Corrosion Behaviour of High-Strength Al 7005 Alloy and Its Composites Reinforced with Industrial Waste-Based Fly Ash and Glass Fibre: Comparison of Stir Cast and Extrusion Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experiment details
2.2. Gravimetric Corrosion Tests
2.3. Electrochemical Measurements
3. Results and Discussion
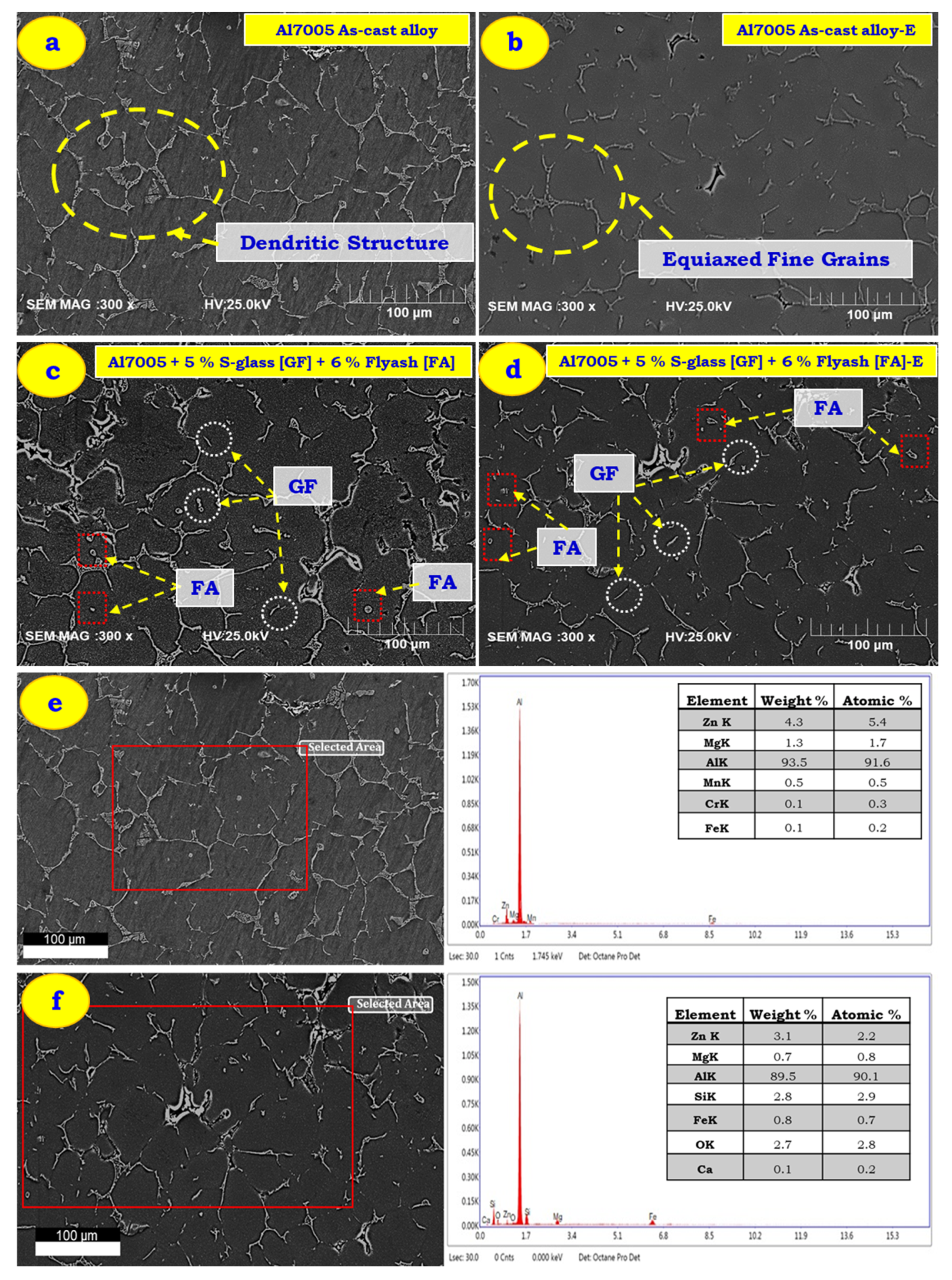
3.1. Microstructure Characterization of As-Cast and Extruded Samples
3.2. Gravimetric Corrosion Studies
3.3. Potentiodynamic Polarization Studies
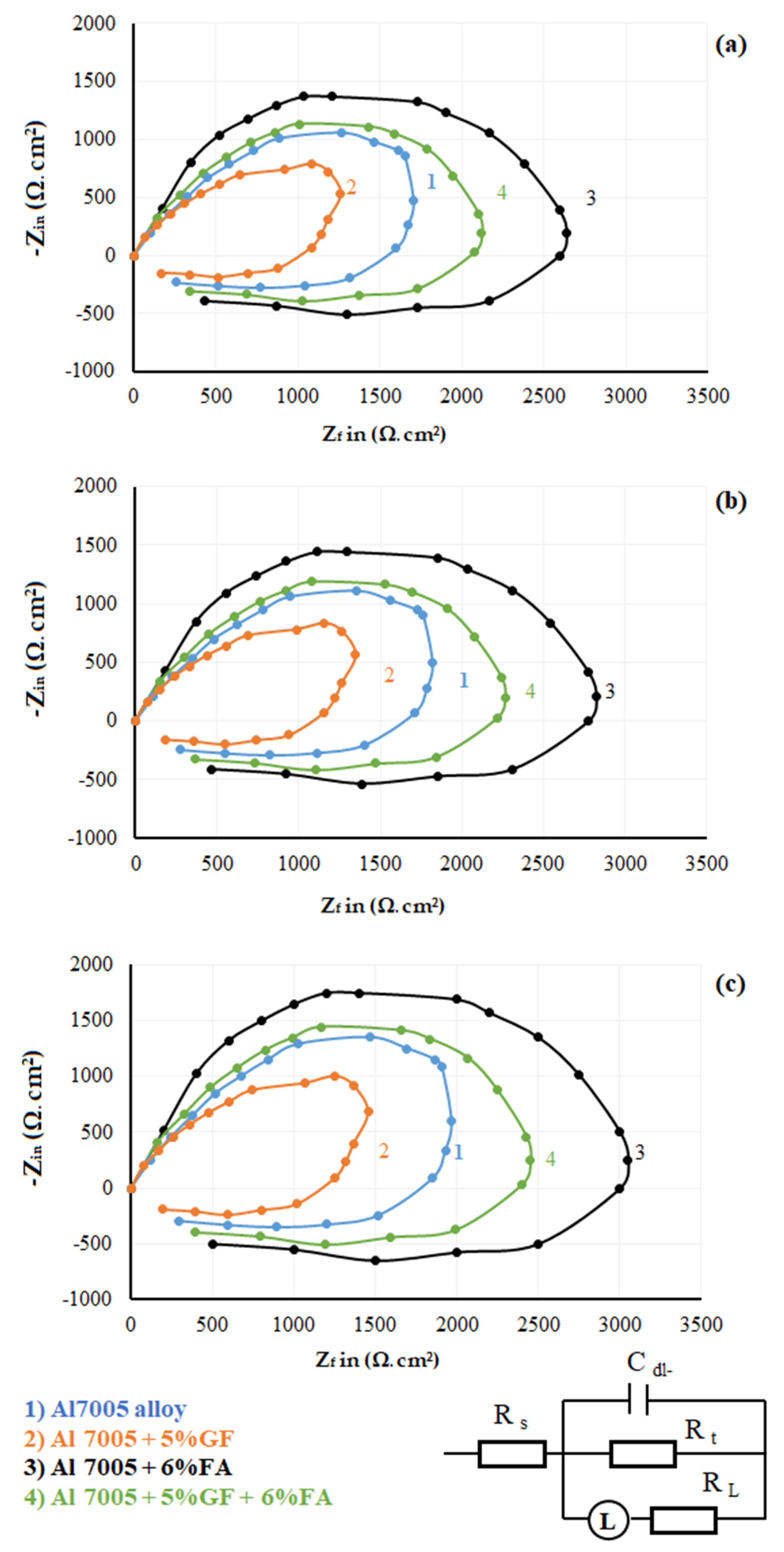
3.4. Electrochemical Impedance Spectroscopy Studies
4. Conclusions
- The presence of voids or porosities were observed in Al 7005 alloy stir cast conditions, which are reduced subjected to extrusion pressure. The glass fibre breaks and refines the grain structure of as-cast composite (Al 7005 + 5% GF + 6% FA) parts subjected to extrusion.
- The gravimetric corrosion behaviour of Al 7005 and its composites in an HCl environment showed decreased corrosion rate with increased testing duration, due to the passive layer deposited on the surface of the specimen. The corrosion rate of Al 7005 composites showed mixed behaviour for fly ash with a lower corrosion rate, but higher in the case of glass fibre.
