On the Electrochemical Migration Mechanism of Gold in Electronics—Less Reliable Than Expected?
Abstract
:1. Introduction
- Primary negative complex ion formation by anodic corrosion induced by halogen contaminants (see Equation (1)).
- A multi-step chemical process resulting in metal ions or secondary complex cations (Equations (2) and (3)).
- Cation migration through the electrolyte under the electrical field toward the cathode.
- Electrochemical deposition at the cathode forming metallic dendrites.
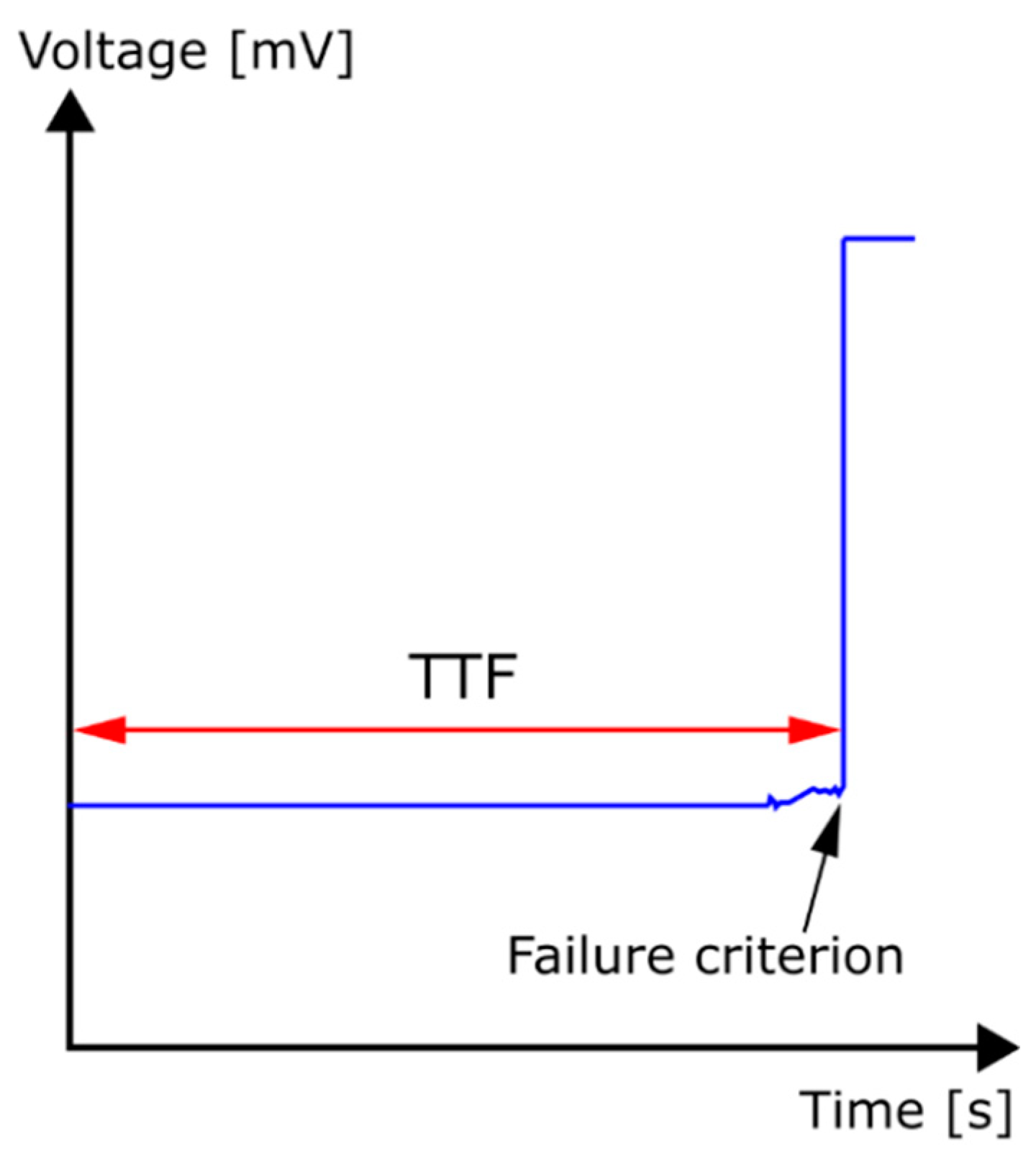
2. Experimental
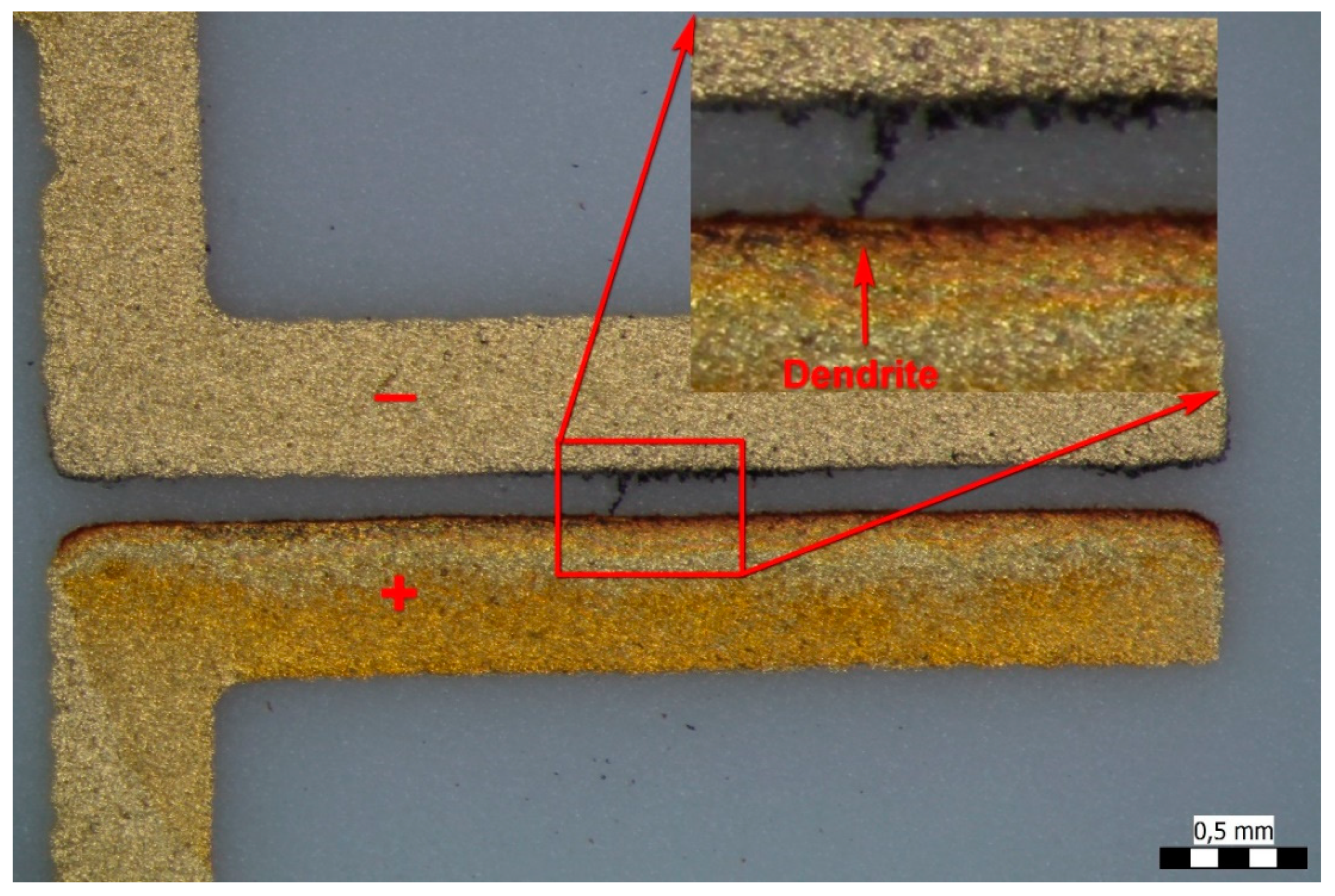
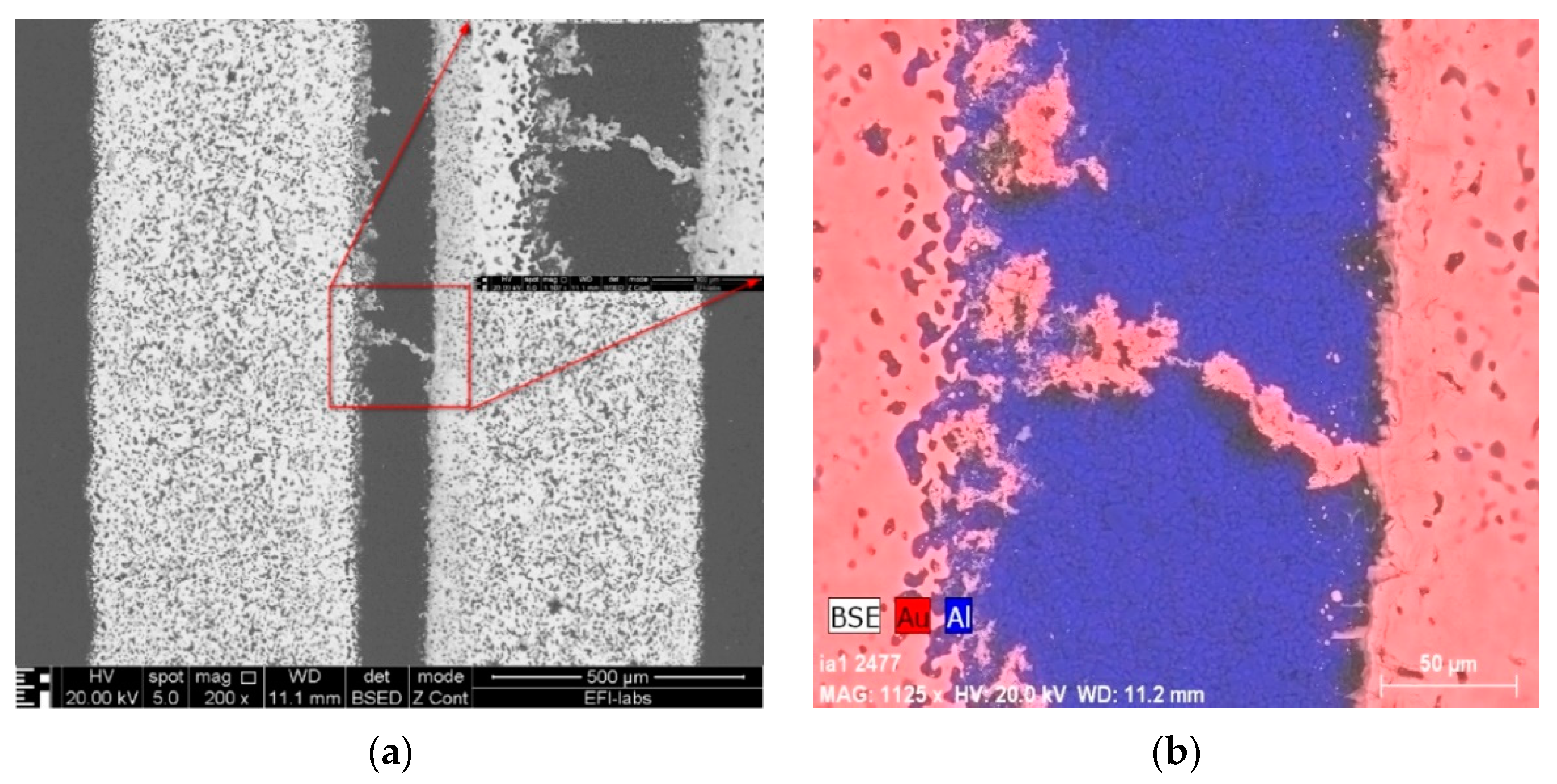
