Effect of TiO2 on the Sintering Behavior of Low-Grade Vanadiferous Titanomagnetite Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
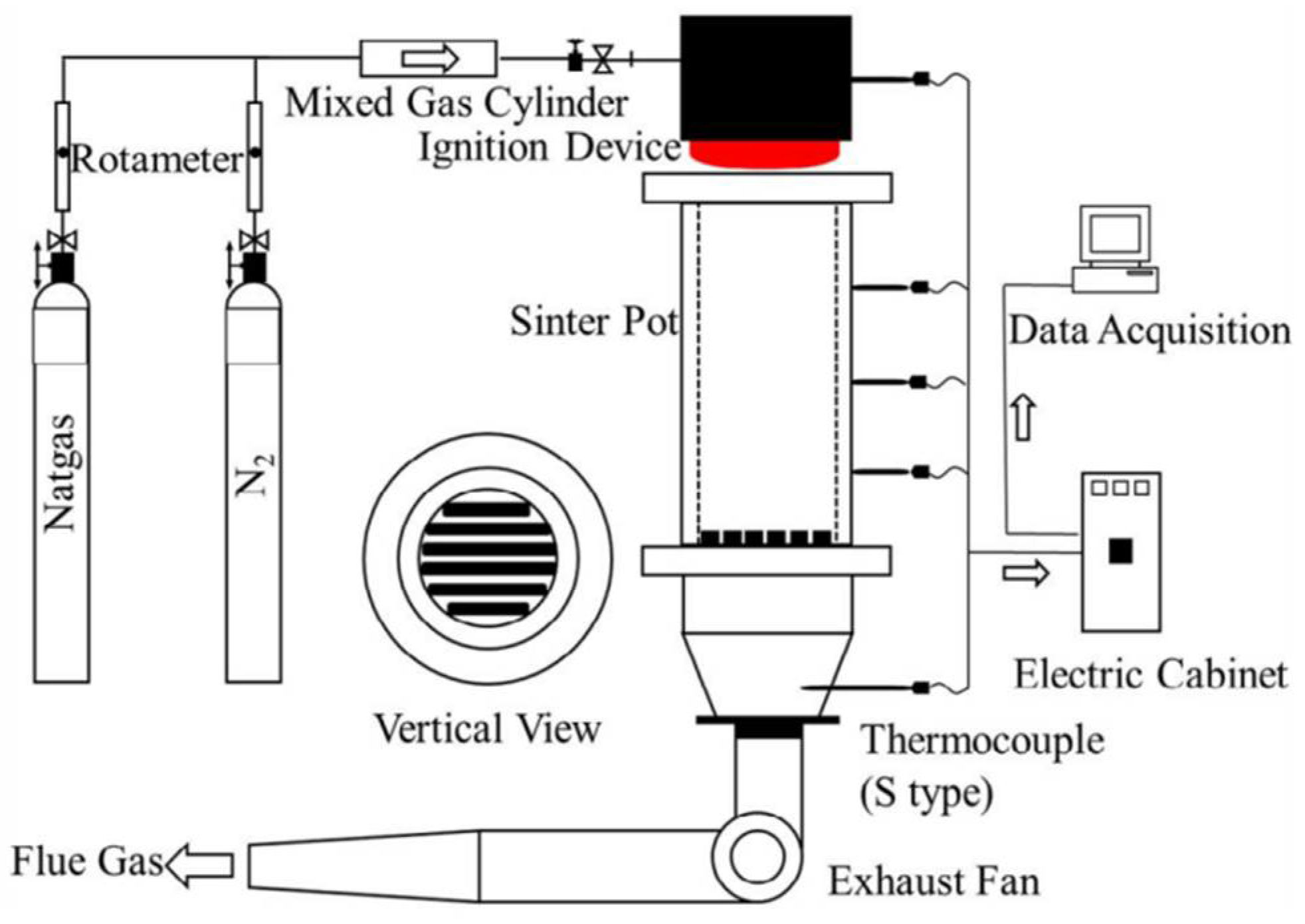
2.2. Sintering Pot Test
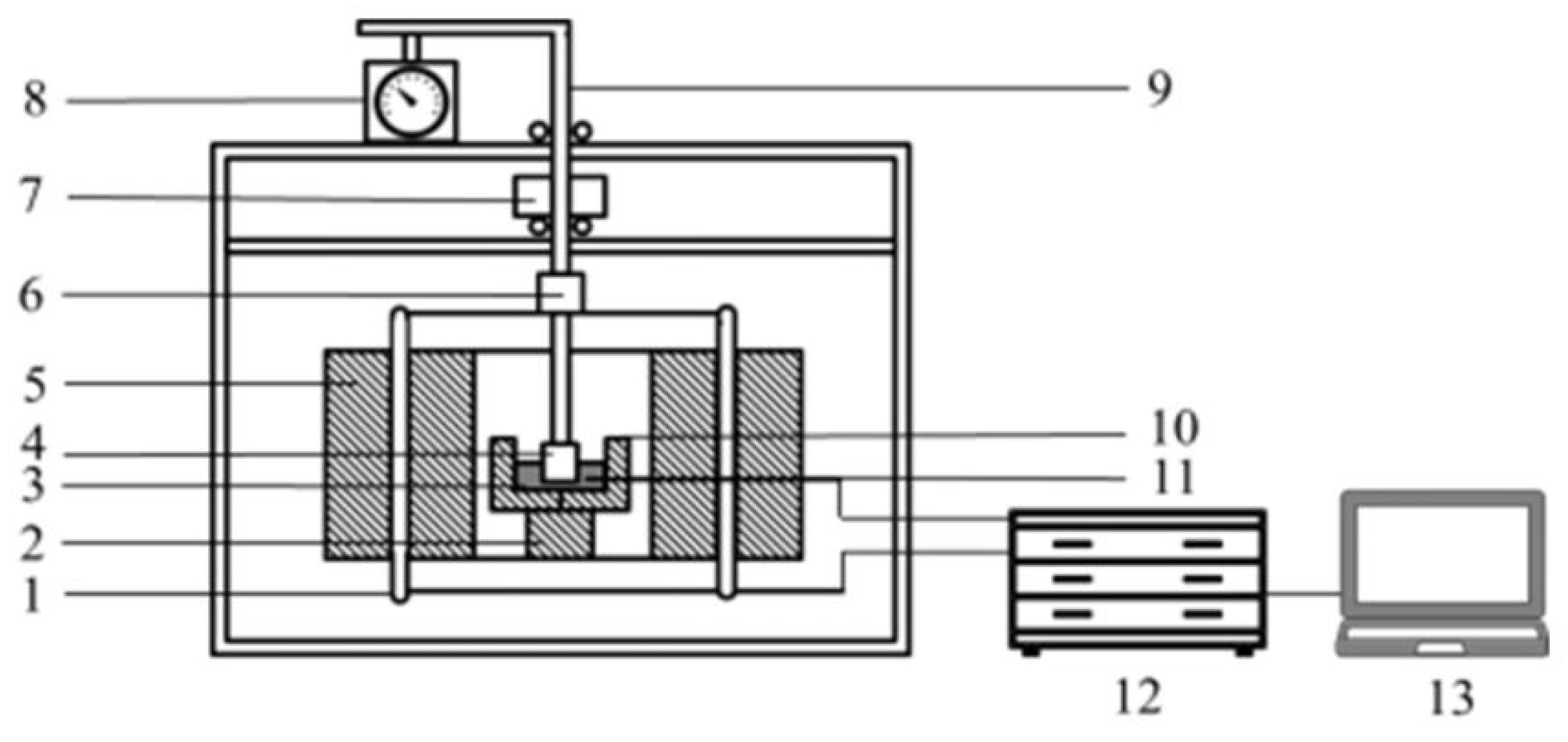
2.3. Metallurgical Properties Test and Mineral Phase Analysis