- Polarization potentiodynamic studies showed that Al 7005 resulted in the highest corrosion rate, followed by Al 7005 + 5% GF, Al 7005 + 5% GF + 6% FA, and Al 7005 + 6% FA. High destructive corrosion current was observed with GF reinforcement, which might be due to the synergetic effect of reduction and oxidation of electrochemical process occurs at the interface of glass fibre and aluminium matrix. Furthermore, GF undergoes fibre degradation or disrupts the continuity of the glass network as a result of fibre leaching, hydrolysis and the fibre matrix interface debonding phenomenon.
- FA particles reinforced to the aluminium matrix showed improved corrosion resistance property, which might be due to the gaps or discontinuities in the form of pits or cracks filled with FA. These FA particles act as a potential site to resist corrosion by creating a surface film.
- The corrosion rate increasing with GF might be due to the formation of pits or discontinuities around the fibre particle. The area around the GF particle serves as a potential pit-initiating site.
- The impedance studies show the same nature of the behaviour of gravimetric corrosion, but a lesser significant change in corrosion behaviour. Higher diameter curves are observed with 6% FA reinforced to Al 7005 alloy (i.e., Al 7005 + 6% FA) which ensures higher corrosion resistance. FA addition ensures insulation of metal and solution interface by creating the surface film. This film contributes towards an increase in charge transfer resistance, which offers higher corrosion resistance.
