Capture of Fullerenes in Cages and Rings by Forming Metal-π Bond Arene Interactions
Abstract
:1. Introduction
2. Methods
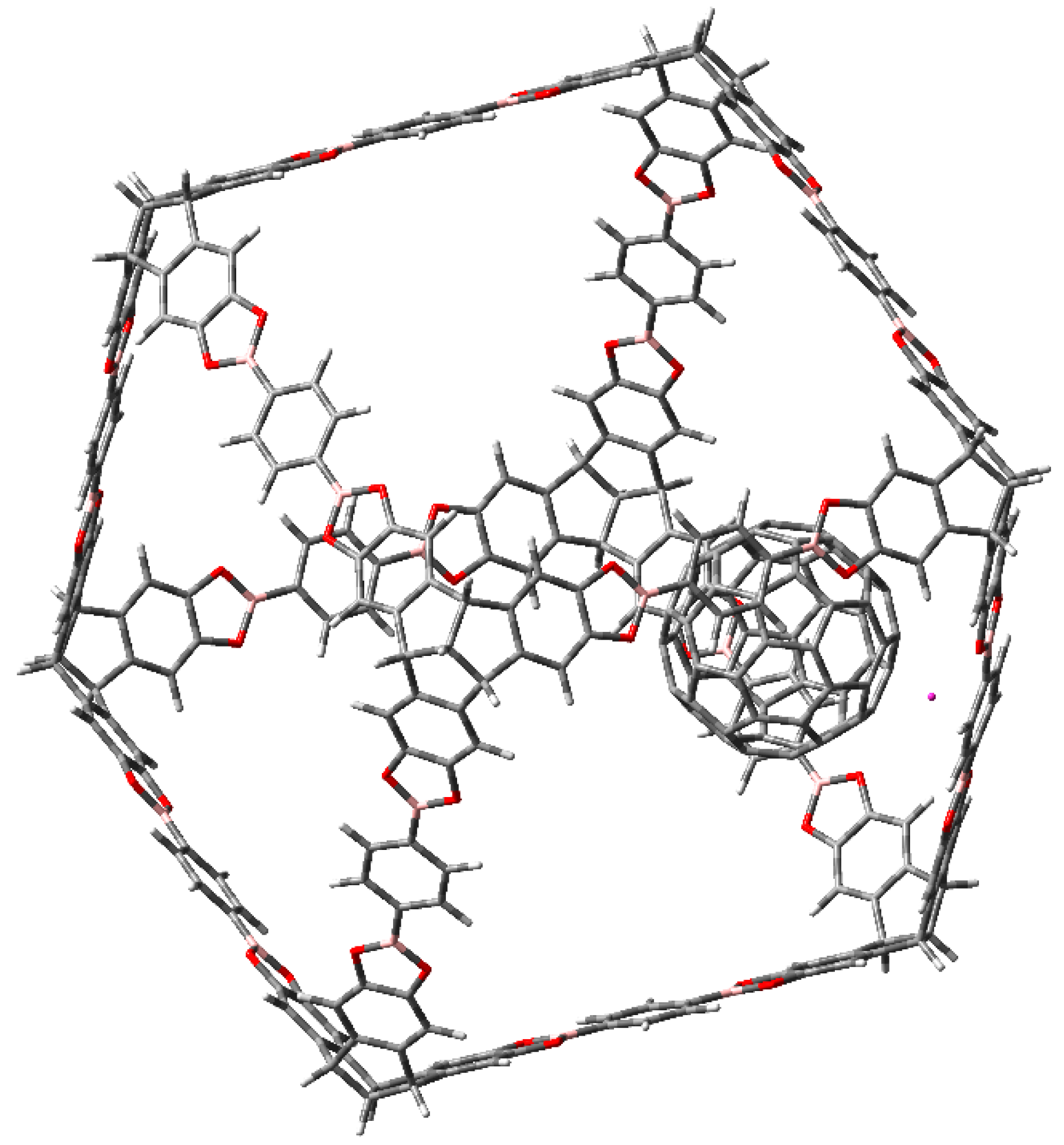
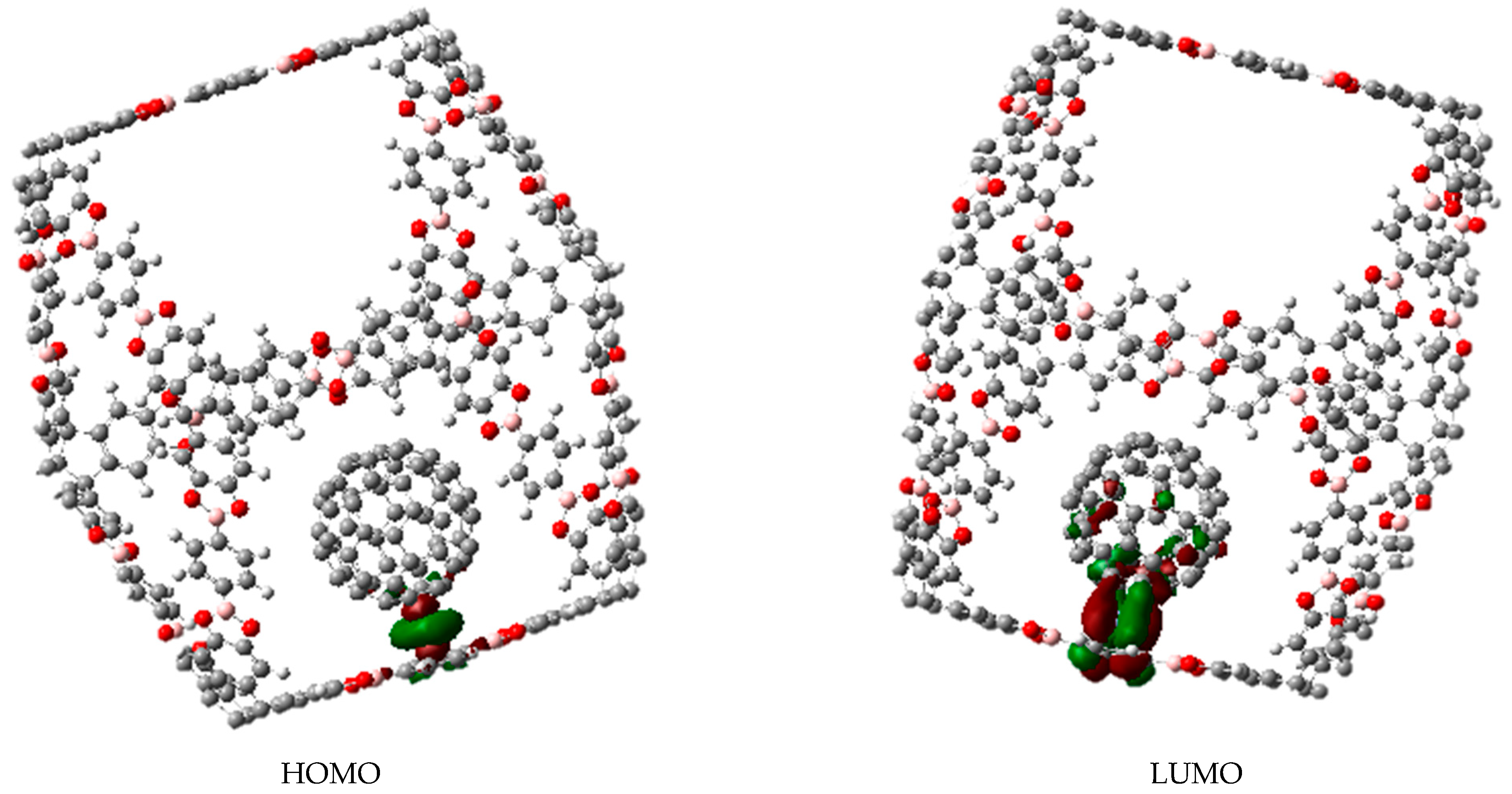
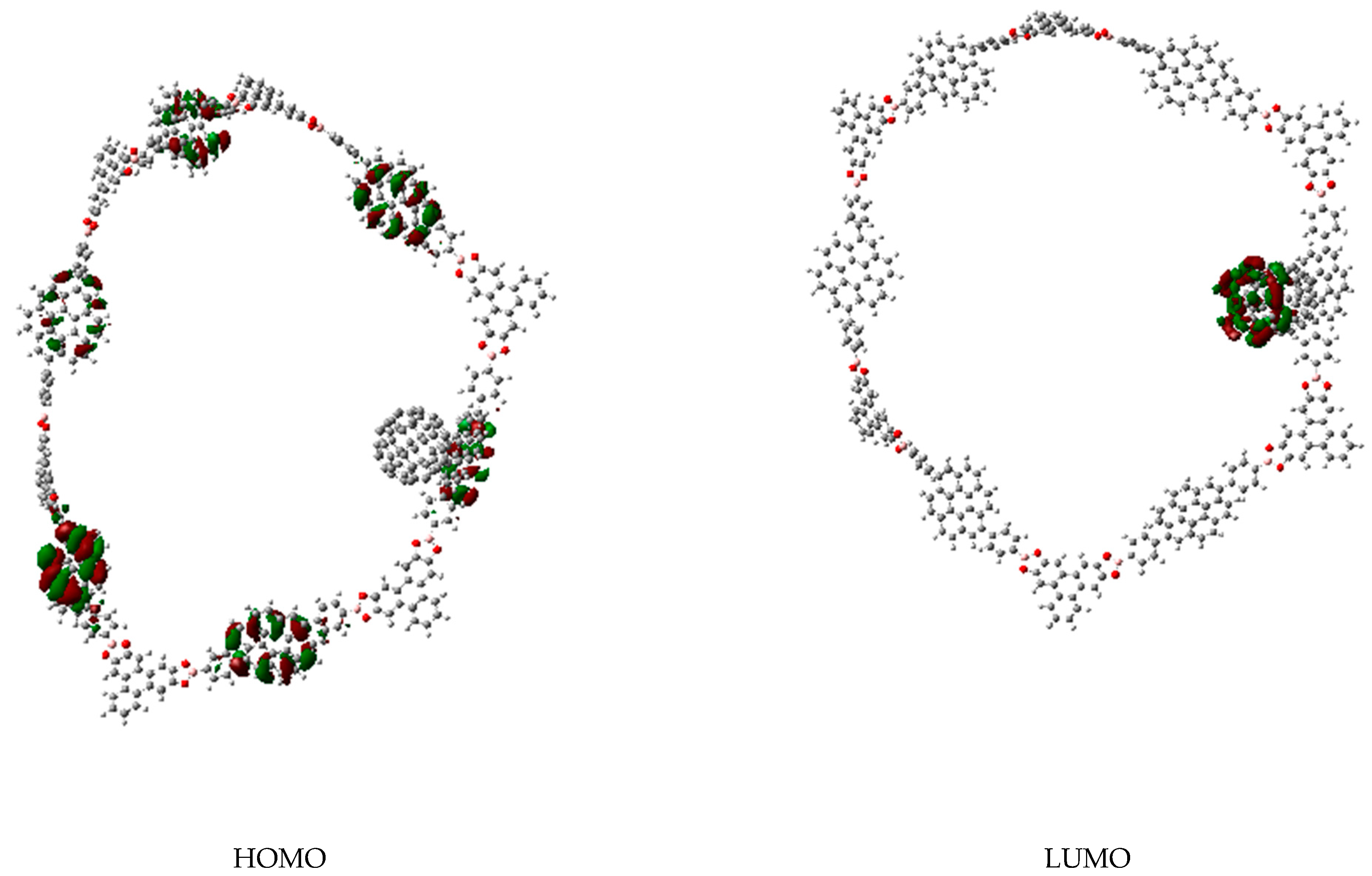
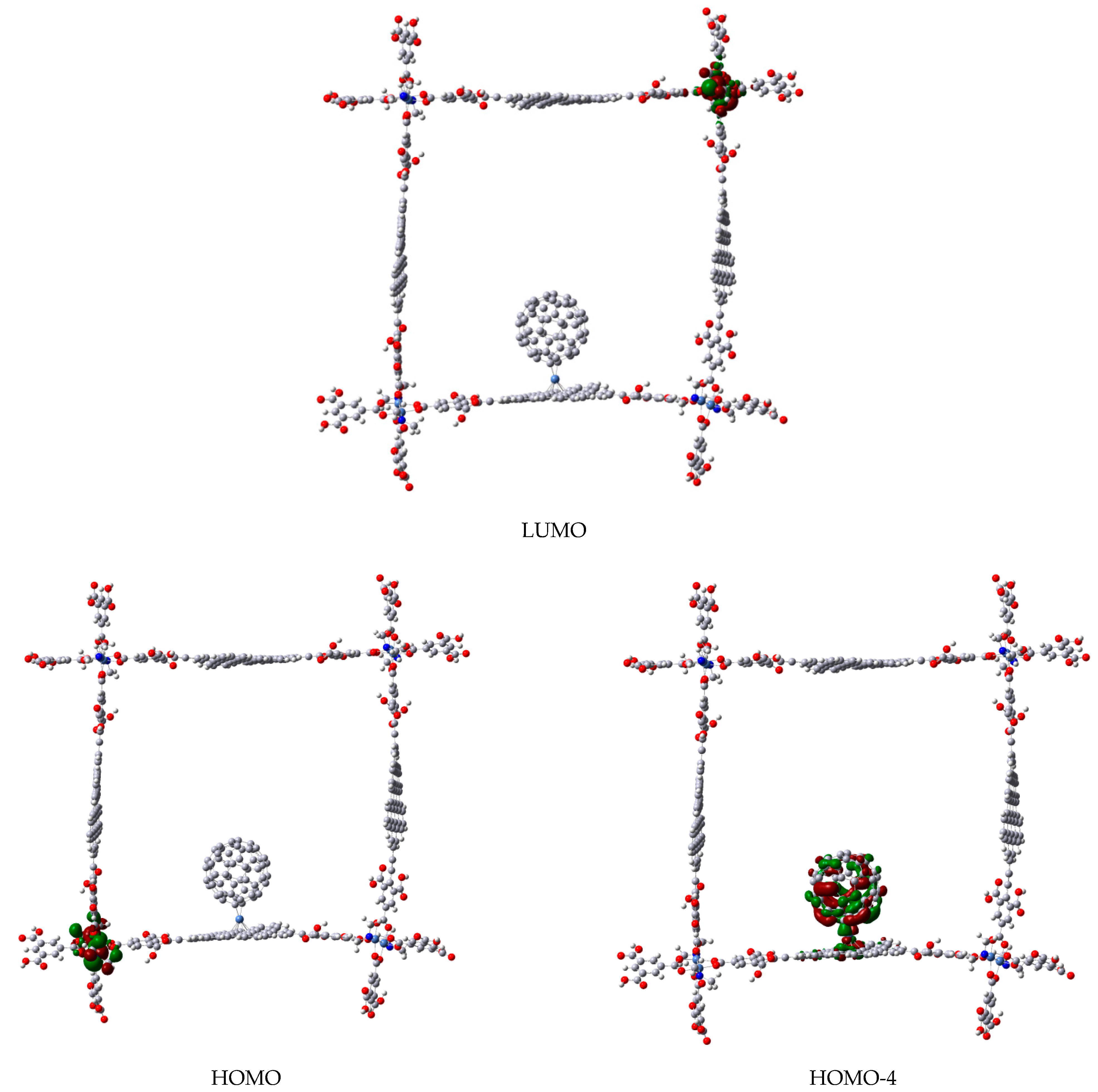
3. Results and Discussion
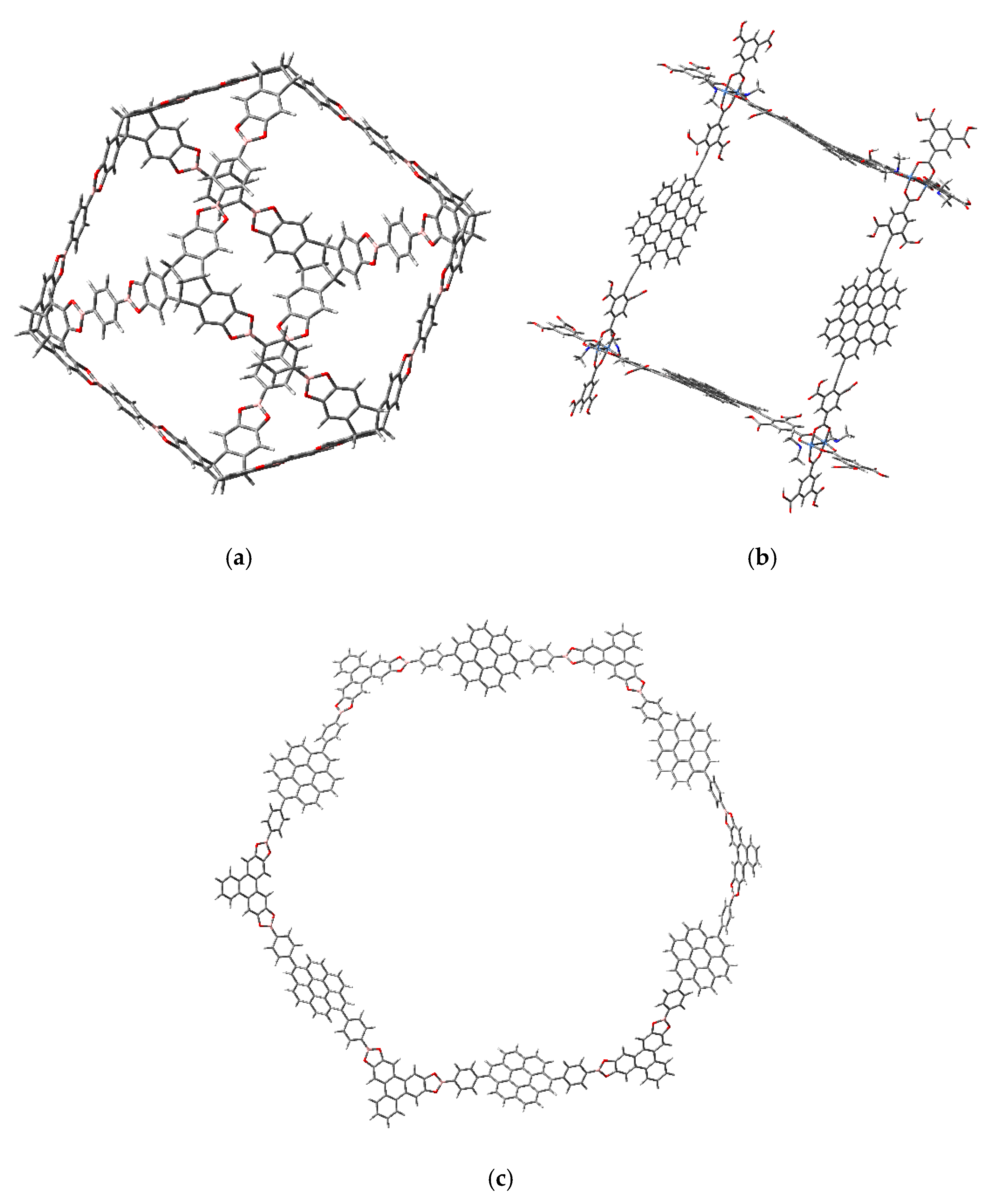
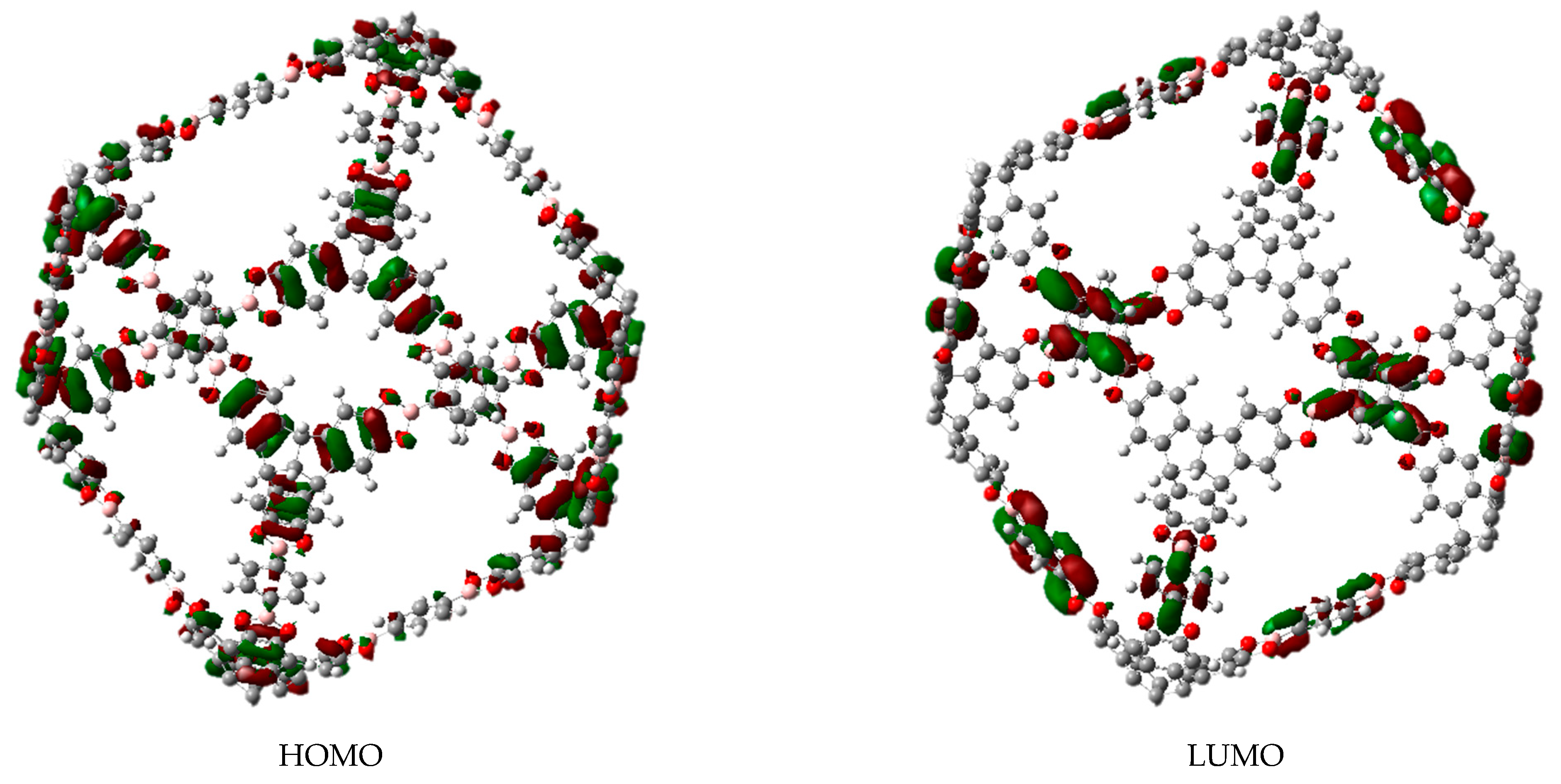
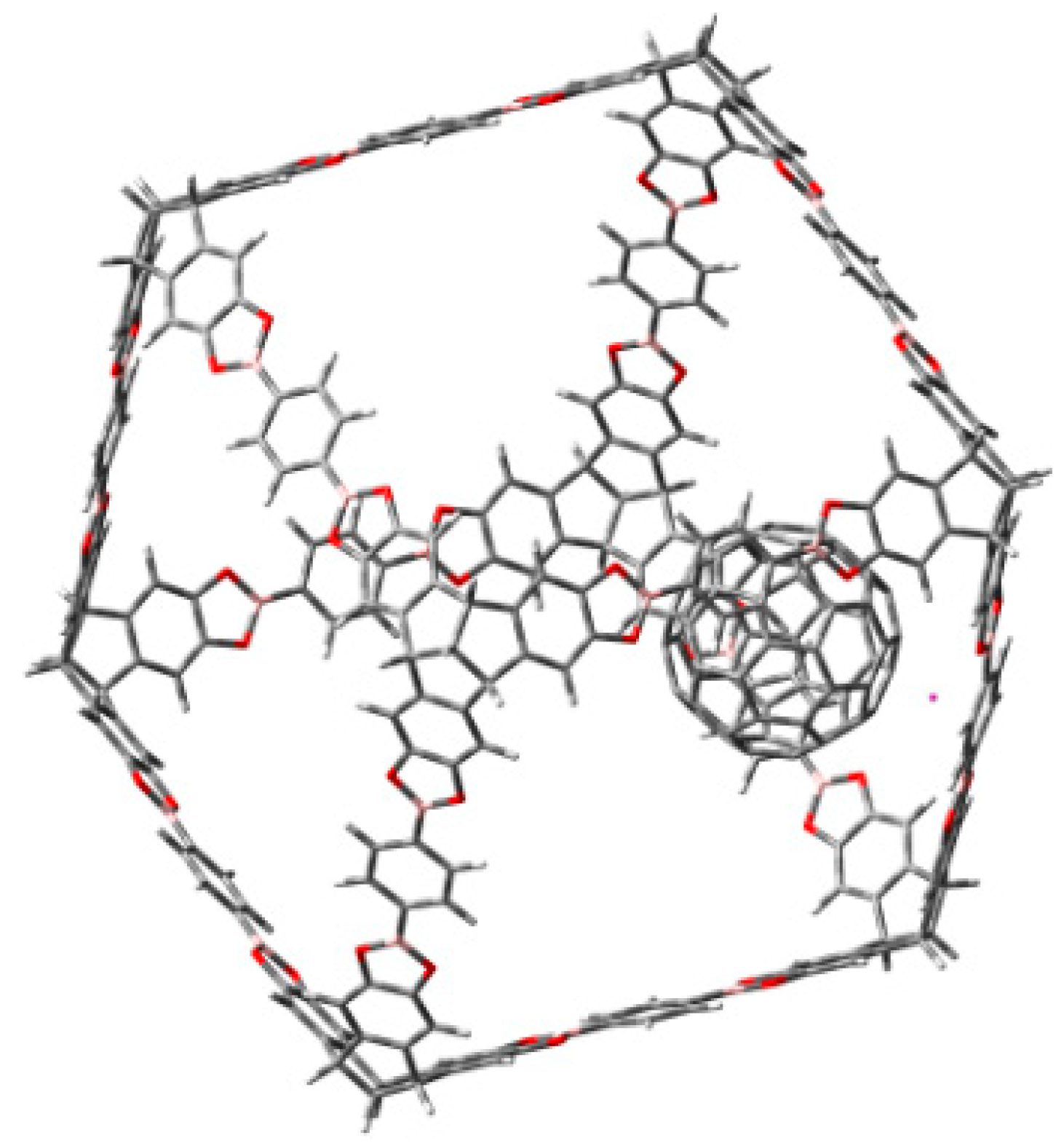
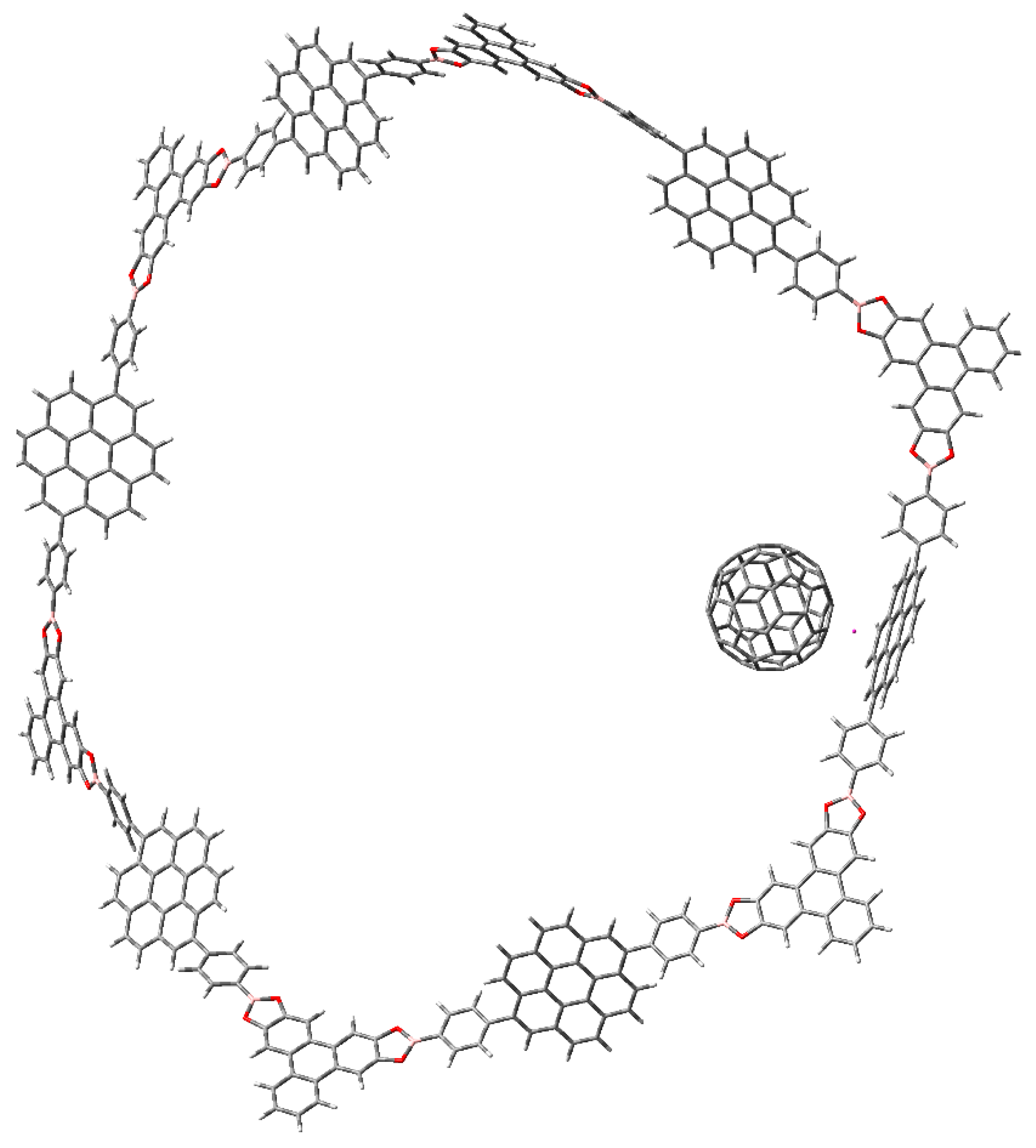
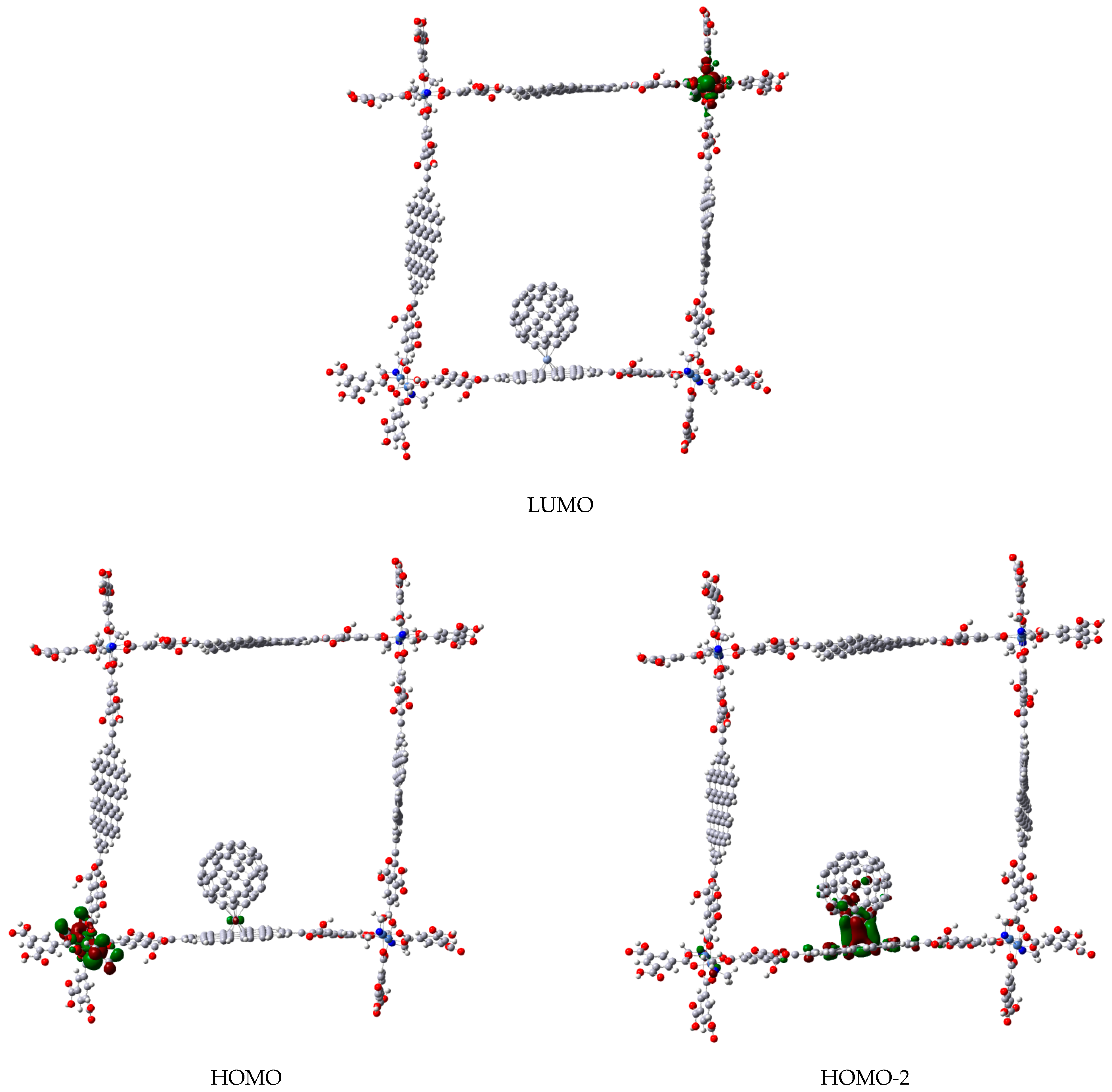
3.1. Π-Stacking
3.2. Formation of Metallocenes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60 buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Murayama, H.; Tomonoh, S.; Alford, J.M.; Karpuk, M.E. Fullerene production in tons and more: From science to industry. Fuller. Nanotub. Carbon Nanostructures 2004, 12, 1–9. [Google Scholar] [CrossRef]
- Delgado, J.R.; Fillipone, S.; Giacolone, F.; Herranz, M.A.; Illescas, B.; Perez, E.M.; Martin, N. Bucckyballs, Polyarenes II. In Topics in Current Chemistry; Siegel, J.S., Wu, Y.T., Eds.; Springer: Berlin, Germany, 2014; Volume 350, pp. 1–64. [Google Scholar]
- Acquah, S.F.A.; Penkova, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhardt, B.E.; Magi, J.M. Review—The beautiful molecule: 30 years of C60 and its derivatives. ECS J. Sol. State Sci. Tech. 2017, 6, M3155–M3162. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cells applications. Sol. Energy Mater. Sol. Cell 2017, 161, 102–148. [Google Scholar] [CrossRef]
