Cu Patterning Using Femtosecond Laser Reductive Sintering of CuO Nanoparticles under Inert Gas Injection
Abstract
1. Introduction
2. Experimental Methods
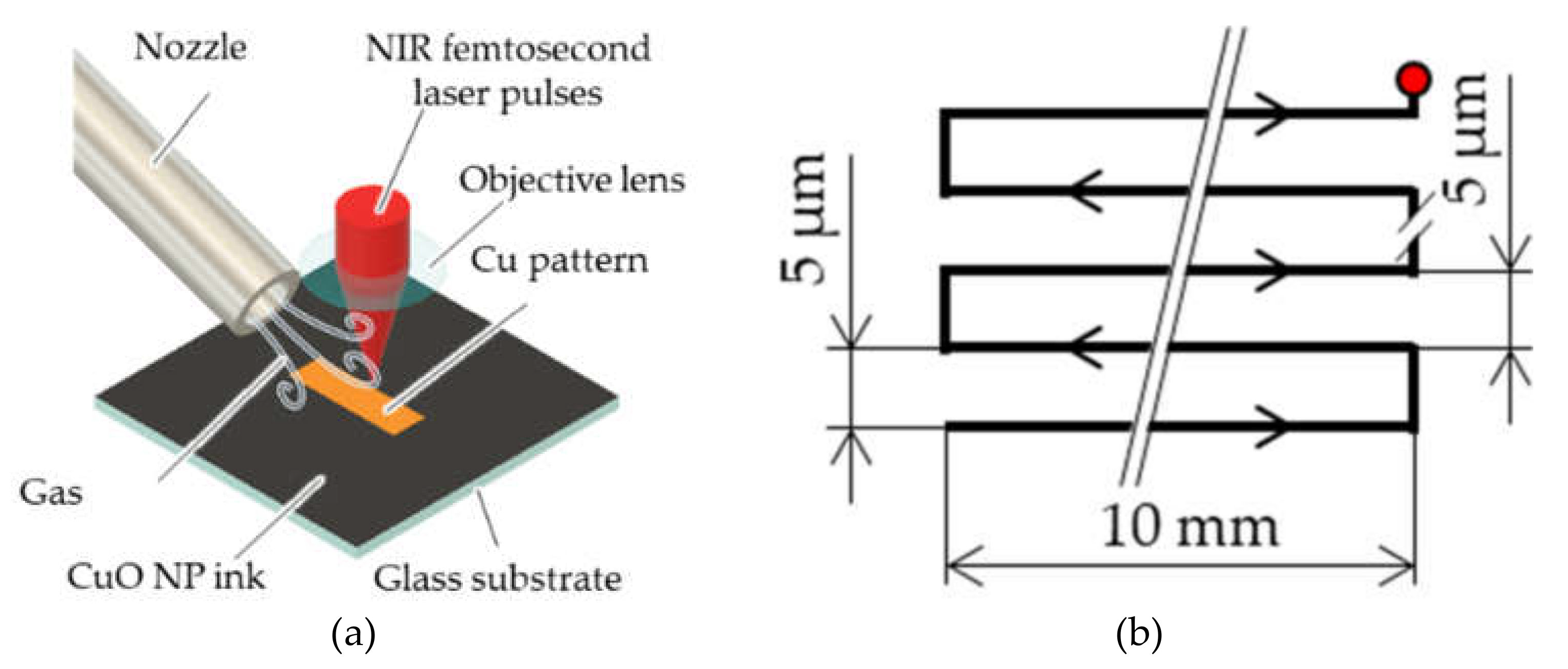
2.1. Cu Patterning Based on Femtosecond Laser Reductive Sintering of CuO NPs under a Controlled Atmosphere
2.2. Evaluation Methods
3. Results
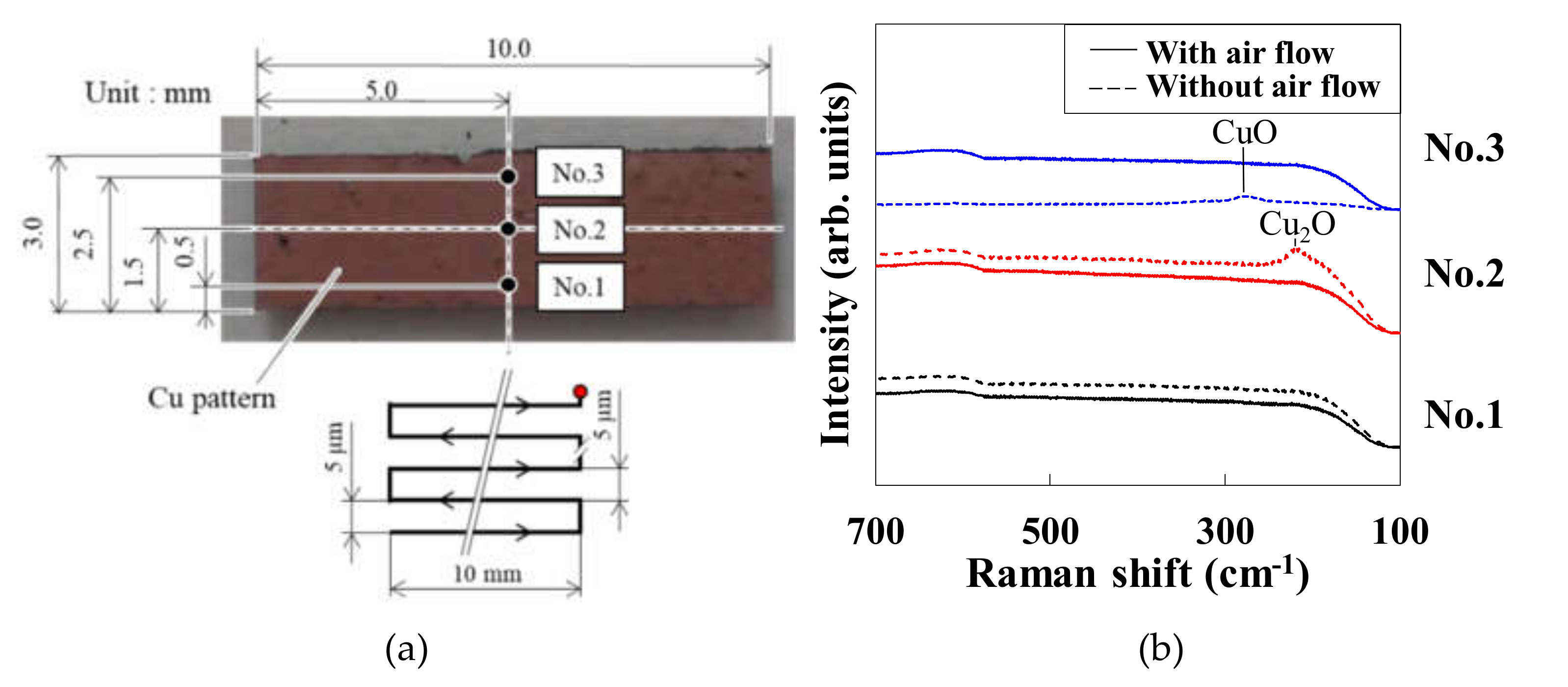
3.1. Effect of Air Injection on the Pattern Uniformity
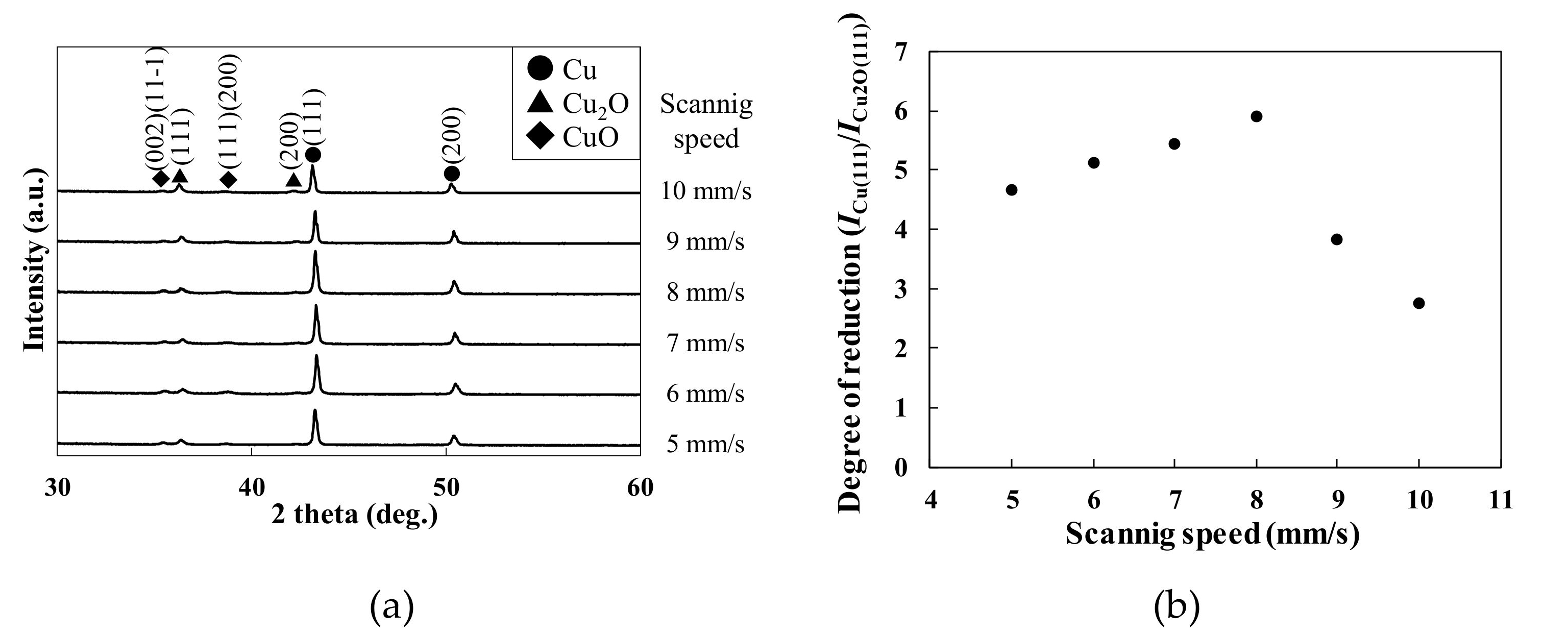
3.2. Effect of Scan Speed on the Crystal Structures of the Patterns
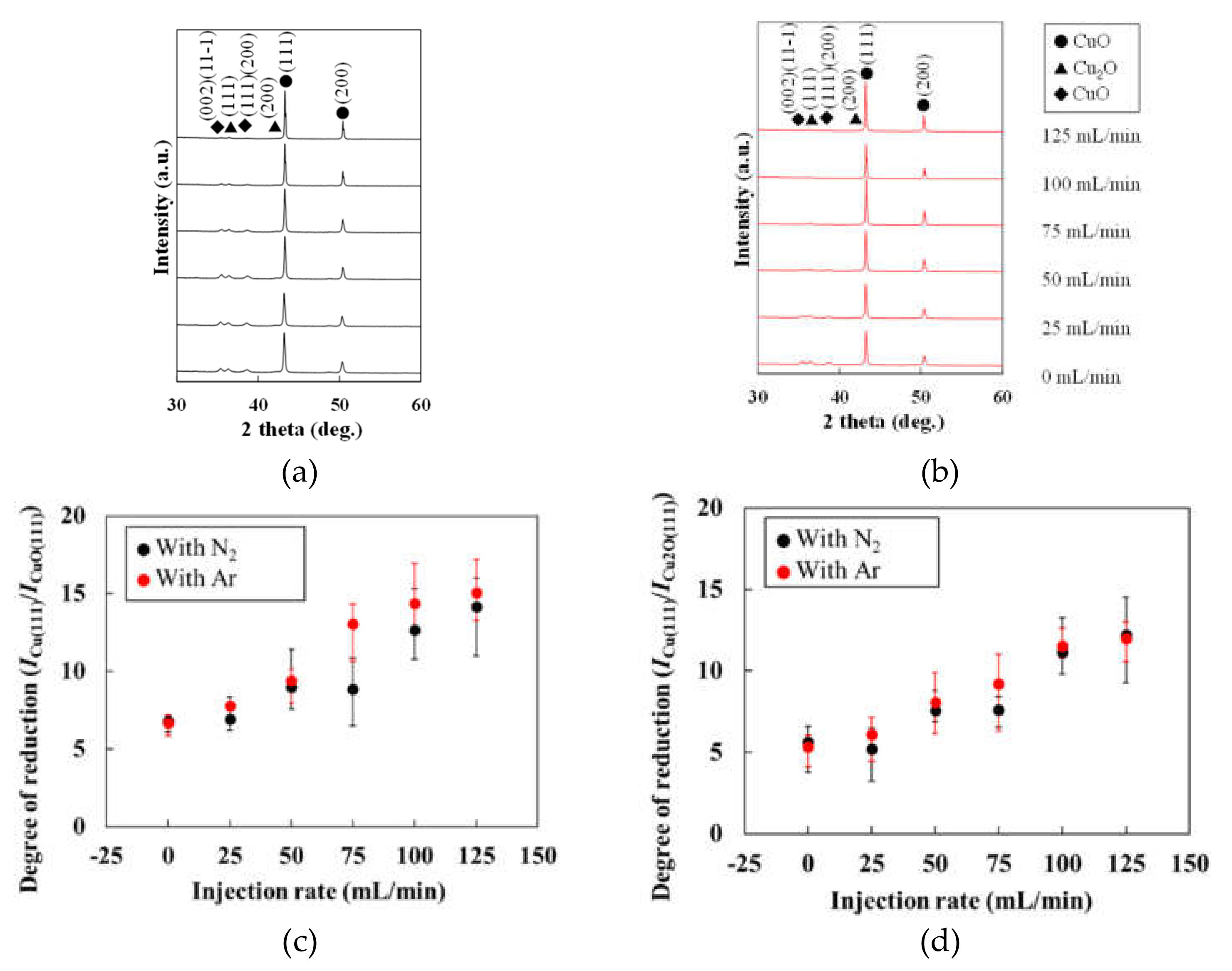
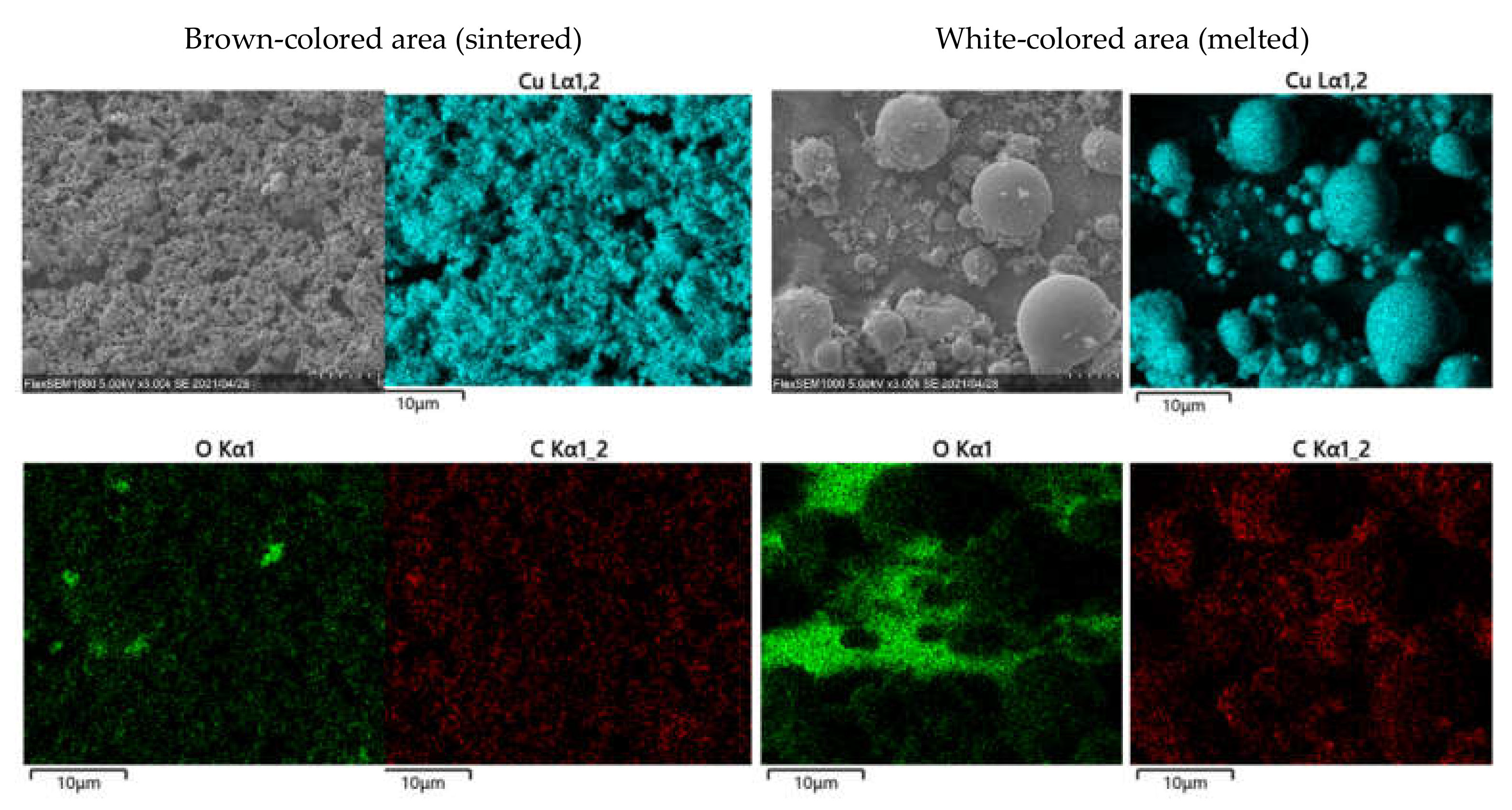
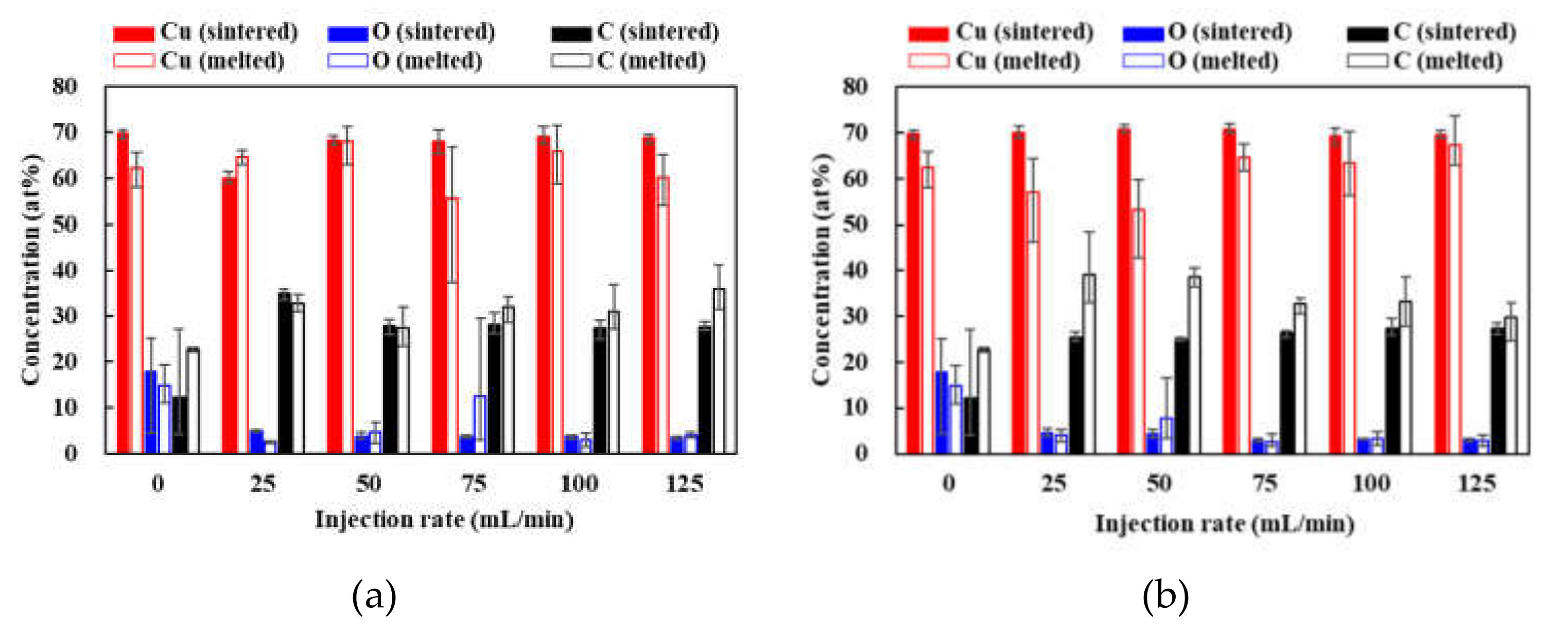
3.3. Crystal Structures of the Patterns Fabricated under Various Gas Injections
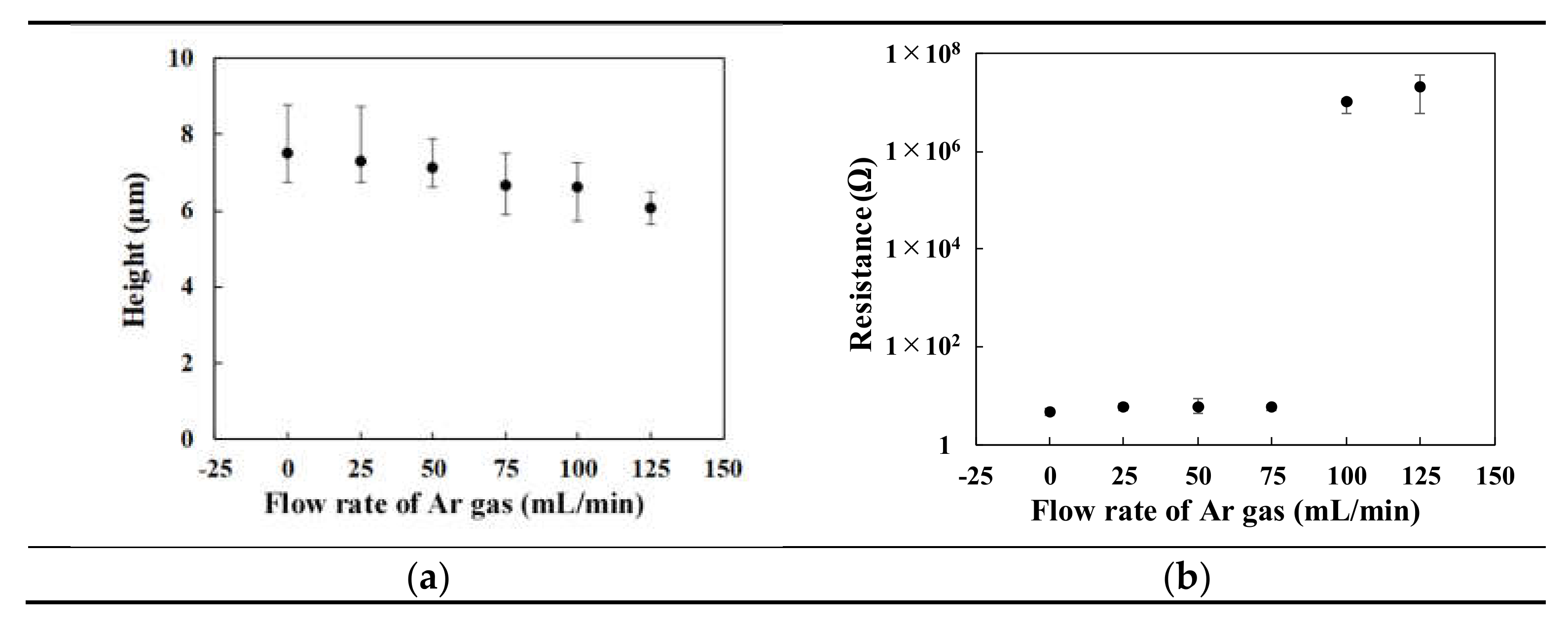
3.4. Surface Morphology