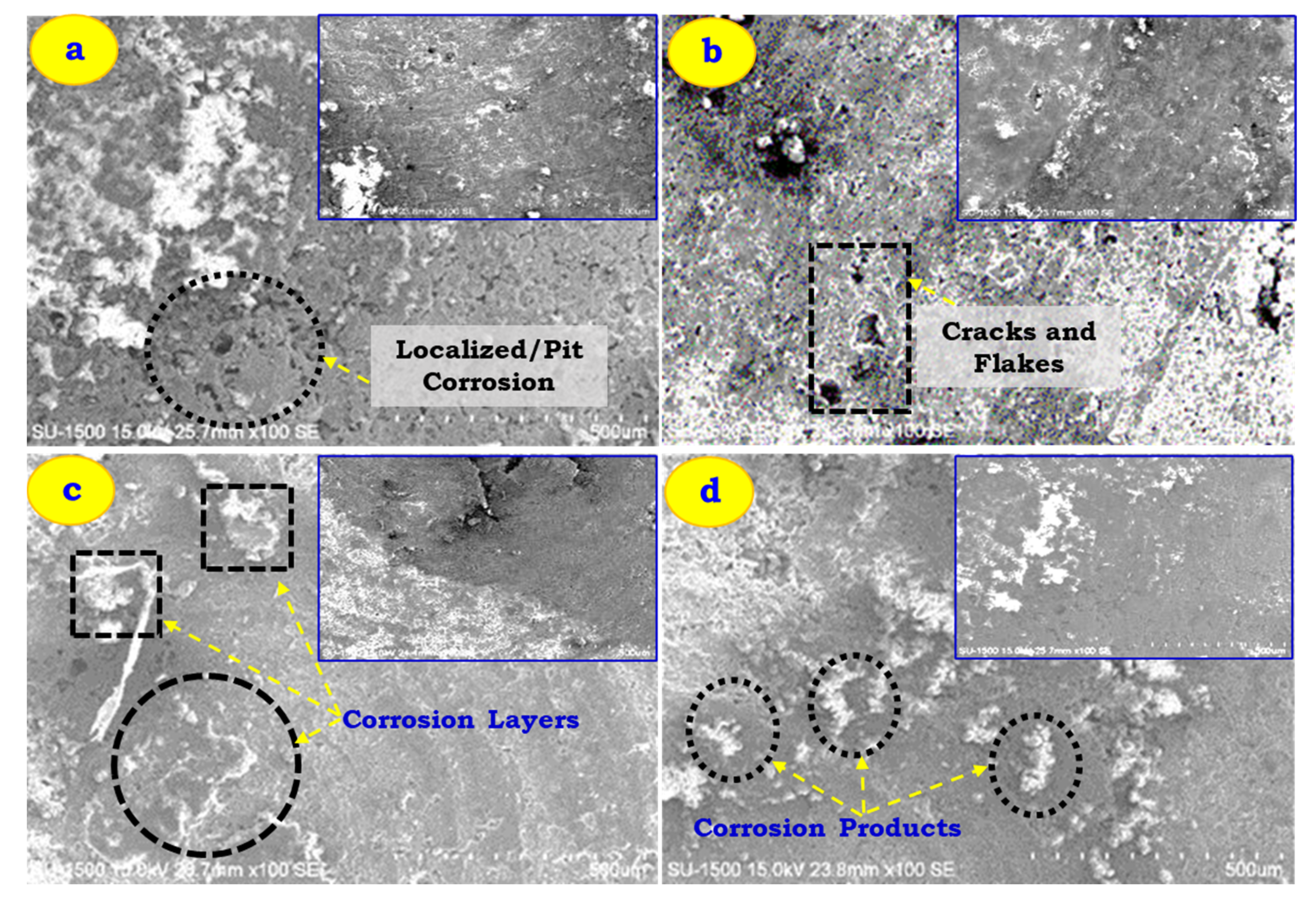
- The corrosion morphology study reveals that the corrosion layer, pits, cracks, and flakes are major contributors to the removal of material from the host material during corrosion testing. Al 7005 + 6% FA resulted in lesser corrosion products than that obtained for other samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avinash, L.; Kumar, H.; Parthasarathy, A.; Varun Kumar, K.N.; Sajjan, B. The effect of ceramic reinforcement on the microstructure, mechanical properties and tribological behavior of Al–7.5% Si–0.5% Mg alloy. Appl. Mech. Mater. 2017, 867, 3–9. [Google Scholar] [CrossRef]
- Soliman, M.S.; El Rayes, M.M.; Abbas, A.T.; Pimenov, D.Y.; Erdakov, I.N.; Junaedi, H. Effect of tensile strain rate on high-temperature deformation and fracture of rolled Al-15 vol% B4C composite. Mater. Sci. Eng. A 2019, 749, 129–136. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, S.; Roy, M. Recent developments in magnesium metal–matrix composites for biomedical applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 4748–4773. [Google Scholar] [CrossRef]
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic self-healing epoxies in polymer matrix composites (PMCs) for aerospace applications. Polymers 2021, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Boaretto, J.; Fotouhi, M.; Tende, E.; Aver, G.F.; Marcon, V.R.R.; Cordeiro, G.L.; Bergmann, C.P.; Vannucchi de Camargo, F. Biomimetics and composite materials toward efficient mobility: A Review. J. Compos. Sci. 2021, 5, 22. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Mohamed, F.A.; Lavernia, E.J. Particulate reinforced metal matrix composites—A review. J. Mat. Sci. 1991, 26, 1137–1156. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Huda, M.D.; Hashmi, M.S.J.; El-Baradie, M.A. MMCs: Materials, manufacturing and mechanical properties. Key Eng. Mater. 1995, 104, 37–64. [Google Scholar] [CrossRef]
- Kainer, K.U. (Ed.) Basics of metal matrix composites. In Metal Matrix Composites: Custom-Made Materials for Automotive and Aerospace Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–54. [Google Scholar] [CrossRef]
- Abbas, A.T.; Pimenov, D.Y.; Erdakov, I.N.; Taha, M.A.; El Rayes, M.M.; Soliman, M.S. Artificial intelligence monitoring of hardening methods and cutting conditions and their effects on surface roughness, performance, and finish turning costs of solid-state recycled Aluminum alloy 6061 chips. Metals 2018, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A review on the production of metal matrix composites through stir casting–Furnace design, properties, challenges, and research opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Lakshmikanthan, A.; Udayagiri, S.B.; Koppad, P.G.; Gupta, M.; Munishamaiah, K.; Bontha, S. The effect of heat treatment on the mechanical and tribological properties of dual size SiC reinforced A357 matrix composites. J. Mater. Res. Technol. 2020, 9, 6434–6452. [Google Scholar] [CrossRef]
- Lakshmikanthan, A.; Bontha, S.; Krishna, M.; Koppad, P.G.; Ramprabhu, T. Microstructure, mechanical and wear properties of the A357 composites reinforced with dual sized SiC particles. J. Alloys Compd. 2019, 786, 570–580. [Google Scholar] [CrossRef]
- Jeong, H.T.; Kim, W.J. Comparison of hot deformation behavior characteristics between as-cast and extruded Al-Zn-Mg-Cu (7075) aluminum alloys with a similar grain size. Materials 2019, 12, 3807. [Google Scholar] [CrossRef] [Green Version]
