An Innovative Nanobody-Based High-Biocompatibility Gold Interdigitated Microelectrode Electrochemical Bioimpedance Sensor for the Ultrasensitive Detection of Difenacoum in Human Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatuses
2.2. Fabrication and Preparation of the Bioimpedance Sensor
2.2.1. Micro-Nanofabrication of Interdigitated Microelectrode (IDME)
2.2.2. Preparation of the Bioimpedance Sensor
2.3. Measurements of the Electrochemical Impedance Spectroscopy (EIS)
2.4. Analysis of DIF (3-(3-Biphenyl-4-Yl-1,2,3,4-Tetrahydro-L-Naphthyl)-4-Hydroxycoumarin) in the Human Serum Samples
3. Results and Discussion
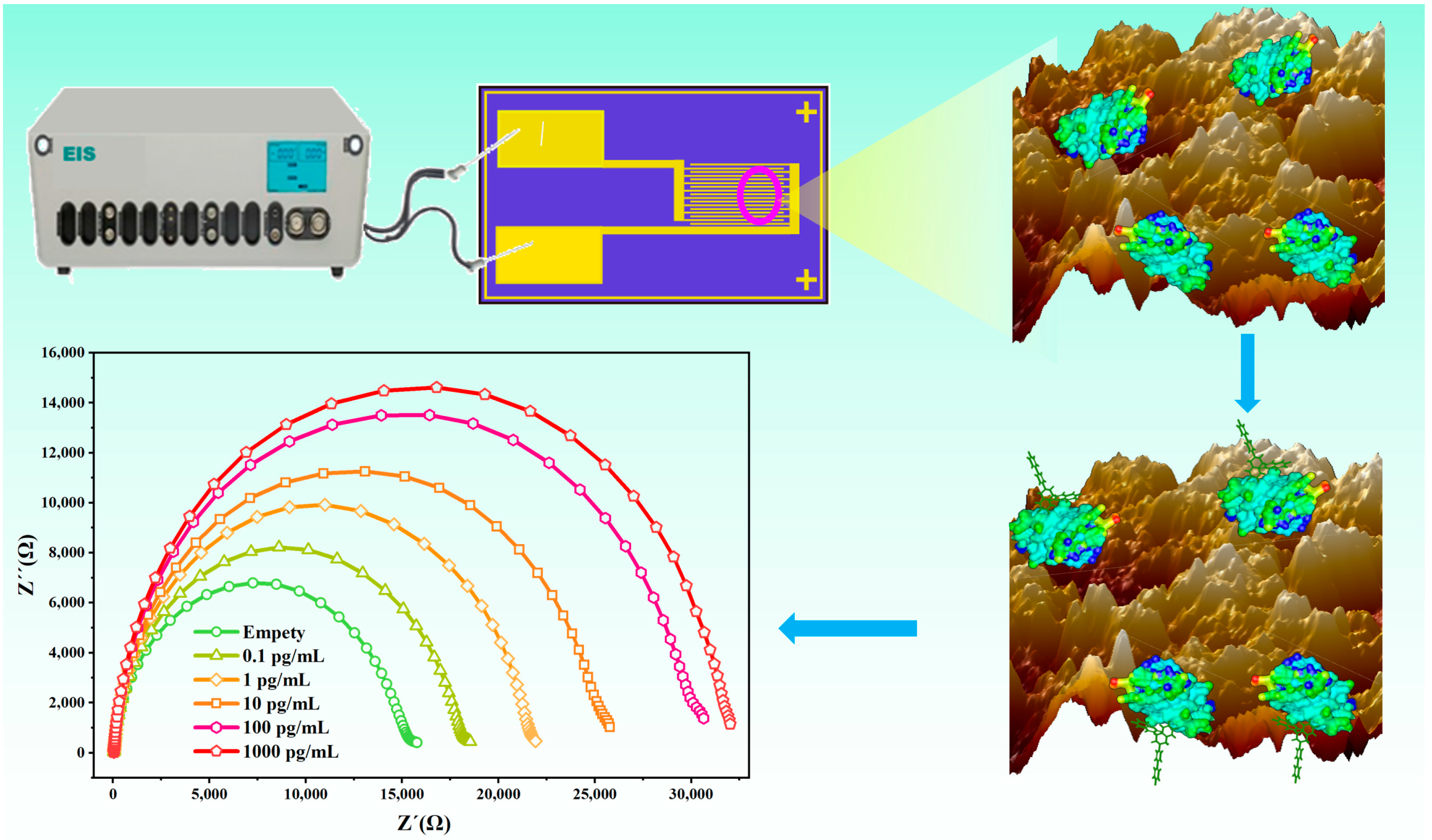
3.1. Principle and Construction of the Sensor
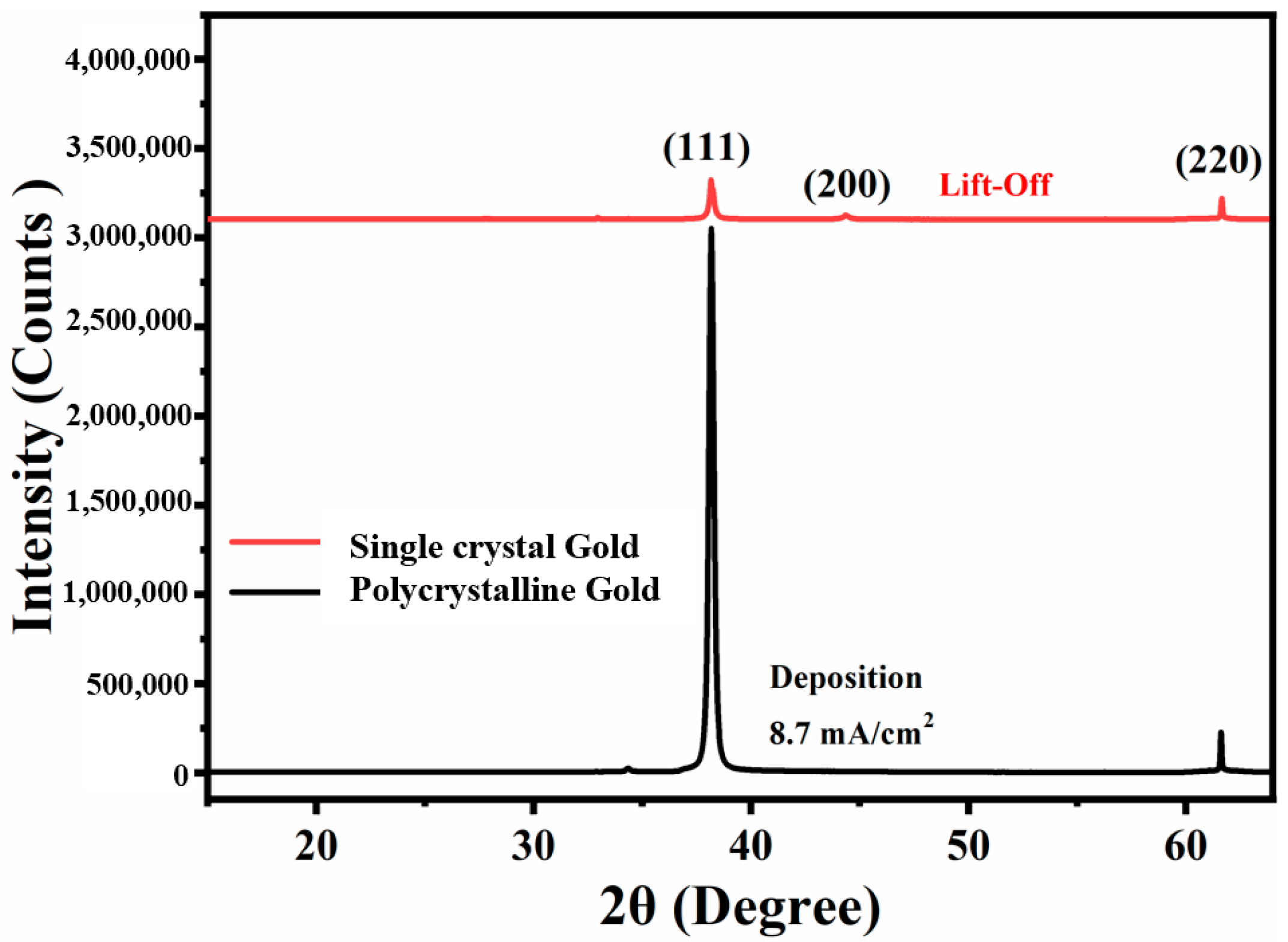
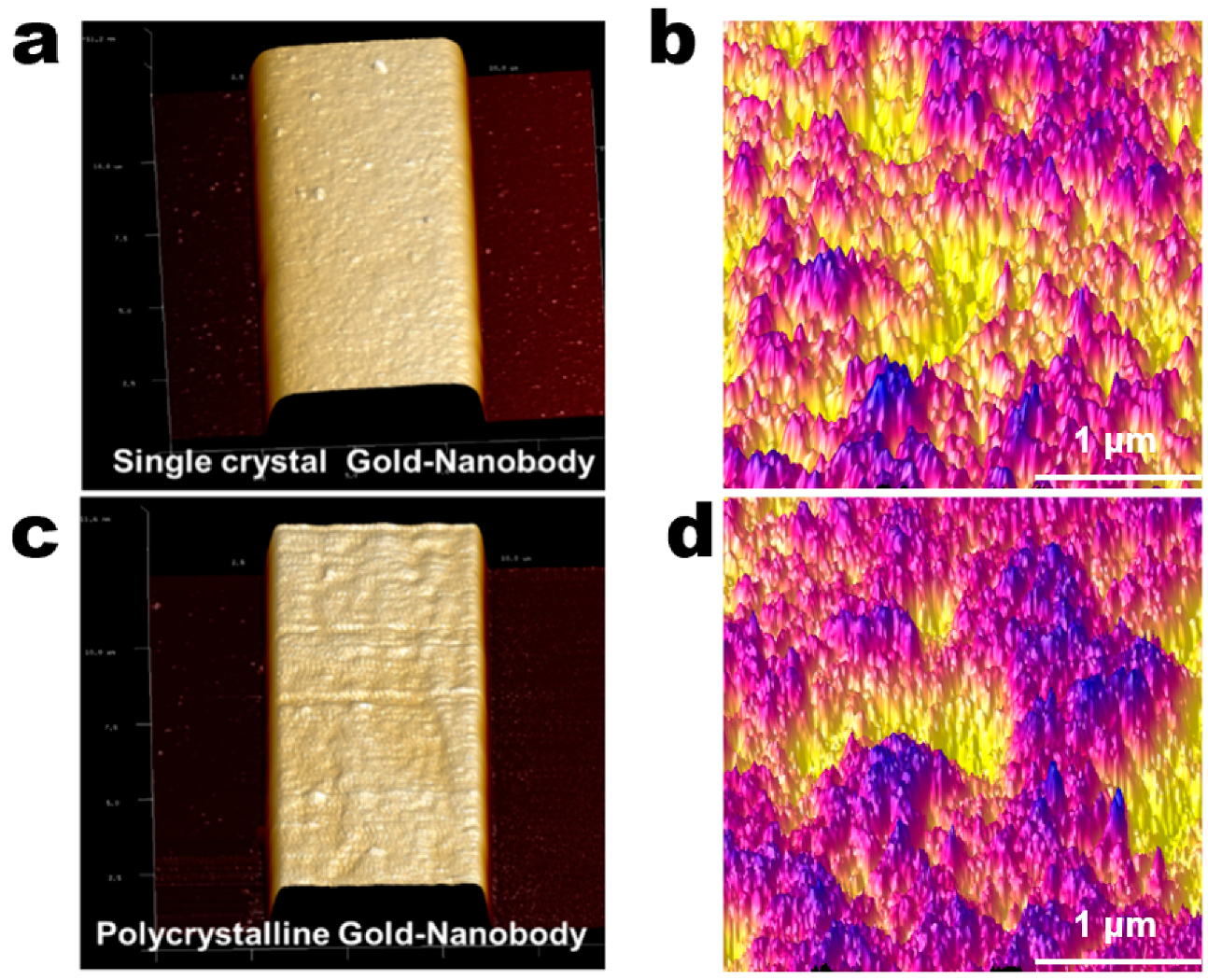
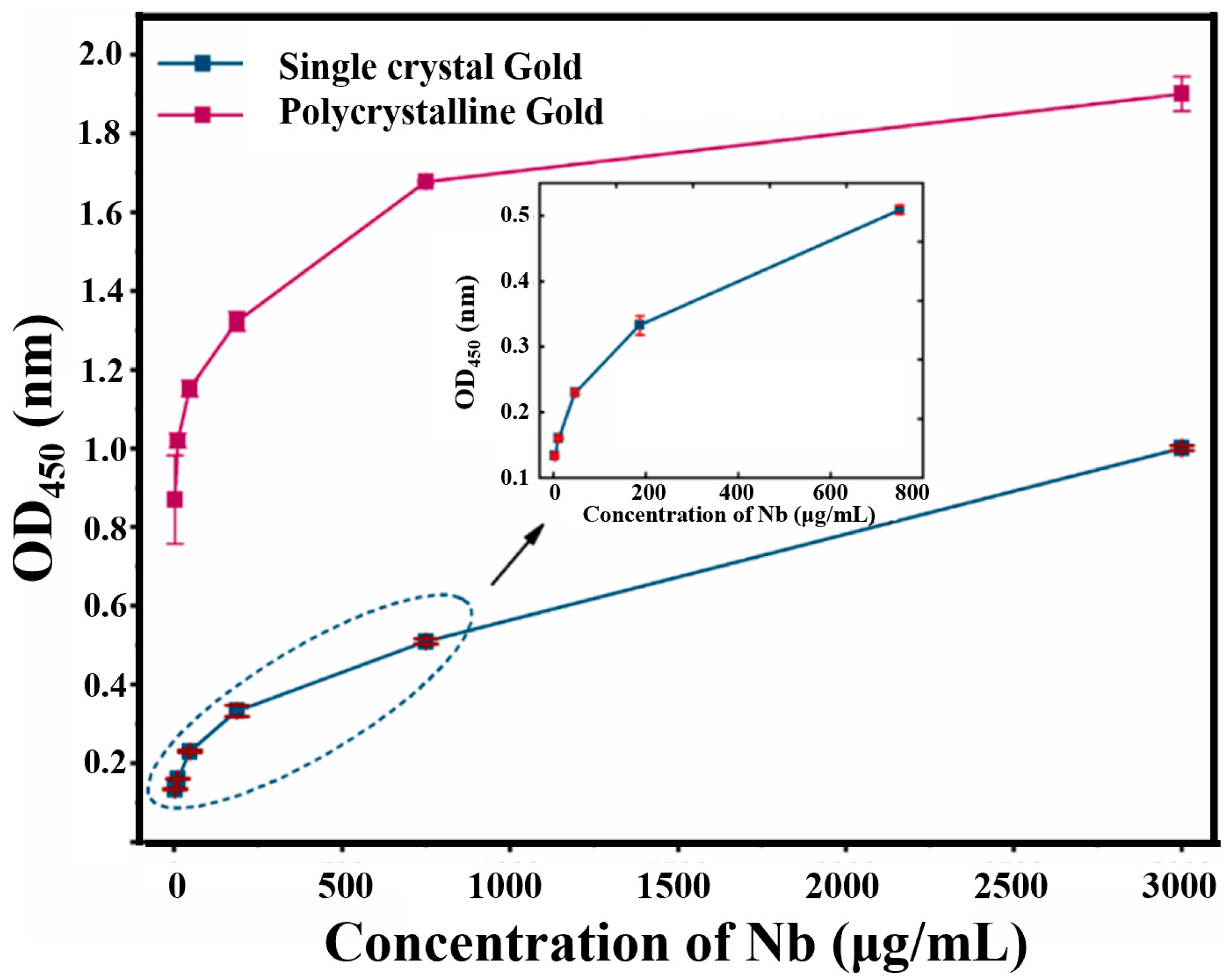
3.2. Controlled Orientation-Growth of the Gold Nanofilms Electrode
3.3. Characteristic and Optimization of the Bioimpedance Sensor
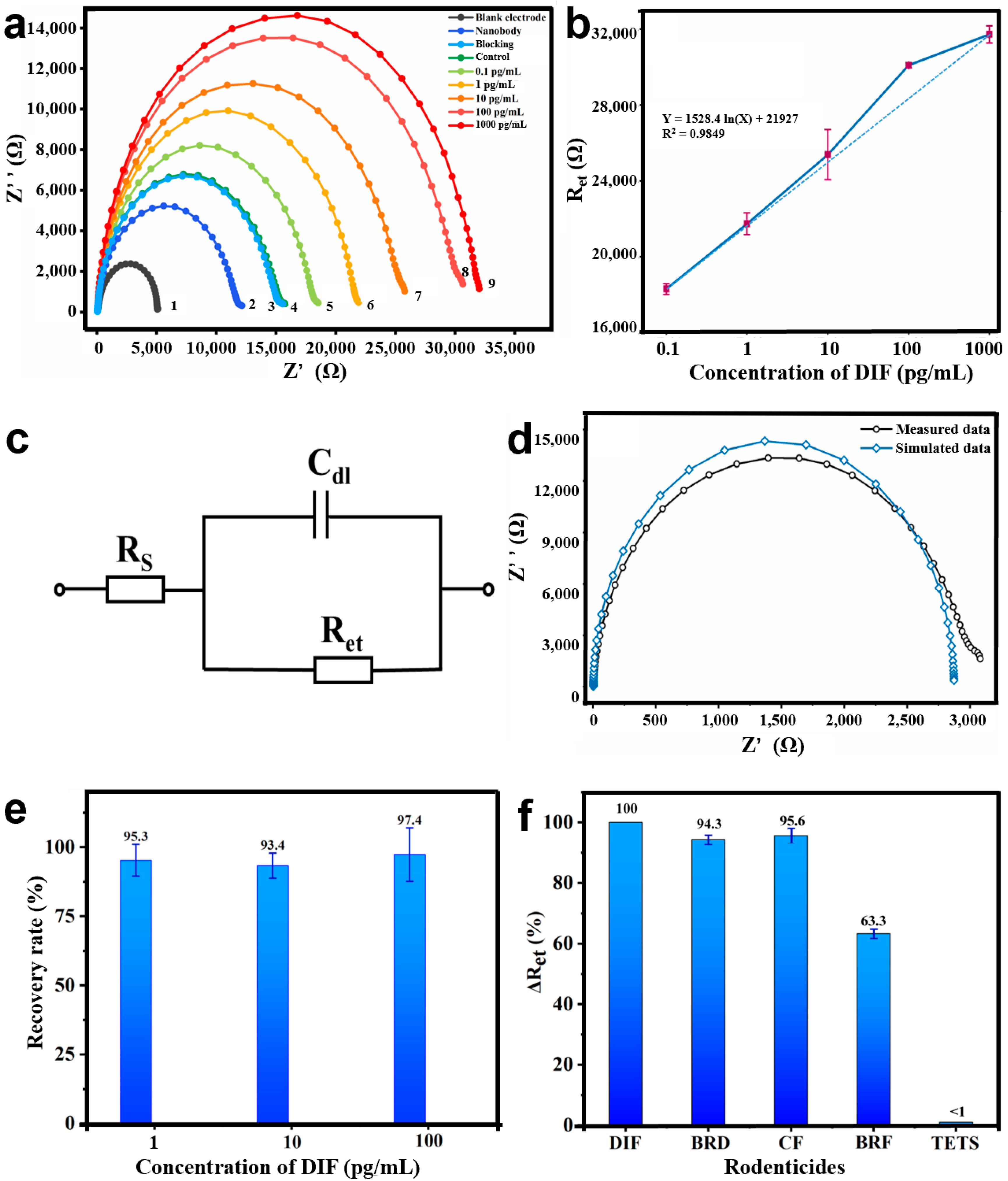
3.4. Development of the Bioimpedance Sensor for DIF
3.5. Application of the Bioimpedance Sensor in Human Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadler, M.R.; Shadbolt, R.S. Novel 4-hydroxy-coumarin anticoagulants active against resident rats. Nature 1975, 253, 5489. [Google Scholar] [CrossRef] [PubMed]
