Experimental Study on Solidification and Stabilization of Heavy-Metal-Contaminated Soil Using Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.2. Sample Preparation
2.3. Experiment Method
2.3.1. Unconfined Compressive Strength Test
2.3.2. Toxicity Leaching Test
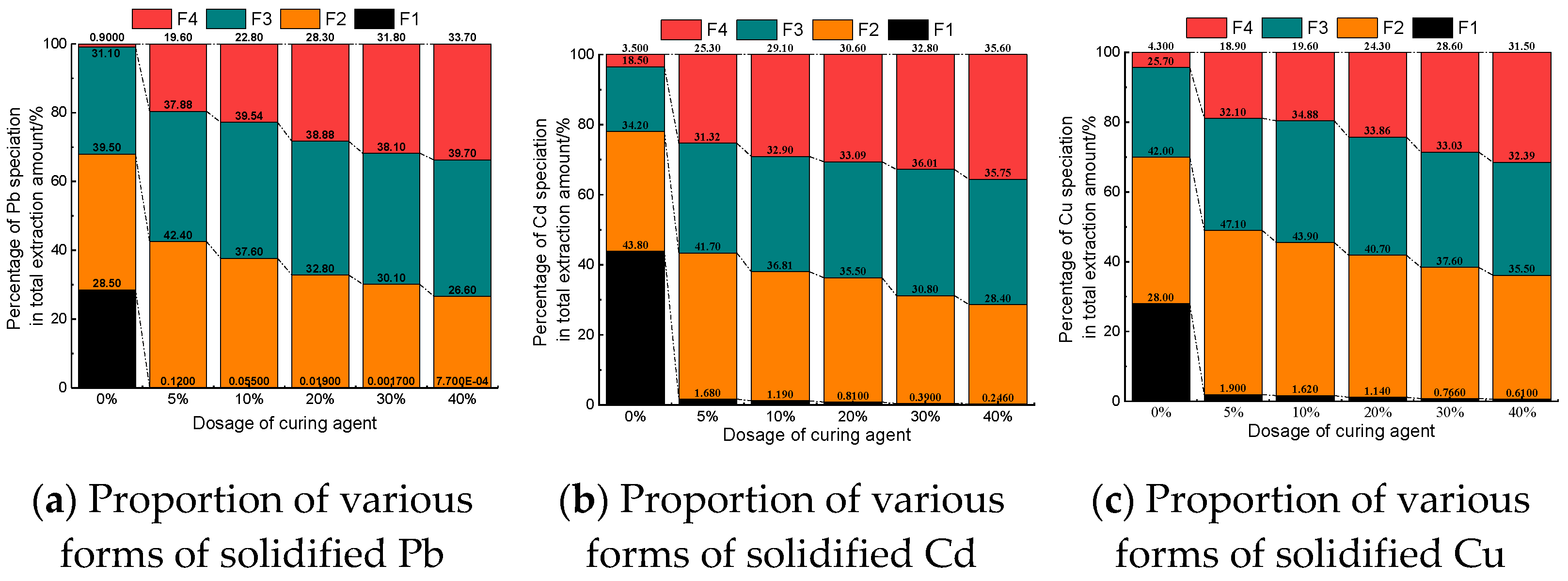
2.3.3. Contaminated Soil Sample Heavy Metal Form Test
- (1)
- Adding 1 mol/L MgCl2 solution to the selected samples and adjusting pH to 7.0, oscillating continuously for 1 h at 25 °C, and centrifuging for 20 min. The supernatant was taken and diluted to 25 mL as the exchangeable solution to be tested.
- (2)
- Adding 16 mL of 1 mol/L NaAc solution to the residue in the previous step, adjusting pH to 5.0, followed by continuous oscillation at 25 °C for 8 h. After centrifugation for 20 min, the supernatant was taken and diluted to 25 mL as the carbonate-bound solution.
- (3)
- Adding 16 mL of 25% HAC solution of 0.04 mol/L NH2OH HCl to the residue in the previous step, oscillating intermittently at 96 °C for 4 h and centrifuging for 20 min. The supernatant was taken and diluted to 25 mL as the Fe-Mn oxide bound solution.
- (4)
- Adding 3 mL of 0.01 mol/L HNO3 and 5 mL of 30% H2O2 to the residue, adjusting the pH value to 2.0, water-bath heating to 85 °C for 2 h and cooling to 25 °C. Then, 5 mL of 20% HNO3 solution of 3.2 mol/L NH4Ac was diluted to 20 mL, oscillated for 30 min and centrifuged for 20 min. The supernatant was taken out and diluted to 25 mL as the organic bound solution.