- Montajabnia, A.; Pourbahari, B.; Emamy, M. The microstructure and tensile properties of a newly developed Mg–Al/Mg 3 Sb 2 in situ composite in as-cast and extruded conditions. Met. Mater. Int. 2018, 24, 1099–1111. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, B.; Zhang, S. The advancement of 7XXX series aluminum alloys for aircraft structures: A Review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Kim, D.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloys Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Naik, H.R.; Manjunath, L.H.; Malik, V.; Patel, G.C.M.; Saxena, K.K.; Lakshmikanthan, A. Effect of microstructure, mechanical and wear on Al-CNTs/graphene hybrid MMC’S. Adv. Mater. Process. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Naik, M.H.; Manjunath, L.H.; Koti, V.; Lakshmikanthan, A.; Koppad, P.G.; Kumaran, S.P. Al/Graphene/CNT hybrid composites: Hardness and sliding wear studies. FME Trans. 2021, 49, 414–421. [Google Scholar] [CrossRef]
- Kareem, A.; Qudeiri, J.A.; Abdudeen, A.; Ahammed, T.; Ziout, A. A review on AA 6061 metal matrix composites produced by stir casting. Materials 2021, 14, 175. [Google Scholar] [CrossRef]
- Bhoi, N.K.; Singh, H.; Pratap, S. Developments in the aluminum metal matrix composites reinforced by micro/nano particles—A review. J. Compos. Mater. 2020, 54, 813–833. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, J.; Liu, Y.; Nie, J.; Yang, Z.; Kang, Y. Strengthening and weakening effects of particles on strength and ductility of SiC particle reinforced Al-Cu-Mg alloys matrix composites. Materials 2021, 14, 1219. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Zhang, F.; Ji, G.; Wang, M.; Ma, Y.; Ji, V.; Zhong, S.; Wu, Y.; Wang, H. Simultaneously increasing strength and ductility of nanoparticles reinforced Al composites via accumulative orthogonal extrusion process. Mater. Res. Lett. 2018, 6, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Yuan, L.; Xu, F.; Zheng, Z.; Shan, D. Refining whisker size of 2024Al/Al18B4O33w composite through extrusion and its effects on the material’s micro-structures and mechanical properties. Mater. Charact. 2018, 138, 98–106. [Google Scholar] [CrossRef]
- Kumar, S.N.; Keshavamurthy, R.; Haseebuddin, M.R.; Koppad, P.G. Mechanical properties of aluminium-graphene composite synthesized by powder metallurgy and hot extrusion. Trans. Indian Inst. Met. 2017, 70, 605–613. [Google Scholar] [CrossRef]
- Karamış, M.B.; Nair, F. Effects of reinforcement particle size in MMCs on extrusion die wear. Wear 2008, 265, 1741–1750. [Google Scholar] [CrossRef]
- Singh, J.; Alpas, A.T. Elevated temperature wear of Al6061 and Al6061–20% Al2O3. Scr. Metall. Mater. 1995, 32, 1099–1110. [Google Scholar] [CrossRef]
- Chen, J.; Yu, W.; Zuo, Z.; Li, Y.; Chen, D.; An, Q.; Wang, H.; Chen, M. Tribological properties and tool wear in milling of in-situ TiB2/7075 Al composite under various cryogenic MQL conditions. Tribol. Int. 2021, 160, 107021. [Google Scholar] [CrossRef]
- Sharma, A.; Joshi, S.S.; Datta, D.; Balasubramaniam, R. Modeling and analysis of tool wear mechanisms in diamond turning of copper beryllium alloy. J. Manuf. Process. 2020, 56, 439–450. [Google Scholar] [CrossRef]
- Behrens, B.A.; Klose, C.; Chugreev, A.; Heimes, N.; Thürer, S.E.; Uhe, J. A numerical study on co-extrusion to produce coaxial aluminum-steel compounds with longitudinal weld seams. Metals 2018, 8, 717. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Lu, S.; Liu, P.; Li, W.; Liu, X.; Chen, X.; Zhang, K.; Pan, D. Evolution of strength and fibers orientation of a short-fibers reinforced Ti-matrix composite after extrusion. Mater. Des. 2017, 126, 297–304. [Google Scholar] [CrossRef]
