Evaluation of the Composite Mechanism of Nano-Fe2O3/Asphalt Based on Molecular Simulation and Experiments
Abstract
:1. Introduction
2. Experimental and Simulation Calculation Details
2.1. Experimental Details
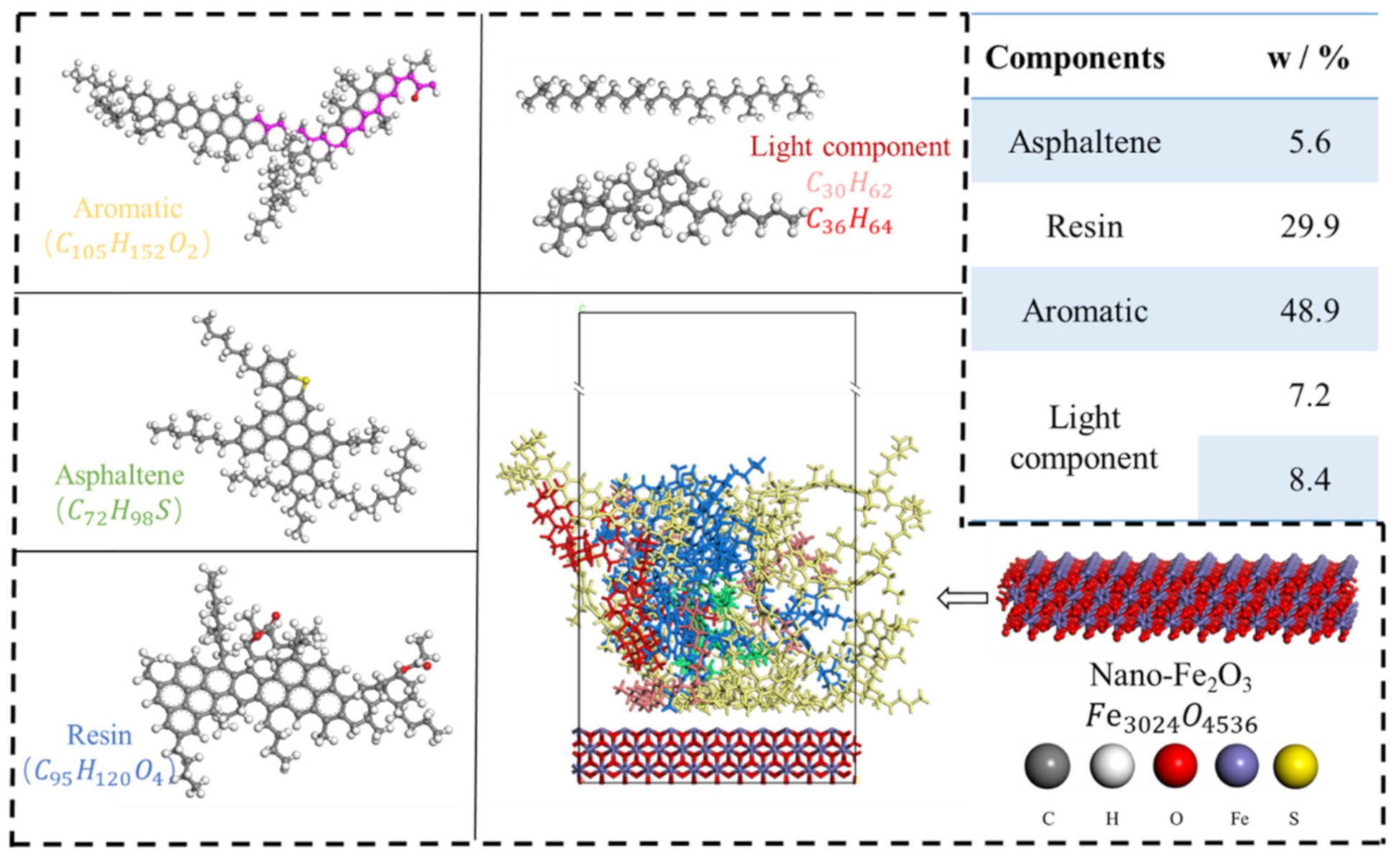
2.2. Simulation Calculation Details
3. Results and Discussion
3.1. Evaluation of Basic Physical Properties
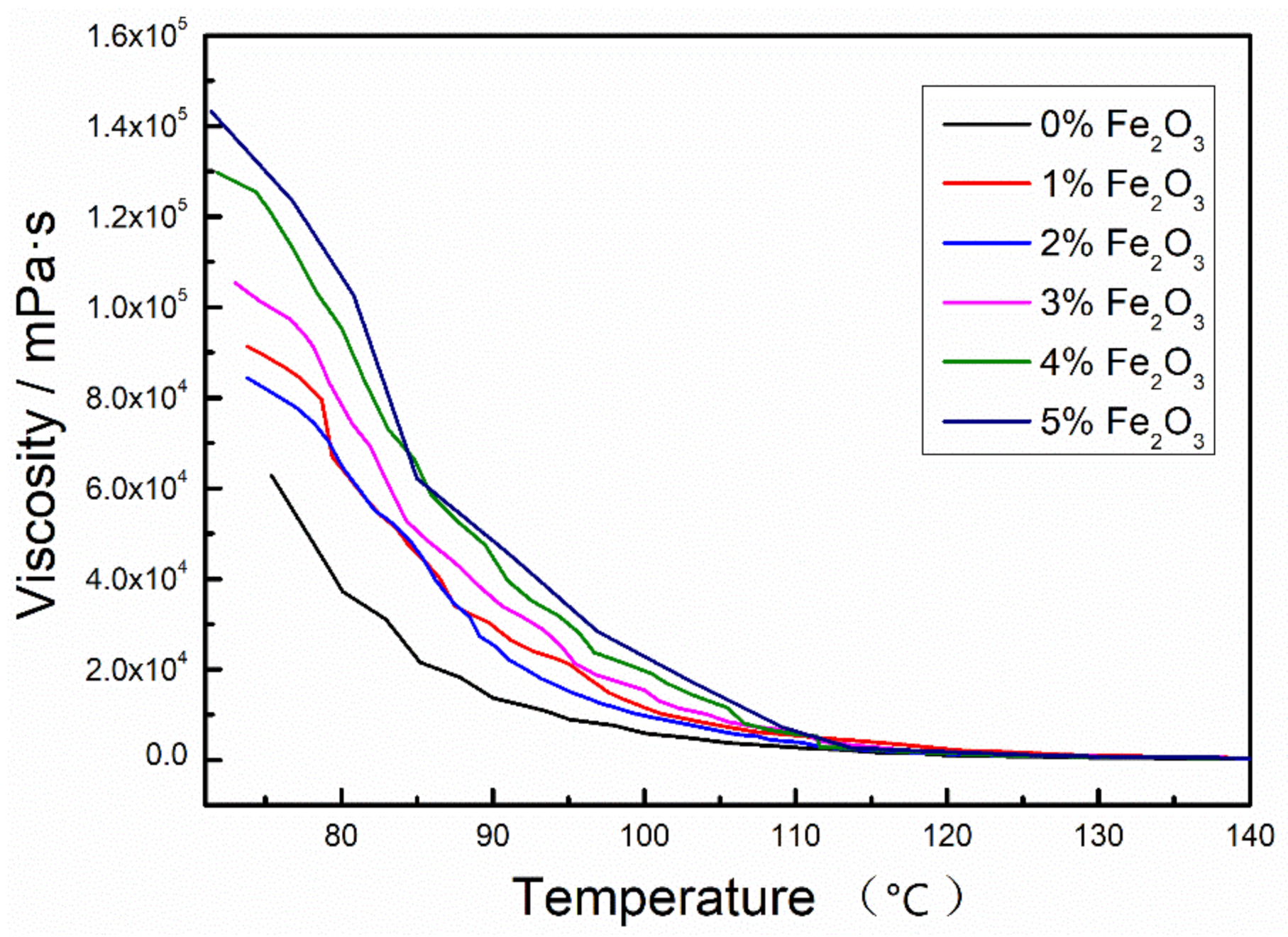
3.2. Viscosity
3.3. Activation Energy of Viscous Flow
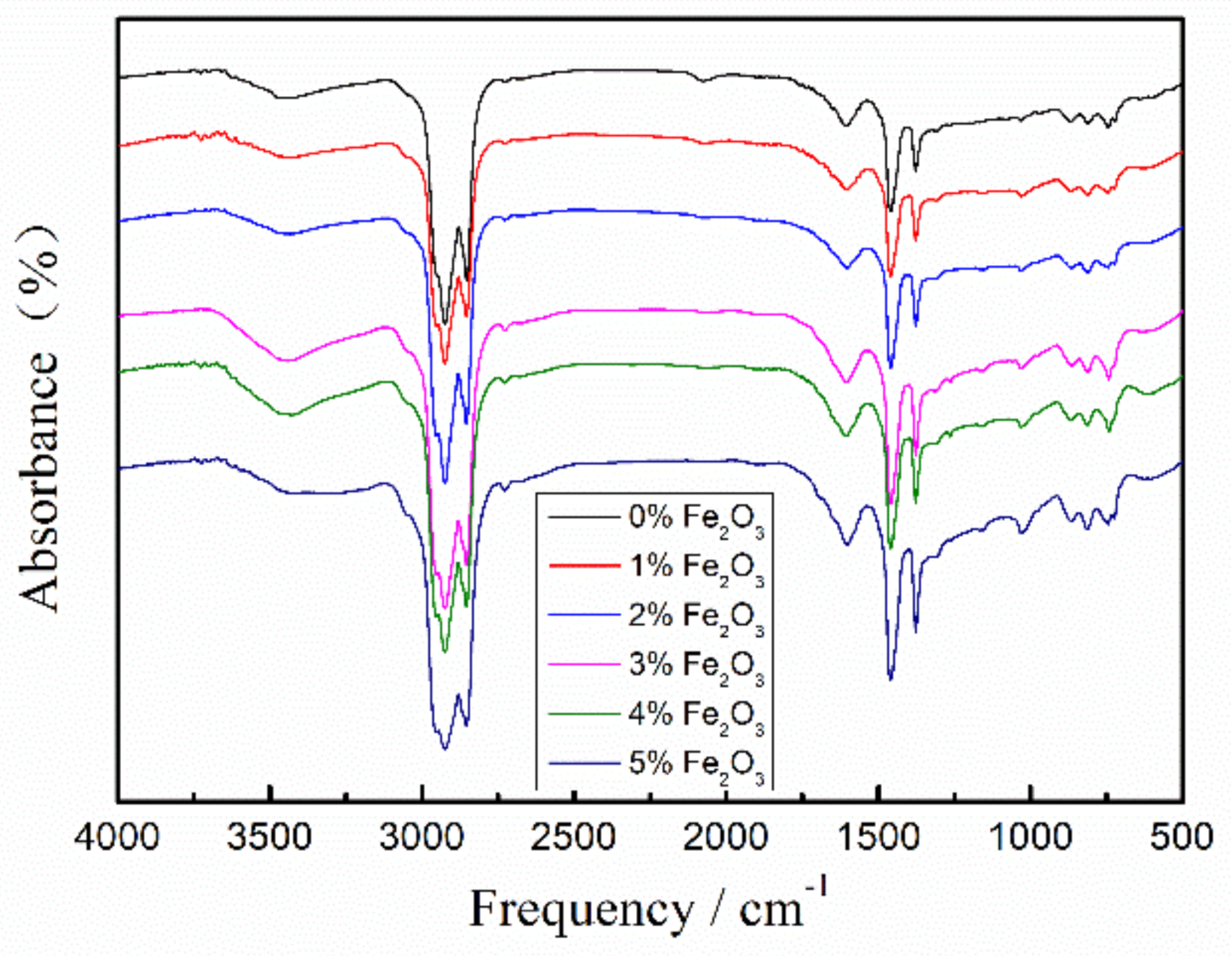
3.4. FTIR Analysis
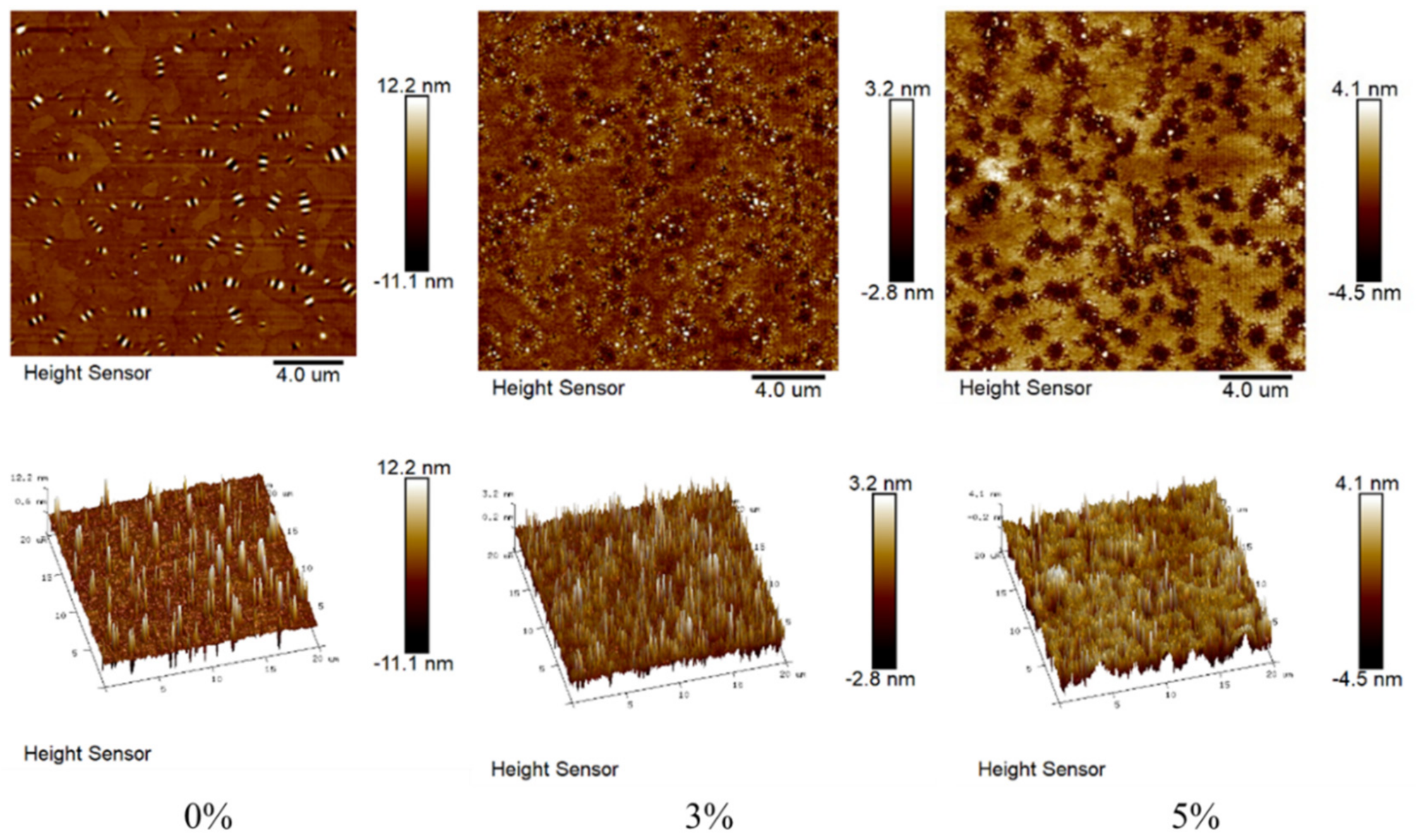
3.5. Microstructure Analysis of AFM
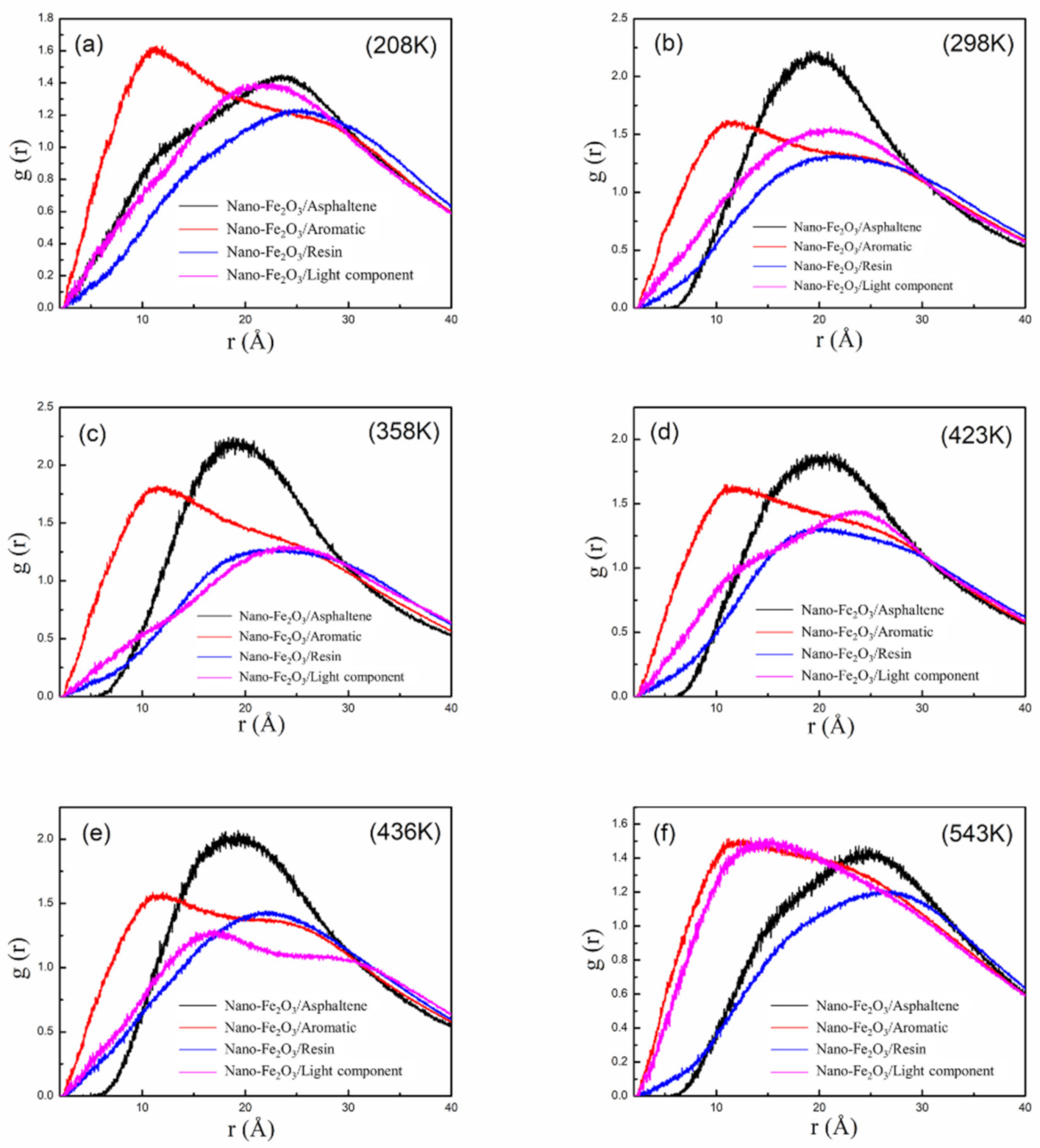
3.6. RDF Analysis Based on Molecular Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Tighe, S. A Review of Advances of Nanotechnology in Asphalt Mixtures. Procedia Soc. Behav. Sci. 2013, 96, 1269–1276. [Google Scholar] [CrossRef] [Green Version]
