2Pt0.9Fe11.3 |
| Al63Cu24Pt2Fe12 | i | Al63.2Cu23.8Pt1.7Fe11.3 |
| Al63Cu22Pt3Fe12 | i | Al63.1Cu22.3Pt2.6Fe12 |
| Samples | κt,300K | Δκt,300K/κt,300K | κph,300K | Δκph,300K/κph,300K |
| (W m−1 K−1) | (%) | (W m−1 K−1) | (%) | |
| x = 0 | 1.42 | - | 1.12 | - |
| Au: x = 1 | 1.41 | −0.7 | 0.96 | −14.3 |
| Au: x = 2 | 1.46 | 2.8 | 1.06 | −5.4 |
| Pt: x = 1 | 1.33 | −6.3 | 0.93 | −17.0 |
| Pt: x = 2 | 1.35 | −4.9 | 1.03 | −8.0 |
| Pt: x = 3 | 1.31 | −7.7 | 1.03 | −8.0 |
| Samples | ΔCP,300K/CP,300K | Δvs/vs | κmin,300K | |
| (%) | (%) | (W m−1 K−1) | ||
| x = 0 | - | - | 1.11 | |
| Au: x = 1 | −3.9 | −0.2 | 1.11 | |
| Au: x = 2 | −4.5 | −5.2 | 1.08 | |
| Pt: x = 1 | −8.7 | −0.2 | 1.13 | |
| Pt: x = 2 | −13 | −2.3 | 1.12 | |
| Pt: x = 3 | −11 | 0 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagiwa, Y.; Maeda, R.; Ohhashi, S.; Tsai, A.-P. Reduction of Thermal Conductivity for Icosahedral Al-Cu-Fe Quasicrystal through Heavy Element Substitution. Materials 2021, 14, 5238. https://doi.org/10.3390/ma14185238
Takagiwa Y, Maeda R, Ohhashi S, Tsai A-P. Reduction of Thermal Conductivity for Icosahedral Al-Cu-Fe Quasicrystal through Heavy Element Substitution. Materials. 2021; 14(18):5238. https://doi.org/10.3390/ma14185238
Chicago/Turabian StyleTakagiwa, Yoshiki, Ryota Maeda, Satoshi Ohhashi, and An-Pang Tsai. 2021. "Reduction of Thermal Conductivity for Icosahedral Al-Cu-Fe Quasicrystal through Heavy Element Substitution" Materials 14, no. 18: 5238. https://doi.org/10.3390/ma14185238
APA StyleTakagiwa, Y., Maeda, R., Ohhashi, S., & Tsai, A.-P. (2021). Reduction of Thermal Conductivity for Icosahedral Al-Cu-Fe Quasicrystal through Heavy Element Substitution. Materials, 14(18), 5238. https://doi.org/10.3390/ma14185238