- (5)
- Taking out the residue in the previous step and completely digesting with graphite digester. The solution was canceled as the residual state to be measured.
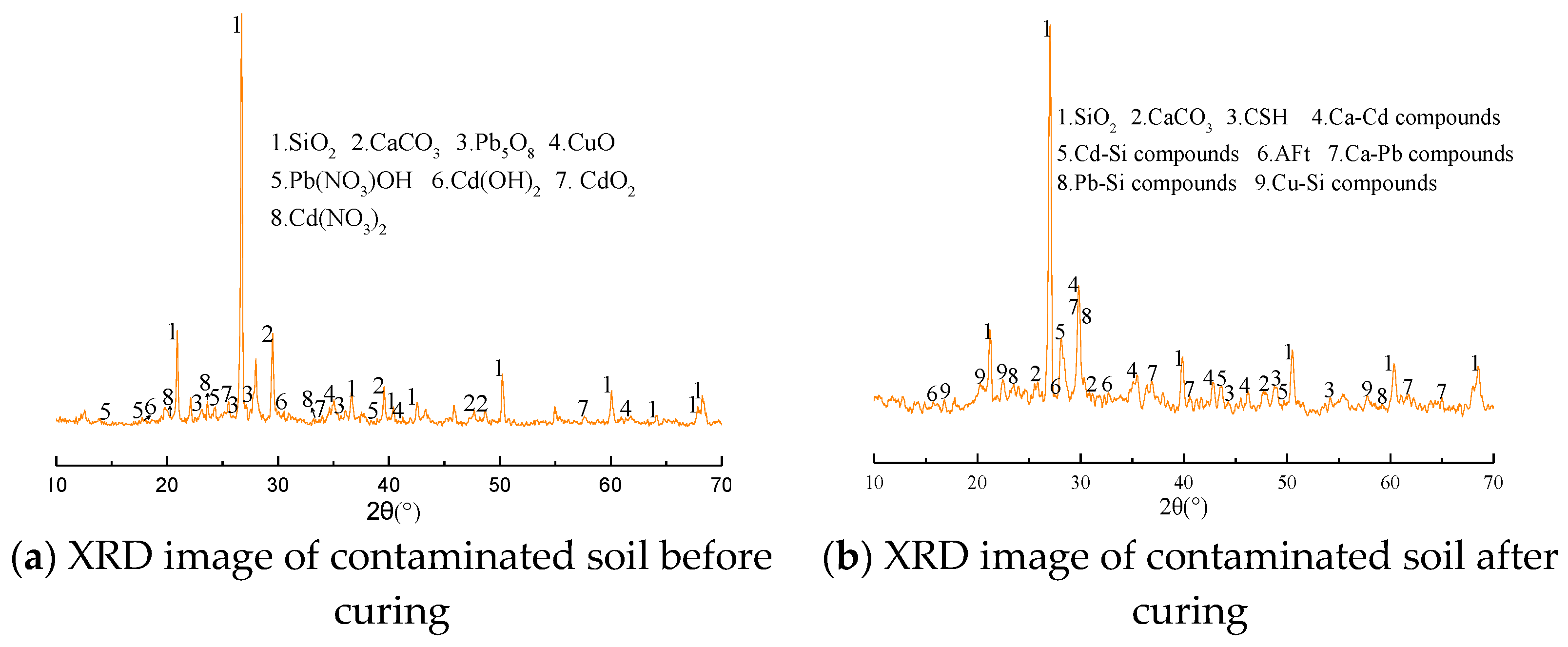
2.3.4. X-ray Diffraction (XRD)
2.3.5. Scanning Electron Microscope Test (SEM-EDS)