3. Results and Discussion
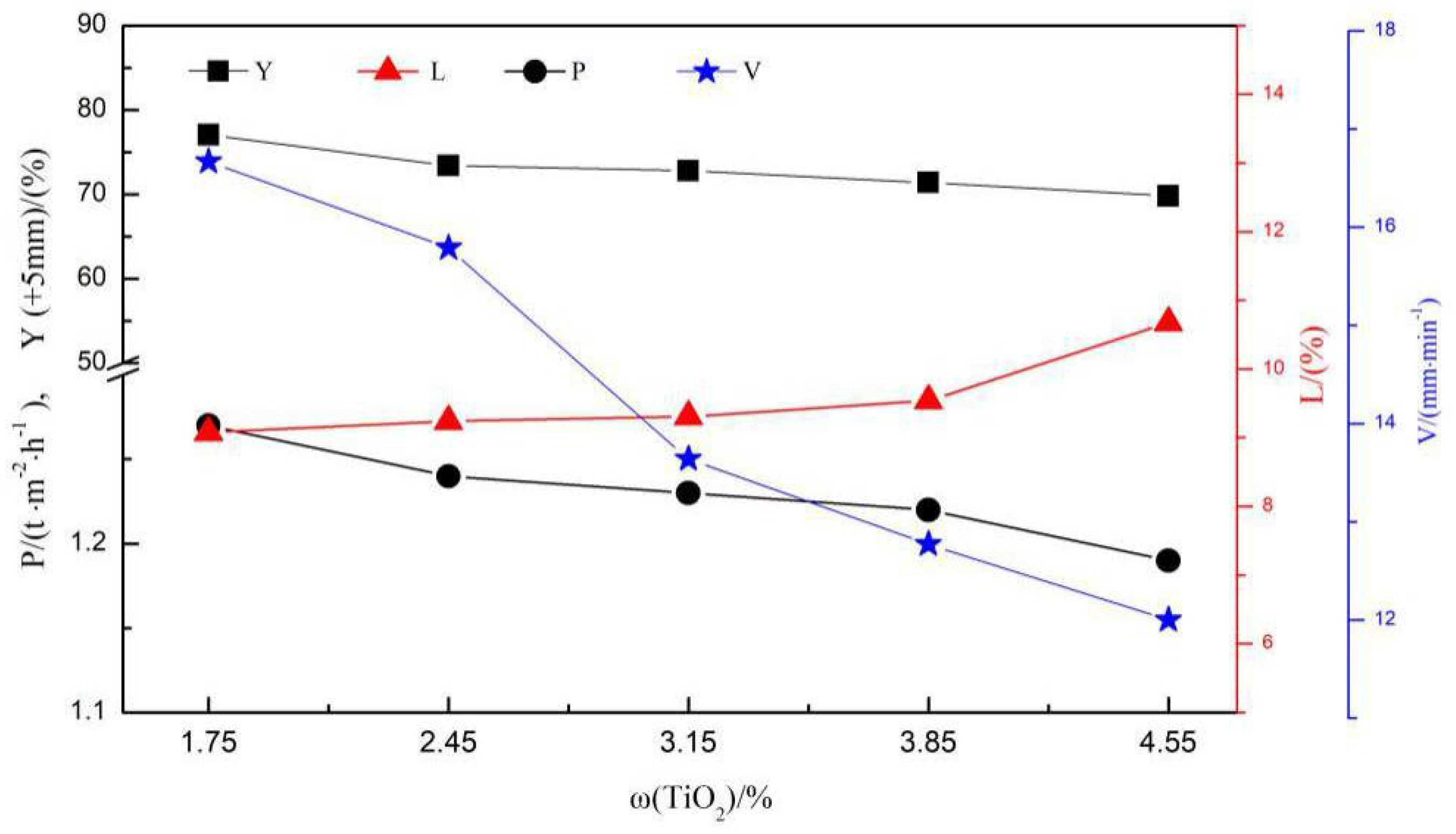
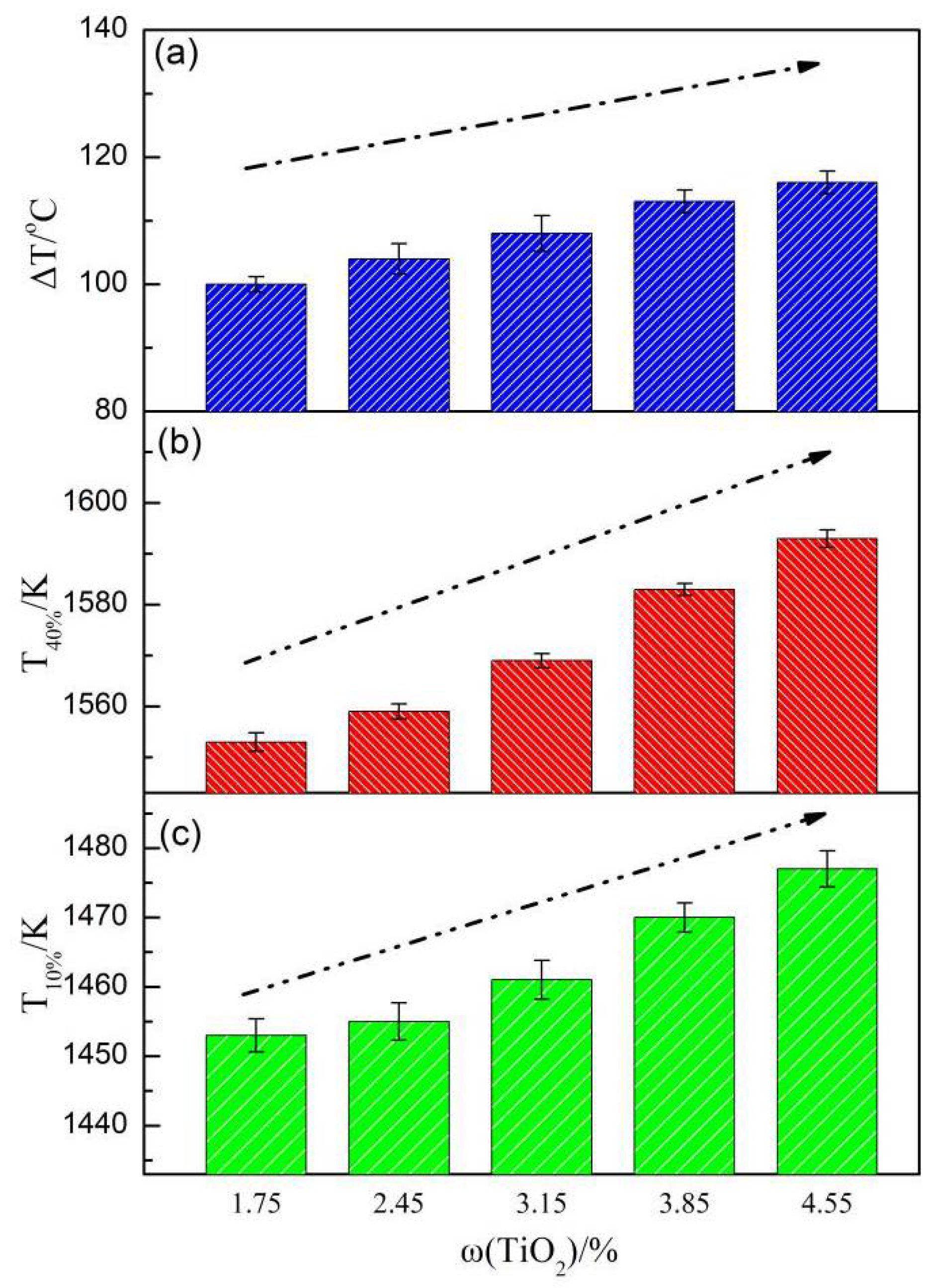
3.1. Sintering Process Parameters
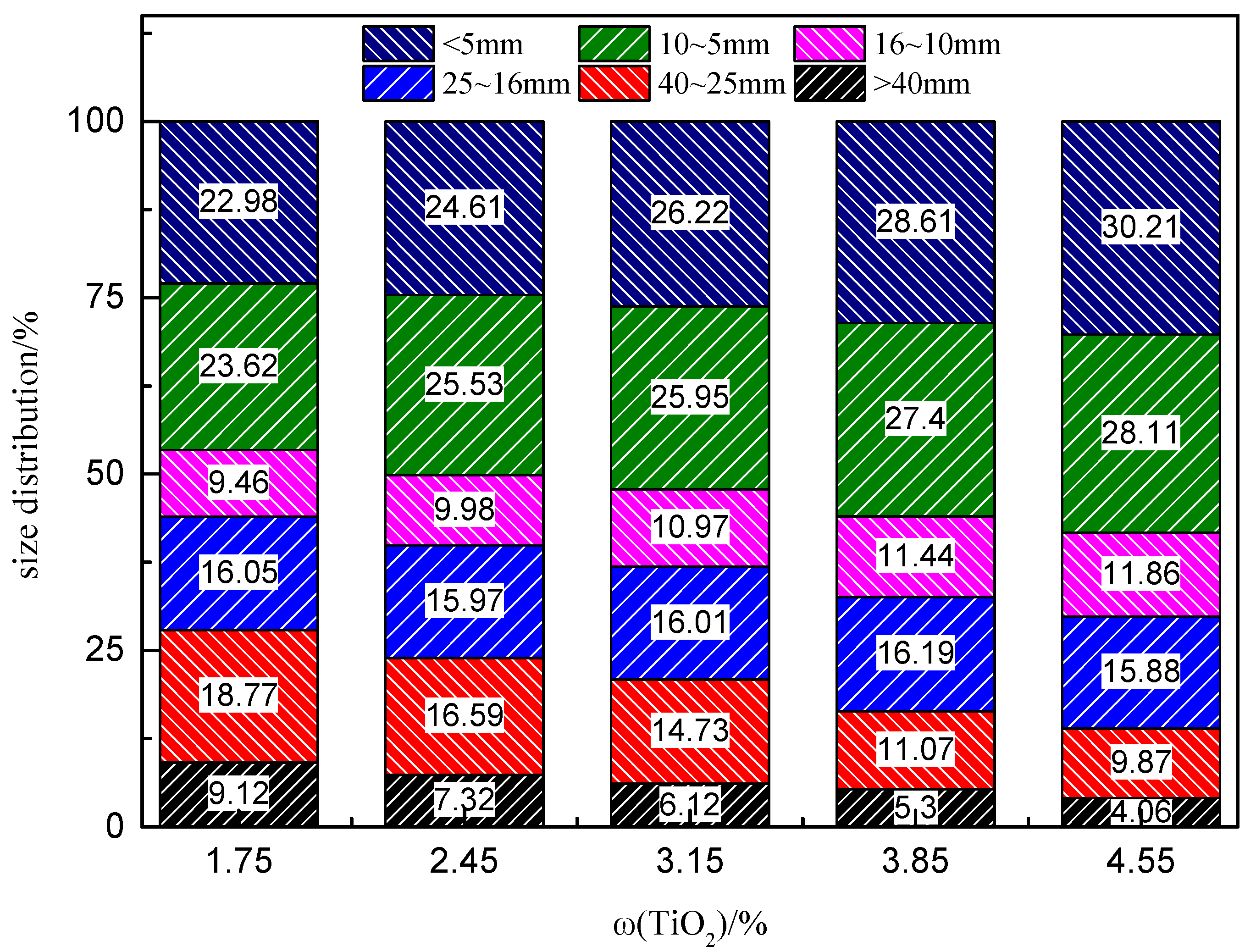