- Coro, J.; Suarez, M.; Silva, L.S.R.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Fullerene applications in fuel cells: A review. Int. J. Hydrog. Energy 2016, 41, 17944–17959. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotech. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Hernández-García, A.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Sivaraman, N.; Dhamodaran, R.; Kaliappan, I.; Srinivasan, T.G.; Vasudeva Rao, P.R.; Mathews, C.K. Solubility of C60 in organic solvents. J. Org. Chem. 1992, 57, 6077–6079. [Google Scholar] [CrossRef]
- Astefanei, A.; Nunez, O.; Galceran, M.T. Characterization and determination of fullerenes; a critical review. Anal. Chim. Acta 2015, 882, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Simón, C.; García-Borras, M.; Gómez, L.; Parella, T.; Osuna, S.; Juanhuix, J.; Imaz, I.; Maspoch, D.; Costas, M. Sponge-like molecular cage for purification of fullerenes. Nat. Comm. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef]
- García-Simón, C.; Costas, M.; Ribas, X. Metallosupraamolecular receptors for fullerene binding and release. Chem. Soc. Rev. 2015, 45, 40–62. [Google Scholar] [CrossRef]
- Ubasart, E.; Borodin, O.; Fuertes-Espinosa, C.; Xu, Y.; García-Simón, C.; Gómez, L.; Juanhuix, J.; Gándara, F.; Imaz, I.; Maspoch, D.; et al. A three-shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60. Nat. Chem. 2021, 13, 420–427. [Google Scholar] [CrossRef]