- Hadler, M.R.; Redfern, R.; Rowe, F.P. Laboratory evaluation of difenacoum as a rodenticide. J. Hyg. 1975, 74, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greaves, J.H.; Shepherd, D.S.; Gill, J.E. An investigation of difenacoum resistance in Norway rat populations in Hampshire. Ann. Appl. Biol. 1982, 100, 581–587. [Google Scholar] [CrossRef]
- McCarthy, P.T.; Cox, A.D.; Harrington, D.J.; Evely, R.S.; Hampton, E.; Al-Sabah, A.; Massey, E.; Jackson, H.; Ferguson, T. Covert poisoning with difenacoum: Clinical and toxicological observations. Hum. Exp. Toxicol. 1997, 16, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Jacolot, M.; Moebs-Sanchez, S.; Popowycz, F. Enantioselective rhodium-catalyzed hydroacylation to access the four stereoisomers of anti-rodent difenacoum. Tetrahedron Lett. 2017, 58, 1636–1639. [Google Scholar] [CrossRef]
- Jacolot, M.; Moebs-Sanchez, S.; Popowycz, F. Vkorc1 variation in house mice during warfarin and difenacoum field trials. Pest Manag. Sci. 2013, 69, 409–413. [Google Scholar] [CrossRef]
- Armentano, A.; Iammarino, M.; Magro, S.L.; Muscarella, M. Validation and application of multi-residue analysis of eight anticoagulant rodenticides by high-performance liquid chromatography with fluorimetric detection. J. Vet. Diagn. Investig. 2012, 24, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gül, N.; Yiğit, N.; Saygılı, F.; De Mirel, E.; Geniş, C. Comparison of the effects of difenacoum and brodifacoum on the ultrastructure of rat liver cells. Arch. Ind. Hyg. Toxicol. 