3.5. Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zacharatos, F.; Theodorakos, I.; Karvounis, P.; Tuohy, S.; Braz, N.; Melamed, S.; Kabla, A.; De la Vega, F.; Andritsos, K.; Hatziapostolou, A.; et al. Selective Laser Sintering of Laser Printed Ag Nanoparticle Micropatterns at High Repetition Rates. Materials 2018, 11, 2142. [Google Scholar] [CrossRef]
- Yang, L.; Gan, Y.; Zhang, Y.; Chen, J.K. Molecular dynamics simulation of neck growth in laser sintering of different-sized gold nanoparticles under different heating rates. Appl. Phys. A 2011, 106, 725–735. [Google Scholar] [CrossRef]
- Back, S.; Kang, B. Low-cost optical fabrication of flexible copper electrode via laser-induced reductive sintering and adhesive transfer. Opt. Lasers Eng. 2018, 101, 78–84. [Google Scholar] [CrossRef]
- Halonen, E.; Heinonen, E.; Mäntysalo, M. The Effect of Laser Sintering Process Parameters on Cu Nanoparticle Ink in Room Conditions. Opt. Photonics J. 2013, 3, 40–44. [Google Scholar] [CrossRef]
- Zenou, M.; Ermak, O.; Saar, A.; Kotler, Z. Laser sintering of copper nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 025501. [Google Scholar] [CrossRef]
- Cheng, C.W.; Chen, J.K. Femtosecond laser sintering of copper nanoparticles. Appl. Phys. A 2016, 122, 289. [Google Scholar] [CrossRef]
- Soltani, A.; Khorramdel Vahed, B.; Mardoukhi, A.; Mantysalo, M. Laser sintering of copper nanoparticles on top of silicon substrates. Nanotechnology 2016, 27, 035203. [Google Scholar] [CrossRef]
- Ohishi, T.; Kimura, R. Fabrication of Copper Wire Using Glyoxylic Acid Copper Complex and Laser Irradiation in Air. Mater. Sci. Appl. 2015, 6, 799–808. [Google Scholar] [CrossRef]