- Gopalraj, S.K.; Kärki, T. A review on the recycling of waste carbon fibre/glass fibre-reinforced composites: Fibre recovery, properties and life-cycle analysis. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Devanathan, R.; Ravikumar, J.; Boopathi, S.; Selvam, D.C.; Anicia, S.A. Influence in mechanical properties of stir cast aluminium (AA6061) hybrid metal matrix composite (HMMC) with silicon carbide, fly ash and coconut coir ash reinforcement. Mater. Today Proc. 2020, 22, 3136–3144. [Google Scholar] [CrossRef]
- Mamanpush, S.H.; Li, H.; Englund, K.; Tabatabaei, A.T. Recycled wind turbine blades as a feedstock for second generation composites. Waste Manag. 2018, 76, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Z.; Zhang, F.S. Advanced degradation of brominated epoxy resin and simultaneous transformation of glass fiber from waste printed circuit boards by improved supercritical water oxidation processes. Waste Manag. 2016, 56, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Ferreira, M.; Almeida, M.F. A wet dismantling process for the recycling of computer printed circuit boards. Resour. Conserv. Recy. 2018, 132, 71–76. [Google Scholar] [CrossRef]
- Mativenga, P.T. Sustainable location identification decision protocol (SuLIDeP) for determining the location of recycling centres in a circular economy. J. Clean. Prod. 2019, 223, 508–521. [Google Scholar] [CrossRef]
- Sharma, S.C.; Girish, B.M.; Satish, B.M.; Kamath, R. Mechanical properties of As-cast and heat-treated ZA-27 alloy/short glass fiber composites. J. Mater. Eng. Perform. 1997, 7, 93–99. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shakery, A.; Salahinejad, E. Aluminum-matrix composites reinforced with E-glass fibers by cross accumulative roll bonding process. J. Alloys Compd. 2019, 804, 450–456. [Google Scholar] [CrossRef]
- Sharma, S.C.; Krishna, M.; Shashishankar, A.; Vizhian, S.P. Damping behaviour of aluminium/short glass fibre composites. Mater. Sci. Eng. A 2004, 364, 109–116. [Google Scholar] [CrossRef]
- Patel, S.; Rana, R.S.; Singh, S.K. Study on mechanical properties of environment friendly Aluminium E-waste Composite with Fly ash and E-glass fiber. Mater. Today Proc. 2017, 4, 3441–3450. [Google Scholar] [CrossRef]
- Vinayaka, N.; Avinash, L.; Manjunath Patel, G.C.; Pon, C.; Vikram Kumar, S.; Jain, S.A. Srinivasan & Harsha hm (2021): Mechanical, Microstructure and Wear properties of Al 6113 Fly Ash reinforced Composites: Comparison of as-cast and Heat-treated Conditions. Adv. Mater. Process. Technol. 2021. [Google Scholar] [CrossRef]
- Matli, P.R.; Fareeha, U.; Shakoor, R.A.; Yusuf, M.; Mohamed, A.M.A.; Gupta, M. Fabrication and mechanical properties of extruded Al-SiC nanocomposites. NH Nano Hybrids. 2017, 16, 9–12. [Google Scholar] [CrossRef]
- Reddy, M.P.; Ubaid, F.; Shakoor, R.A.; Parande, G.; Manakari, V.; Mohamed, A.M.A.; Gupta, M. Effect of reinforcement concentration on the properties of hot extruded Al-Al2O3 composites synthesized through microwave sintering process. Mater. Sci. Eng. A 2017, 696, 60–69. [Google Scholar] [CrossRef]
- Esawi, A.M.; Morsi, K.; Sayed, A.; Gawad, A.A.; Borah, P. Fabrication and properties of dispersed carbon nanotube–aluminum composites. Mater. Sci. Eng. A 2009, 508, 167–173. [Google Scholar] [CrossRef]
- Reddy, M.P.; Shakoor, R.A.; Parande, G.; Manakari, V.; Ubaid, F.; Mohamed, A.M.A.; Gupta, M. Enhanced performance of nano-sized SiC reinforced Al metal matrix nanocomposites synthesized through microwave sintering and hot extrusion techniques. Prog. Nat. Sci. 2017, 27, 606–614. [Google Scholar] [CrossRef]
- Turan, M.E. Investigation of mechanical properties of carbonaceous (MWCNT, GNPs and C60) reinforced hot-extruded aluminum matrix composites. J. Alloys Compd. 2019, 788, 352–360. [Google Scholar] [CrossRef]
