In Vivo Study of Osteochondral Defect Regeneration Using Innovative Composite Calcium Phosphate Biocement in a Sheep Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biocements
2.1.1. C Cement
2.1.2. CAL Cement
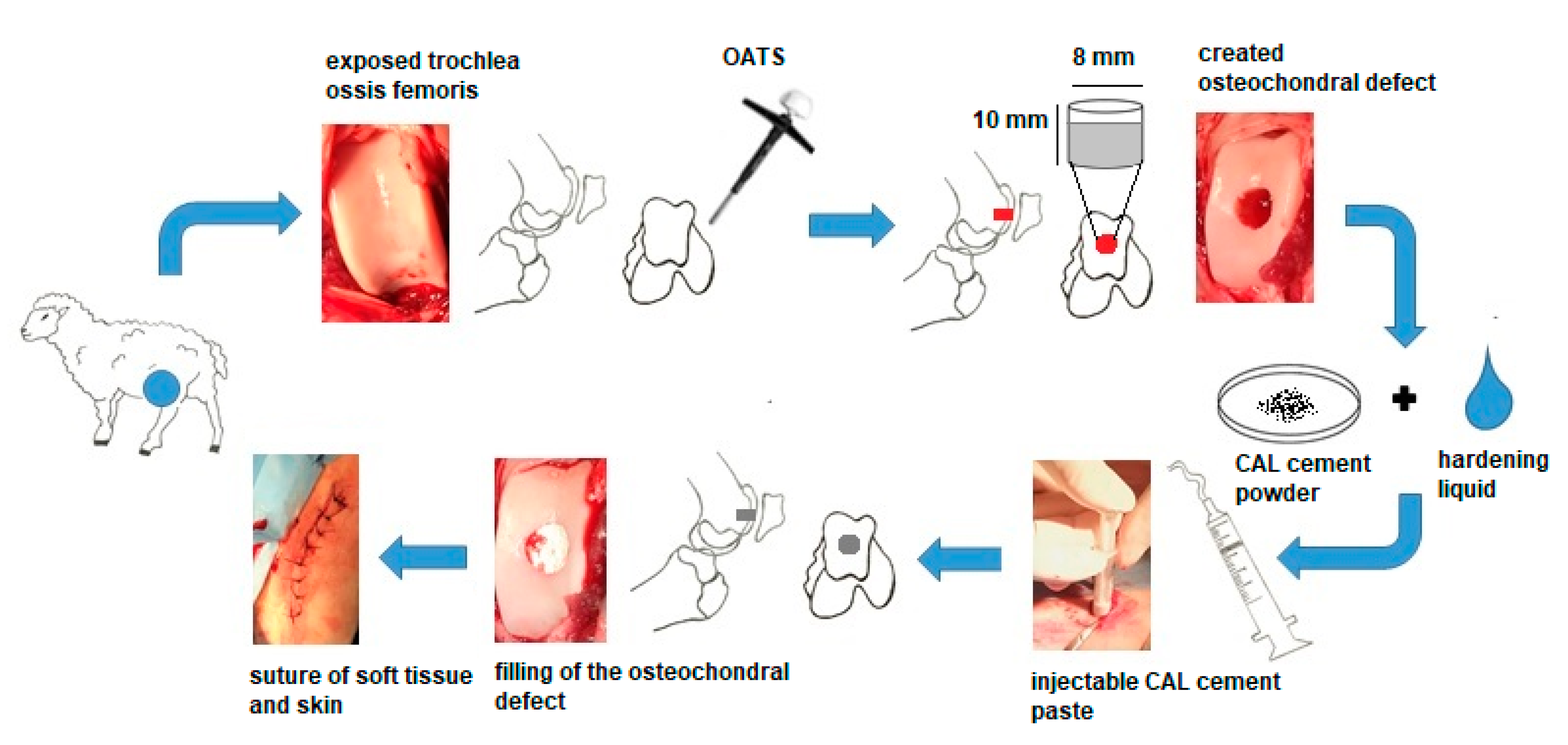
2.2. In Vivo Models for Regeneration of Osteochondral Defects and Surgical Technique
2.3. Postoperative Management
2.4. Gross Characteristic and Histological Analysis
2.5. Microscopic Evaluations
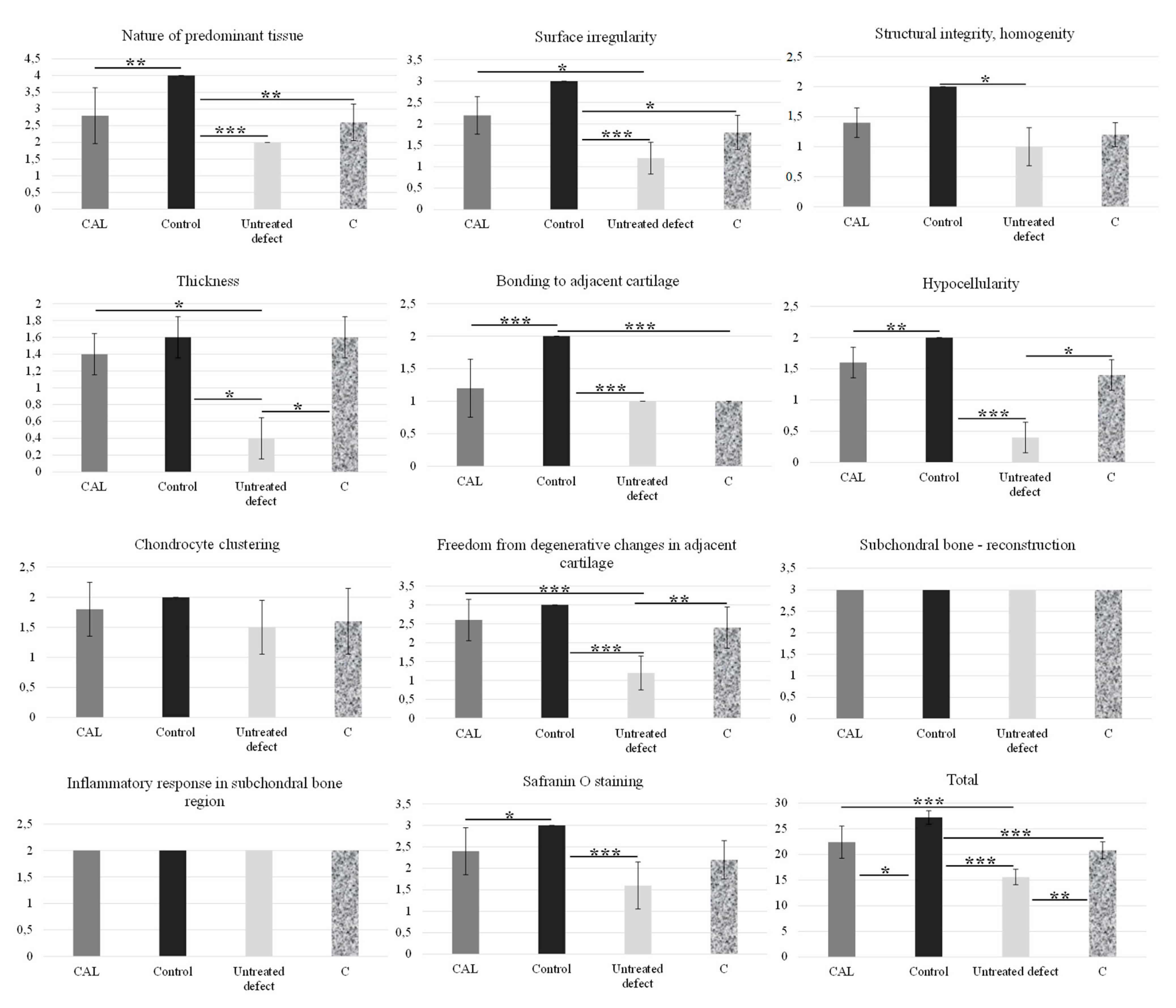
2.5.1. Pineda Histological Scoring System
2.5.2. Modified O’Driscoll Scoring System
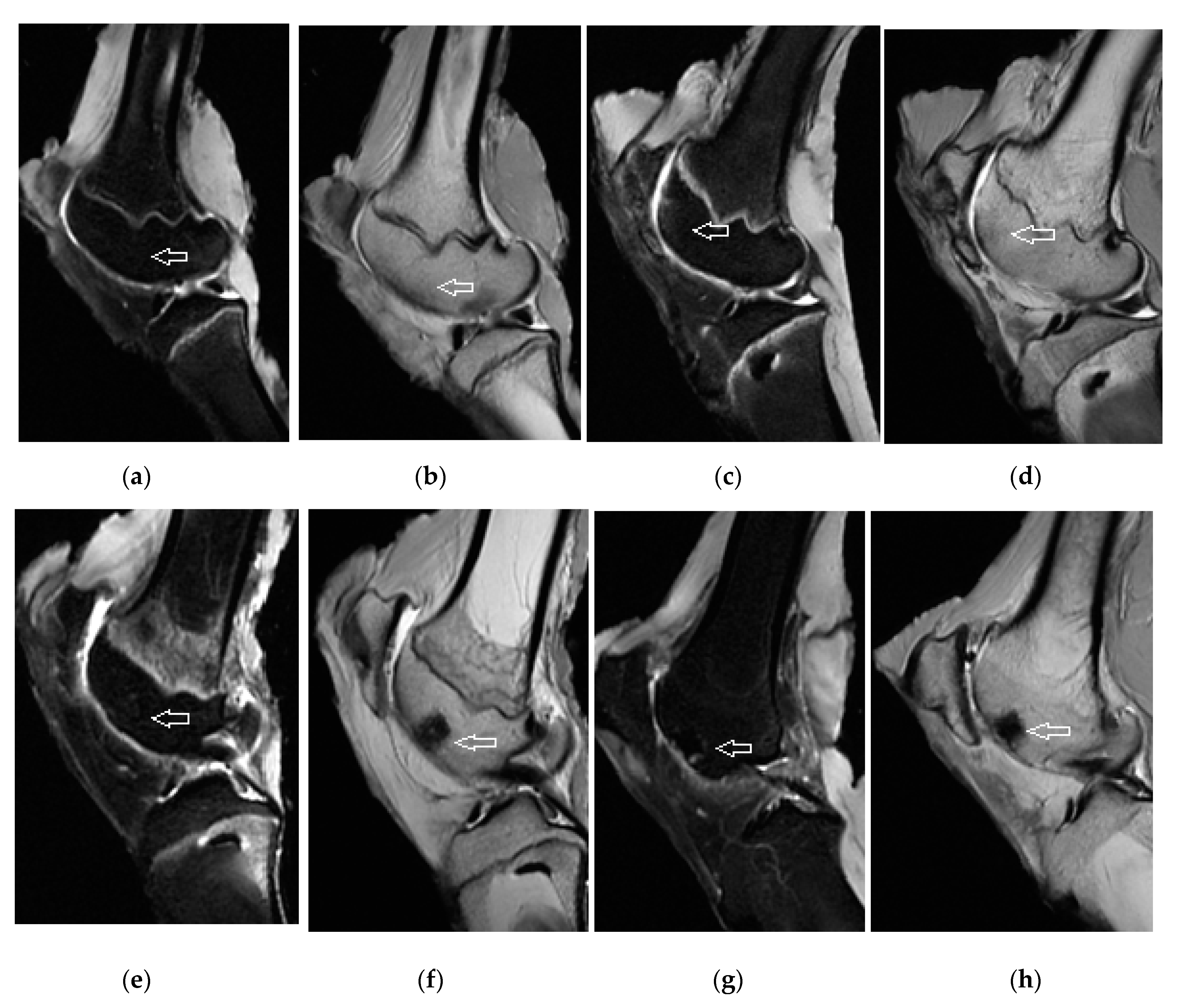
2.6. X-ray, Micro-CT, and MR Imaging
2.7. Statistical Analysis
3. Results
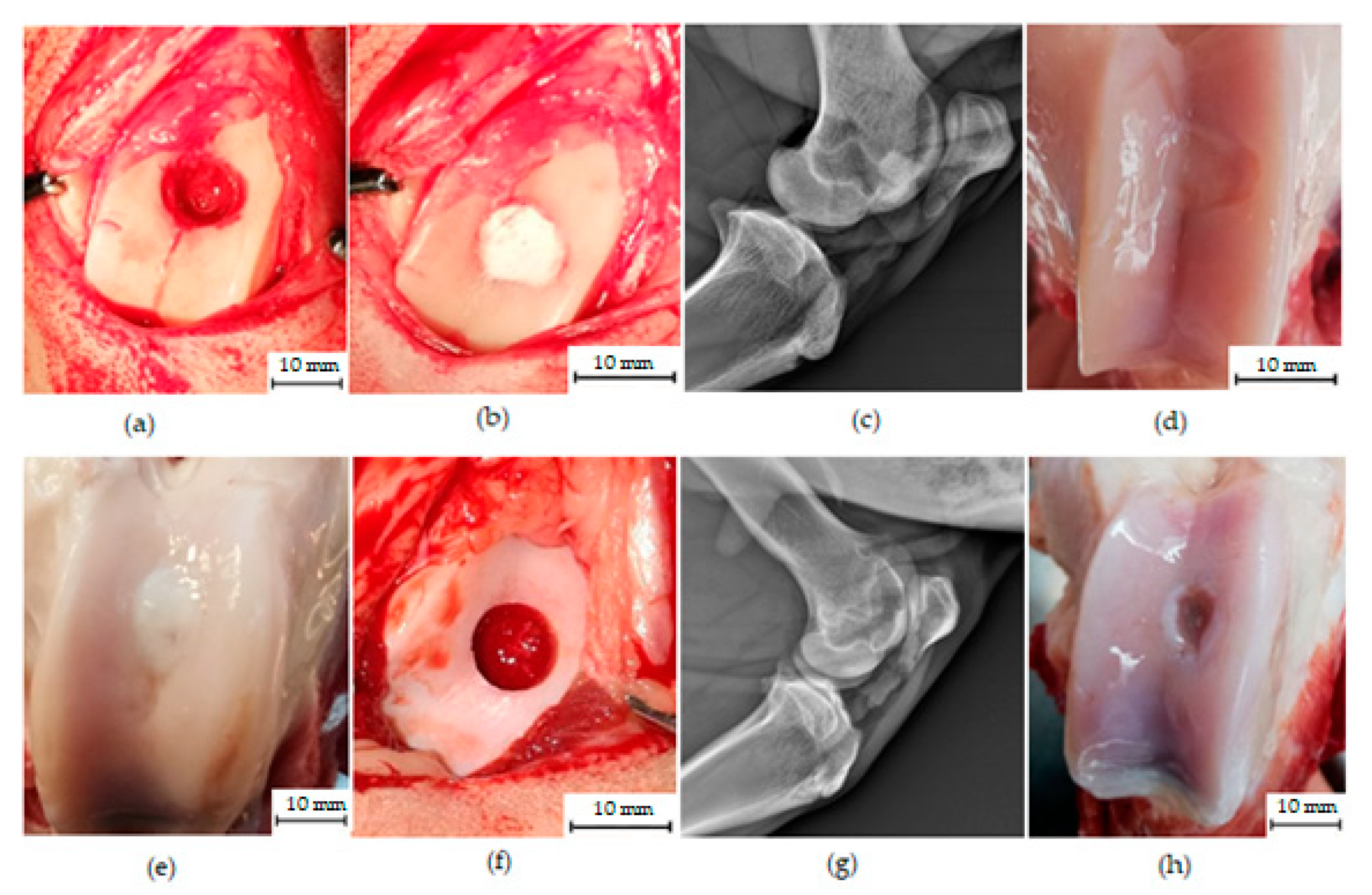
3.1. Macroscopic Analysis of In Vivo Regenerated Articular Cartilage of Trochlea Humeri in Osteochondral Defects
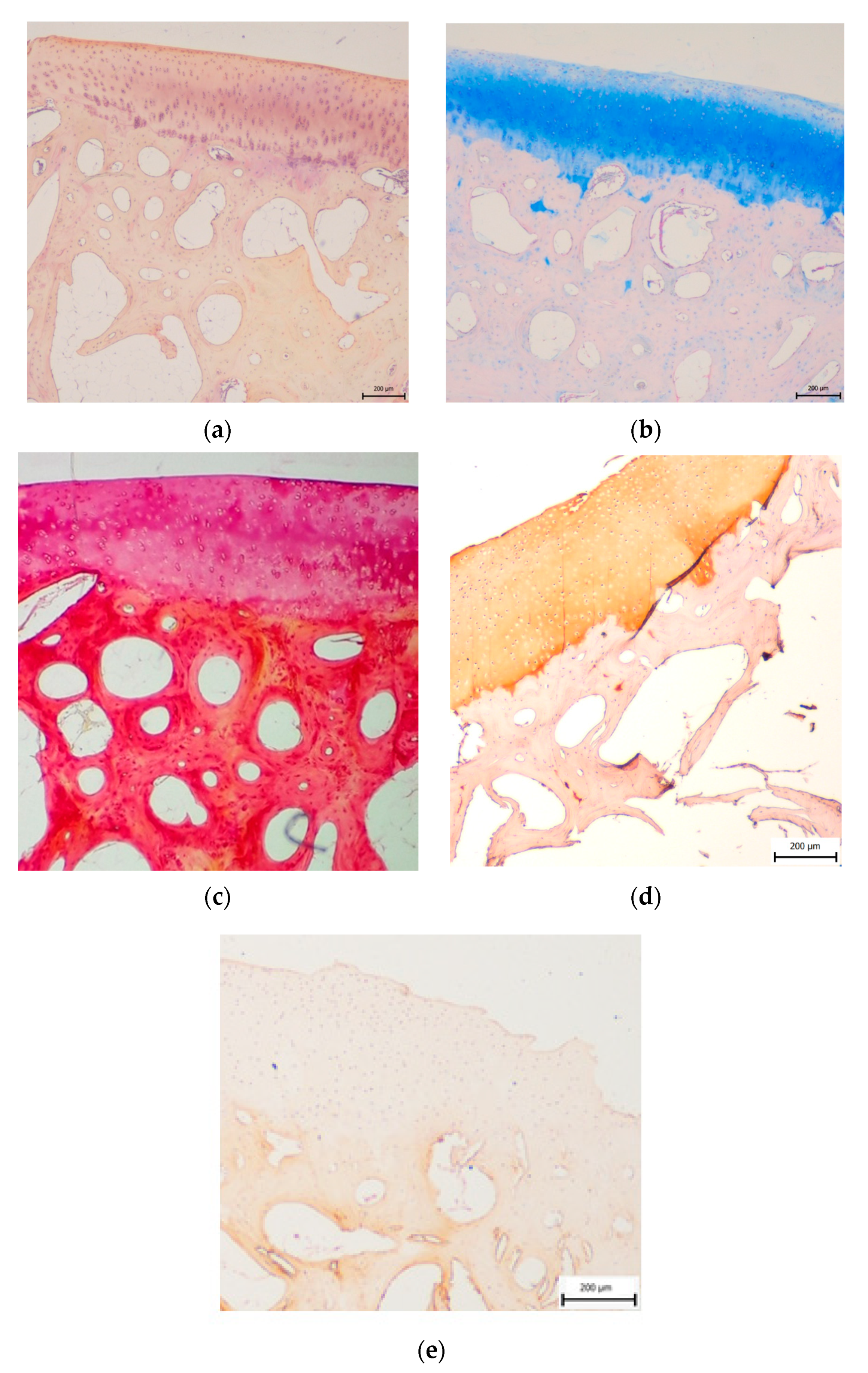
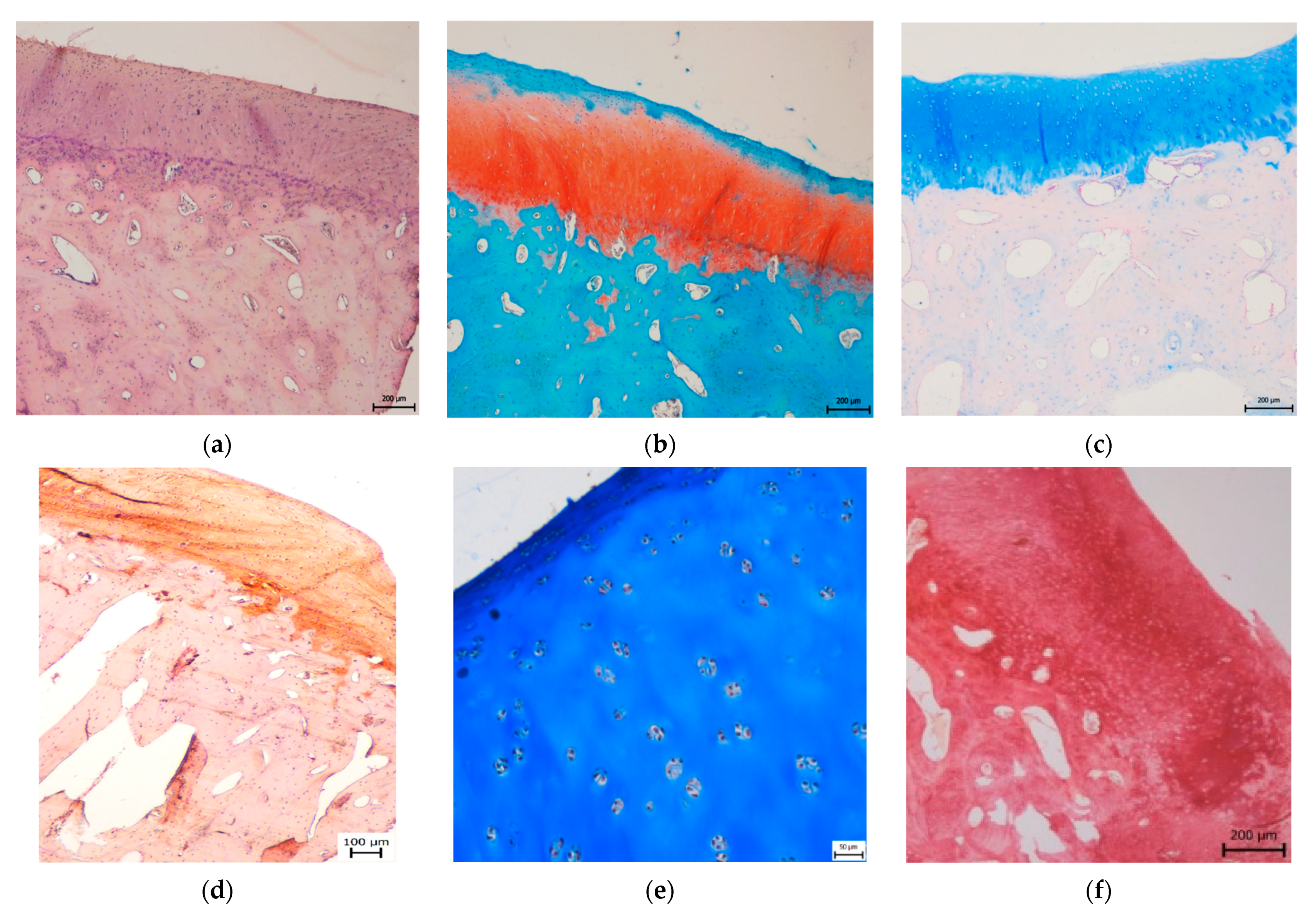
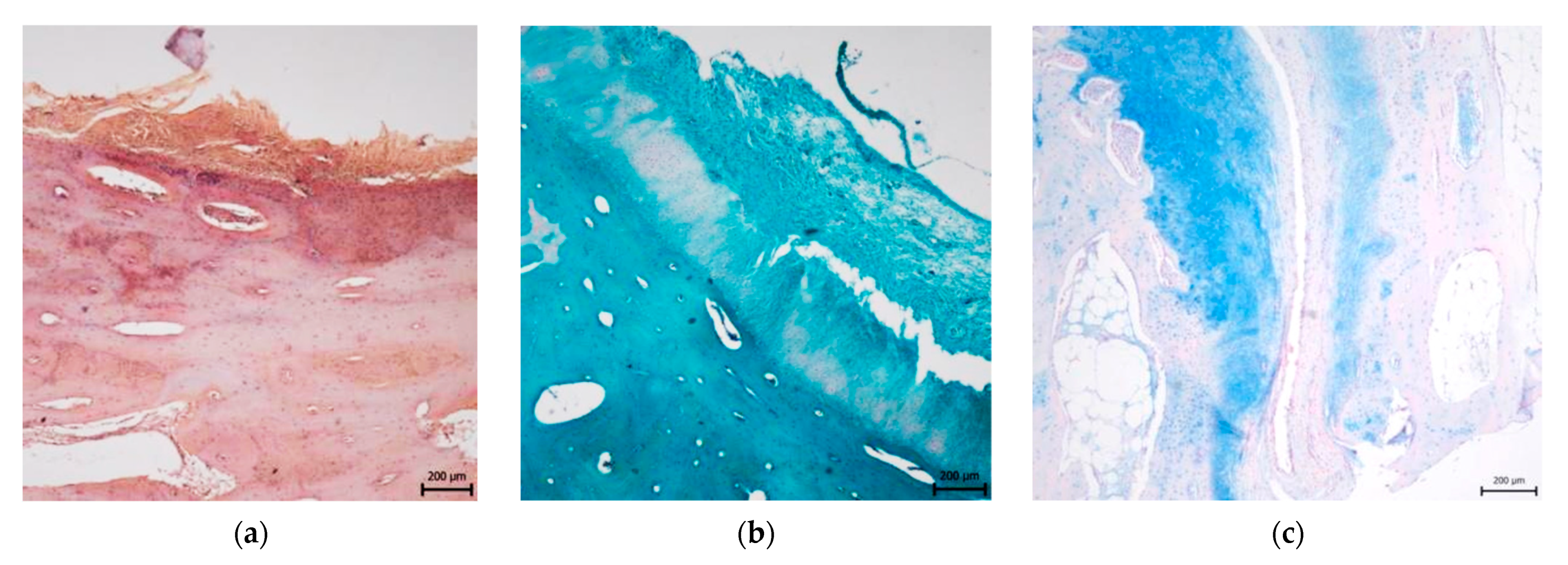
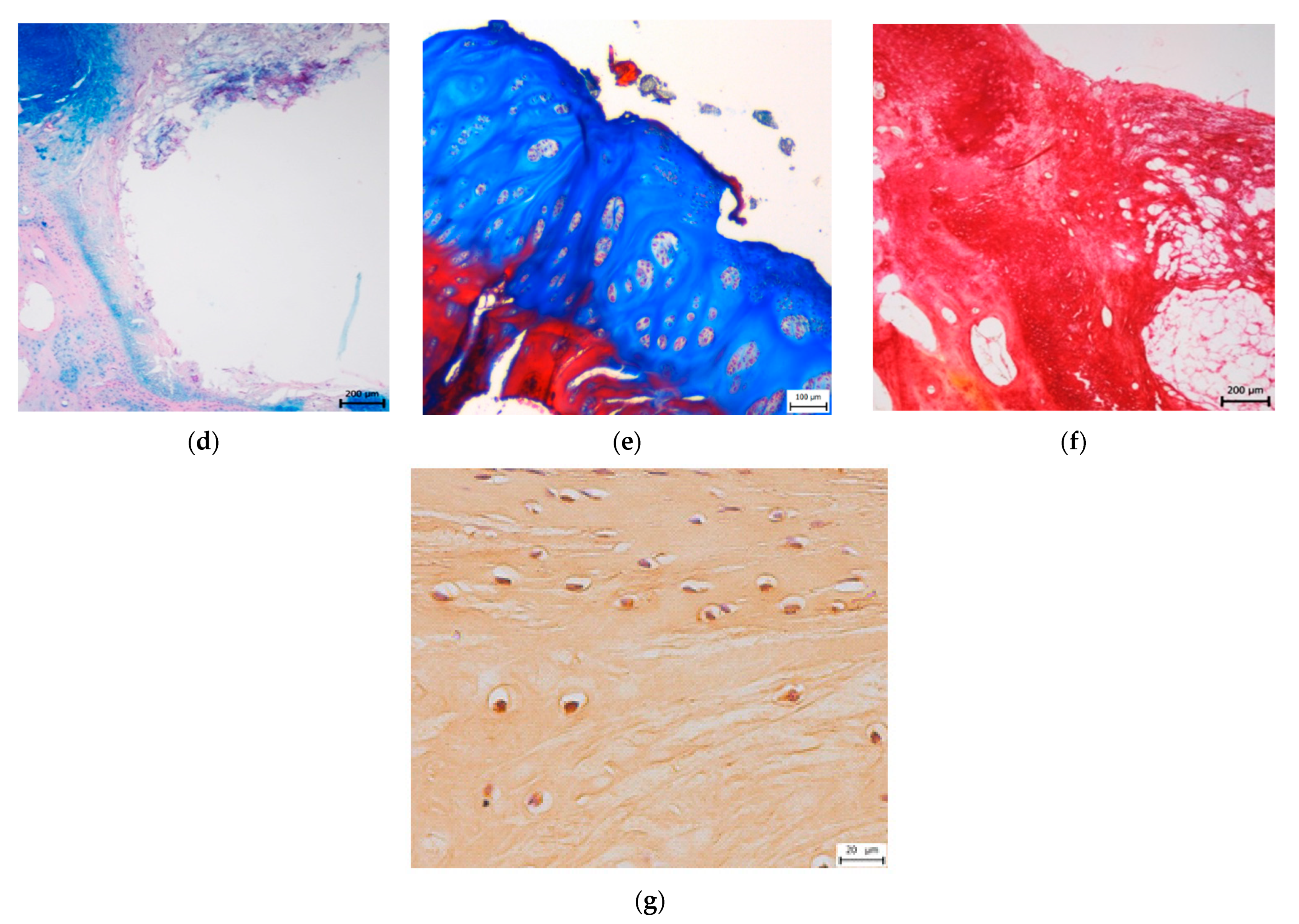
3.2. Histological Evaluation
3.2.1. Treated Osteochondral Defects
3.2.2. Untreated Osteochondral Defects
3.3. Magnetic Resonance Imaging Assessment of Cartilage Repair Tissue, Micro-CT and X-ray Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of Three-Dimensional Chitosan-Agarose-Gelatin Cryogel Scaffold for the Repair of Subchondral Cartilage Defects: An In Vivo Study in a Rabbit Model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Chhabra, A.B.; Thawait, G.K.; Andreise, G. Pre and Postoperative Imaging of Knee Articular Cartilage. In Articular Cartilage of the Knee; Gahunia, H., Gross, A., Pritzker, K., Babyn, P., Murnaghan, L., Eds.; Springer: New York, NY, USA, 2020; pp. 329–342. [Google Scholar]