3. Test Results and Analysis
3.1. Analysis of the Mechanical Properties of the Cured Body
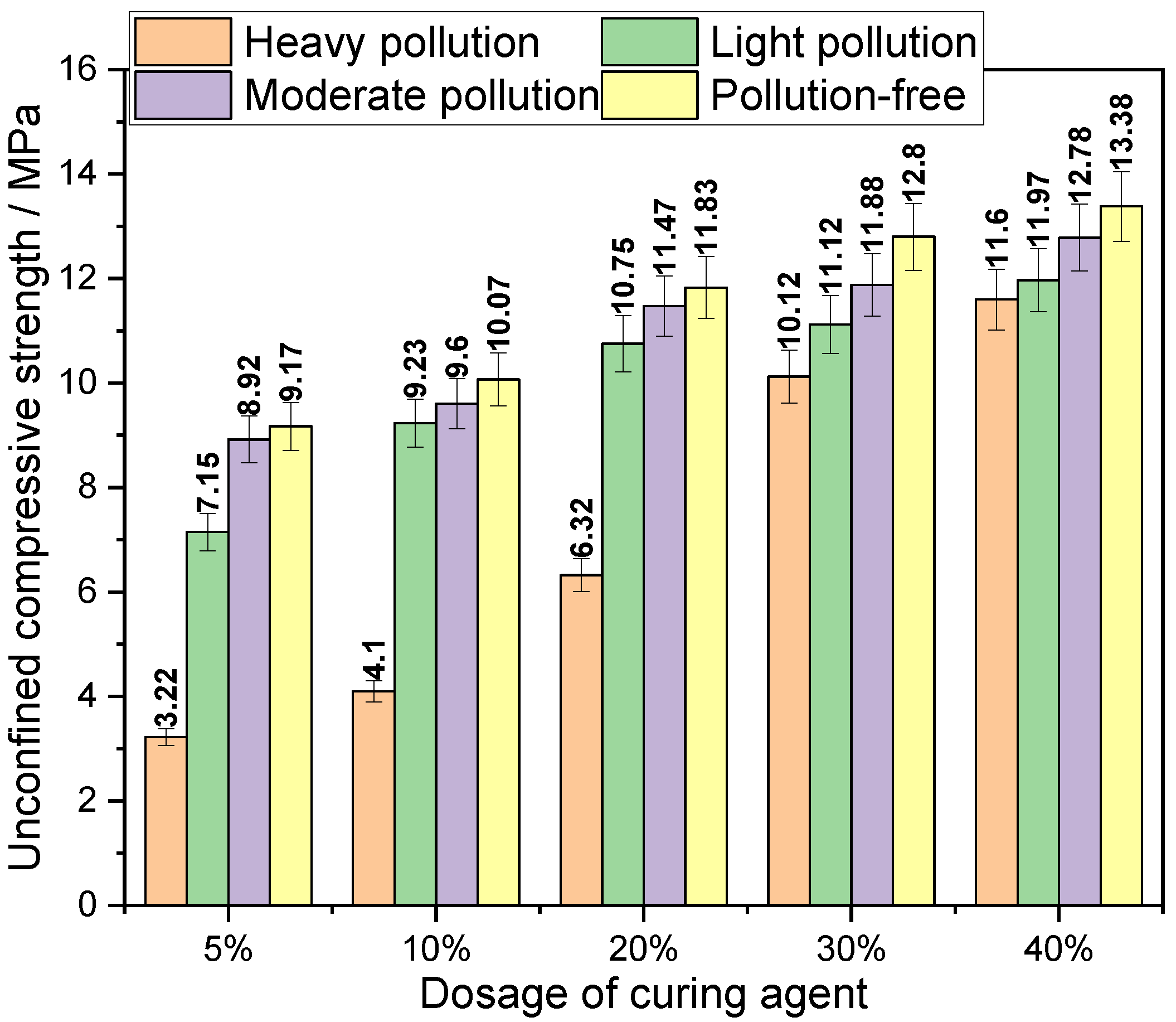
3.1.1. The Effect of Curing Agent Content on Mechanical Properties
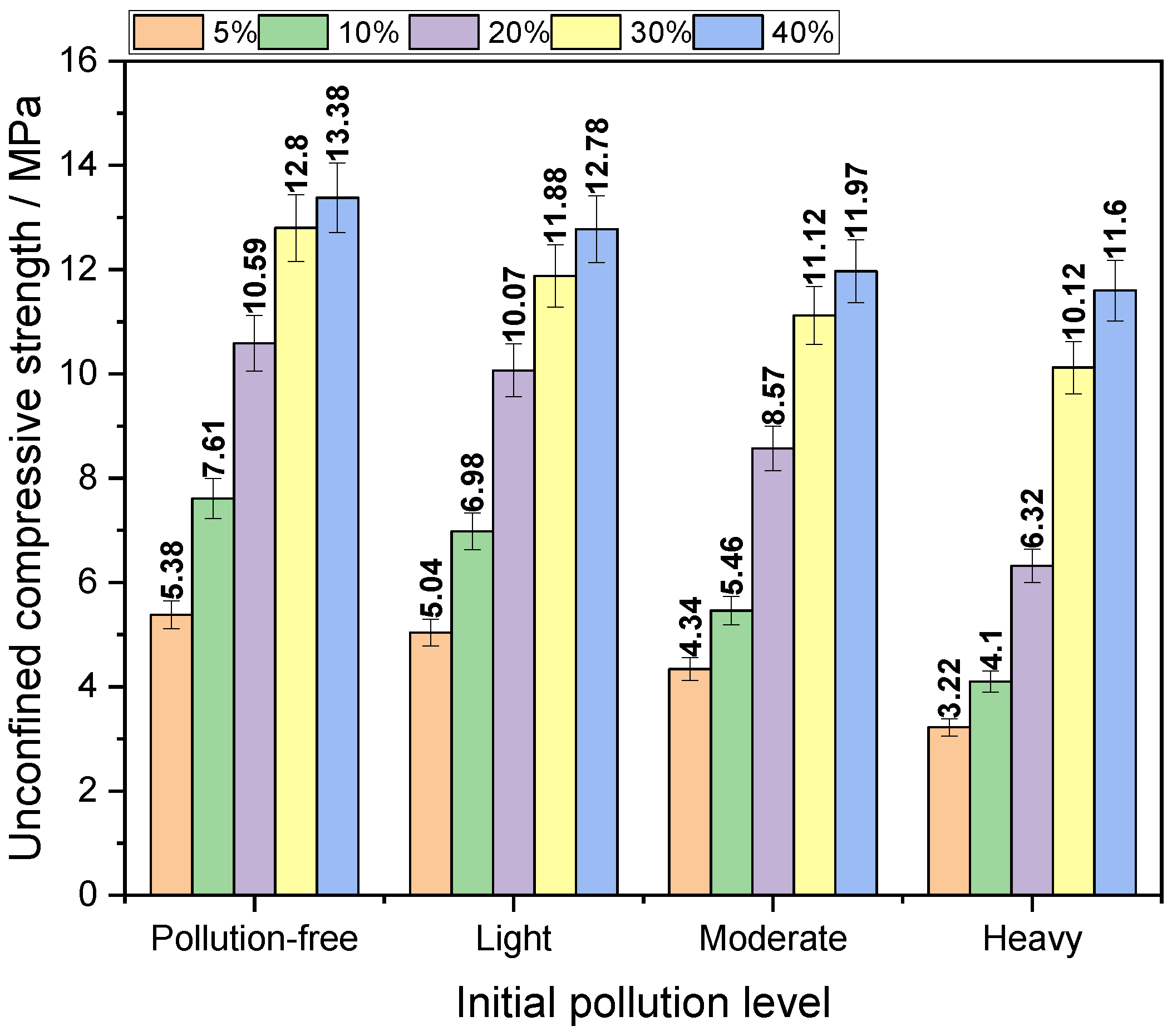