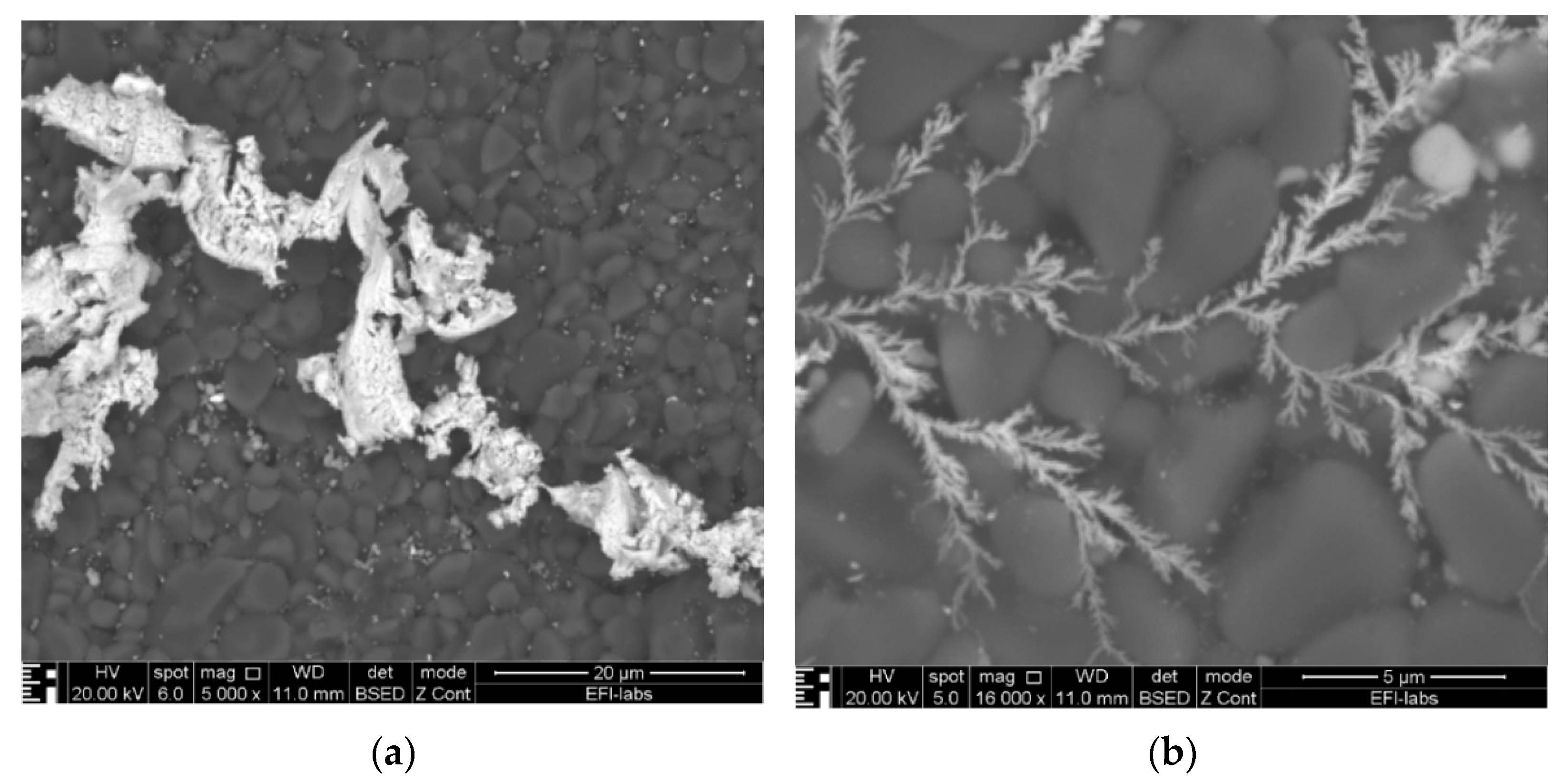
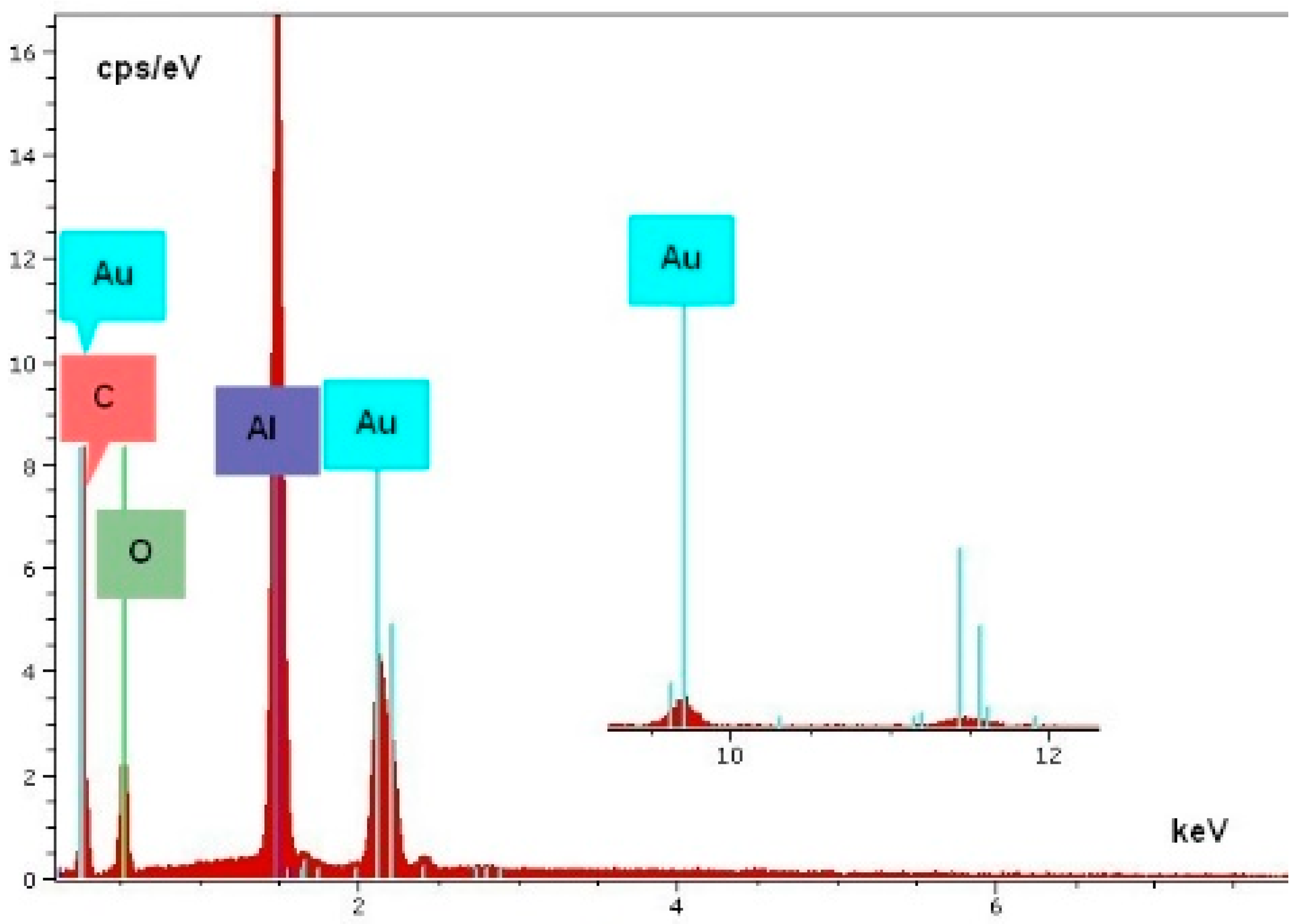
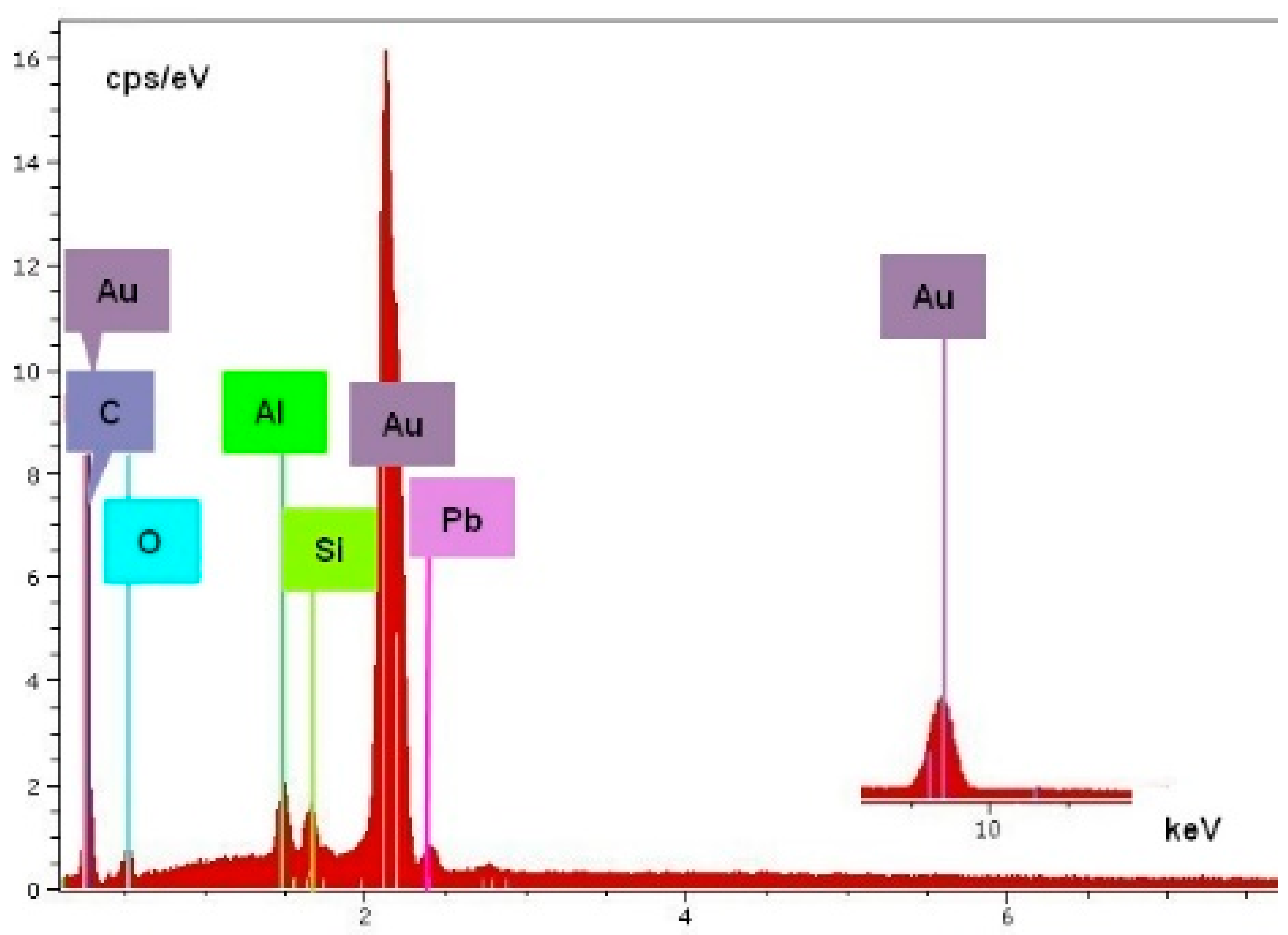
3. Results of the WD and SEM-EDS Investigations
4. Discussion
- Au ion formation by anodic corrosion:
- 2.
- Cation migration through the electrolyte under an electrical field toward the cathode.
- 3.
