Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
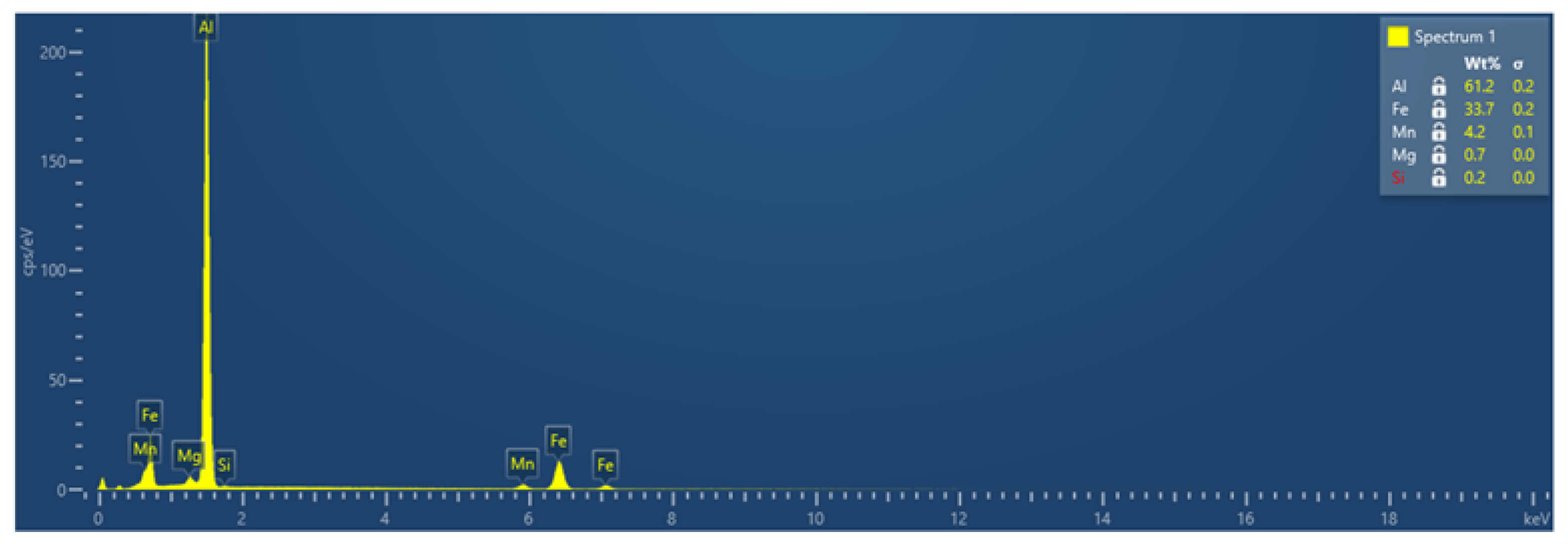
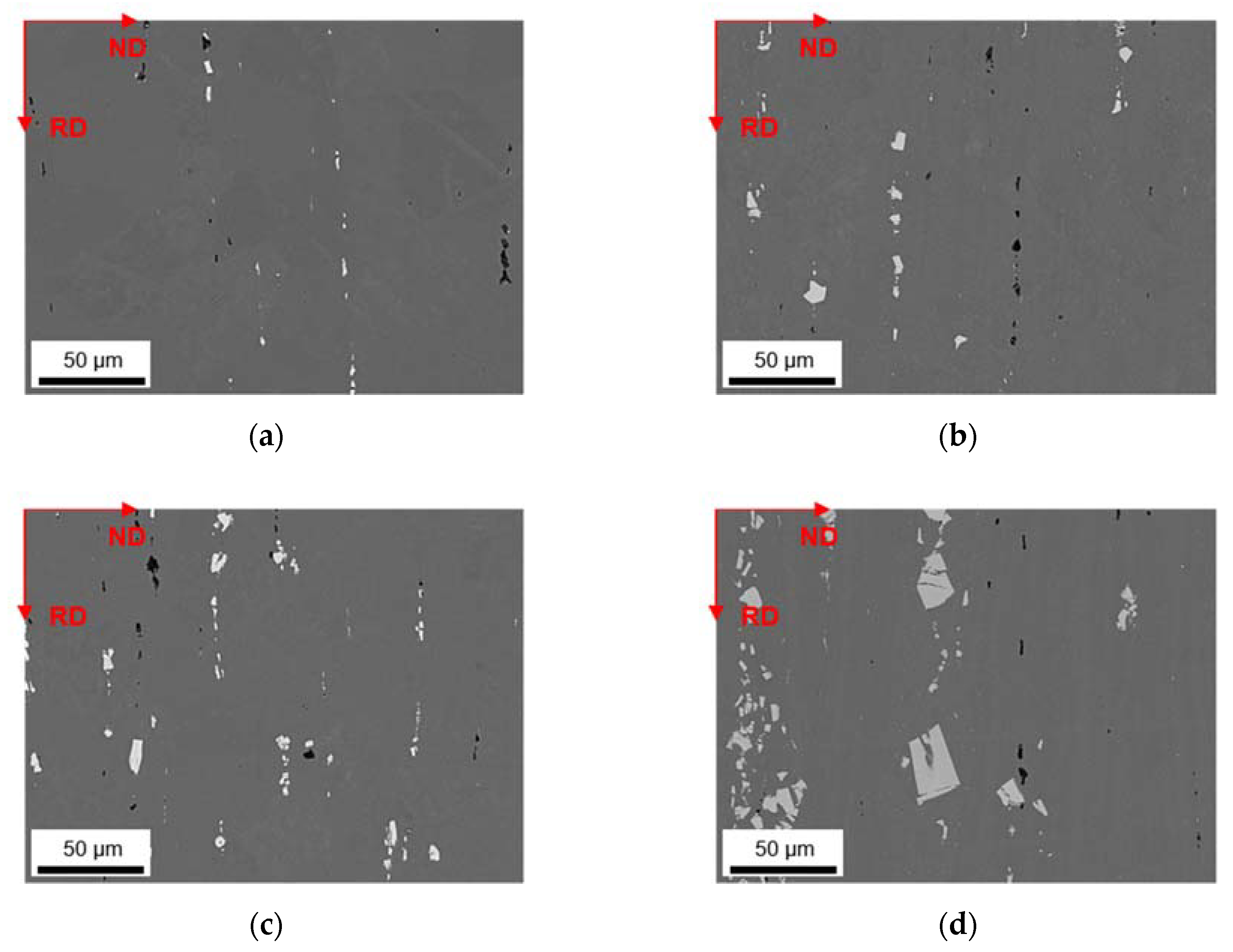
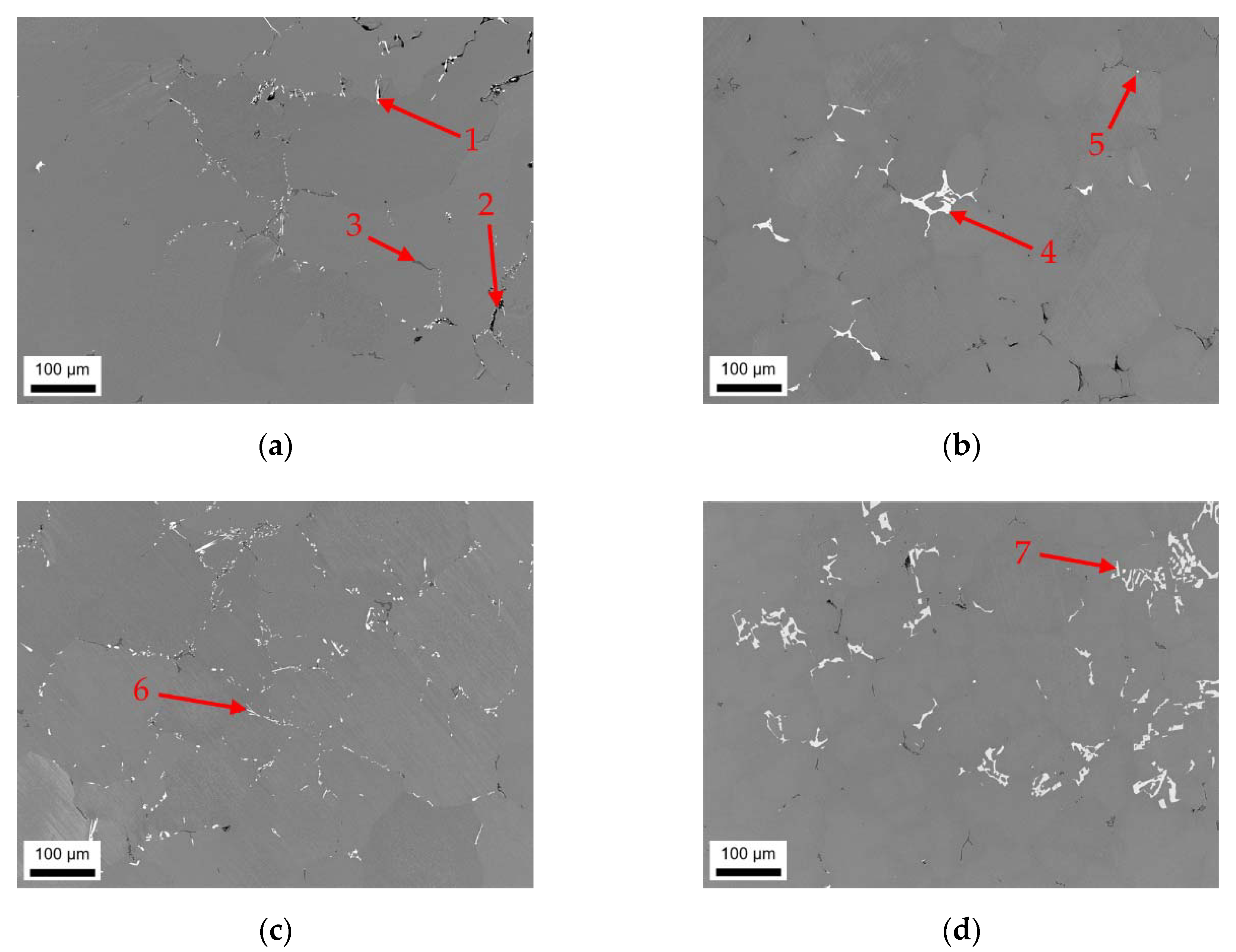