3.1.2. Influence of Initial Pollution Degree on Mechanical Strength
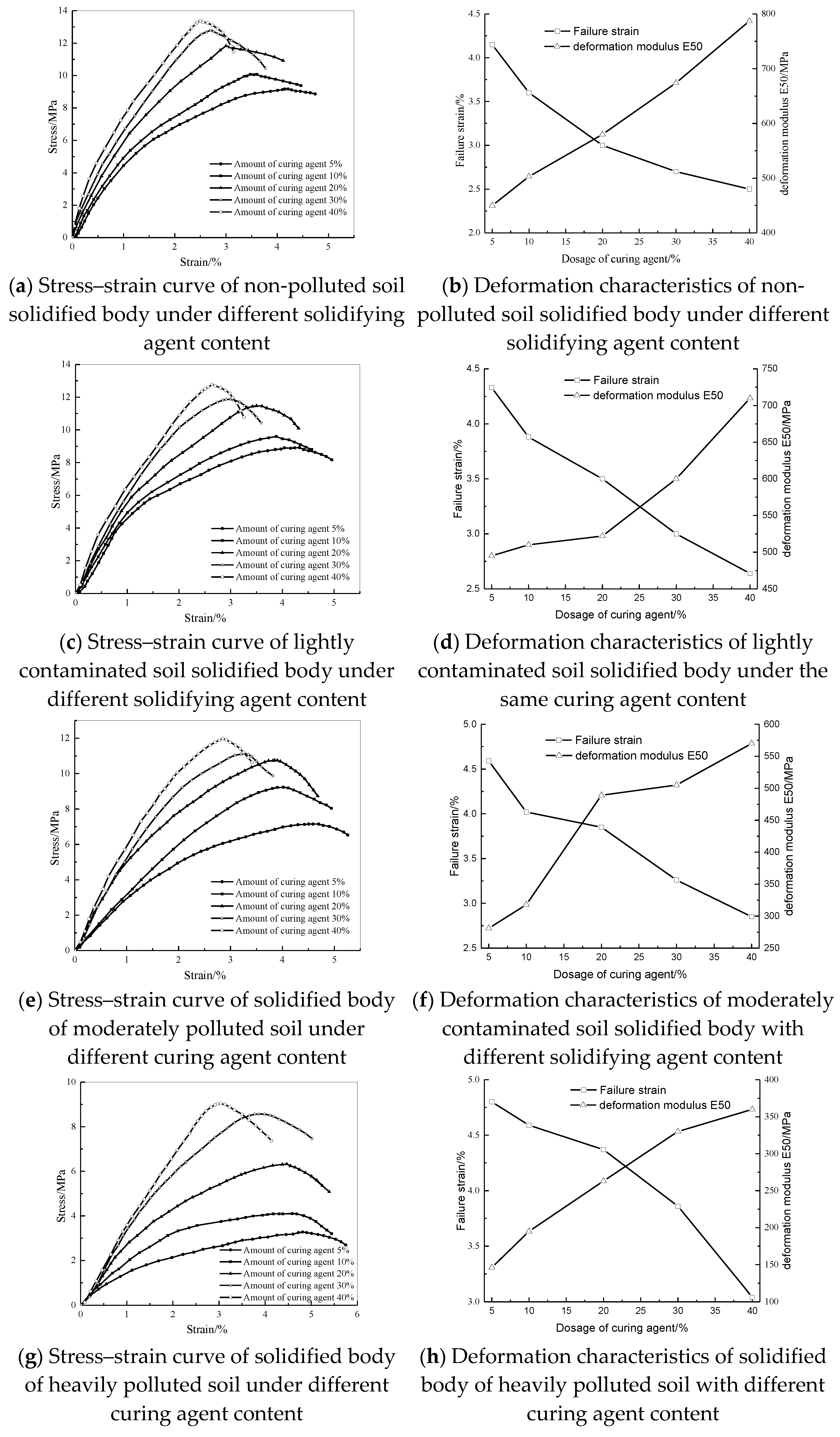
3.2. Analysis of the Deformation Characteristics of the Solidified Body
3.3. Analysis on Leaching Characteristics of Solidified Form
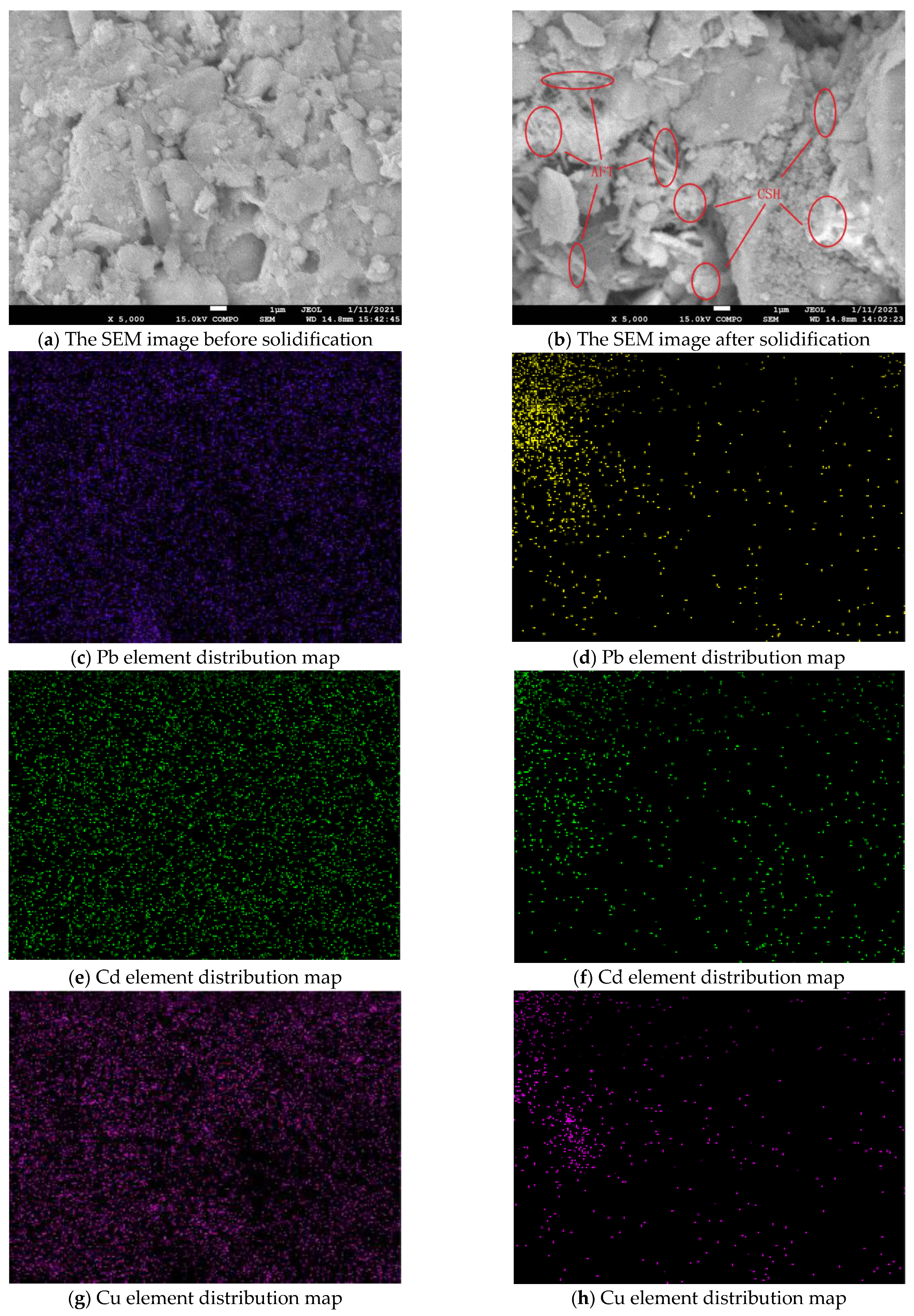