- Electrochemical deposition at the cathode forming metallic dendrites:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdingovas, V.; Jellesen, M.S.; Ambat, R. Solder Flux Residues and Humidity-Related Failures in Electronics: Relative Effects of Weak Organic Acids Used in No-Clean Flux Systems. J. Electron. Mater. 2015, 44, 1116–1127. [Google Scholar] [CrossRef]
- Pour, Z.S.; Ghaemy, M.; Bordbar, S.; Karimi-Maleh, H. Effects of surface treatment of TiO2 nanoparticles on the adhesion and anticorrosion properties of the epoxy coating on mild steel using electrochemical technique. Prog. Org. Coat. 2018, 119, 99–108. [Google Scholar] [CrossRef]
- Harsányi, G. Copper may destroy chip-level reliability: Handle with care—Mechanism and conditions for copper migrated resistive short formation. IEEE Electron. Device Lett. 1999, 20, 5–8. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, S.; Chen, L.; Hu, J.; Zhang, Z. Test methods for electrochemical migration: A review. J. Mater. Sci. Mater. Electron. 2017, 28, 2279–2289. [Google Scholar] [CrossRef]
- Harsanyi, G. Electrochemical Processes Resulting in Migrated Short Failures in Microcircuits. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1995, 18, 602–610. [Google Scholar] [CrossRef]
- Zhan, S.; Azarian, M.H.; Pecht, M. Reliability of printed circuit boards processed using no-clean flux technology in temperature-humidity-bias conditions. IEEE Trans. Device Mater. Reliab. 2008, 8, 426–434. [Google Scholar] [CrossRef]
- Warren, G.W.; Wynblatt, P.; Zamanzadeh, M. The Role of Electrochemical Migration and Moisture Adsorption on the Reliability of Metallized Ceramic Substrates. J. Electron. Mater. 1989, 18, 339–353. [Google Scholar] [CrossRef]
- Ripka, G.; Harsanyi, G. Electrochemical Migration in Thick-Film Ic-S. Electrocompon. Sci. Technol. 1985, 11, 281–290. [Google Scholar] [CrossRef]
- DerMarderosian, A. The Electrochemical Migration of Metals. In Proceedings of the 11th International Microelectronics Symposium, Minneapolis, MN, USA, 25–27 September 1978; pp. 134–141. [Google Scholar]
- Sbar, N.L. Bias-Humidity Performance of Encapsulated and Unencapsulated Ti-Pd-Au Thin-Film Conductors in an Environment Contaminated with Cl2. IEEE Trans. Parts Hybrids Packag. 1976, 12, 176–181. [Google Scholar] [CrossRef]
- Christou, A.; Griffith, J.R.; Wilkins, B.R. Reliability of Hybrid Encapsulation Based on Fluorinated Polymeric Materials. IEEE Trans. Electron Devices 1979, 26, 77–83. [Google Scholar] [CrossRef]
- Grunthaner, F.; Griswold, T.W.; Clendening, P.J. Migratory Gold Resistive Shorts: Chemical Aspects of a Failure Mechanism. In Proceedings of the 13th IEEE International Reliability Physics Symposium, Las Vegas, NV, USA, 1–3 April 1975; pp. 99–106. [Google Scholar]
- Wright, J.C. Reliability Improvements of Plastic Semiconductors using Gold Metallization. In Proceedings of the 11th IEEE International Reliability Physics Symposium, Las Vegas, NV, USA, 3–5 April 1973; pp. 224–229. [Google Scholar]
- Shumka, A.; Piety, R.R. Migrated-Gold Resistive Shorts in Microcircuits. In Proceedings of the 13th IEEE International Reliability Physics Symposium, Las Vegas, NV, USA, 1–3 April 1975; Volume 3, pp. 93–98. [Google Scholar]
- Hakim, E.B.; Shappiro, I.R. Failure Mechanism in Gold Metalized Sealed Junction Devices. Solid State Technol. 1975, 4, 66–68. [Google Scholar]
- Harsányi, G. Irregular effect of chloride impurities on migration failure reliability: Contradictions or understandable? Microelectron. Reliab. 1999, 39, 1407–1411. [Google Scholar] [CrossRef]
- Gaur, J.N.; Schmid, G.M. Electrochemical behavior of gold in acidic chloride solutions. J. Electroanal. Chem. 1970, 24, 279–286. [Google Scholar] [CrossRef]