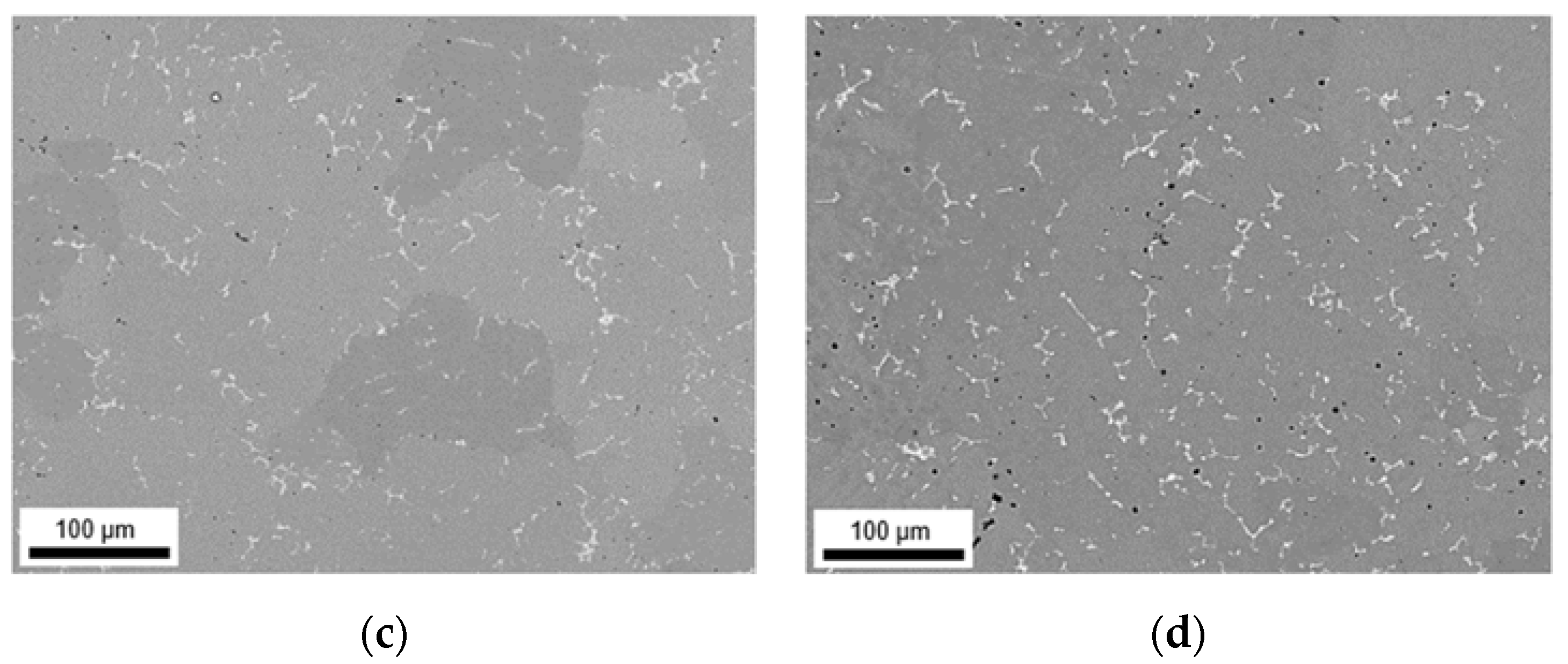
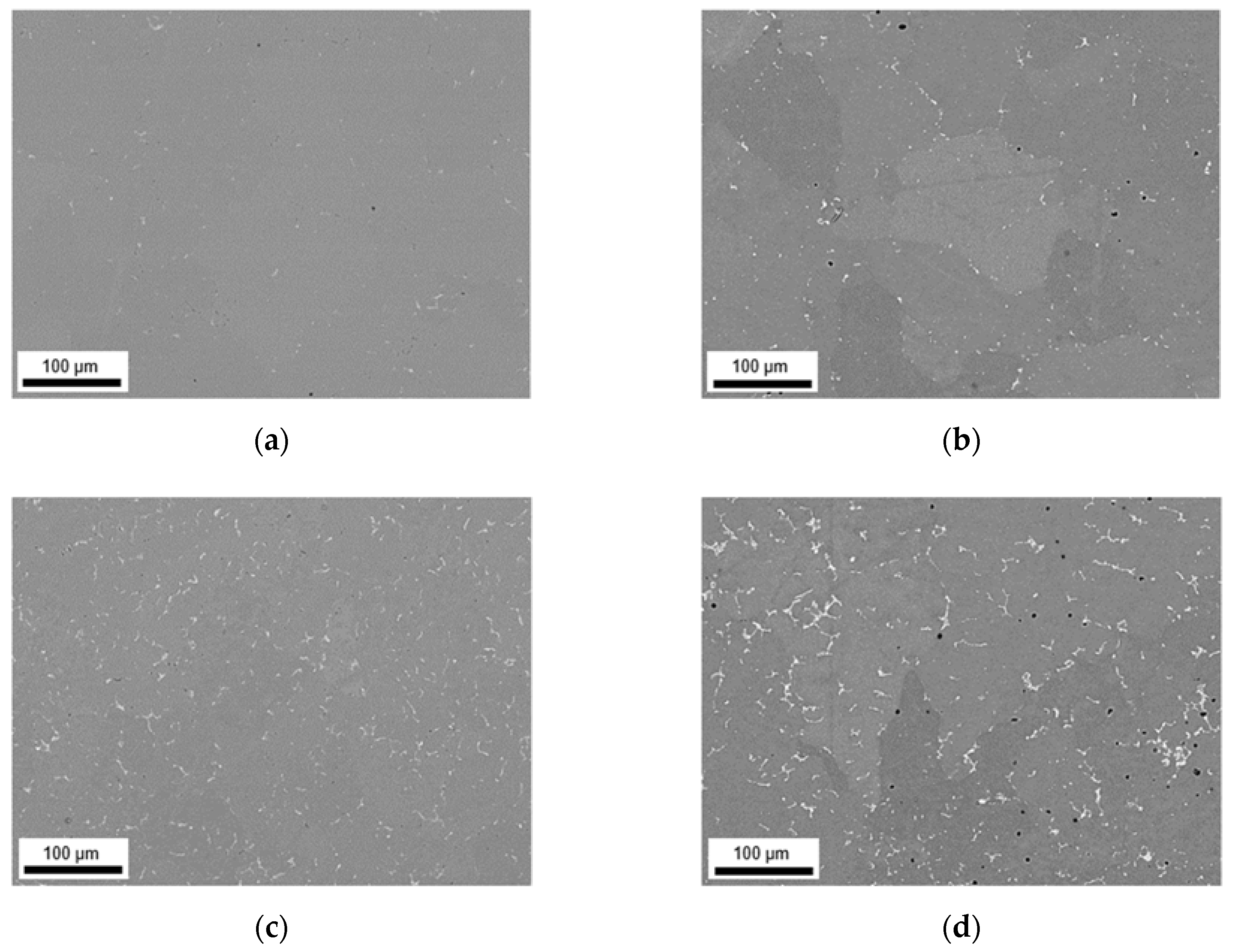
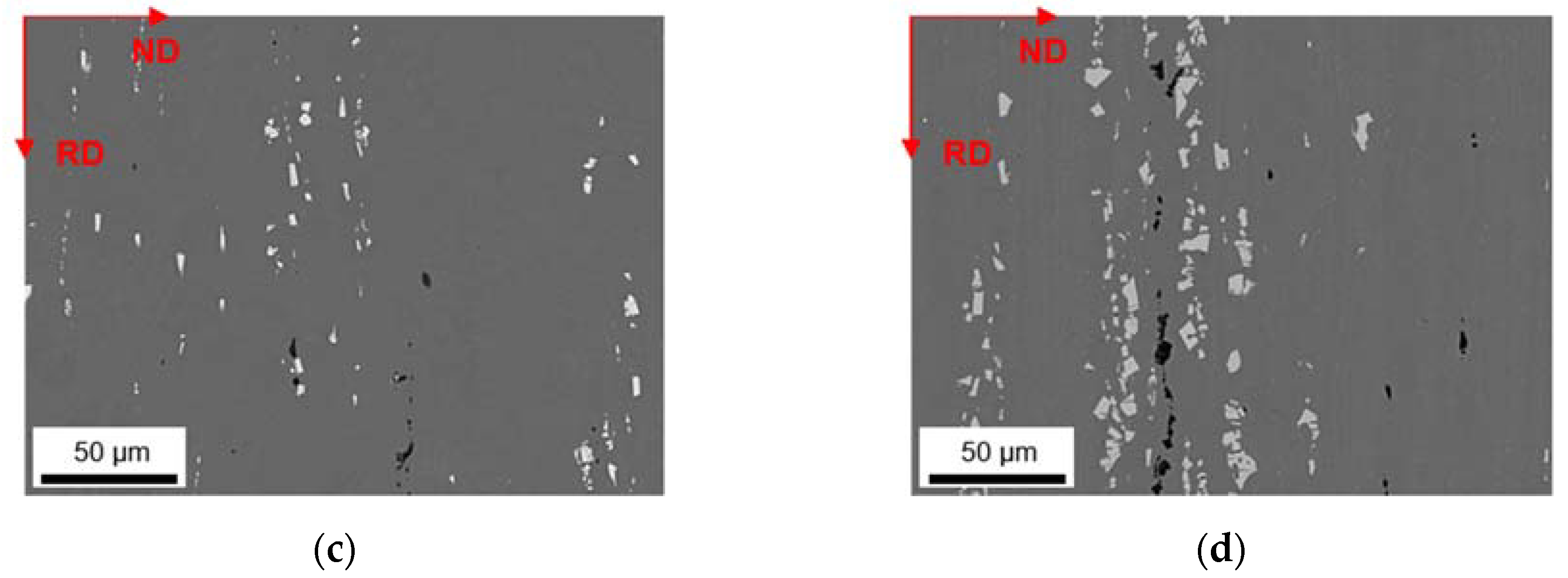
3.1. Microstructures and Intermetallic Phases in As-Cast State