3.2. Size Distribution
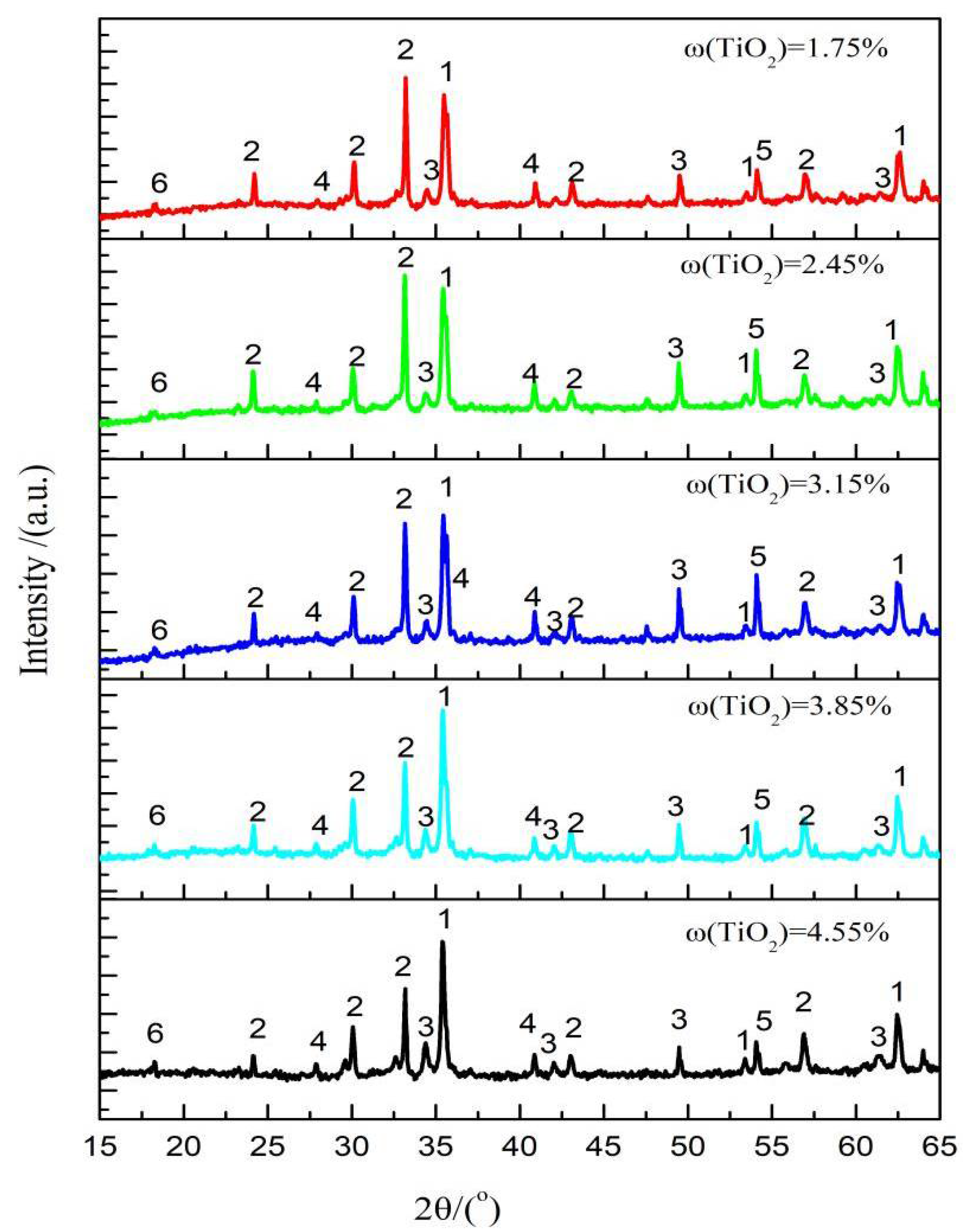
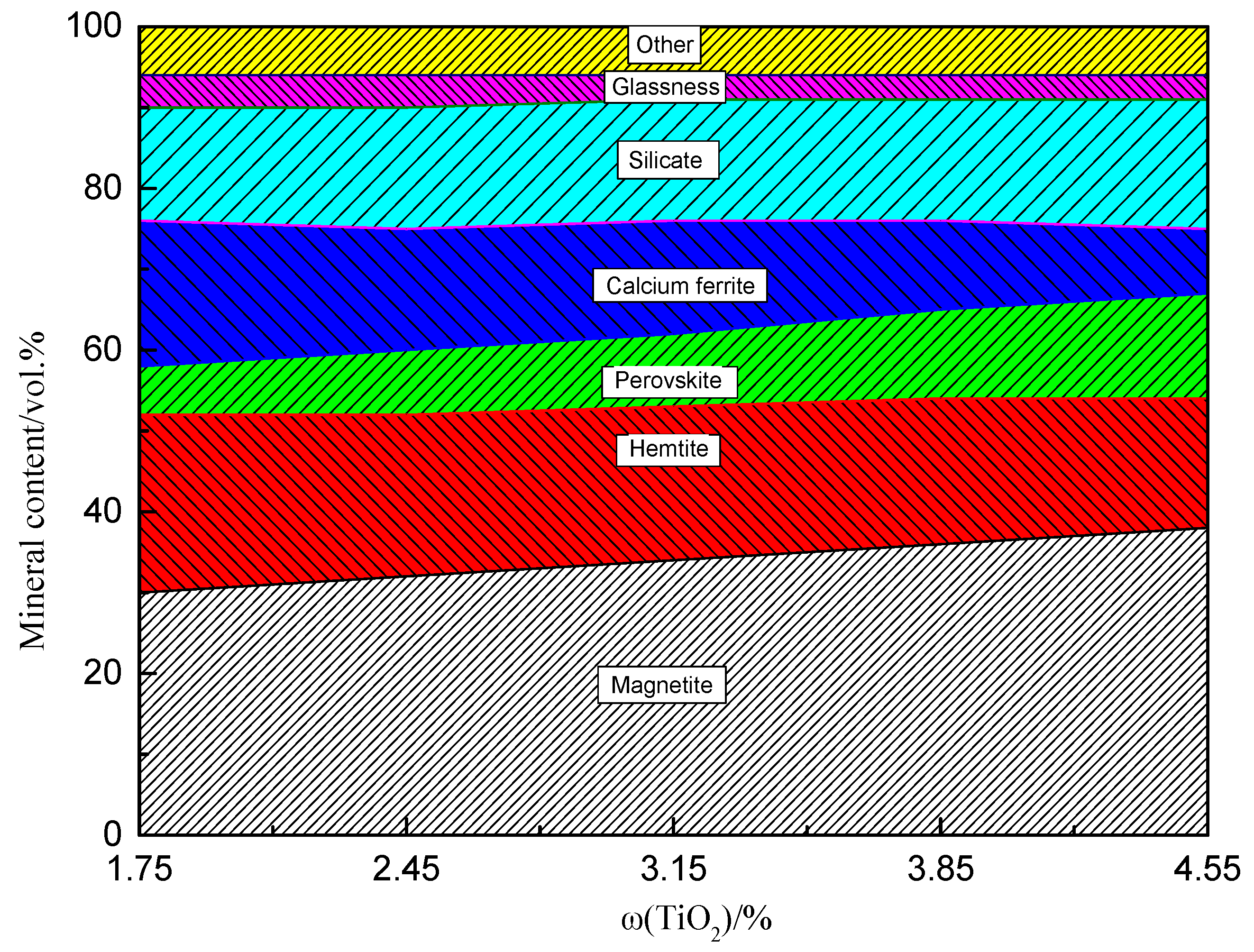
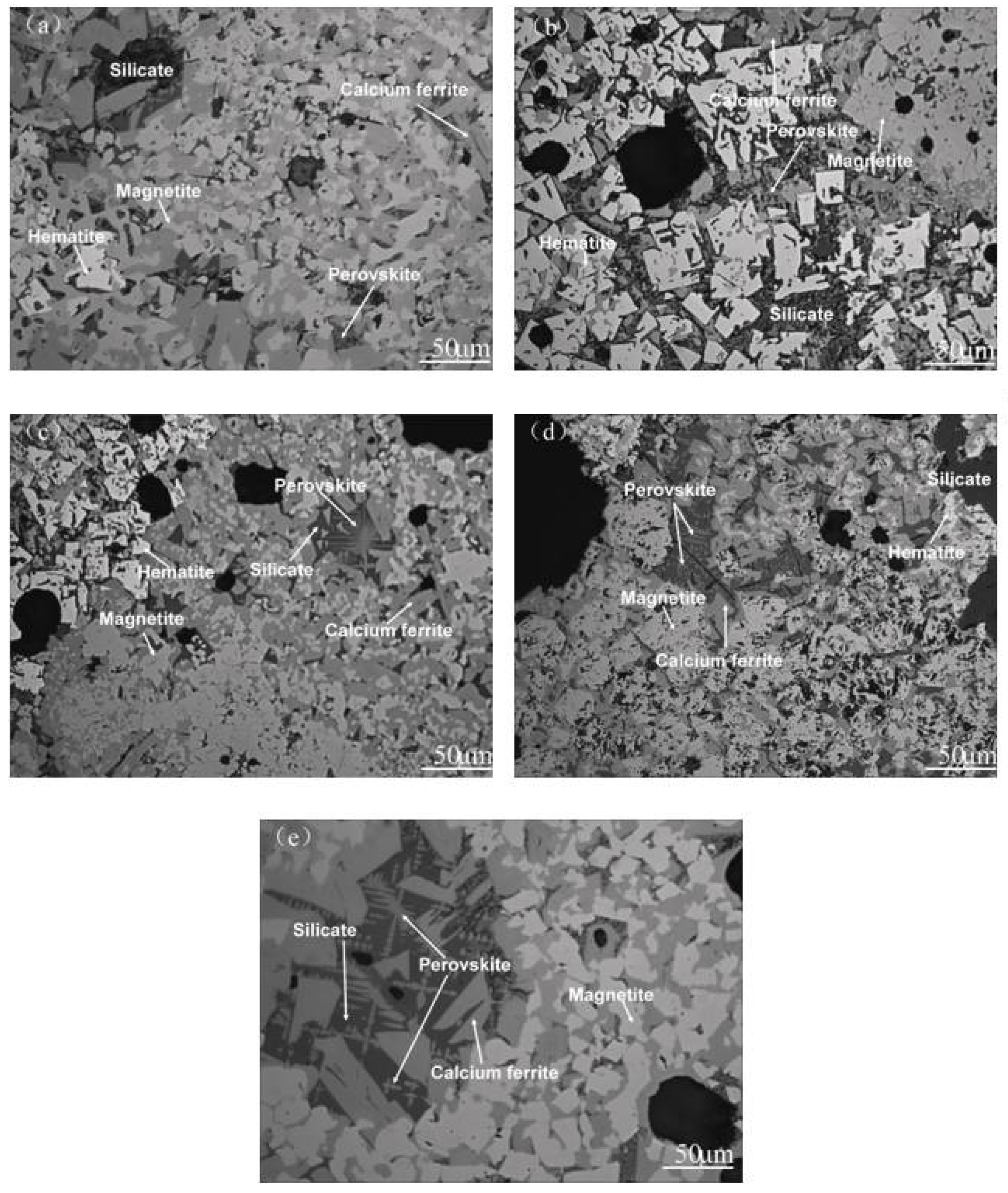