- Liao, G.; Huang, X.; Sang, D.B. Antiultraviolet Aging and Antithermal-Oxygen Aging Tests of Asphalts Adapting to Environment of Tibetan Plateau of China. In Proceedings of the Transportation Research Board 87th Annual Meeting, Washington, DC, USA, 13–17 January 2008. [Google Scholar]
- Hao, X.H.; Zhang, A.Q.; Yang, W. Study on the Performance of Nano Calcium Carbonate Modified Asphalt Concrete AC-13. Adv. Mater. Res. 2012, 450–451, 503–507. [Google Scholar] [CrossRef]
- Steyn, W.J. Applications of Nanotechnology in Road Pavement Engineering. In Nanotechnology in Civil Infrastructure; Gopalakrishnan, K., Birgisson, B., Taylor, P., Attoh-Okine, N.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 49–83. [Google Scholar]
- Hamedi, G.H.; Nejad, F.M.; Oveisi, K. Estimating the moisture damage of asphalt mixture modified with nano zinc oxide. Mater. Struct. 2015, 49, 1165–1174. [Google Scholar] [CrossRef]
- ĀBele, A.; Merijs-Meri, R.; Bērziņa, R.; Zicāns, J.; Haritonovs, V.; Ivanova, T. Effect of bio-oil on rheological and calorimetric properties of RTFOT aged bituminous compositions. Int. J. Pavement Res. Technol. 2021, 14, 537–542. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.-J.; Tan, Y.; Liu, Z.-Y. Investigating the Interactions of the Saturate, Aromatic, Resin, and Asphaltene Four Fractions in Asphalt Binders by Molecular Simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar] [CrossRef]
- Hung, A.M.; Mousavi, M.; Fini, E.H. Implication of wax on hindering self-healing processes in bitumen. Appl. Surf. Sci. 2020, 523, 146449. [Google Scholar] [CrossRef]
- Corbett, L.W. Composition of asphalt based on generic fractionation, using solvent deasphaltening, elution-adsorption chromatography, and densimetric characterization. Anal. Chem. 2002, 41, 576–579. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moulthrop, J.S. The SHRP Materials Reference Library; National Academy of Science: Washington, DC, USA, 1993. [Google Scholar]
- Claudy, P.; Letoffe, J.; King, G.N.; Brule, B.; Planche, J. Caracterisation Des Bitumes Routiers Par Analyse Calorimetrique Differentielle. Bull. de Liaison des Lab. des Ponts et Chaussees 1990, 165, 85–92. [Google Scholar]
- Pieri, N. Etude Du Vieillissement Simulé et in Situ des Bitumes Routiers par IRTF et Fluorescence UV en Excitation-Émission Synchrones: Détermination des Relations Structures Chimiques-Propriétés Rhéologiques par Analyse en Composantes Principales. Ph.D. Thesis, Aix-Marseille University, Marseille, France, 1994. [Google Scholar]
- Callaway, J.; March, N.H. Density Functional Methods: Theory and Applications. Solid State Phys. 1984, 38, 135–221. [Google Scholar]
- Mullins, O.C. Optical Interrogation of Aromatic Moieties in Crude Oils and Asphaltenes. In Structures and Dynamics of Asphaltenes; Metzler, J.B., Ed.; Springer: Boston, MA, USA, 1998; pp. 21–77. [Google Scholar]
- Scotti, R.; Montanari, L. Molecular structure and intermolecular interaction of asphaltenes by FT-IR, NMR, EPR. In Structures and Dynamics of Asphaltenes; Metzler, J.B., Ed.; Springer: Boston, MA, USA, 1998; pp. 79–113. [Google Scholar]