- Mukesh, Y.B.; Bharathesh, T.P.; Saravanan, R.; Keshavamurthy, R. Effect of hot extrusion on mechanical behaviour of boron nitride reinforced aluminium 6061-based metal matrix composites. Int. J. Mater. Eng. Innov. 2019, 10, 135–151. [Google Scholar] [CrossRef]
- Megahed, M.; Attia, M.A.; Abdelhameed, M.; El-Shafei, A.G. Tribological characterization of hybrid metal matrix composites processed by powder metallurgy. Acta Met. Sin. Eng. 2017, 30, 781–790. [Google Scholar] [CrossRef]
- Koli, D.K.; Agnihotri, G.; Purohit, R. Advanced aluminium matrix composites: The critical need of automotive and aerospace engineering fields. Mater. Today Proc. 2015, 2, 303–3041. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A.; Sharma, S.; Srivastava, A.K.; Maurya, N.K. Influence of SAC and eggshell addition in the physical, mechanical and thermal behaviour of Cr reinforced aluminium based composite. Int. J. Cast Met. Res. 2021, 34, 43–55. [Google Scholar] [CrossRef]
- Brotzu, A.; De Lellis, G.; Felli, F.; Pilone, D. Study of defect formation in Al 7050 alloys. Procedia Struct. Integr. 2017, 3, 246–252. [Google Scholar] [CrossRef]
- Deng, K.K.; Wang, X.J.; Zheng, M.Y.; Wu, K. Dynamic recrystallization behavior during hot deformation and mechanical properties of 0.2 μm SiCp reinforced Mg matrix composite. Mater. Sci. Eng. A 2013, 560, 824–830. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Nilsson, J.O. Corrosion behaviour of AA6082 Al-Mg-Si alloy extrusion: The influence of quench cooling rate. Corros Sci. 2019, 150, 100–109. [Google Scholar] [CrossRef]
- Jiang, B.; Xiang, Q.; Atrens, A.; Song, J.; Pan, F. Influence of crystallographic texture and grain size on the corrosion behaviour of as-extruded Mg alloy AZ31 sheets. Corros Sci. 2017, 126, 374–380. [Google Scholar] [CrossRef]
- Wang, S.; Luo, B.; Bai, Z.; He, C.; Tan, S.; Jiang, G. Effect of Zn/Mg ratios on microstructure and stress corrosion cracking of 7005 alloy. Materials 2019, 12, 285. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.G.; Lin, P.; Man, C.; Cui, J.; Wang, H.B.; Dong, C.F.; Li, X.G. Prediction model for atmospheric corrosion of 7005-T4 aluminum alloy in industrial and marine environments. Int. J. Miner. Metall. Mater. 2018, 25, 1313–1319. [Google Scholar] [CrossRef]
- Qin, C.; Gou, G.Q.; Che, X.L.; Chen, H.; Chen, J.; Li, P.; Gao, W. Effect of composition on tensile properties and fracture toughness of Al–Zn–Mg alloy (A7N01S-T5) used in high speed trains. Mater. Des. 2016, 91, 278–285. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Yang, X.K.; Zhang, L.W.; Zhang, S.Y.; Liu, M.; Zhou, K.; Mu, X.L. Properties degradation and atmospheric corrosion mechanism of 6061 aluminum alloy in industrial and marine atmosphere environments. Mater. Corros. 2017, 68, 529–535. [Google Scholar] [CrossRef]
- Blücher, D.B.; Svensson, J.E.; Johansson, L.G. Influence of ppb levels of SO2 on the atmospheric corrosion of aluminum in the presence of NaCl. J. Electrochem. Soc. 2005, 152, B397. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, Q.; Li, D.; Wen, J. Long-term atmospheric corrosion behaviour of aluminium alloys 2024 and 7075 in urban, coastal and industrial environments. Corros Sci. 2009, 51, 719–727. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Strzępek, P.; Mamala, A.; Noga, P. Reinforcement of Aluminium-Matrix Composites with Glass Fibre by Metallurgical Synthesis. Materials 2020, 13, 5441. [Google Scholar] [CrossRef]
- Kumar, S.P.; Shantharaja, M.; Vinyas, M. The effect of reinforcements on mechanical properties of A7005 hybrid MMCs. AIP Conf. Proc. 2020, 2204, 040021. [Google Scholar] [CrossRef]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing and Design, 3rd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Debnath, S.; Lancaster, L.; Lung, M.H. Utilization of agro-industrial waste in metal matrix composites: Towards sustainability. World Acad. Sci. Eng. Technol. 2013, 7, 1136–1144. [Google Scholar]