3.3. Microstructure and Mineralogy
3.4. Metallurgical Performance
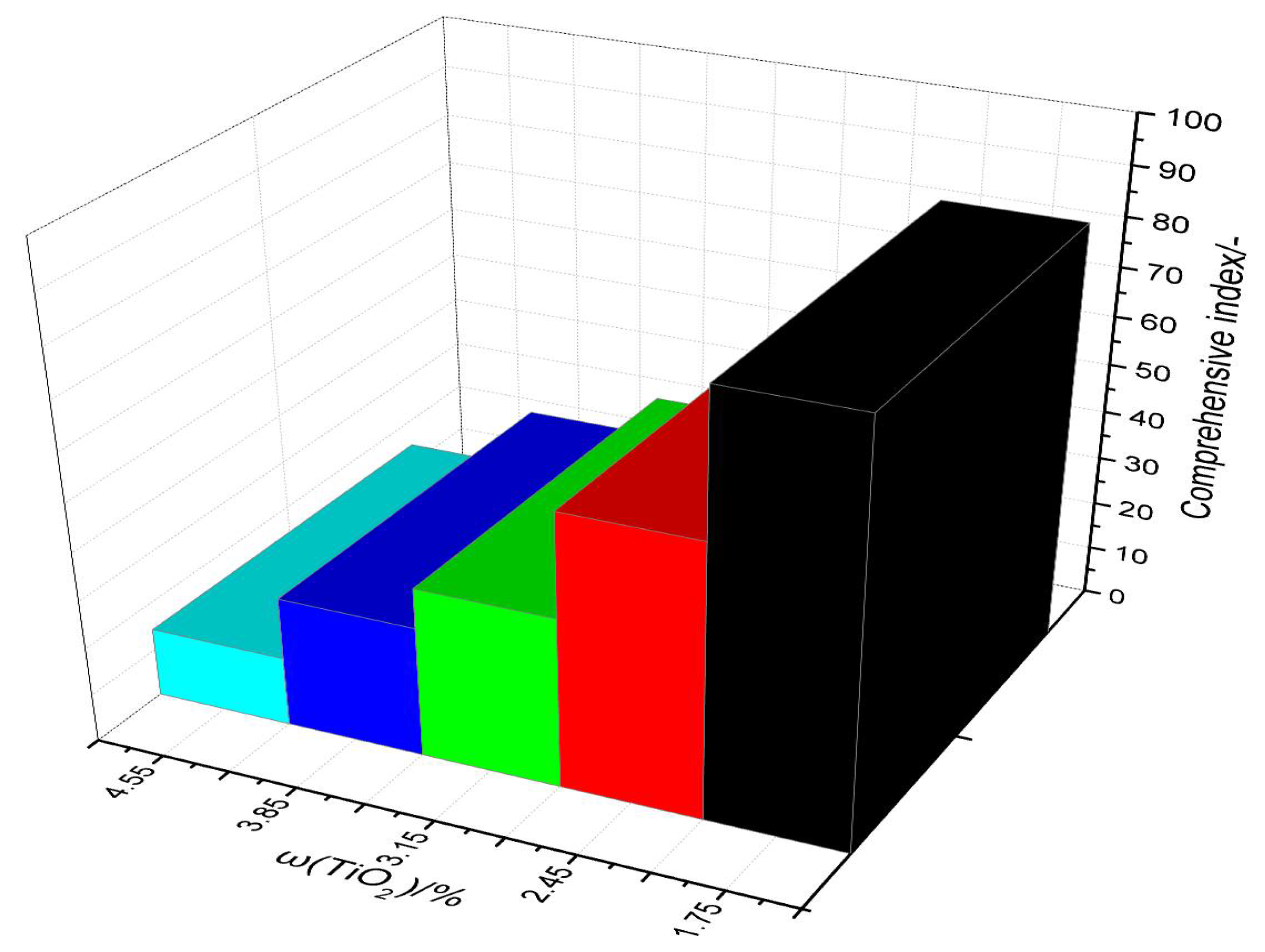
3.5. Comprehensive Index
4. Conclusions
- When the LVTM sinter TiO2 content increased from 1.75% to 4.55%, the flame front speed, P, Y, TI, RDI, and softening properties decreased and RI improved.