- Carbone, A.; Rodeo, S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J. Orthop. Res. 2017, 35, 397–405. [Google Scholar] [CrossRef]
- Ruvinov, E.; Re’em, T.T.; Witte, F.; Cohen, S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: A 6-month study in a mini-pig model of osteochondral defects. J. Orthop. Translat. 2019, 16, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Goh, J.C.; Hutmacher, D.W.; Lee, E.H.; Zigang, G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006, 12, 1539–1551. [Google Scholar] [CrossRef]
- Grassel, S.; Ahmed, N. Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front. Biosci. 2007, 12, 4946–4956. [Google Scholar] [CrossRef]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.; Nöth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007, 9, 213–222. [Google Scholar] [CrossRef]
- Mano, J.F.; Reis, R.L. Osteochondral defects: Present situation and tissue engineering approaches. J. Tissue Engin. Reg. Med. 2007, 1, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Scheer, M.J.; Simon, T.M. Cartilage substitutes: Overview of basic science and treatment options. J. Am. Acad. Orthop. Surg. 2001, 9, 37–52. [Google Scholar] [CrossRef][Green Version]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998, 41, 1331–1342. [Google Scholar] [CrossRef]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Joint. Surg. Am. 1993, 75, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Pilichi, S.; Rocca, S.; Pool, R.R.; Dattena, M.; Masala, G.; Mara, L.; Sanna, D.; Casu, S.; Manunta, M.L.; Manunta, A.; et al. Treatment with embryonic stem-like cells into osteochondral defects in sheep femoral condyles. BMC Vet. Res. 2014, 10, 301. [Google Scholar] [CrossRef]
- Endo, J.; Watanabe, A.; Sasho, T.; Yamaguchi, S.; Saito, M.; Akagi, R.; Muramatsu, Y.; Mukoyama, S.; Katsuragi, J.; Akatsu, Y.; et al. Utility of T2 mapping and dGEMRIC for evaluation of cartilage repair after allograft chondrocyte implantation in a rabbit model. Osteoarthr. Cartil. 2015, 23, 280–288. [Google Scholar] [CrossRef][Green Version]
- Ivkovic, A.; Pascher, A.; Hudetz, D.; Maticic, D.; Jelic, M.; Dickinson, S.; Loparic, M.; Haspl, M.; Windhager, R.; Pecina, M. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010, 17, 779–789. [Google Scholar] [CrossRef]
- Murata, D.; Akieda, S.; Misumi, K.; Nakayam, K. Osteochondral Regeneration with a Scaffold-Free Three-Dimensional Construct of Adipose Tissue-Derived Mesenchymal Stromal Cells in Pigs. Tissue Eng. Regen. Med. 2018, 15, 101–113. [Google Scholar]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Getgood, A.M.J.; Kew, S.J.; Brooks, R.; Aberman, H.; Simon, T.; Lynn, A.K.; Rushton, N. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen–glycosaminoglycan biopolymer in a caprine model. Knee 2012, 19, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, R.L.; Kinard, L.A.; Lam, J.; Needham, C.J.; Lu, S.; Kasper, F.K.; Mikos, A.G. Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials 2014, 35, 7460–7469. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Brogini, S.; Pagani, S.; Giavaresi, G.; Tschon, M. Current Trends in the Evaluation of Osteochondral Lesion Treatments: Histology, Histomorphometry, and Biomechanics in Preclinical Models. Bio. Med. Res. Int. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- Cherng, A.; Takagi, S.; Chow, L.C. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J. Biomed. Mater. Res. A 1997, 35, 273–277. [Google Scholar] [CrossRef]
- Fukase, Y.; Eanes, E.D.; Takagi, S.; Chow, L.C.; Brown, W.E. Setting reactions and compressive strengths of calcium phosphate cements. J. Dent. Res. 1990, 69, 1852–1856. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015. [Google Scholar] [CrossRef]
- Ozbek, Y.Y.; Bastan, F.E.; Canikoglu, N.; Ozsarac, J. The experimental study of titanium-ions into hydroxyapatite by chemical precipitation. J. Therm. Anal. Calorim. 2016, 125, 651–658. [Google Scholar] [CrossRef]
- Dorozhin, S.V. Calcium orthophospates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Moreau, J.L.; Xu, H.H. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Yuan, H.; Li, Y.; de Bruijn, J.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef]
- Medvecky, L.; Giretova, M.; Stulajterova, R.; Danko, J.; Vdoviakova, K.; Kresakova, L.; Zert, Z.; Petrovova, E.; Holovska, K.; Varga, M.; et al. Characterization of Properties, In Vitro and In Vivo Evaluation of Calcium Phosphate/Amino Acid Cements for Treatment of Osteochondral Defects. Materials 2021, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Medvecky, L.; Giretova, M.; Sopcak, T. Preparation and properties of tetracalcium phosphate–monetite biocement. Mater. Lett. 2013, 100, 137–140. [Google Scholar] [CrossRef]