- Luo, Y.; Zhang, Z.; Li, B.; Gao, M.; Qiu, Y.; He, M. Effects of annular electromagnetic stirring coupled with intercooling on grain refinement and homogeneity during direct chill casting of large-sized 7005 alloy billet. JOM 2017, 69, 2640–2643. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Krishna, M.; Vijayalakshmi, V.T.; Uchil, J. Corrosion behaviour of garnet particulate reinforced LM13 Al alloy MMCs. Corros Sci. 2002, 44, 917–925. [Google Scholar] [CrossRef]
- Bobić, B.; Mitrovic, S.; Babic, M.; Bobić, I. Corrosion of aluminium and zinc-aluminium alloys based metal-matrix composites. Tribol. Ind. 2009, 31, 44–53. [Google Scholar]
- Seah, K.H.W.; Sharma, S.C.; Girish, B.M. Corrosion characteristics of ZA-27-graphite particulate composites. Corros Sci. 1997, 39, 1–7. [Google Scholar] [CrossRef]
- Sharma, S.C.; Somashekar, D.R.; Satish, B.M. A note on the corrosion characterisation of ZA-27/zircon particulate composites in acidic medium. J. Mater. Process. Technol. 2001, 118, 62–64. [Google Scholar] [CrossRef]
- Le Bozec, N.; Blandin, N.; Thierry, D. Accelerated corrosion tests in the automotive industry: A comparison of the performance towards cosmetic corrosion. Mater. Corros. 2008, 59, 889–894. [Google Scholar] [CrossRef]
- Verma, R.; Randhawa, J.S.; Kant, S.; Suri, N.M. Characterization Studies of Slurry-Sprayed Mullite–Nickel Coatings on ASTM 1018 Steel. Arab. J. Sci. Eng. 2019, 44, 5897–5919. [Google Scholar] [CrossRef]
- Mehra, R.; Soni, A. Cast iron deterioration with time in various aqueous salt solutions. Bull. Mater. Sci. 2002, 25, 53–58. [Google Scholar] [CrossRef]
- Dasgupta, R.; Das, S.; Chaturvedi, S.; Jha, A.K. Effect of extrusion on properties of Al-based composite. Trans. Nonferrous Met. Soc. China 2010, 20, 2229–2233. [Google Scholar] [CrossRef]
- Trdan, U.; Grum, J. Evaluation of corrosion resistance of AA6082-T651 aluminium alloy after laser shock peening by means of cyclic polarisation and ElS methods. Corros. Sci. 2012, 59, 324–333. [Google Scholar] [CrossRef]
- Almomani, M.; Hayajneh, M.T.; Draidi, M. Corrosion investigation of zinc−aluminum alloy matrix (ZA-27) reinforced with alumina (Al2O3) and fly ash. Part. Sci. Technol. 2017, 35, 439–447. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, Z.; Tian, Z.; Huang, W.; Wu, J.; Fan, Z. Corrosion behaviour of low dielectric glass fibres in hydrochloric acid. J. Non Cryst. Solids 2019, 511, 212–218. [Google Scholar] [CrossRef]
- Sharma, B.; Chhibber, R.; Mehta, R. Seawater ageing of glass fiber reinforced epoxy nanocomposites based on silylated clays. Polym. Degrad. Stab. 2018, 147, 103–114. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Chen, B. Effect of aging on the corrosion behavior of 6005 Al alloys in 3.5 wt% NaCl aqueous solution. J. Mater. Res. 2018, 33, 1830–1838. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, N.; Wu, J.; Jiang, Y.; Li, J. Effect of surface roughness on pitting corrosion of 2205 duplex stainless steel investigated by electrochemical noise measurements. Materials 2019, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
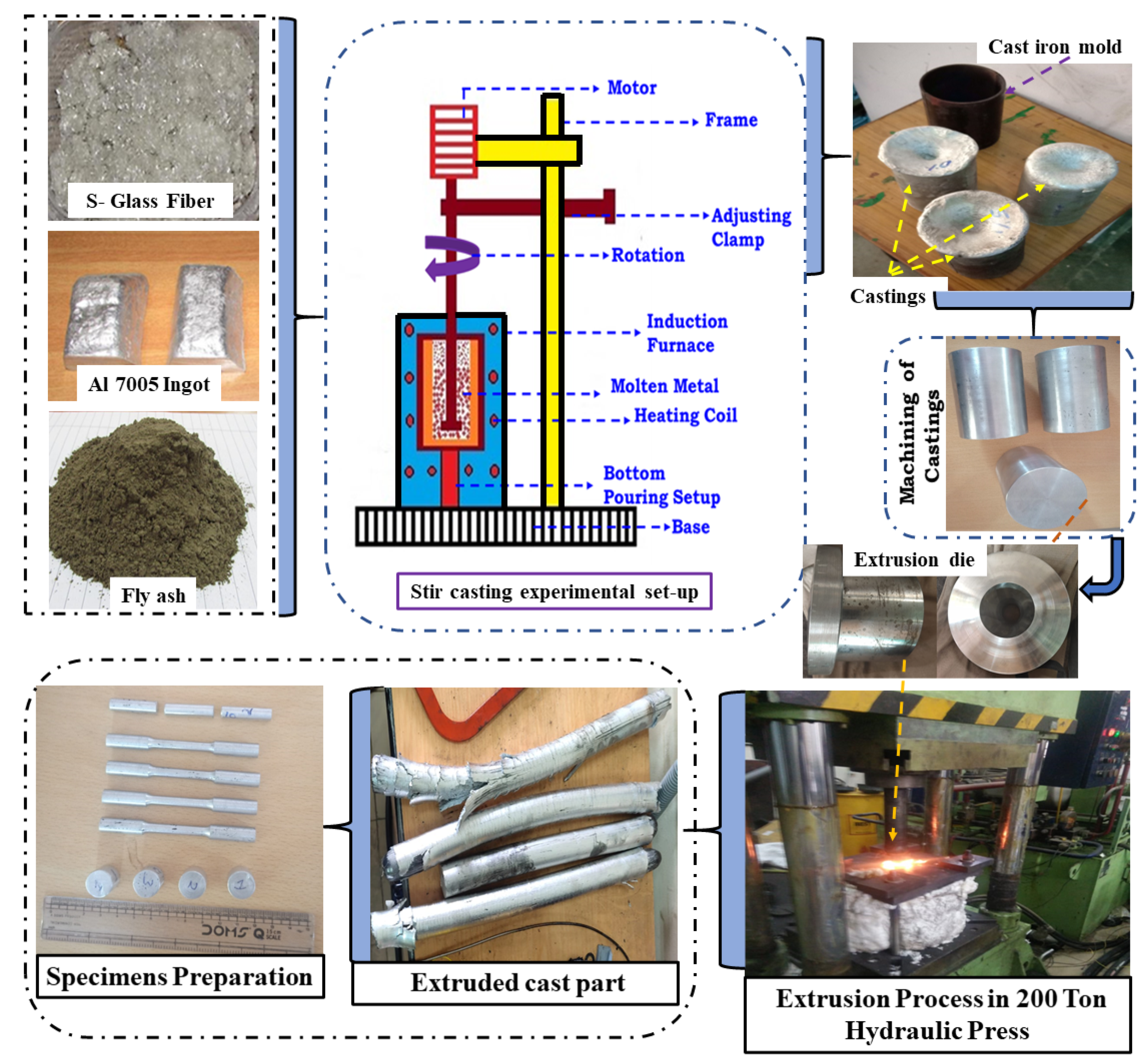
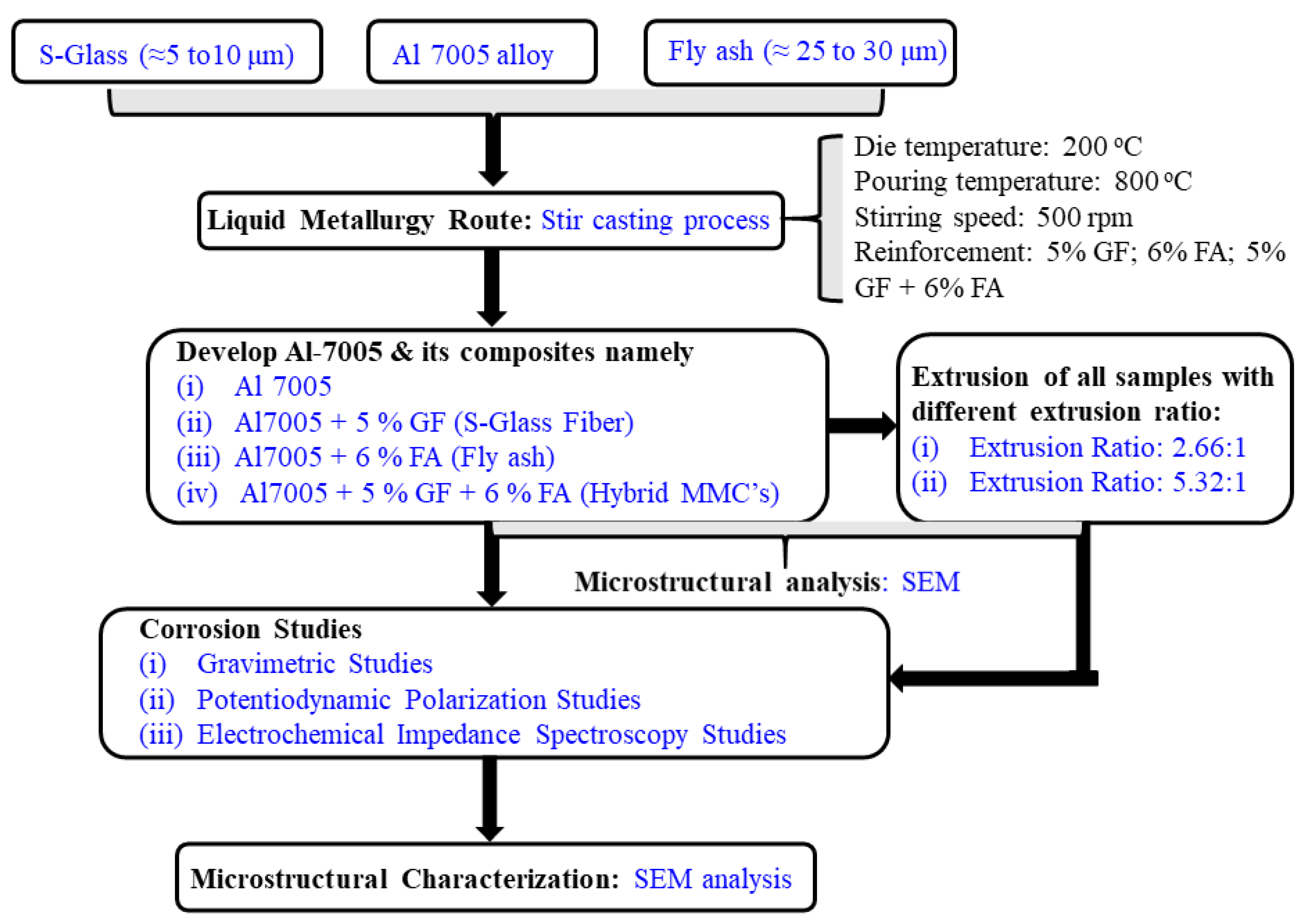


| Matrix | Reinforcement Particles and Amount | Size of Reinforcements | Remark | Reference |
|---|---|---|---|---|
| Pure Al | SiC and 0–1.5% vol. | 15 nm and ER: 20.25:1 | ↑ 121% H and ↑ 11% TS | [43] |
| Pure Al | Al2O3 and 5–15% vol. | 45 µm and ER: 20.25:1 | ↑ 59.78% H and ↑ 24.67% TS | [44] |