- The mineral composition of the LVTM sinter changed considerably when the TiO2 content was varied. As the TiO2 content increased, the magnetite and perovskite phases increased, while the hematite and calcium ferrite phases decreased.
- As the TiO2 content increased, the comprehensive index of the sinter decreased. In this study, the appropriate TiO2 content was 1.75%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takano, C.; Zambrano, A.P.; Nogueira, A.E.A.; Mourao, M.B.; Iguchi, Y. Chromites reduction reaction mechanisms in carbon-chromites composite agglomerates at 1773K. ISIJ Int. 2007, 47, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.Y.; Zou, X.L.; Lu, X.G.; Xie, X.L.; Zheng, K.; Xiao, W.; Cheng, H.W.; Li, G.S. Reductive kinetics of panzhihua ilmenite with hydrogen. Trans. Nonferr. Met. Soc. 2016, 26, 3266–3273. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, manitoba, canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Cheng, G.; Gao, Z.; Yang, H.; Xue, X. Effect of calcium oxide on the crushing strength, reduction, and smelting performance of high-chromium vanadium–titanium magnetite pellets. Metals 2017, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.J.; Xue, X.X.; Jiang, T.; Duan, P.N. Effect of TiO2 on the crushing strength and smelting mechanism of high-chromium vanadium–titanium magnetite pellets. Metall. Trans. B 2016, 47, 1713–1726. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Jiang, T.; Wang, Y.J.; Xue, X.X. Study on Sintering Characteristics of Ultra-Poor Vanadium-Titanium Magnetite. Minerals 2021, 11, 515. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Tang, W.D.; Jiang, T.; Xue, X.X.; Zhang, W.J. Influence of coke ratio on the sintering behavior of high-chromium vanadium–titanium magnetite. Minerals 2017, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Tang, W.D.; Zhou, M.; Jiang, T.; Xue, X.X.; Zhang, W.J. Effects of dolomite on mineral compositions and metallurgical properties of chromium-bearing vanadium–titanium magnetite sinter. Minerals 2017, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Zhou, M.; Jiang, T.; Wang, Y.J.; Xue, X.X. Effect of basicity on sintering behavior of low-titanium vanadium–titanium magnetite. Trans. Nonferr. Met. Soc. 2015, 25, 2087–2094. [Google Scholar] [CrossRef]
- Glazer, A.M. The Classification of tilted octahedral in perovskites. Struct. Sci. 1972, 28, 3384–3392. [Google Scholar]
- Glazer, A.M. The Simple ways of determining perovskite Strueture. Found. Crystallogr. 1975, 31, 756–762. [Google Scholar]
- Umadevi, T.; Nelson, K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of magnesia on iron ore sinter properties and productivity. Ironmak. Steelmak. 2009, 36, 515–520. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Wu, L.S.; Su, B.X.; Xing, X.D.; Zhu, G.Y. Effect of TiO2 on equilibrium phase sinter at oxygen partial pressure of 5 × 1003 atm. Ironmak. Steelmak. 2013, 41, 132–137. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Dwarapudi, S.; Ranjan, M. Influence of oxide and silicate melt phases on the RDI of iron ore pellets suitable for shaft furnace of direct reduction process. ISIJ Int. 2010, 50, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Bristow, N.J.; Loo, C.E. Sintering properties of iron ore mixes containing titanium. ISIJ Int. 1992, 32, 819–828. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.T.; Jiang, T.; Xue, X.X. Influence of MgO in form of magnesite on properties and mineralogy of high chromium, vanadium, titanium magnetite sinters. Ironmak. Steelmak. 2015, 42, 217–225. [Google Scholar] [CrossRef]
- Yin, Z.K.; Li, J.S.; Yang, S.F. Sintering pot test on improving TiO2–containing ore’s allocated proportion. Adv. Mater. Res. 2011, 311-313, 850–853. [Google Scholar] [CrossRef]
- Xue, M.; Guo, X. Effect of Al2O3 and SiO2 on formation and crystal structure of calcium ferrite containing Al2O3 and SiO2. J. Chin. Rare Earth Soc. 2008, 26, 205. [Google Scholar]