- Pineda, S.; Pollack, A.; Stevenson, S.; Goldberg, V.; Caplan, A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. 1992, 143, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Südkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; Eisenhart-Rothe, R.; Bohner, M.; Görz, L.; et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490–7505. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; Kon, E.; Perdisa, F.; Fini, M.; Di Martino, A.; Parrilli, A.; Salamanna, F.; Sandri, M.; Sartori, M.; Sprio, S.; et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. Int. J. Mol. Sci. 2019, 20, 2227. [Google Scholar] [CrossRef]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes in vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef]
- Zhou, R.; Ni, H.; Peng, J.; Liu, N.; Chen, S.; Shao, J.; Fu, Q.; Liu, J.; Chen, F.; Qian, Q. The mineralization, drug release and in vivo bone defect repair properties of calcium phosphates/PLA modified tantalum scaffolds. RSC Adv. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Vindas Bolanos, R.A.; Cokelaere, S.M.; Estrada McDermott, J.M.; Benders, K.E.M.; Gbureck, U.; Plomp, S.G.M.; Weinans, H.; Groll, J.; van Weeren, P.R.; Malda, J. The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: The importance of long-term studies in a large animal model. Osteoarthr. Cartil. 2017, 25, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef]
- Forni, M.; Bernardini, C.; Zamparini, F.; Zannoni, A.; Salaroli, R.; Ventrella, D.; Parchi, G.; Degli Esposti, M.; Polimeni, A.; Fabbri, P.; et al. Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (α-hydroxy) Acids Scaffolds for Bone Regeneration. Nanomaterials 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Dorigo, E.D.S.; Prati, C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1412–1422. [Google Scholar] [CrossRef]
- Gandolfi, T.P.; Modena, E.; Siboni, F.; Prati, C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeek, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- de Paz-Lugo, P.; Lupiáñez, J.A.; Meléndez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Takarada, T.; Osawa, M.; Hinoi, E.; Nakamura, Y.; Yoneda, Y. Differential regulation of cellular maturation in chondrocytes and osteoblasts by glycine. Cell Tissue Res. 2008, 333, 91–103. [Google Scholar] [CrossRef]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R. Human osteopenic bone-derived osteoblasts: Essential amino acids treatment effects. Artif. Cells Blood Substit. Biotechnol. 2003, 31, 35–46. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Li, G. Molecular insights into lysyl oxidases in cartilage regeneration and rejuvenation. Front. Bioeng. Biotechnol. 2020, 8, 359. [Google Scholar]
- Bullough, P.G.; Jagannath, A. The morphology of the calcification front in articular cartilage. J. Bone Joint Surg. 1983, 65, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef]