- Ohishi, T.; Takahashi, N. Preparation and Properties of Copper Fine Wire on Polyimide Film in Air by Laser Irradiation and Mixed-Copper-Complex Solution Containing Glyoxylic Acid Copper Complex and Methylamine Copper Complex. Mater. Sci. Appl. 2018, 9, 859–872. [Google Scholar] [CrossRef][Green Version]
- Yu, J.H.; Rho, Y.; Kang, H.; Jung, H.S.; Kang, K.-T. Electrical behavior of laser-sintered Cu based metal-organic decomposition ink in air environment and application as current collectors in supercapacitor. Int. J. Precis. Eng. Manuf.-Green Technol. 2015, 2, 333–337. [Google Scholar] [CrossRef][Green Version]
- Mizoshiri, M.; Kondo, Y. Direct writing of Cu-based fine micropatterns using femtosecond laser pulse-induced sintering of Cu2O nanospheres. Jpn. J. Appl. Phys. 2019, 58, SDDF05. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Kondo, Y. Direct writing of two- and three-dimensional Cu-based microstructures by femtosecond laser reductive sintering of the Cu2O nanospheres. Opt. Mater. Express 2019, 9, 2828–2837. [Google Scholar] [CrossRef]
- Lee, H.; Yang, M. Effect of solvent and PVP on electrode conductivity in laser-induced reduction process. Appl. Phys. A 2015, 119, 317–323. [Google Scholar] [CrossRef]
- Kang, B.; Han, S.; Kim, J.; Ko, S.; Yang, M. One-Step Fabrication of Copper Electrode by Laser-Induced Direct Local Reduction and Agglomeration of Copper Oxide Nanoparticle. J. Phys. Chem. C 2011, 115, 23664–23670. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Ito, Y.; Arakane, S.; Sakurai, J.; Hata, S. Direct fabrication of Cu/Cu2O composite micro-temperature sensor using femtosecond laser reduction patterning. Jpn. J. Appl. Phys. 2016, 55, 06GP05. [Google Scholar] [CrossRef]