- Noh, B.I.; Lee, J.B.; Jung, S.B. Effect of surface finish material on printed circuit board for electrochemical migration. Microelectron. Reliab. 2008, 48, 652–656. [Google Scholar] [CrossRef]
- Noh, B.I.; Yoon, J.W.; Hong, W.S.; Jung, S.B. Evaluation of electrochemical migration on flexible printed circuit boards with different surface finishes. J. Electron. Mater. 2009, 38, 902–907. [Google Scholar] [CrossRef]
- Medgyes, B. Electrochemical migration of Ni and ENIG surface finish during Environmental test contaminated by NaCl. J. Mater. Sci. Mater. Electron. 2017, 28, 18578–18584. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.S.; Oh, C. Lifetime Prediction of Electrochemical Ion Migration with Various Surface Finishes of Printed Circuit Boards. J. Electron. Mater. 2019, 49, 48–58. [Google Scholar] [CrossRef]
- Yi, P.; Xiao, K.; Ding, K.; Dong, C.; Li, X. Electrochemical migration behavior of copper-clad laminate and electroless nickel/immersion gold printed circuit boards under thin electrolyte layers. Materials 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsányi, G. Material design aspects of high reliability, high density interconnections. Mater. Chem. Phys. 1996, 45, 120–123. [Google Scholar] [CrossRef]
- Harsányi, G. Dendritic Growth from Dielectric Constituents in Thick Film ICs. Microelectron. Int. 1992, 9, 54–59. [Google Scholar] [CrossRef]
- Matthew, L.C.; Rath, D.L. The waterdrop test—Highly accelerated migration testing. In Proceedings of the Materials Development in Microelectronic Packaging Conference, Montreal, QC, Canada, 19–22 August 1991; pp. 353–357. [Google Scholar]
- Medgyes, B.; Illés, B.; Berényi, R.; Harsányi, G. In situ optical inspection of electrochemical migration during THB tests. J. Mater. Sci. Mater. Electron. 2011, 22, 694–700. [Google Scholar] [CrossRef]
- Burke, L.D.; Nugent, P.F. The electrochemistry of gold: I. The redox behaviour of the metal in aqueous media. Gold Bull. 1997, 30, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.J. The anodic behaviour of gold—Part I—Oxidation in acidic solutions. Gold Bull. 1980, 13, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.J. The anodic behaviour of gold—Part II—Oxidation in alkaline solutions. Gold Bull. 1980, 13, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Oesch, U.; Janata, J. Electrochemical study of gold electrodes with anodic oxide films—I. Formation and reduction behaviour of anodic oxides on gold. Electrochim. Acta 1983, 28, 1237–1246. [Google Scholar] [CrossRef]
- Massey, A.G.; Thompson, N.R.; Johnson, B.F.G.; Davis, R. The Chemistry of Copper, Silver and Gold; Pergamon Press: New York, NY, USA, 1975; pp. 28–29. [Google Scholar]
- Van Muylder, J.; Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: New York, NY, USA, 1974; pp. 399–405. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medgyes, B.; Gharaibeh, A.; Rigler, D.; Harsányi, G. On the Electrochemical Migration Mechanism of Gold in Electronics—Less Reliable Than Expected? Materials 2021, 14, 5237. https://doi.org/10.3390/ma14185237
Medgyes B, Gharaibeh A, Rigler D, Harsányi G. On the Electrochemical Migration Mechanism of Gold in Electronics—Less Reliable Than Expected? Materials. 2021; 14(18):5237. https://doi.org/10.3390/ma14185237
Chicago/Turabian StyleMedgyes, Bálint, Ali Gharaibeh, Dániel Rigler, and Gábor Harsányi. 2021. "On the Electrochemical Migration Mechanism of Gold in Electronics—Less Reliable Than Expected?" Materials 14, no. 18: 5237. https://doi.org/10.3390/ma14185237
APA StyleMedgyes, B., Gharaibeh, A., Rigler, D., & Harsányi, G. (2021). On the Electrochemical Migration Mechanism of Gold in Electronics—Less Reliable Than Expected? Materials, 14(18), 5237. https://doi.org/10.3390/ma14185237