3.1.1. Slow Cooling during Solidification (S-C)
3.1.2. Near Rapid Cooling during Solidification (NR-C Cast)
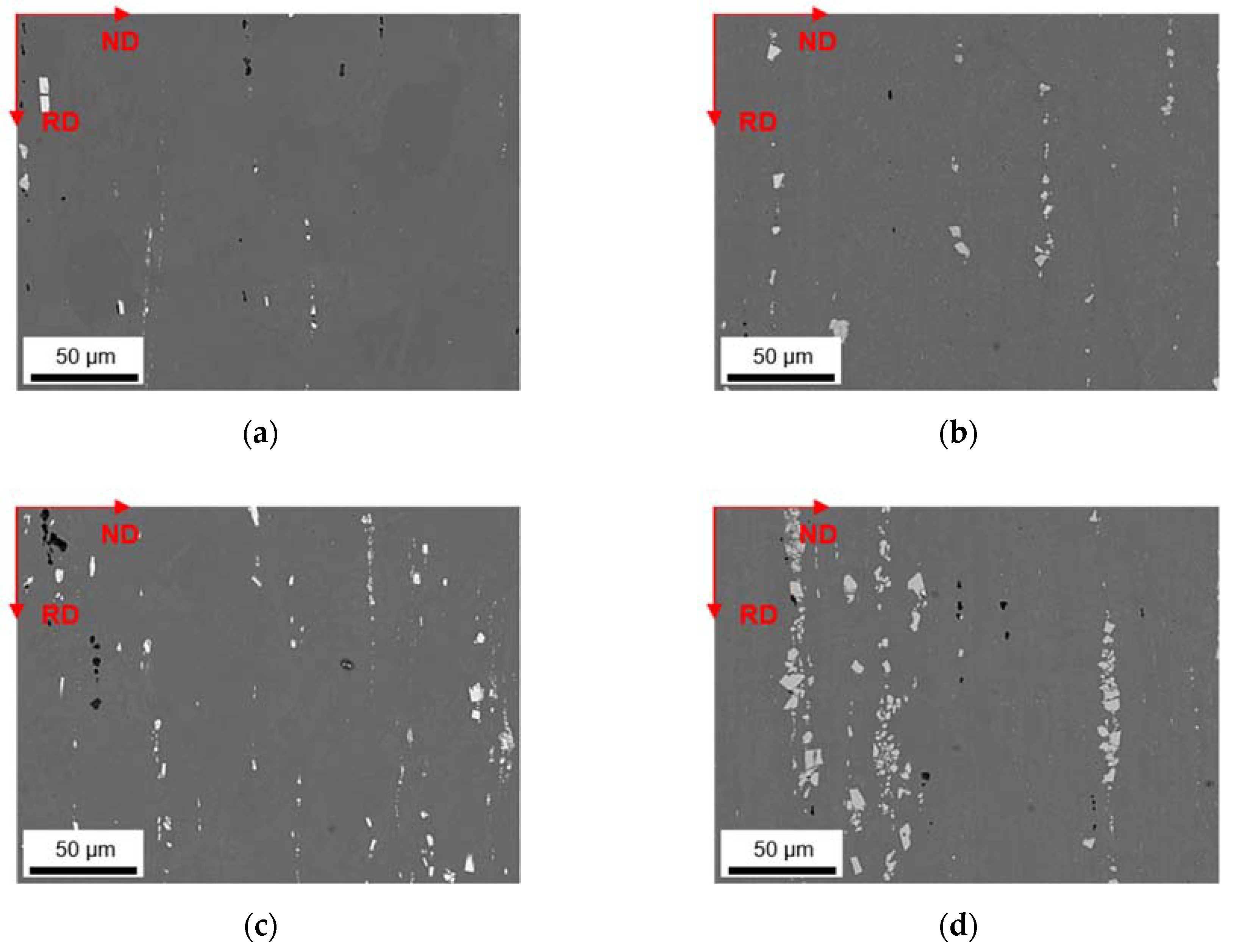