- Zhang, G.L.; Wu, S.L.; Su, B.; Que, Z.G.; Hou, C.G.; Jiang, Y. Influencing factor of sinter body strength and its effects on iron ore sintering indexes. Int. J. Miner. Metall. Mater. 2015, 22, 553–561. [Google Scholar] [CrossRef]
- Wang, Z.; Pinson, D.; Chew, S.; Rogers, H.; Monaghan, B.J.; Pownceby, M.I.; Webster, N.A.S.; Zhang, G. Behavior of New Zealand ironsand during iron ore sintering. Metall. Trans. B 2015, 47, 330–343. [Google Scholar] [CrossRef]
- Löfmark, A.; Mårtensson, G. Validation of the tool assessment of clinical education (AssCE): A study using Delphi method and clinical experts. Nurse Educ. Today 2016, 50, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, S.T.; Jiang, T.; Jiang, L.H.; Zhang, J.T.; Xue, X.X. Effects of carbon content on the sintering behavior of low-titanium vanadium-titanium magnetite. Res. Technol. 2016, 113, 612–622. [Google Scholar] [CrossRef]

| Item | TFe | SiO2 | CaO | MgO | Al2O3 | TiO2 | V2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|
| LVTM | 63.50 | 3.96 | 1.46 | 1.25 | 1.57 | 2.18 | 0.50 | 0.08 |
| HT ore | 59.61 | 4.11 | 0.87 | 1.68 | 2.26 | 6.22 | 0.70 | - |
| Indian ore | 56.06 | 5.57 | 0.06 | 0.15 | 5.63 | 0.21 | - | - |
| Malaysian ore | 51.71 | 6.57 | 0.21 | 0.15 | 8.48 | 0.33 | - | - |
| Zirong ore | 65.55 | 3.04 | 0.46 | 3.50 | 0.65 | 0.09 | - | - |
| Gas ash | 33.28 | 7.26 | 5.65 | 1.98 | 4.55 | 1.32 | 0.25 | - |
| V tailings | 30.68 | 16.97 | 2.44 | 2.82 | 1.53 | 9.81 | 1.22 | - |
| Dolomite | - | 2.38 | 45.14 | 32.05 | - | - | - | - |
| Quicklime | - | 2.49 | 83.17 | 3.48 | - | - | - | - |
| Fixed Carbon | Total Sulfur | Volatile | Ash (14.00) | ∑ | |||||
|---|---|---|---|---|---|---|---|---|---|
| FeO | Al2O3 | SiO2 | CaO | MgO | Others | ||||
| 84.00 | 0.50 | 1.50 | 0.14 | 2.72 | 7.50 | 0.48 | 0.15 | 2.89 | 100.00 |
| Item | Parameter | Item | Parameter |
|---|---|---|---|
| Sintering pot size | Φ320 mm × 700 mm | Ignition suction | 6.0 kPa |
| Sintering suction | 10.0 kPa | Granulation time | 10 min |
| Ignition temperature | 1373 K | Ignition time | 1200 S |
| Moisture | 7.5 ± 0.3% |
| Item | w (TiO2)/% | Mixed Sinter Materials/(100%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LVTM | HT Ore | Indian Ore | Malaysian Ore | Zirong Ore | Return Fine | Ash | V Tailings | Dolomite | ||
| 1 | 1.75 | 64.0 | 0 | 5 | 4 | 5 | 18 | 1 | 2 | 1 |
| 2 | 2.45 | 48.7 | 15.3 | 5 | 4 | 5 | 18 | 1 | 2 | 1 |
| 3 | 3.15 | 33.7 | 30.3 | 5 | 4 | 5 | 18 | 1 | 2 | 1 |
| 4 | 3.85 | 18.8 | 45.2 | 5 | 4 | 5 | 18 | 1 | 2 | 1 |
| 5 | 4.55 | 4.0 | 60 | 5 | 4 | 5 | 18 | 1 | 2 | 1 |
| Item | Parameter | ||
|---|---|---|---|
| Temperature | Room temperature–1173 K | 1173–1293 K | 1293–1823 K |
| Time | 87.5 min | 40 min | 106 min |
| Atmosphere | 3 L/min N2 | 9 L/min N2, 3.9 L/min CO, and 2.1 L/min CO2 | 10.5 L/min N2 and 4.5 L/min CO |
| Items | TiO2 | TFe | CaO | MgO | SiO2 | Al2O3 | V2O5 |
|---|---|---|---|---|---|---|---|
| 1 | 1.75 | 56.35 | 7.562 | 2.08 | 3.98 | 1.61 | 0.32 |
| 2 | 2.45 | 56.13 | 7.657 | 2.09 | 4.03 | 1.64 | 0.36 |
| 3 | 3.15 | 56.04 | 7.885 | 2.11 | 4.15 | 1.73 | 0.39 |
| 4 | 3.85 | 55.57 | 8.094 | 2.13 | 4.26 | 2.11 | 0.44 |
| 5 | 4.45 | 55.37 | 8.398 | 2.19 | 4.42 | 2.35 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Tang, W.; Xue, X. Effect of TiO2 on the Sintering Behavior of Low-Grade Vanadiferous Titanomagnetite Ore. Materials 2021, 14, 4376. https://doi.org/10.3390/ma14164376
Yang S, Tang W, Xue X. Effect of TiO2 on the Sintering Behavior of Low-Grade Vanadiferous Titanomagnetite Ore. Materials. 2021; 14(16):4376. https://doi.org/10.3390/ma14164376
Chicago/Turabian StyleYang, Songtao, Weidong Tang, and Xiangxin Xue. 2021. "Effect of TiO2 on the Sintering Behavior of Low-Grade Vanadiferous Titanomagnetite Ore" Materials 14, no. 16: 4376. https://doi.org/10.3390/ma14164376
APA StyleYang, S., Tang, W., & Xue, X. (2021). Effect of TiO2 on the Sintering Behavior of Low-Grade Vanadiferous Titanomagnetite Ore. Materials, 14(16), 4376. https://doi.org/10.3390/ma14164376