2016, 67, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Kotthoff, M.; Rüdel, H.; Jürling, H.; Severin, K.; Hennecke, S.; Friesen, A.; Koschorreck, J. First evidence of anticoagulant rodenticides in fish and suspended particulate matter: Spatial and temporal distribution in German freshwater aquatic systems. Environ. Sci. Pollut. Res. 2018, 26, 7315–7325. [Google Scholar] [CrossRef] [Green Version]
- Bankes, J.; Garthwaite, D. Pesticide Usage Survey Report 175: Rodenticide Use on Farms in Great Britain Growing Arable Crops 2000; DEFRA: London, UK, 2000.
- Sergei, B.; Kim, G.; Mark, D.; Thanh, D.; Desdemona, A.; Naren, G. A validated LC-MS-MS method for simultaneous identification and quantitation of rodenticides in blood. J. Anal. Toxicol. 2015, 39, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhu, L.; Zhuo, X.; Shen, M.; Xiang, P. Anticoagulant rodenticide intoxication in east China: A three-year analysis. Forensic Sci. Res. 2016, 1, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Damin-Pernik, M.; Espana, B.; Besse, S.; Fourel, I.; Caruel, H.; Benoit, F.P.; Lattard, V. Development of an Ecofriendly Anticoagulant Rodenticide Based on the Stereochemistry of Difenacoum. Drug Metab. Dispos. 2016, 44, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Shimshoni, J.A.; Soback, S.; Cuneah, O.; Shlosberg, A.; Britzi, M. New validated multiresidue analysis of six 4-hydroxy-coumarin anticoagulant rodenticides in hen eggs. J. Vet. Diagn. Investig. 