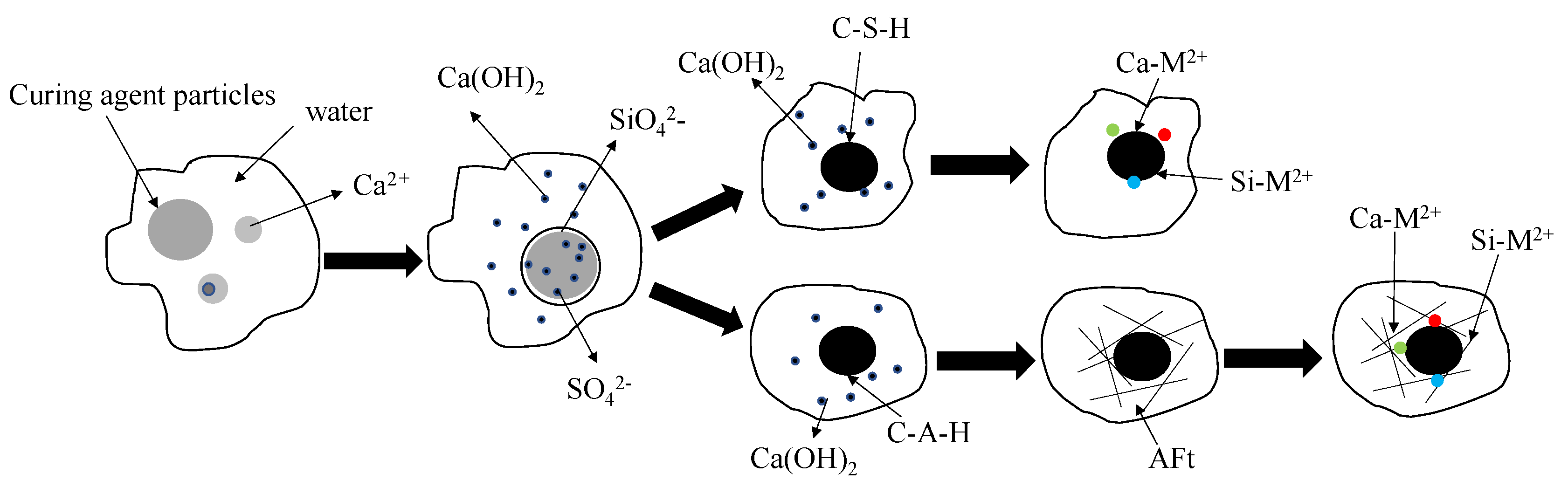
3.4. Microstructure Analysis of Solidified Body
3.5. Morphology Analysis of Solidified Metal
3.6. Solid Phase Analysis
4. Conclusions
- (1)
- The mechanical properties of the contaminated soil solidified by the new curing agent A are superior, and the maximum unconfined compressive strength is 33.45 MPa, while the minimum is 3.22 MPa. However, simply adding more curing agent to improve the mechanical properties of the solidified body may not achieve the desired effect.