- Purba, P.C.; Maity, M.; Bhatacharyya, S.; Mukherjee, P.S. A self-assembled palladium(II) barrel for binding of fullerenes and photosensitization ability of the fullerene-encapsulated barrel. Angew. Chem. 2021. [Google Scholar] [CrossRef]
- Ray, D.; Goswami, S.; Duan, J.; Hupp, J.T.; Cramer, C.J.; Gagliardi, L. Tuning the conductivity of hexa-zirconium(IV) metal–organic frameworks by encapsulating heterofullerenes. Chem. Mater. 2021, 33, 1182–1189. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Meng, H.; Nie, M.; Li, Q.; Xiang, J.; Zhang, Z.; Wang, C.; Wang, T. Size-selective encapsulation of metallofullerenes by cycloparaphenylene and dissociation using metal-organic framework. Carbon 2020, 161, 694–701. [Google Scholar] [CrossRef]
- Villalva, J.; Develioglu, A.; Montenegro-Pholhammer, N.; Sánchez de Armas, R.; Gamonal, A.; Rial, E.; García-Hernández, M.; Ruiz-González, L.; Sánchez-Costa, J.; Calzado, C.J.; et al. Spin-state-dependent electrical conductivity in single-walled carbon nanotubes encapsulating spin-crossover molecules. Nat. Commun. 2021, 12, 1578–1585. [Google Scholar] [CrossRef]