- Koots, J.A.; Speight, J.G. Relation of petroleum resins to asphaltenes. Fuel 1975, 54, 179–184. [Google Scholar] [CrossRef]
- Bergmann, U.; Mullins, O.C.; Cramer, S.P. X-ray Raman spectroscopy of carbon in asphaltene: Light element characterization with bulk sensitivity. Anal. Chem. 2000, 72, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Michon, L.; Martin, D.; Planche, J.-P.; Hanquet, B. Estimation of average structural parameters of bitumens by 13C nuclear magnetic resonance spectroscopy. Fuel 1997, 76, 9–15. [Google Scholar] [CrossRef]
- Sheremata, J.M.; Gray, M.R.; Dettman, H.D.; McCaffrey, W.C. Quantitative Molecular Representation and Sequential Optimization of Athabasca Asphaltenes. Energy Fuels 2004, 18, 1377–1384. [Google Scholar] [CrossRef]
- Zhao, S.; Kotlyar, L.S.; Sparks, B.D.; Woods, J.R.; Gao, J.; Chung, K.H. Solids contents, properties and molecular structures of asphaltenes from different oilsands. Fuel 2001, 80, 1907–1914. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]

| Nano-Fe2O3 (%) | Softening Point (°C) | Penetration Degree (25 °C, 0.1 mm) | Ductility (5 °C, cm) |