- (2)
- With the increase in the amount of curing agent, the resistance to deformation of solidified soil is significantly enhanced, which is manifested as the decrease in failure strain and the increase in the deformation modulus E50 under compressive load, and the trend of solidified soil with different initial pollution degree is consistent.
- (3)
- From the aspect of leaching characteristics, the new curing agent has an excellent solidifying effect on the composite heavy-metal-contaminated soil. To achieve the purpose of safe disposal, the dosage of curing agent should reach 10%, which only applies for the heavily polluted soil. For other soils of different pollution degrees, 5% of the dosage is satisfying. This study, however, only discusses the properties of solidified polluted soil which were cured for 7 days, and its long-term environmental safety and mechanical properties need to be further studied.
- (4)
- The content of weak acid-extractable heavy metals can be significantly reduced and effectively transformed into residual state by the new curing agent A. Increasing the amount of curing agent can further reduce the content of weak acid extraction form and improve the content of residual form. After solidification, there are hydrated products such as calcium silicate hydrate (CSH) and ettringite (AFT), which can inhibit the leaching performance of heavy metal ions by adsorption, encapsulation and ion exchange.
Author Contributions
Funding
Conflicts of Interest
References
- Du, Y.-J.; Liu, S.-Y.; Liu, Z.-B.; Chen, L.; Zhang, F.; Jin, F. An Overview of Stabilization/Solidification Technique for Heavy Metals Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2010; pp. 760–766. [Google Scholar]
- Liu, S.-J.; Jiang, J.-Y.; Wang, S.; Guo, Y.-P.; Ding, H. Assessment of water-soluble thiourea-formaldehyde (WTF) resin for stabilization/solidification (S/S) of heavy metal contaminated soils. J. Hazard. Mater. 2018, 346, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.S.; Ok, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, L.; Cui, J.-L.; Poon, C.S.; Beiyuan, J.; Tsang, D.C.W.; Li, X.-D. Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: Spectroscopic investigation and leaching tests. Sci. Total. Environ. 2018, 631–632, 1486–1494. [Google Scholar] [CrossRef]
- Jiang, N.; Cai, D.; He, L.; Zhong, N.; Wen, H.; Zhang, X.; Wu, Z. A Facile Approach to Remediate the Microenvironment of Saline-Alkali Soil. ACS Sustain. Chem. Eng. 2015, 3, 374–380. [Google Scholar] [CrossRef]
- Du, Y.-J.; Wei, M.-L.; Reddy, K.R.; Jin, F.; Wu, H.-L.; Liu, Z.-B. New phosphate-based binder for stabilization of soils contaminated with heavy metals: Leaching, strength and microstructure characterization. J. Environ. Manag. 2014, 146, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.-Y.; Feng, Y.-S.; Jin, F.; Zhang, L.-M.; Du, Y.-J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.-S.; Du, Y.-J.; Zhou, A.; Zhang, M.; Li, J.-S.; Zhou, S.-J.; Xia, W.-Y. Geoenvironmental properties of industrially contaminated site soil solidified/stabilized with a sustainable by-product-based binder. Sci. Total. Environ. 2021, 765, 142778. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Kang, B.; Tan, X.; Deng, Y.; Yang, C. Mechanism of stabilized/solidified heavy metal contaminated soils with cement-fly ash based on electrical resistivity measurements. Measurement 2019, 141, 85–94. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Deiranlou, M. Enhancement of cement-based solidification/stabilization of a lead-contaminated smectite clay. J. Hazard. Mater. 2021, 403, 123969. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Soil Environmental Quality Control Standard for Soil Pollution Risk of Agricultural Land (Trial); GB15618-2018; China Standards Press: Beijing, China, 2018. [Google Scholar]
- Liu, X.; Hou, P.; Chen, H. Effects of nanosilica on the hydration and hardening properties of slag cement. Constr. Build. Mater. 2021, 282, 122705. [Google Scholar] [CrossRef]