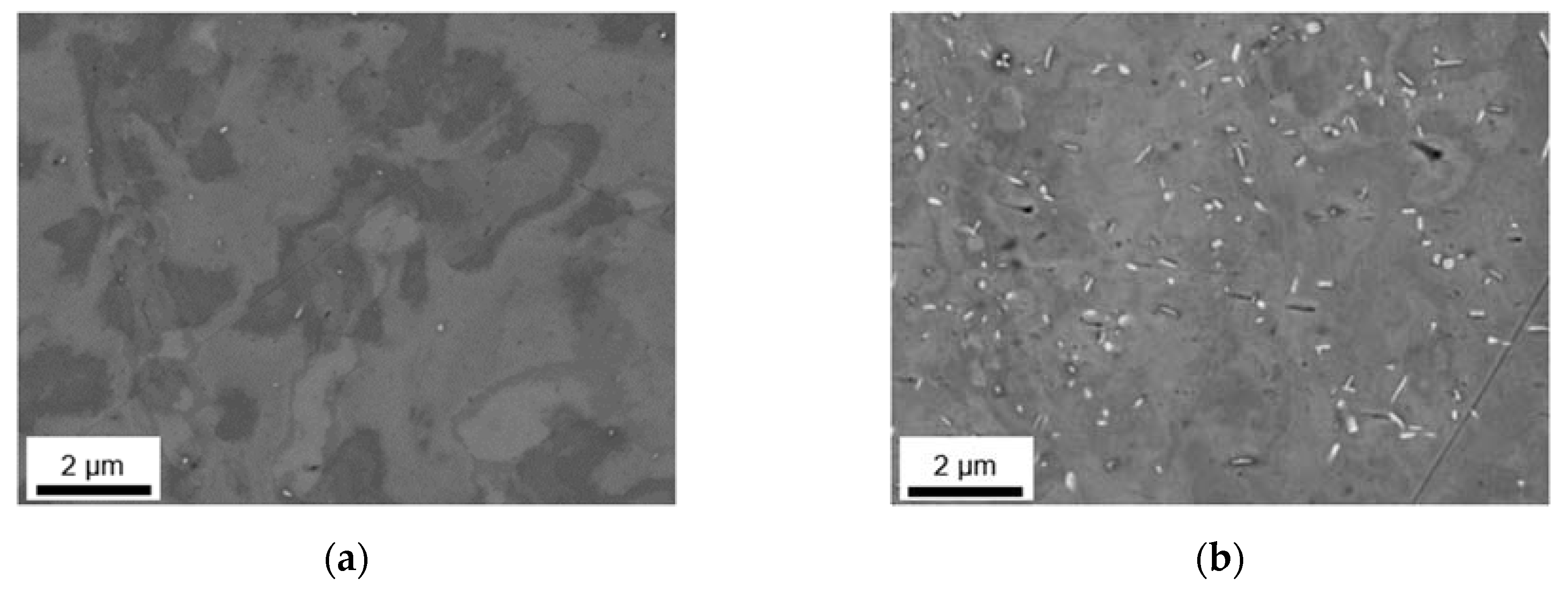
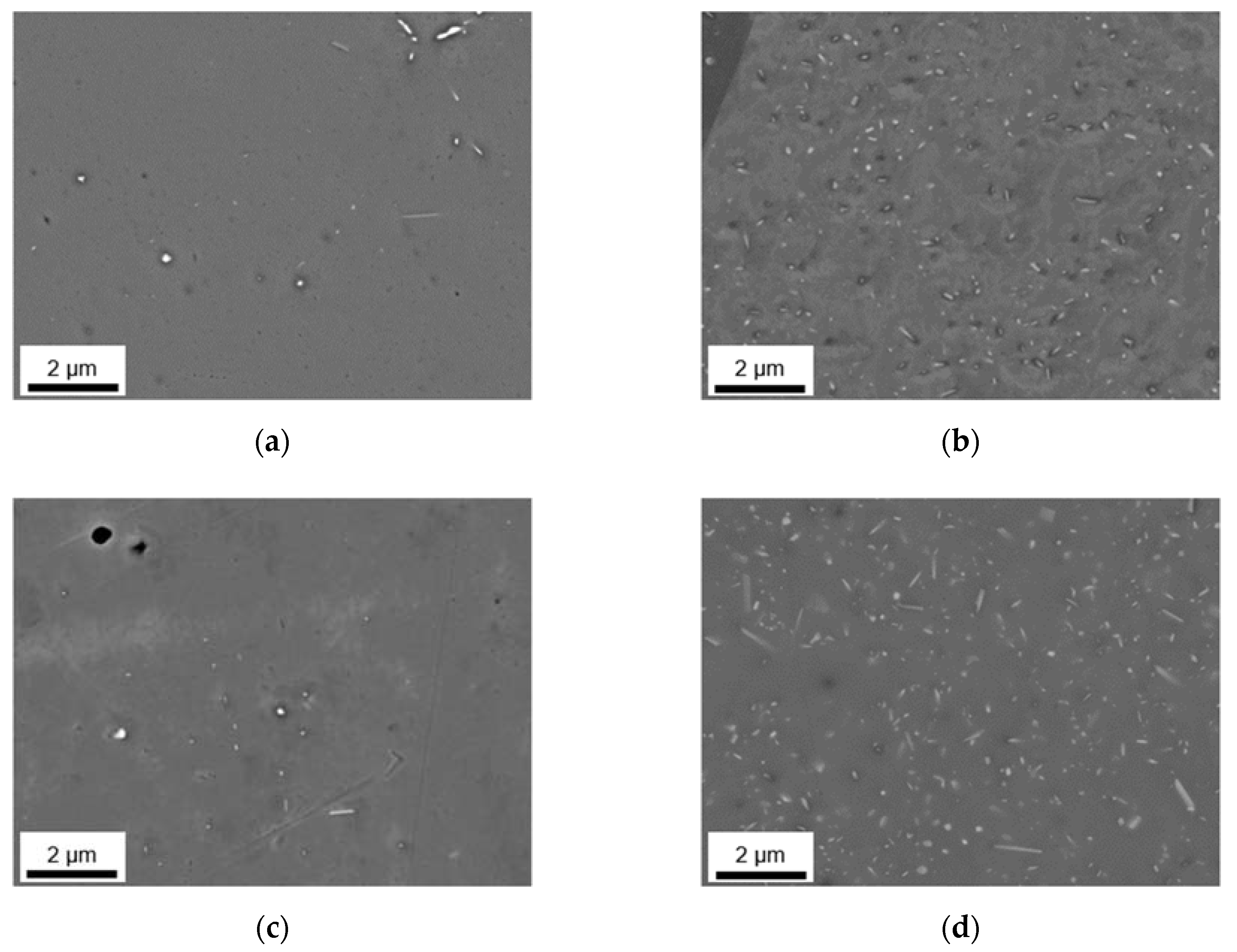
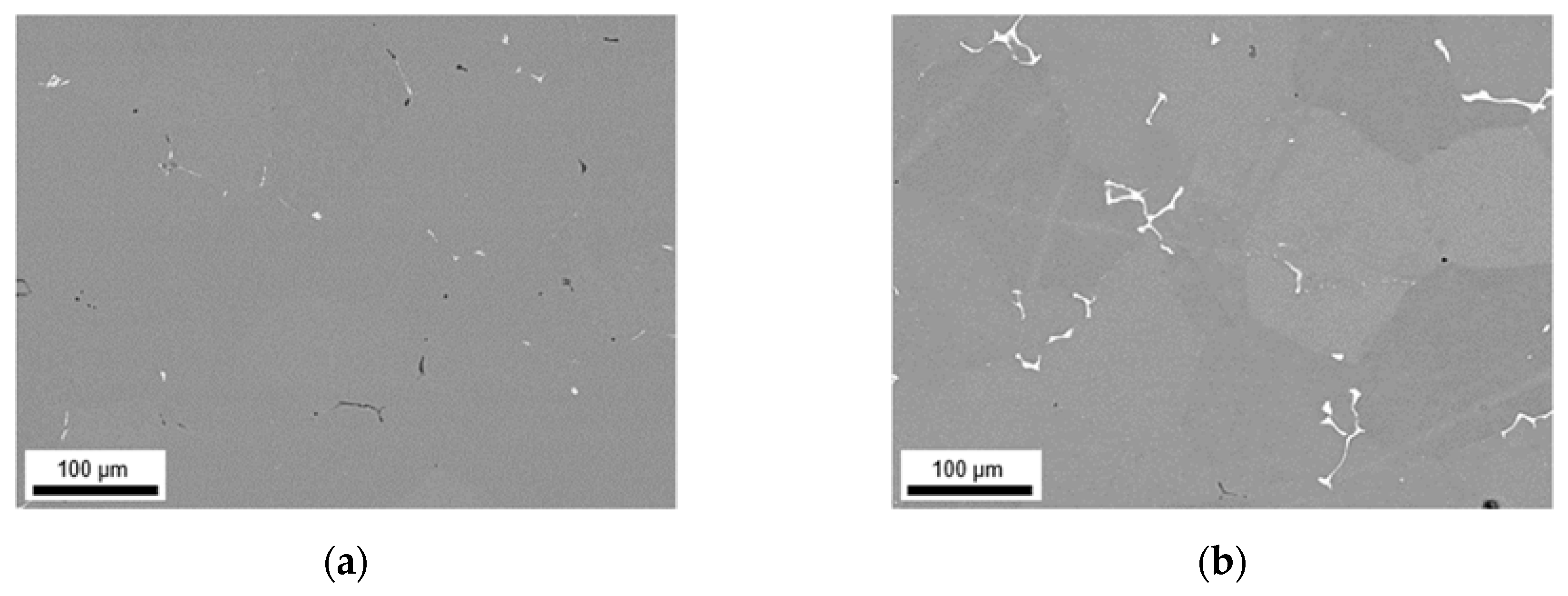
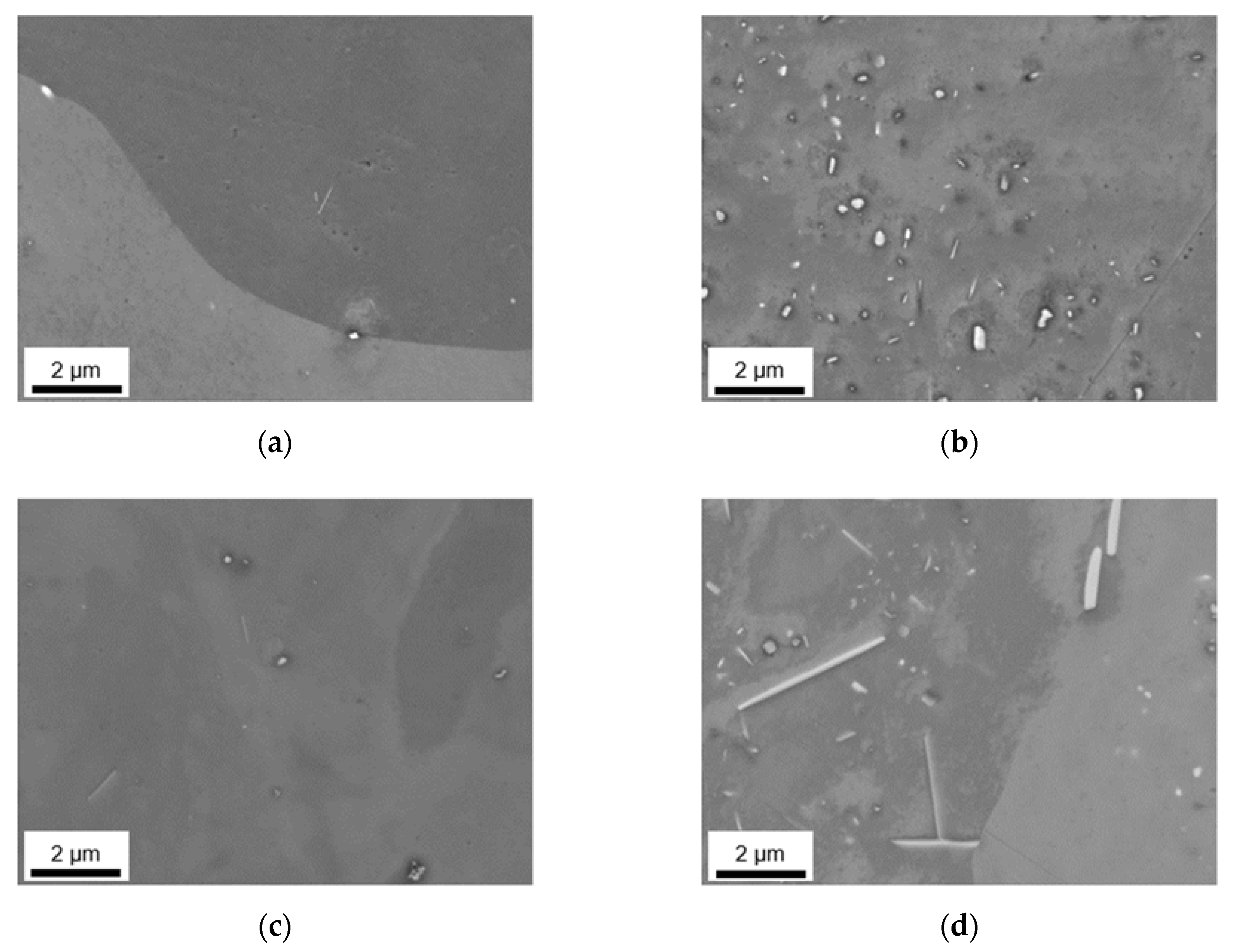