2013, 25, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dong, B.; Dou, L.; Yu, W.; Yu, X.; Wen, K.; Ke, Y.; Shen, J.; Wang, Z. Fluorescent lateral flow immunoassay for highly sensitive detection of eight anticoagulant rodenticides based on cadmium-free quantum dot-encapsulated nanospheres. Sens. Actuators B Chem. 2020, 324, 128771. [Google Scholar] [CrossRef]
- Wei, L.; Liu, L.; Kang, H.; Liu, S.; Wang, G.; Hu, X.; Wang, C. Development of a Disposable Label-Free Impedance Immunosensor for Direct and Sensitive Clenbuterol Determination in Pork. Food Anal. Methods 2015, 9, 1781–1788. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Kunz, R.R. Large-area interdigitated array microelectrodes for electrochemical sensing. Sens. Actuators B Chem. 2000, 62, 23–29. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, R.; Wang, Y.; Li, Y. A portable impedance biosensor for detection of multiple avian influenza viruses. Sensors 2013, 1–4. [Google Scholar] [CrossRef]
- Rocha, G.S.; Silva, M.K.L.; Cesarino, I. Reduced Graphene Oxide-Based Impedimetric Immunosensor for Detection of Enterotoxin A in Milk Samples. Materials 2020, 13, 1751. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, X.; Meng, Q.; Zheng, P.; Zhang, J.; He, Z.; Jiang, H. Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosens. Bioelectron. 2021, 183, 113186. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.; Hamers, R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994, 7, 1129–1135. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.; Hamers, R. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Muyldermans, S.; Baral, T.; Retamozzo, V.C.; De Baetselier, P.; De Genst, E.; Kinne, J.; Leonhardt, H.; Magez, S.; Nguyen, V.; Revets, H.; et al. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 2009, 128, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; McCoy, M.R.; Majkova, Z.; DeChant, J.E.; Gee, S.J.; Rosa, S.T.-D.; Gonzalez-Sapienza, G.; Hammock, B.D. Isolation of Alpaca Anti-Hapten Heavy Chain Single Domain Antibodies for Development of Sensitive Immunoassay. Anal. Chem. 2011, 84, 1165–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wen, Y.; Wang, W.; Zhao, Z.; Wang, D. Nanobody-based electrochemical competitive immunosensor for the detection of AFB1 through AFB1-HCR as signal amplifier. Microchim. Acta 2020, 187, 352. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Em, A.; Jz, B.; Kp, C.; D, K.K.; Adm, A.; Em, D. Electrochemical immunosensor functionalized with nanobodies for the detection of the toxic microalgae Alexandrium minutum using glassy carbon electrode modified with gold nanoparticles. Biosens. Bioelectron. 2020, 154, 112052. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Wei, S.; Zhang, J.; Baklanov, M.R.; Fanta, A. Influence of Current Density on Orientation-Controllable Growth and Characteristics of Electrochemically Deposited Au Films. J. Electrochem. Soc. 2019, 166, D3232–D3237. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Y.; Li, H.; Guo, Y.; Chu, J.; Guo, H.; Zhao, Q. Simultaneous determination of nine anticoagulant rodenticides by ultra-performance liquid chromatography–tandem mass spectrometry with ultrasound-assisted low–density solvent dispersive liquid–liquid microextraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1092, 453–458. [Google Scholar] [CrossRef]
- Qiao, Z.; Xiang, P.; Shen, B.; Shen, M.; Yan, H. Simultaneous Determination of 13 Anticoagulant Rodenticidesin Human Blood by Liquid Chromatography-Tandem Mass Spectrometry and its Application in Three Poisoning Cases. J. Forensic Sci. 2018, 63, 784–792. [Google Scholar] [CrossRef]
- Seljetun, K.O.; Eliassen, E.; Karinen, R.; Moe, L.; Vindenes, V. Quantitative method for analysis of six anticoagulant rodenticides in faeces, applied in a case with repeated samples from a dog. Acta Vet. Scand. 2018, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiaoli, C.; Xiaoqian, Y.; Zhong, L.; Haitao, J.; Suhua, L. Rapid Simultaneous Screening and Detection of 12 Anticoagulant Rodenticides in Food by Ultra-performance Liquid Chromatography-Triple Quadrupole/Linear Ion Trap Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 3538–3547. [Google Scholar] [CrossRef]