- Arakane, S.; Mizoshiri, M.; Hata, S. Direct patterning of Cu microstructures using femtosecond laser-induced CuO nanoparticle reduction. Jpn. J. Appl. Phys. 2015, 54, 06FP07. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Arakane, S.; Sakurai, J.; Hata, S. Direct writing of Cu-based micro -temperature detectors using femtosecond laser reduction of CuO nanoparticles. Appl. Phys. Express 2016, 9, 036701. [Google Scholar] [CrossRef]
- Arakane, S.; Mizoshiri, M.; Sakurai, J.; Hata, S. Direct writing of three-dimensional Cu-based thermal flow sensors using femtosecond laser-induced reduction of CuO nanoparticles. J. Micromech. Microeng. 2017, 27, 055013. [Google Scholar] [CrossRef]
- Roth, G.-L.; Haubner, J.; Kefer, S.; Esen, C.; Hellmann, R. Fs-laser based hybrid micromachining for polymer micro-opto electrical systems. Opt. Lasers Eng. 2021, 137, 106362. [Google Scholar] [CrossRef]
- Rahman, M.K.; Lu, Z.; Kwon, K.-S. Green laser sintering of copper oxide (CuO) nano particle (NP) film to form Cu conductive lines. AIP Adv. 2018, 8, 095008. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, X.; Li, M.; Xu, M.; Long, J. Copper circuits fabricated on flexible polymer substrates by a high repetition rate femtosecond laser-induced selective local reduction of copper oxide nanoparticles. Opt. Express 2021, 29, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Mizoshiri, M.; Iijima, Y.; Hata, S. Preparation of Nonspherical Monodisperse Polydimethylsiloxane Microparticles for Self-assembly Fabrication of Periodic Structures. IEEJ Trans. Sens. Micromach. 2019, 139, 132–136. [Google Scholar] [CrossRef]

| Injection Rate (mL/min) | 0 | 25 | 50 | 75 | 100 | 125 |
|---|---|---|---|---|---|---|
| N2 gas | 95.1% | 94.5% | 94.0% | 93.2% | 81.8% | 68.8% |
| Ar gas | 95.9% | 96.4% | 96.6% | 95.7% | 84.9% | 76.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoshiri, M.; Yoshidomi, K. Cu Patterning Using Femtosecond Laser Reductive Sintering of CuO Nanoparticles under Inert Gas Injection. Materials 2021, 14, 3285. https://doi.org/10.3390/ma14123285
Mizoshiri M, Yoshidomi K. Cu Patterning Using Femtosecond Laser Reductive Sintering of CuO Nanoparticles under Inert Gas Injection. Materials. 2021; 14(12):3285. https://doi.org/10.3390/ma14123285
Chicago/Turabian StyleMizoshiri, Mizue, and Kyohei Yoshidomi. 2021. "Cu Patterning Using Femtosecond Laser Reductive Sintering of CuO Nanoparticles under Inert Gas Injection" Materials 14, no. 12: 3285. https://doi.org/10.3390/ma14123285
APA StyleMizoshiri, M., & Yoshidomi, K. (2021). Cu Patterning Using Femtosecond Laser Reductive Sintering of CuO Nanoparticles under Inert Gas Injection. Materials, 14(12), 3285. https://doi.org/10.3390/ma14123285