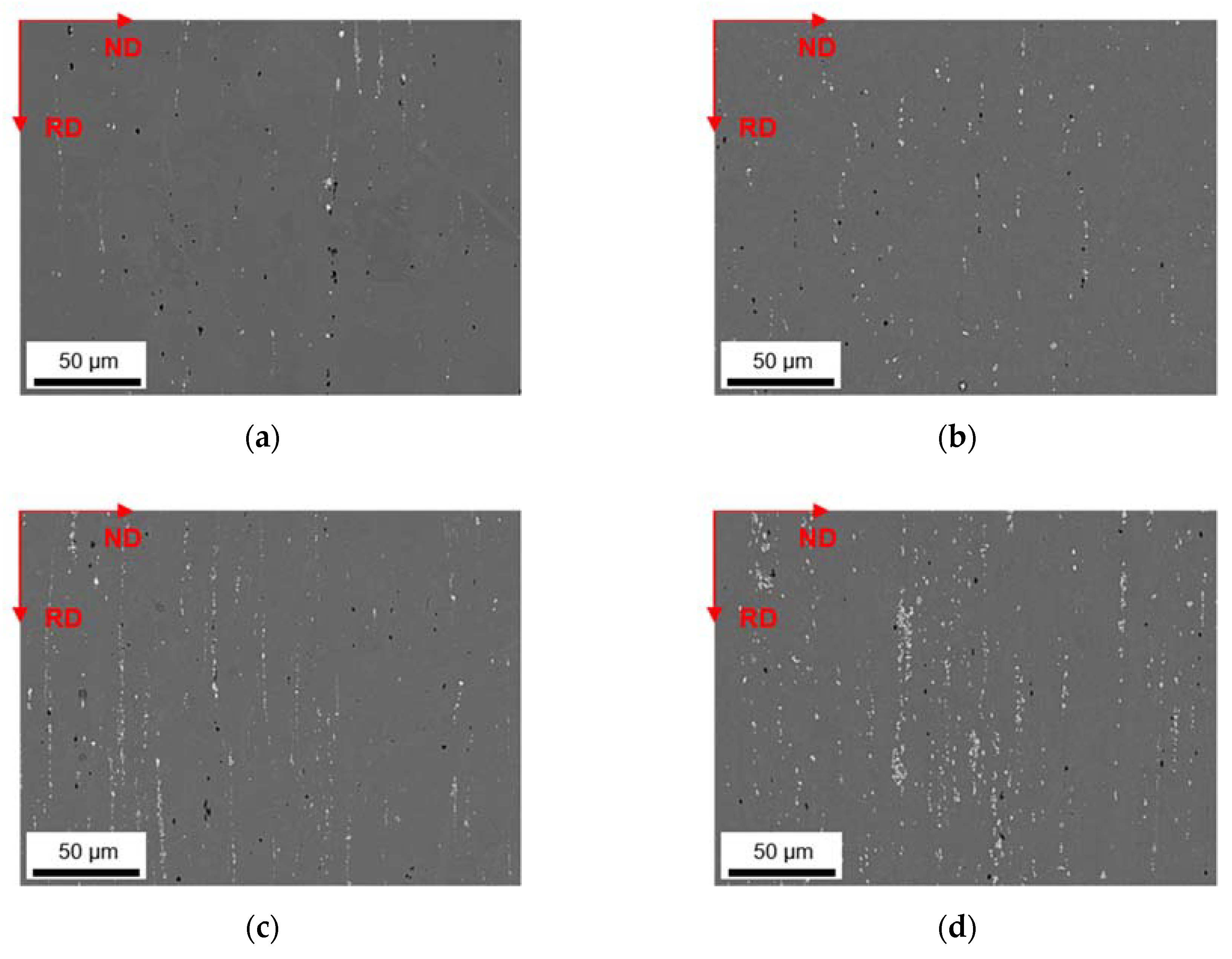
3.2. Microstructural Evolution during Homogenization
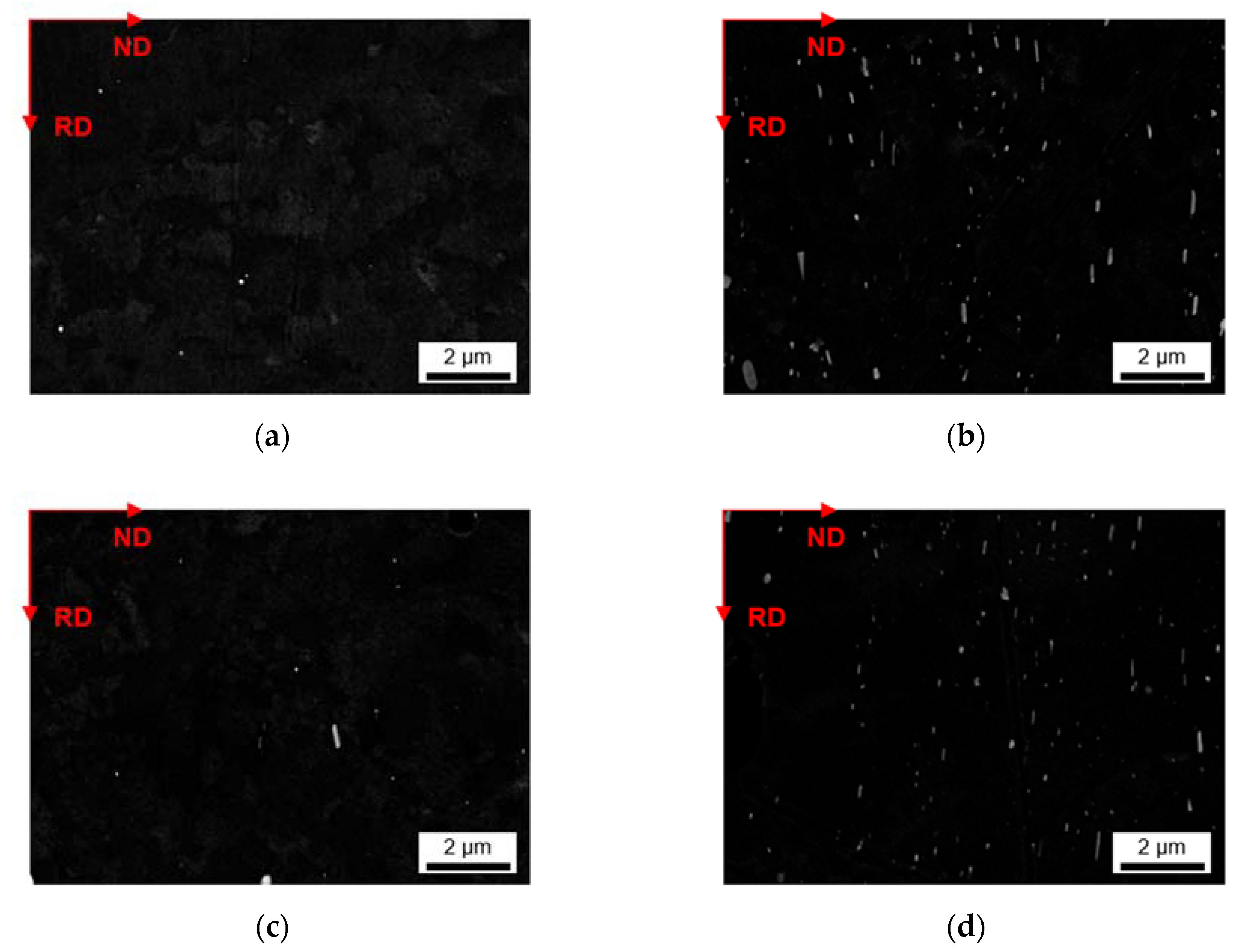
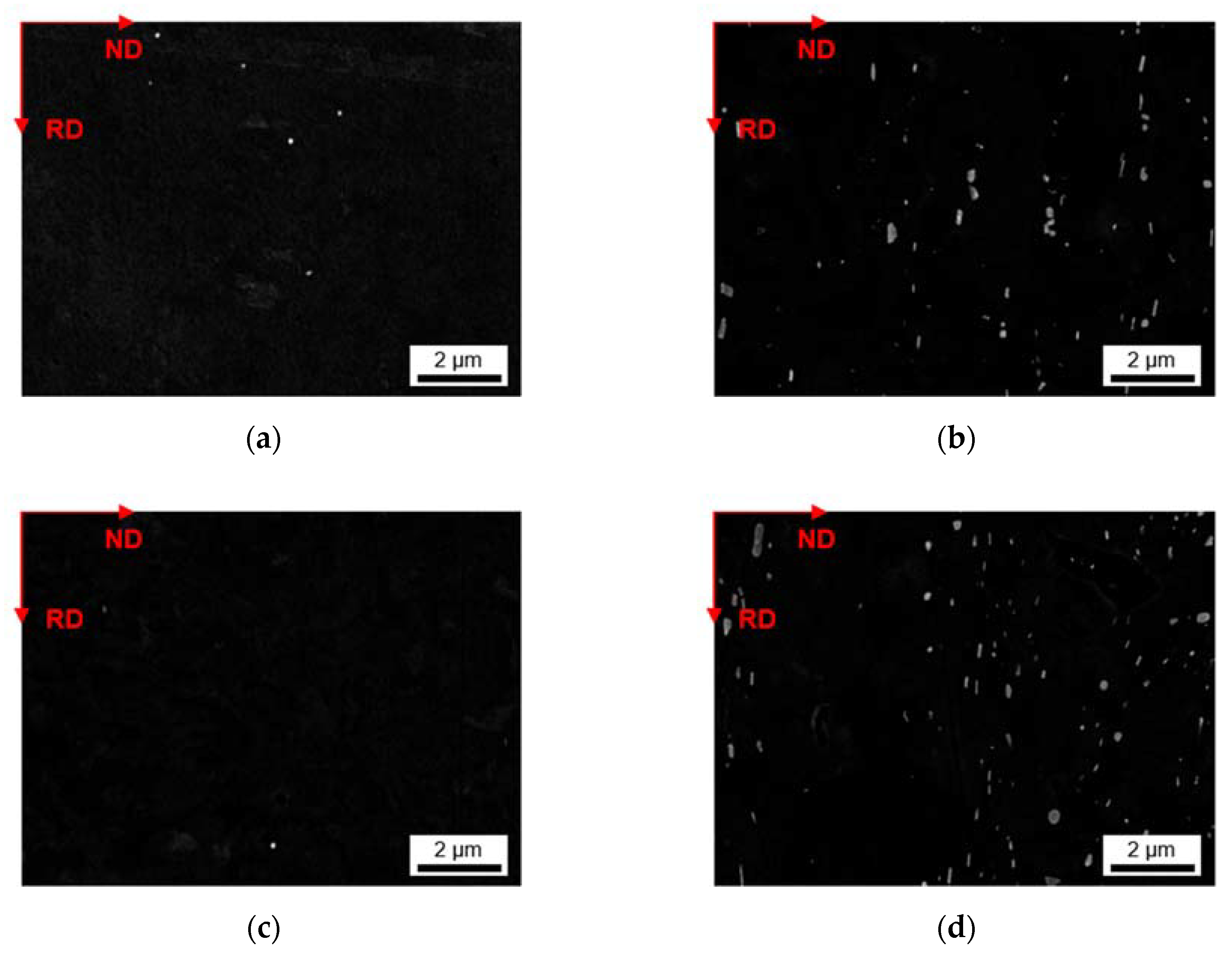