- Fang, K.; Wang, D.; Zhao, J.; Zhang, M. Utilization of ladle furnace slag as cement partial replacement: Influences on the hydration and hardening properties of cement. Constr. Build. Mater. 2021, 299, 124265. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Y.; Wang, Y.; He, H.; Luo, S.; Huang, C. Synergistic use of sodium bicarbonate and aluminum sulfate to enhance the hydration and hardening properties of Portland cement paste. Constr. Build. Mater. 2021, 299, 124248. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Qian, J.; Shen, X. Effect of protogenetic alkali sulfates on the hydration and hardening of cement with different tricalcium aluminate content. Constr. Build. Mater. 2020, 256, 119475. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Yang, C.; Chu, C.; Tan, X. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Guo, G.-L.; Yan, X.-L.; Li, C.-P.; Arulrajah, A.; Wang, F.; Wang, S. Field evaluation of a new hydroxyapatite based binder for ex-situ solidification/stabilization of a heavy metal contaminated site soil around a Pb-Zn smelter. Constr. Build. Mater. 2019, 210, 278–288. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Li, C.-P.; Yan, X.-L.; Arulrajah, A.; Wang, F.; Song, D.-J. In-situ solidification/stabilization of heavy metals contaminated site soil using a dry jet mixing method and new hydroxyapatite based binder. J. Hazard. Mater. 2019, 369, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total. Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef]
- He, Y.; Lu, L.; Struble, L.J.; Rapp, J.L.; Mondal, P.; Hu, S. Effect of calcium–silicon ratio on microstructure and nanostructure of calcium silicate hydrate synthesized by reaction of fumed silica and calcium oxide at room temperature. Mater. Struct. 2014, 47, 311–322. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Huang, H.; Yin, S. Effects of Ca/Si Ratio, Aluminum and Magnesium on the Carbonation Behavior of Calcium Silicate Hydrate. Materials 2019, 12, 1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Natural Moisture Content w/% | Natural Density ρ/(g/cm3) | Plastic Limit wp/% | Liquid Limit wL/% | Cohesion c/kPa |
|---|---|---|---|---|
| 16.3 | 1.67 | 17.4 | 37.2 | 60 |
| Reagent Name | Chemical Formula | Manufacturer | Purity |
|---|---|---|---|
| Lead nitrate | Pb(NO3)2 | Tianjin Beilian Fine Chemicals Development Co., Ltd. | Analytically pure |
| Cadmium nitrate | Cd(NO3)2·4H2O | Tianjin Guangfu Fine Chemical Research Institute | Analytically pure |
| Copper nitrate | Cu(NO3)2·3H2O | Tianjin Damao Chemical Reagent Factory | Analytically pure |
| Heavy Metal Pollution Level | Heavy Metal Content/(mg·kg−1) | ||
|---|---|---|---|
| Pb | Cd | Cu | |
| Pollution-free | 0 | 0 | 0 |
| Light pollution | 2000 | 8 | 1200 |
| Moderate pollution | 6000 | 24 | 3600 |
| Heavy pollution | 10,000 | 40 | 6000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, R.; Li, H.; Yi, H.; Jing, H. Experimental Study on Solidification and Stabilization of Heavy-Metal-Contaminated Soil Using Cementitious Materials. Materials 2021, 14, 4999. https://doi.org/10.3390/ma14174999
Li X, Yang R, Li H, Yi H, Jing H. Experimental Study on Solidification and Stabilization of Heavy-Metal-Contaminated Soil Using Cementitious Materials. Materials. 2021; 14(17):4999. https://doi.org/10.3390/ma14174999
Chicago/Turabian StyleLi, Xiaojun, Ruizhi Yang, Hao Li, Hao Yi, and Hongjun Jing. 2021. "Experimental Study on Solidification and Stabilization of Heavy-Metal-Contaminated Soil Using Cementitious Materials" Materials 14, no. 17: 4999. https://doi.org/10.3390/ma14174999
APA StyleLi, X., Yang, R., Li, H., Yi, H., & Jing, H. (2021). Experimental Study on Solidification and Stabilization of Heavy-Metal-Contaminated Soil Using Cementitious Materials. Materials, 14(17), 4999. https://doi.org/10.3390/ma14174999