- Smith, L.L.; Liang, B.; Booth, M.C.; Filigenzi, M.S.; Tkachenko, A.; Gaskill, C.L. Development and Validation of Quantitative Ultraperformance Liquid Chromatography–Tandem Mass Spectrometry Assay for Anticoagulant Rodenticides in Liver. J. Agric. Food Chem. 2017, 65, 6682–6691. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, J.; Wu, Y.; Liu, W.; Bu, J.; Zhao, Q. Sensitive and simultaneous determination of nine anticoagulant rodenticides in human blood by UPLC–MS-MS with phospholipid removal pretreatment. J. Anal. Toxicol. 2018, 42, 459–466. [Google Scholar] [CrossRef]
- López-García, M.; Romero-González, R.; Frenich, A.G. Determination of rodenticides and related metabolites in rabbit liver and biological matrices by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2017, 137, 235–242. [Google Scholar] [CrossRef]


| No. | Method of Detection | Detection Results | Sample | Ref |
|---|---|---|---|---|
| 1 | UPLC–MS/MS a | LOD: 0.01 ng/mL, linear range: 0.1–100 ng/mL | Urine | [30] |
| 2 | LC–MS/MS b | LOD c: 0.2 ng/mL, linear range: 0.5–50 ng/mL | Blood | [31] |
| 3 | UHPLC–MS/MS d | LOQ e: 1.5–2.7 ng/g, linear range: 2.2–1111 ng/mL | Faeces | [32] |
| 4 | UPLC–QTrap-MS/MS f | LOD: 0.02 ng/mL, linear range: 0.1–100 ng/mL | Food | [33] |
| 5 | UPLC–MS/MS | LOD: 0.75–25 ng/g, linear range: 50−2500 ng/g | Liver | [34] |
| 6 | UPLC–MS/MS | LOD: 0.25 ng/mL, linear range: 1–2000 ng/mL | Blood | [35] |
| 7 | UHPLC g | LOQ: 0.2 ng/g, linear range: 0.2–150 ng/g | Liver | [36] |
| 8 | QNs–based LFIA h | LOD: 30.6–45.9 (ng/mL, ng/g), linear range: - | Serum, Urine, Wheat | [15] |