- Campanella, C.; Caruso Bavisotto, C.; Logozzi, M.; Marino Gammazza, A.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Sun, J.; Wu, J.; Liu, C.; Chen, F. An in vitro investigation of the mechanical-chemical and biological properties of calcium phosphate/calcium silicate/bismutite cement for dental pulp capping. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 141–14853. [Google Scholar]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Sahin, E. Calcium Phosphate Bone Cements. In Cement Based Materials; Saleh, H.M., Rahman, R.A., Eds.; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Lacourt, M.; Gao, C.; Li, A.; Girard, C.; Beauchamp, G.; Henderson, J.E.; Laverty, S. Relationship between cartilage and subchondral bone lesions in repetetive impact trauma-induced equine osteoarthritis. Osteoarthr. Cartil. 2012, 20, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.N.; Garbout, A.; Fereira, S.A.; Javaheri, B.; Pitsillides, A.A.; Rankin, S.M.; Jeffers, J.R.T.; Hansen, U. Propagation phase-contrast micro-computed tomography allows laboratory-based three-dimensional imaging of articular cartilage down to cellular level. Osteoarthr. Cartil. 2020, 28, 102–111. [Google Scholar] [CrossRef] [PubMed]


| Components of CAL Cement | Quantity (mg) |
|---|---|
| TTCP/monetit | 960/equimolar mixture |
| Aminoacids mixture: | 40 |
| glycine | 17.8 |
| hydroxyproline | 8.9 |
| proline | 8.9 |
| lysine | 4.4 |
| Scoring Systems | Treated Group | Control | Untreated Group | |
|---|---|---|---|---|
| CAL | C | - | ||
| modified O’Driscoll | 22.4 ± 3.1 | 20.8 ± 1.6 | 27.2 ± 1.3 | 15.6 ± 1.5 |