|---|---|---|---|
| 0 | 48.6 | 67.1 | 73 |
| 1 | 50.2 | 59.5 | 65 |
| 2 | 51.2 | 60.6 | 52 |
| 3 | 52 | 60.7 | 47 |
| 4 | 52.2 | 56.4 | 35 |
| 5 | 52.5 | 54.1 | 24 |
| Nano-Fe2O3% | ||
|---|---|---|
| 0 | 7.46 | |
| 1 | 6.96 | |
| 2 | 7.59 | |
| 3 | 7.44 | |
| 4 | 7.93 | |
| 5 | 7.78 |
| Absorption Peaks | Functional Groups |
|---|---|
| 2924 cm−1 | Asymmetric stretching vibration of methylene, (C–H) |
| 2853 cm−1 | Symmetric stretching vibration of methylene, (C–H) |
| 1601 cm−1 | Respiratory vibration of asymmetrically substituted benzene rings |
| 1460 cm−1 | Shear vibration of methylene, (–CH2–) |
| 1377 cm−1 | Umbrella vibration of methyl, (–CH3) |
| 1030 cm−1 | Stretching vibration of sulfoxide group, (S=O) |
| 868 cm−1 | Stretching vibration of benzene ring |
| 813 cm−1 | Stretching vibration of benzene ring |
| 745 cm−1 | Bending vibrations of aromatic branched chains |