- Beuerle, F.; Gole, B. Covalent organic frameworks and cage compounds: Design and applications of polymeric and discrete organic scaffolds. Angew. Chem. 2018, 57, 4850–4878. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scal-mani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Sumimoto, M.; Kawashima, Y.; Hori, K.; Fujimoto, H. Theoretical study on the stability of double-decker type metal phthalocyanines, M(Pc)2 and M(Pc)2+ (M = Ti, Sn and Sc): A critical assessment on the performance of density functionals. Phys. Chem. Chem. Phys. 2015, 17, 6478–6483. [Google Scholar] [CrossRef] [PubMed]
- Masuda, J.D.; Boeré, R.T. Metal carbonyl complexes of phosphaamidines. Coordinative integrity detected in C-amino(λ3,σ2)-phosphaalkene isomers coordinated through n(P) HOMO-1 donor orbitals. Dalton Trans. 2016, 45, 2102–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coimbra, D.F.; Ortolan, A.O.; Orenha, R.P.; da Silva, V.B.; Parreira, R.L.T.; Caramori, G.F. Shedding light on the bonding situation of triangular and square heterometallic clusters: Computational insight. New J. Chem. 2020, 44, 5079–5087. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.J. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, R.F.W.; Nguyen-Dang, T.T.; Tal, Y. A topological theory of molecular structure. Rep. Prog. Phys. 1981, 44, 894–948. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, J.R. The Quantum Theory of Atoms in Molecules. From Solid State to DNA and Drug Design; Chapter 3; Matta, C.F., Boyd, R.J., Eds.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Klotzbach, S.; Scherpf, T.; Beuerle, F. Dynamic covalent assembly of tribenzotriquinacenes into molecular cubes. Chem. Commun. 