| Pure Al | MWCNT and 2% wt. | 140 ± 30 nm outer ⌀, 4–8 nm inner ⌀, and ER: 4:1 | ↑ 3 times higher in H and ↑ 21% higher in TS | [45] |
| Al2024 | Al18B4O33 and 25% vol. | ⌀: 0.5–1 µm, length 10–20 µm, ER: 9:1, 16:1, 25:1 | ↑ 2 times higher in TS, refined grained structure | [24] |
| Al–Zn–Mg–Cu composite | TiB2 and 6% wt. | <100 nm | ↑ 482 MPa to 687 MPa in TS and ↑ ductility from 2% to 14.8%. | [23] |
| Pure Al | SiC and 0.3–1.5% vol. | 15 nm and ER: 20.25:1 | ↑ H from 37 to 86 Hv, CS from 323 to 373 MPa, TS from 133 to 184 MPa | [46] |
| Pure Al | MWCNT, GNPs and C60 and 0.25% wt. | 8–18 nm of MWCNT, 1.5 µm of GNPs, and ER: 16:1 | ↑ H by 17%, 22% and 26% with added MWCNT, GNPs and C60, ↑ TS by 27%, 33% and 48% with added MWCNT, GNPs and C60 | [47] |
| Al 6061 | BN, and 6–9% wt. | ER: 3.06:1 | ↑ H by 17%, ↑ TS by 18.9% | [48] |
| Pure Al | G and 1% wt. | 0.5–20 µm and ER: 9:1 | ↑ H from 37 to 70 VHN, ↓ GS from 30 to 24 µm, ↑ TS by 46% | [25] |
| Pure Al | SiC and 5–30% wt., Al2O3 and 5–25% wt. | SiC: 300 µm, Al2O3: 90 µm and ER: 16:1 | ↑ H and WR was improved | [49] |
| S-Glass [67] | Fly Ash [68] | Al 7005 [69] | |||
|---|---|---|---|---|---|
| Elements | Wt.% | Elements | Wt.% | Elements | Wt.% |
| Al2O3 | 26 | Al2O3 | 29.6 | Zn | 4.44 |
| MgO | 10 | CaO | 0.10 | Mg | 1.38 |
| SiO2 | 64 | Fe2O3 | 0.72 | Mn | 0.54 |
| - | - | K2O | 3.53 | Cr | 0.10 |
| - | - | MgO | 0.34 | Fe | 0.11 |
| - | - | SiO2 | 64.6 | Si | 0.03 |
| - | - | - | - | Cu | 0.01 |
| - | - | - | - | Al | Bal. |
| Extrusion Condition | Test Samples | |||
|---|---|---|---|---|
| Al 7005 | Al 7005 + 5% GF | Al 7005 + 6% FA | Al 7005 + 6% FA + 5% GF | |
| Icorr (mA/cm2) | ||||
| Before Extrusion | 31.623 | 7.079 | 3.162 | 5.129 |
| 2.66:1 Extrusion | 7.943 | 6.310 | 1.230 | 2.512 |
| 5.32:1 Extrusion | 7.586 | 5.623 | 0.794 | 1.778 |
| Corrosion rate, mpy | ||||
| Before Extrusion | 13.56 ± 0.30 | 3.04 ± 0.12 | 1.36 ± 0.22 | 2.20 ± 0.11 |
| 2.66:1 Extrusion | 3.41 ± 0.25 | 2.70 ± 0.15 | 0.53 ± 0.12 | 1.08 ± 0.14 |
| 5.32:1 Extrusion | 3.25 ± 0.23 | 2.41 ± 0.12 | 0.34 ± 0.08 | 0.76 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swamy, P.K.; Mylaraiah, S.; Gowdru Chandrashekarappa, M.P.; Lakshmikanthan, A.; Pimenov, D.Y.; Giasin, K.; Krishna, M. Corrosion Behaviour of High-Strength Al 7005 Alloy and Its Composites Reinforced with Industrial Waste-Based Fly Ash and Glass Fibre: Comparison of Stir Cast and Extrusion Conditions. Materials 2021, 14, 3929. https://doi.org/10.3390/ma14143929
Swamy PK, Mylaraiah S, Gowdru Chandrashekarappa MP, Lakshmikanthan A, Pimenov DY, Giasin K, Krishna M. Corrosion Behaviour of High-Strength Al 7005 Alloy and Its Composites Reinforced with Industrial Waste-Based Fly Ash and Glass Fibre: Comparison of Stir Cast and Extrusion Conditions. Materials. 2021; 14(14):3929. https://doi.org/10.3390/ma14143929
Chicago/Turabian StyleSwamy, Praveen Kumar, Shantharaja Mylaraiah, Manjunath Patel Gowdru Chandrashekarappa, Avinash Lakshmikanthan, Danil Yurievich Pimenov, Khaled Giasin, and Munishamaiah Krishna. 2021. "Corrosion Behaviour of High-Strength Al 7005 Alloy and Its Composites Reinforced with Industrial Waste-Based Fly Ash and Glass Fibre: Comparison of Stir Cast and Extrusion Conditions" Materials 14, no. 14: 3929. https://doi.org/10.3390/ma14143929
APA StyleSwamy, P. K., Mylaraiah, S., Gowdru Chandrashekarappa, M. P., Lakshmikanthan, A., Pimenov, D. Y., Giasin, K., & Krishna, M. (2021). Corrosion Behaviour of High-Strength Al 7005 Alloy and Its Composites Reinforced with Industrial Waste-Based Fly Ash and Glass Fibre: Comparison of Stir Cast and Extrusion Conditions. Materials, 14(14), 3929. https://doi.org/10.3390/ma14143929