| 724 cm−1 | Co-vibration of methylene chain segment, (CH2)n (n ≥ 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zeng, Q.; Liu, Y.; Liu, P.; Zeng, Y.; Xu, Z.; Liu, Q. Evaluation of the Composite Mechanism of Nano-Fe2O3/Asphalt Based on Molecular Simulation and Experiments. Materials 2021, 14, 3425. https://doi.org/10.3390/ma14123425
He Y, Zeng Q, Liu Y, Liu P, Zeng Y, Xu Z, Liu Q. Evaluation of the Composite Mechanism of Nano-Fe2O3/Asphalt Based on Molecular Simulation and Experiments. Materials. 2021; 14(12):3425. https://doi.org/10.3390/ma14123425
Chicago/Turabian StyleHe, Yuhao, Qing Zeng, Yaru Liu, Peng Liu, Yuqin Zeng, Zhenghong Xu, and Qicheng Liu. 2021. "Evaluation of the Composite Mechanism of Nano-Fe2O3/Asphalt Based on Molecular Simulation and Experiments" Materials 14, no. 12: 3425. https://doi.org/10.3390/ma14123425
APA StyleHe, Y., Zeng, Q., Liu, Y., Liu, P., Zeng, Y., Xu, Z., & Liu, Q. (2021). Evaluation of the Composite Mechanism of Nano-Fe2O3/Asphalt Based on Molecular Simulation and Experiments. Materials, 14(12), 3425. https://doi.org/10.3390/ma14123425