2014, 50, 12454–12457. [Google Scholar] [CrossRef]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D.; et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef]
- Chipot, C.; Jaffe, R.; Maigret, B.; Pearlman, D.A.; Kollman, P.A. Benzene dimer: A good model for π-π interactions in proteins? A comparison between the benzene and the toluene dimmers in the gas phase and in an aqueous solution. J. Am. Chem. Soc. 1996, 118, 11217–11224. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Liu, J. π-π stacking interaction: A nondestructive and facile means in material engineering for bioaplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Schwabe, T.; Muck-Lichtenfeld, C. Density functional theory with dispersion corrections for supramolecular structures, aggregates and complexes of (bio) organic molecules. Org. Biomol. Chem. 2007, 5, 741–758. [Google Scholar] [CrossRef]
- Henne, S.; Bredenkötter, B.; Dehghan-Baghi, A.A.; Schmid, R.; Volkmer, D. Advanced buckyball joints: Synthesis, complex formation and computational simulations of centrohexaindane-extended tribenzotriquinacene receptors for C60 fullerene. Dalton Trans. 2012, 41, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L.; Olmstead, M.M. Reactions of transition metal complexes with Fullerenes (C60, C70, etc.) and related materials. Chem. Rev. 1998, 98, 2123–2165. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Salcedo, R. Coordination modes and different hapticities for fullerene organometallic complexes. Molecules 2012, 17, 7151–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axet, M.R.; Dechy-Cabaret, O.; Durand, J.; Gouygou, M.; Serp, P. Coordination chemistry on carbon surfaces. Coord. Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Nakamura, E.; Sawamura, M. Chemistry of η5-fullerene metal complexes. Pure Appl. Chem. 2001, 73, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, E. Bucky ferrocene: Hybrid of ferrocene and fullerene. Pure Appl. Chem. 2003, 75, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, V.I. Fullerenes as a new type of ligands for transition metals. Russ. J. Coord. Chem. 2007, 33, 723–737. [Google Scholar] [CrossRef]
- Salcedo, R. Fullerenocene. Polyhedron 2009, 28, 431–436. [Google Scholar] [CrossRef]
- Molina, B.; Pérez-Manríquez, L.; Salcedo, R. Exohedral complexes of large fullerenes, a theoretical approach. J. Mol. Mod. 2017, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Anafche, M.; Naderi, F.; Khodadadi, Z.; Ektefa, F.; Ghafouri, R.; Zahedi, M. Computational study for the circular redox reaction of N2O with CO catalyzed by fullerometallic cations C60Fe+ and C70Fe+. J. Mol. Graph. Mod. 2017, 72, 50–57. [Google Scholar] [CrossRef]