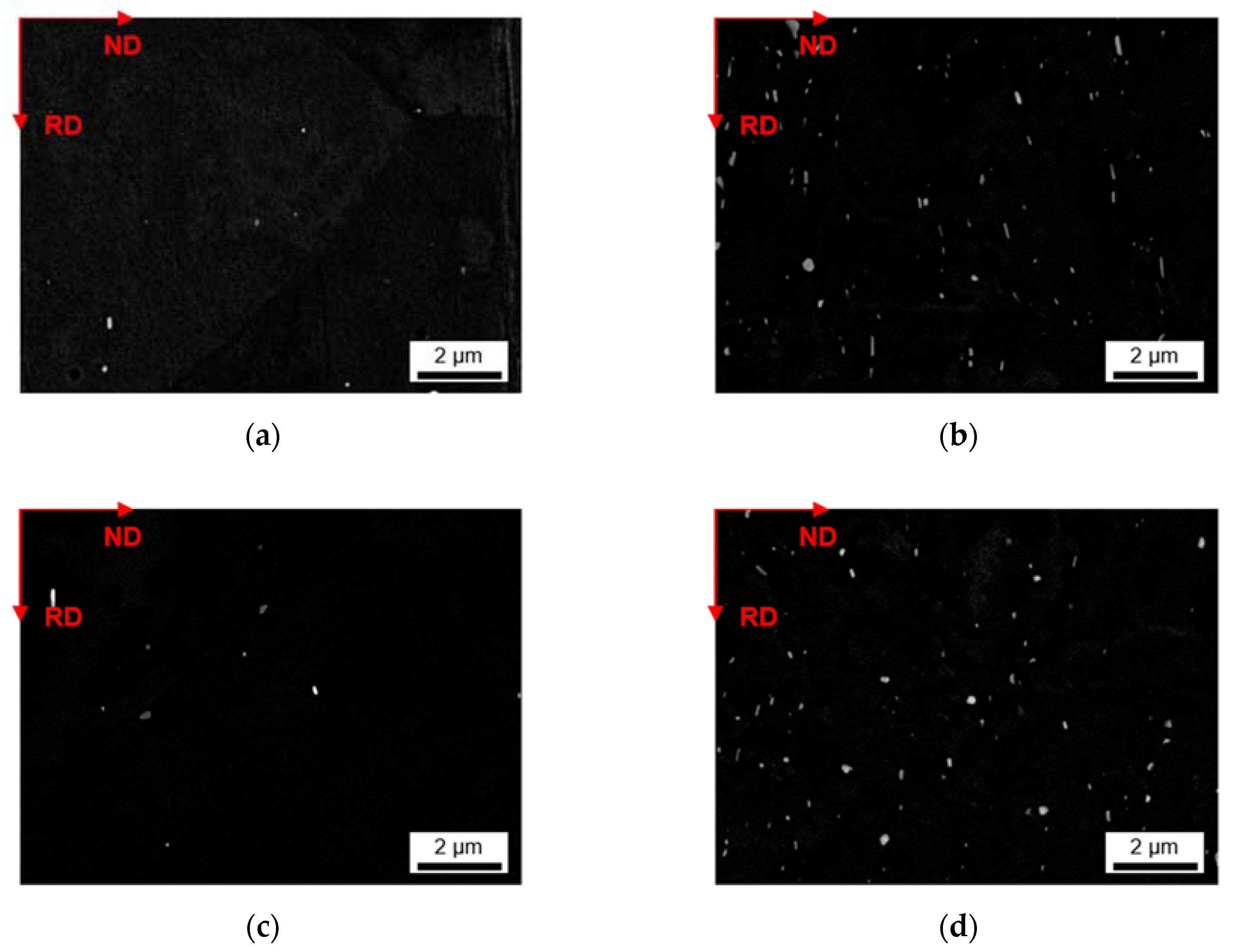
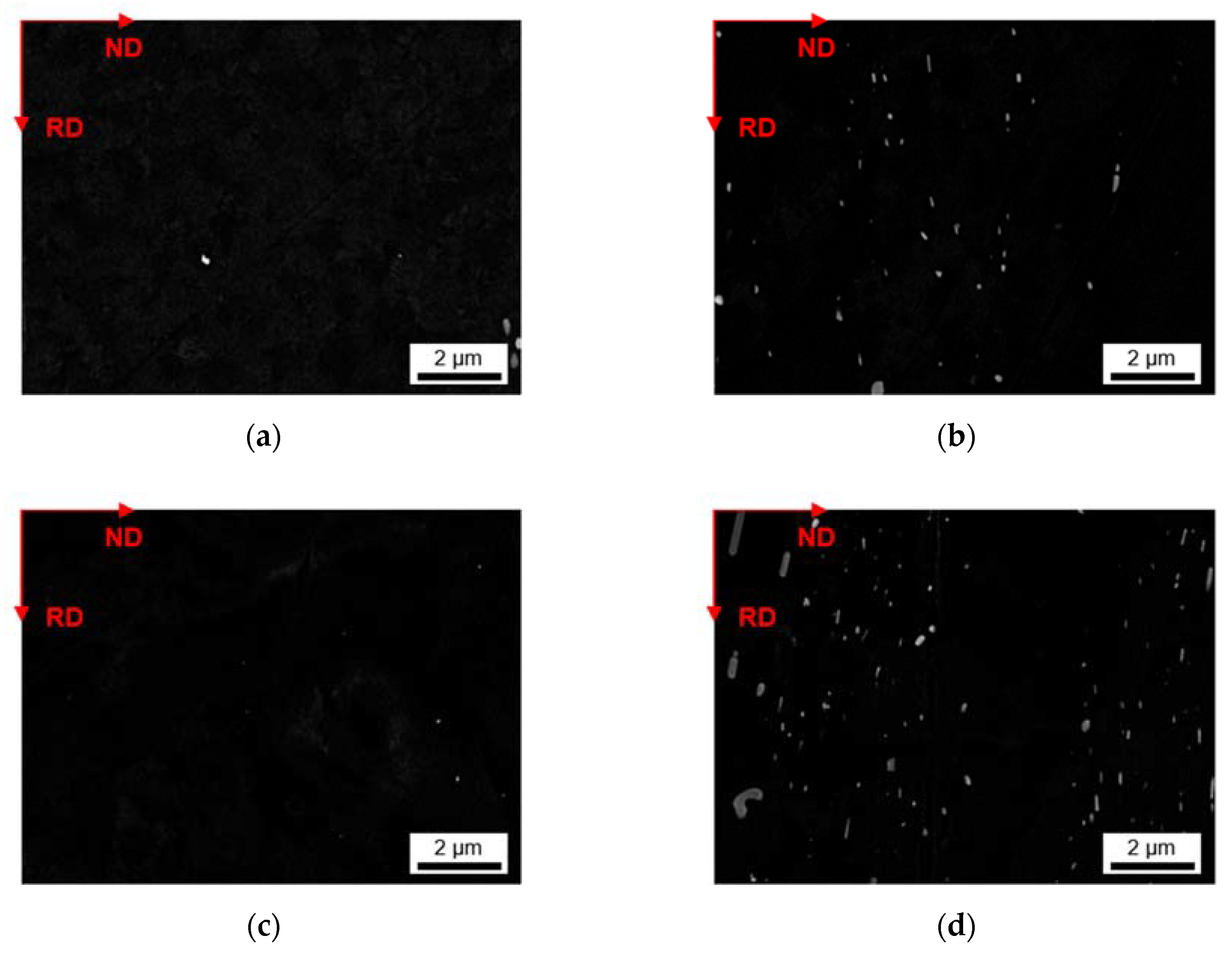
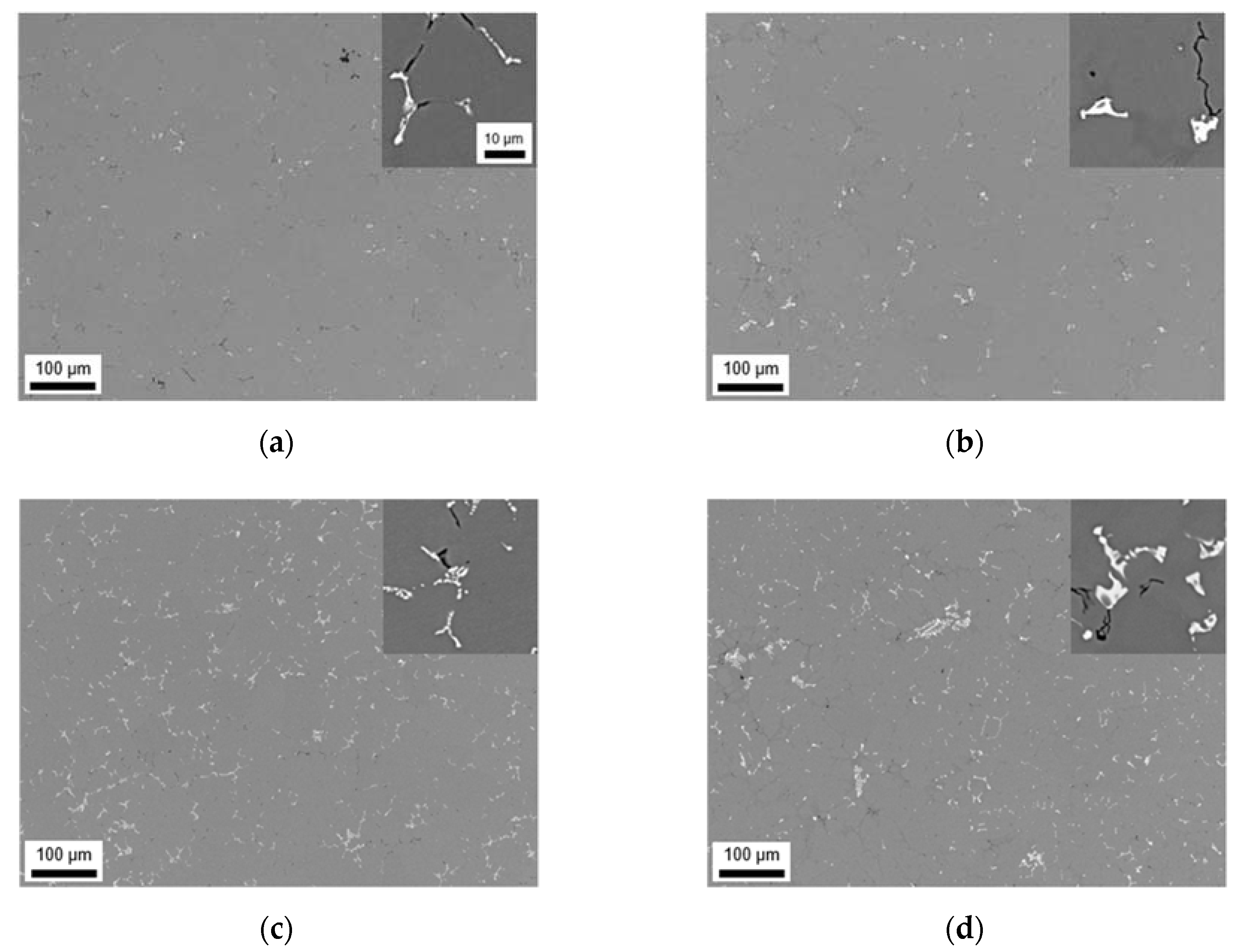
3.2.1. Microstructure after Homogenization at 500 °C of S-C and NR-C Cast Samples