| Pineda | 1 ± 1 | 1.6 ± 0.5 | 0 | 3 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kresakova, L.; Danko, J.; Vdoviakova, K.; Medvecky, L.; Zert, Z.; Petrovova, E.; Varga, M.; Spakovska, T.; Pribula, J.; Gasparek, M.; et al. In Vivo Study of Osteochondral Defect Regeneration Using Innovative Composite Calcium Phosphate Biocement in a Sheep Model. Materials 2021, 14, 4471. https://doi.org/10.3390/ma14164471
Kresakova L, Danko J, Vdoviakova K, Medvecky L, Zert Z, Petrovova E, Varga M, Spakovska T, Pribula J, Gasparek M, et al. In Vivo Study of Osteochondral Defect Regeneration Using Innovative Composite Calcium Phosphate Biocement in a Sheep Model. Materials. 2021; 14(16):4471. https://doi.org/10.3390/ma14164471
Chicago/Turabian StyleKresakova, Lenka, Jan Danko, Katarina Vdoviakova, Lubomir Medvecky, Zdenek Zert, Eva Petrovova, Maros Varga, Tatiana Spakovska, Jozef Pribula, Miroslav Gasparek, and et al. 2021. "In Vivo Study of Osteochondral Defect Regeneration Using Innovative Composite Calcium Phosphate Biocement in a Sheep Model" Materials 14, no. 16: 4471. https://doi.org/10.3390/ma14164471
APA StyleKresakova, L., Danko, J., Vdoviakova, K., Medvecky, L., Zert, Z., Petrovova, E., Varga, M., Spakovska, T., Pribula, J., Gasparek, M., Giretova, M., Stulajterova, R., Kolvek, F., Andrejcakova, Z., Simaiova, V., Kadasi, M., Vrabec, V., Toth, T., & Hura, V. (2021). In Vivo Study of Osteochondral Defect Regeneration Using Innovative Composite Calcium Phosphate Biocement in a Sheep Model. Materials, 14(16), 4471. https://doi.org/10.3390/ma14164471