- Gal’pern, E.G.; Sabirov, A.R.; Stankevich, I.P. C60 fullerene as an η6 ligand in π complexes of transition metals. Phys. Sol. State 2007, 49, 2330–2334. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Manoharan, M.; Sharma, P.K. Exohedral η5 and η6 Transition-Metal Organometallic Complexes of C60 and C70: A theoretical study. Organometallics 2000, 19, 1879–1887. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S. Role of dG/dw and dV/dw in AIM analysis: An approach to the nature of weak to strong interactions. J. Phys. Chem. A 2013, 117, 1795–1803. [Google Scholar] [CrossRef]
- Pilmé, J.; Renault, E.; Bassal, F.; Amaouch, M.; Montavon, G.; Galland, N. QTAIM analysis in the context of quasirelativistic quantum calculations. J. Chem. Theory Comput. 2014, 10, 4830–4841. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical bonds without bonding electron density—Does the difference electron-density analysis suffice for a description of the chemical bond? Angew. Chem. 1984, 23, 627–628. [Google Scholar] [CrossRef]
| Bond Critical Point Number | ρ u.a. | u.a. | G(r) u.a. | |V(r)|/G(r) u.a. |
|---|---|---|---|---|
| Ni-Coronene | C60Ni-Molecule b | |||
| BCP1 | 0.060 | 0.174 | 0.064 | 1.322 |
| BCP2 | 0.059 | 0.1780 | 0.065 | 1.309 |
| BCP3 | 0.061 | 0.179 | 0.066 | 1.322 |
| BCP4 | 0.056 | 0.190 | 0.063 | 1.248 |
| BCP5 | 0.061 | 0.168 | 0.064 | 1.350 |
| BCP6 | 0.057 | 0.188 | 0.064 | 1.265 |
| Ni-Fullerene | ||||
| BCP1 | 0.118 | 0.197 | 0.114 | 1.569 |