| 9 | EIS | LOD: 0.1 pg/mL, linear range: 0.1–1000 pg/mL | Serum | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wang, S.; He, Z.; Zhang, J.; Zhu, X.; Ke, Y.; Jiang, H.; Wang, Z. An Innovative Nanobody-Based High-Biocompatibility Gold Interdigitated Microelectrode Electrochemical Bioimpedance Sensor for the Ultrasensitive Detection of Difenacoum in Human Serum. Materials 2021, 14, 3930. https://doi.org/10.3390/ma14143930
Guo L, Wang S, He Z, Zhang J, Zhu X, Ke Y, Jiang H, Wang Z. An Innovative Nanobody-Based High-Biocompatibility Gold Interdigitated Microelectrode Electrochemical Bioimpedance Sensor for the Ultrasensitive Detection of Difenacoum in Human Serum. Materials. 2021; 14(14):3930. https://doi.org/10.3390/ma14143930
Chicago/Turabian StyleGuo, Liuchuan, Sihan Wang, Zhiwei He, Jing Zhang, Xiaoli Zhu, Yuebin Ke, Haiyang Jiang, and Zhanhui Wang. 2021. "An Innovative Nanobody-Based High-Biocompatibility Gold Interdigitated Microelectrode Electrochemical Bioimpedance Sensor for the Ultrasensitive Detection of Difenacoum in Human Serum" Materials 14, no. 14: 3930. https://doi.org/10.3390/ma14143930
APA StyleGuo, L., Wang, S., He, Z., Zhang, J., Zhu, X., Ke, Y., Jiang, H., & Wang, Z. (2021). An Innovative Nanobody-Based High-Biocompatibility Gold Interdigitated Microelectrode Electrochemical Bioimpedance Sensor for the Ultrasensitive Detection of Difenacoum in Human Serum. Materials, 14(14), 3930. https://doi.org/10.3390/ma14143930