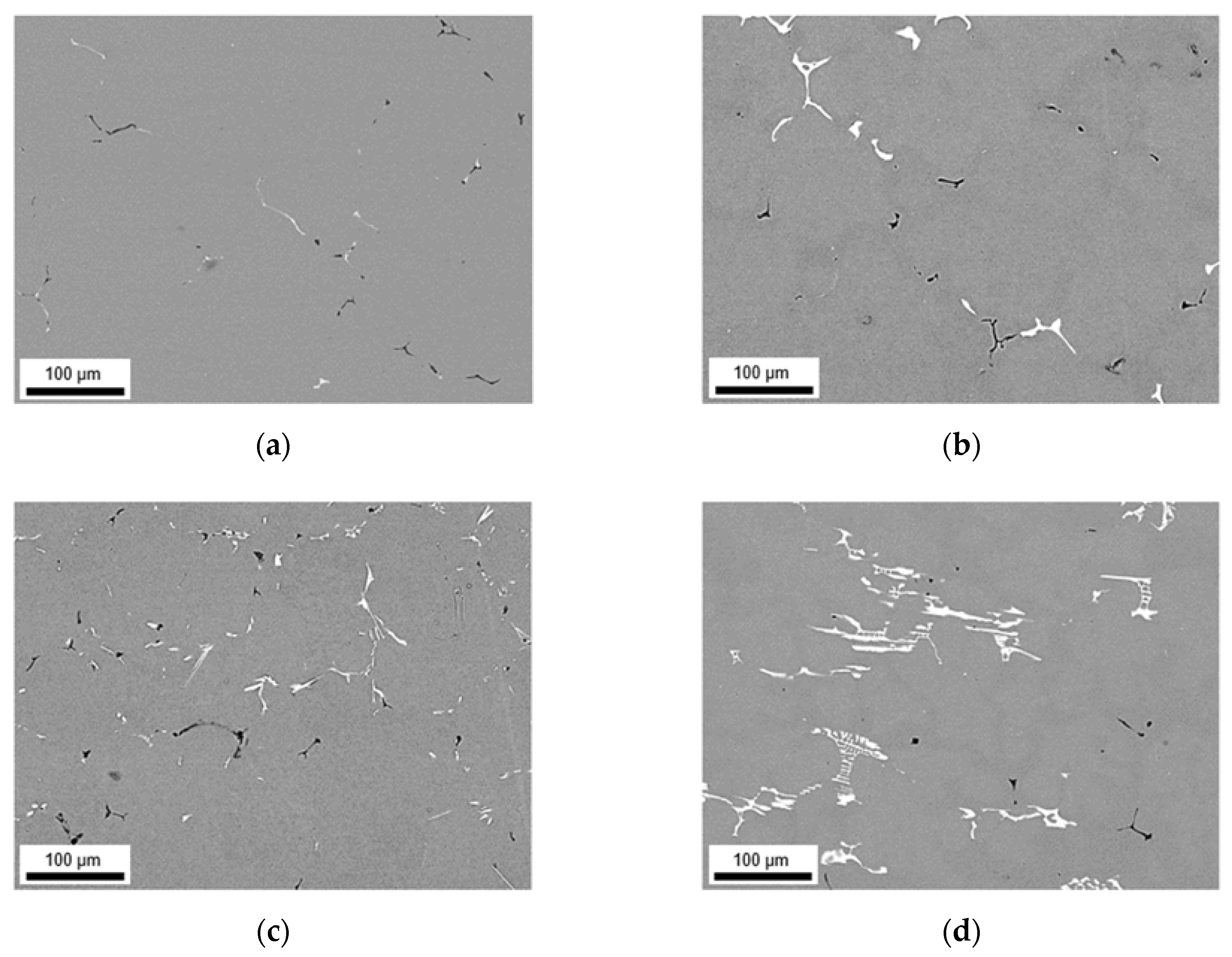
3.2.2. Microstructure after Homogenization at 550 °C of S-C and NR-C Cast Samples
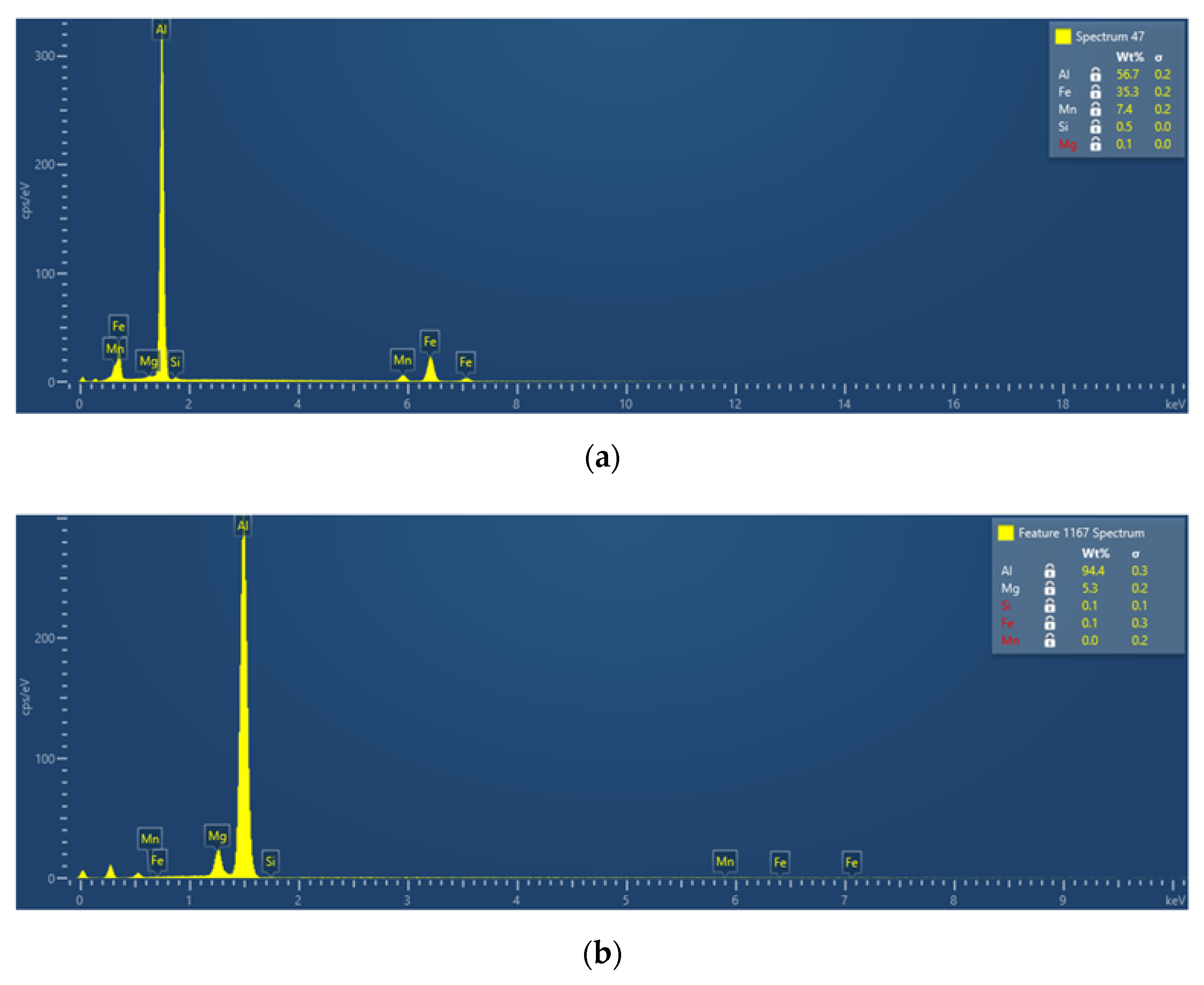