| BCP2 | 0.119 | 0.199 | 0.115 | 1.567 |
| Cr-coronene | C60Cr-Molecule b | |||
| CP1 | 0.063 | 0.246 | 0.072 | 1.143 |
| CP2 | 0.059 | 0.246 | 0.070 | 1.119 |
| CP3 | 0.060 | 0.243 | 0.069 | 1.123 |
| CP4 | 0.062 | 0.245 | 0.071 | 1.138 |
| CP5 | 0.062 | 0.241 | 0.070 | 1.142 |
| CP6 | 0.060 | 0.246 | 0.070 | 1.121 |
| Cr-Fullerene | ||||
| CP1 | 0.054 | 0.223 | 0.063 | 1.114 |
| CP2 | 0.053 | 0.216 | 0.061 | 1.118 |
| CP3 | 0.054 | 0.223 | 0.063 | 1.111 |
| CP4 | 0.052 | 0.212 | 0.060 | 1.118 |
| CP5 | 0.053 | 0.220 | 0.062 | 1.113 |
| CP6 | 0.053 | 0.215 | 0.061 | 1.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, C.; Molina, B.; Salcedo, R. Capture of Fullerenes in Cages and Rings by Forming Metal-π Bond Arene Interactions. Materials 2021, 14, 3424. https://doi.org/10.3390/ma14123424
Rios C, Molina B, Salcedo R. Capture of Fullerenes in Cages and Rings by Forming Metal-π Bond Arene Interactions. Materials. 2021; 14(12):3424. https://doi.org/10.3390/ma14123424
Chicago/Turabian StyleRios, Citlalli, Bertha Molina, and Roberto Salcedo. 2021. "Capture of Fullerenes in Cages and Rings by Forming Metal-π Bond Arene Interactions" Materials 14, no. 12: 3424. https://doi.org/10.3390/ma14123424
APA StyleRios, C., Molina, B., & Salcedo, R. (2021). Capture of Fullerenes in Cages and Rings by Forming Metal-π Bond Arene Interactions. Materials, 14(12), 3424. https://doi.org/10.3390/ma14123424