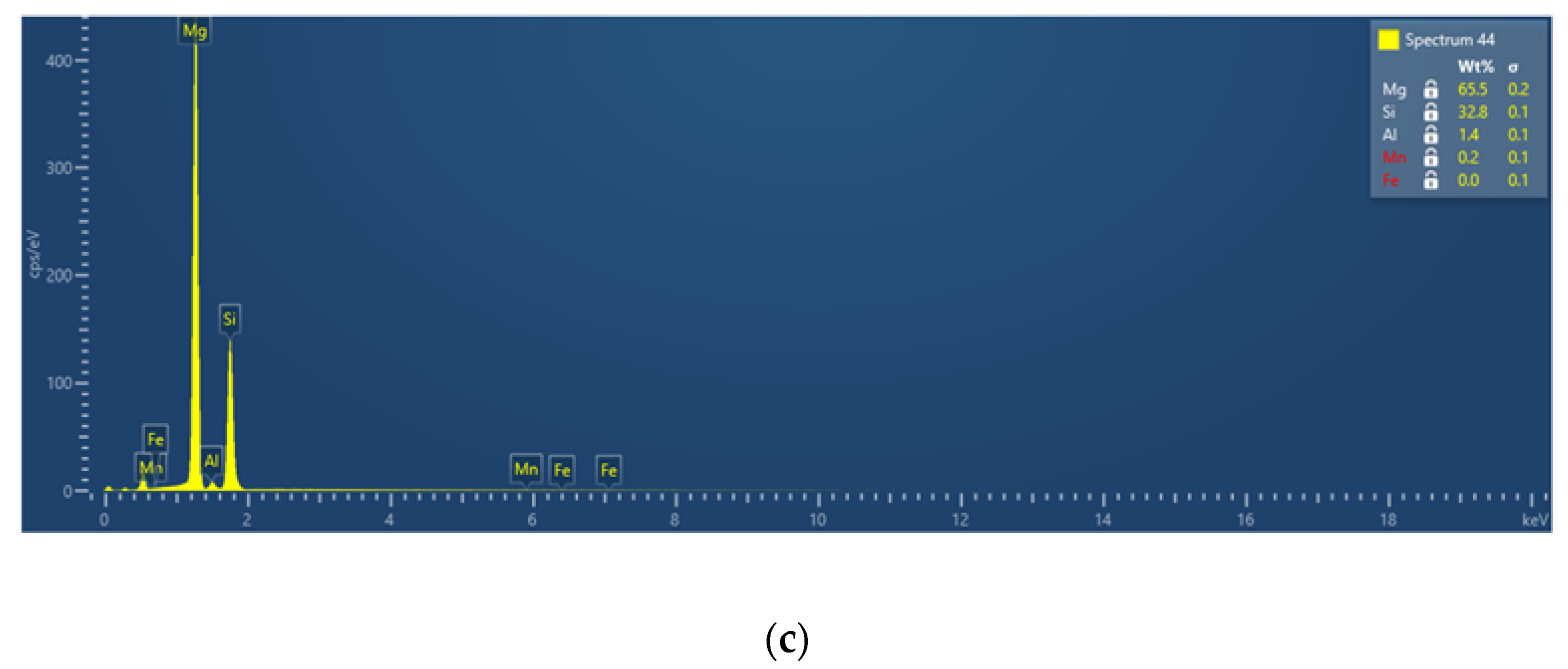
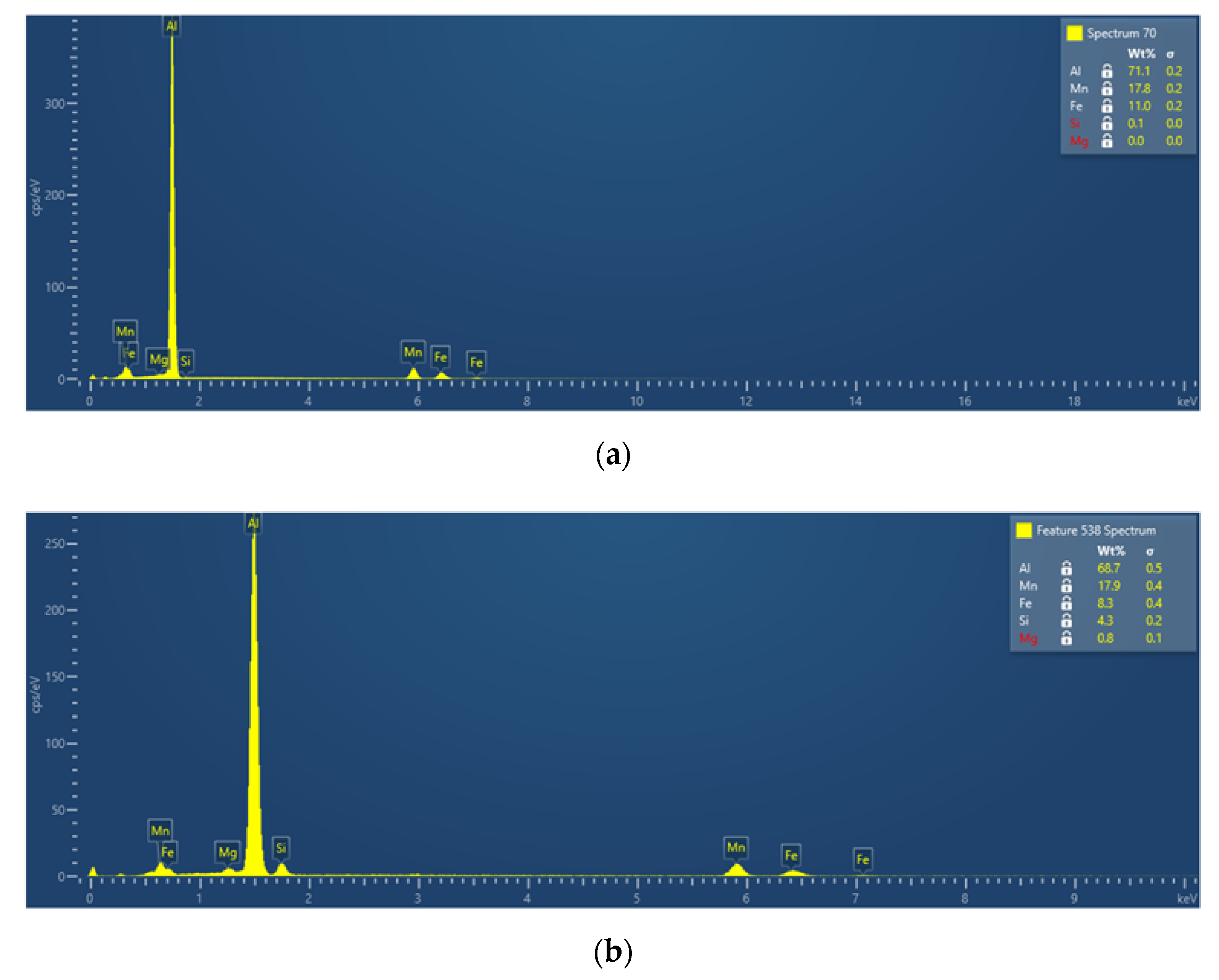
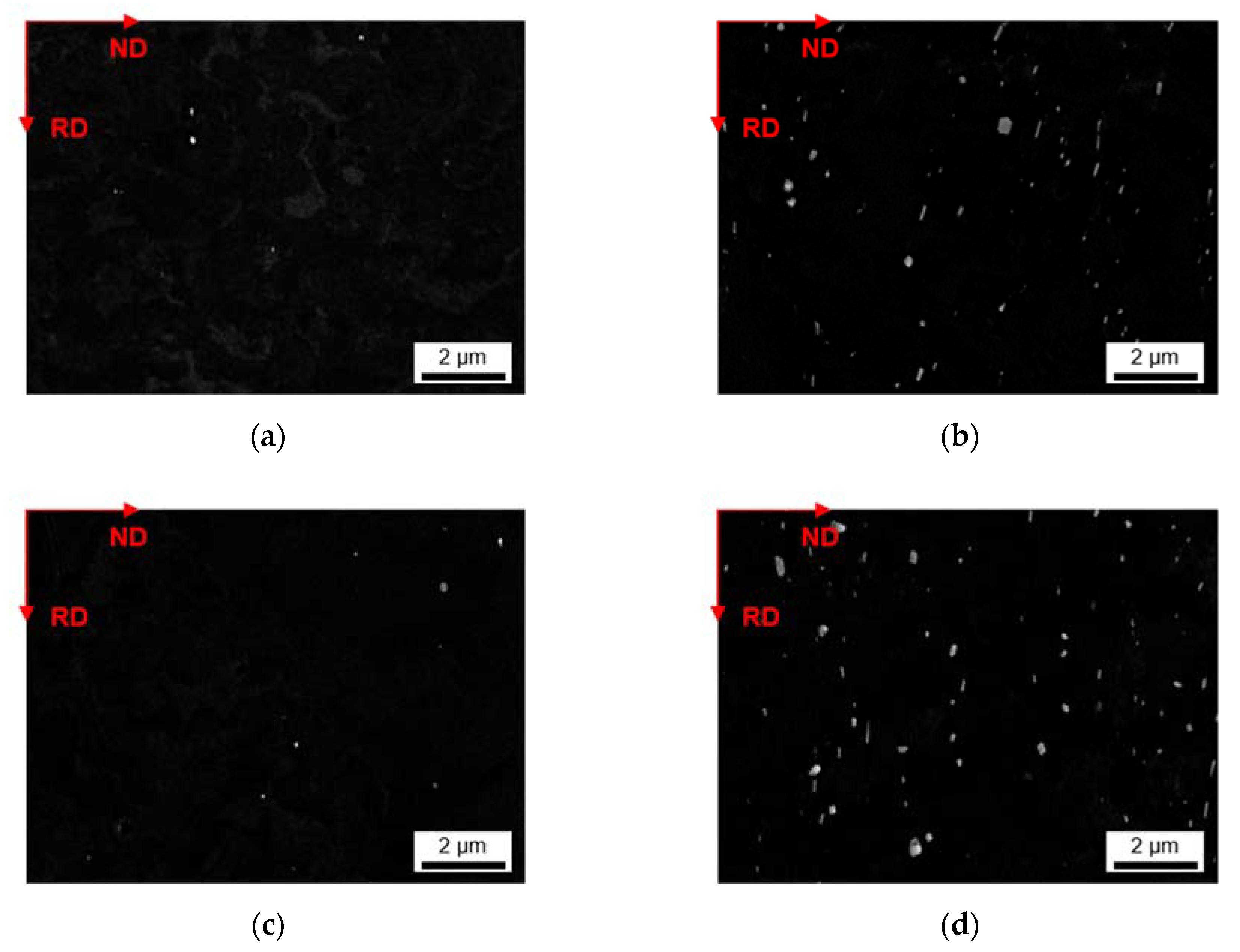
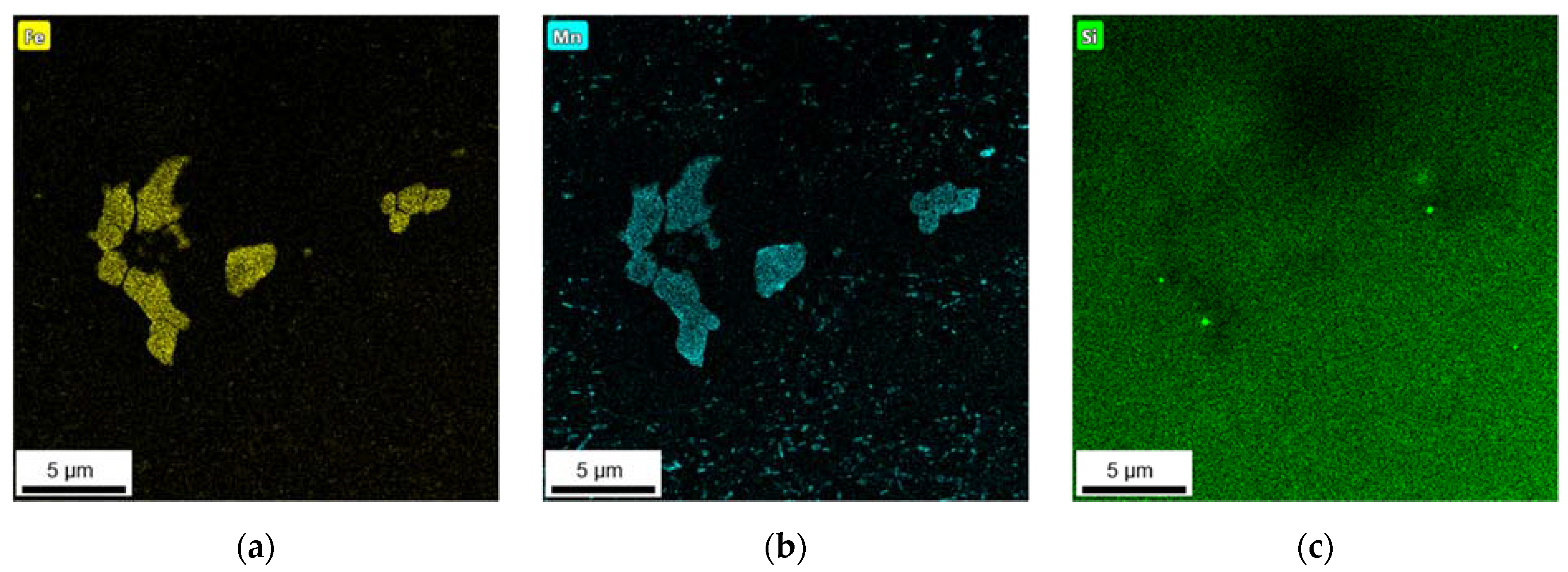
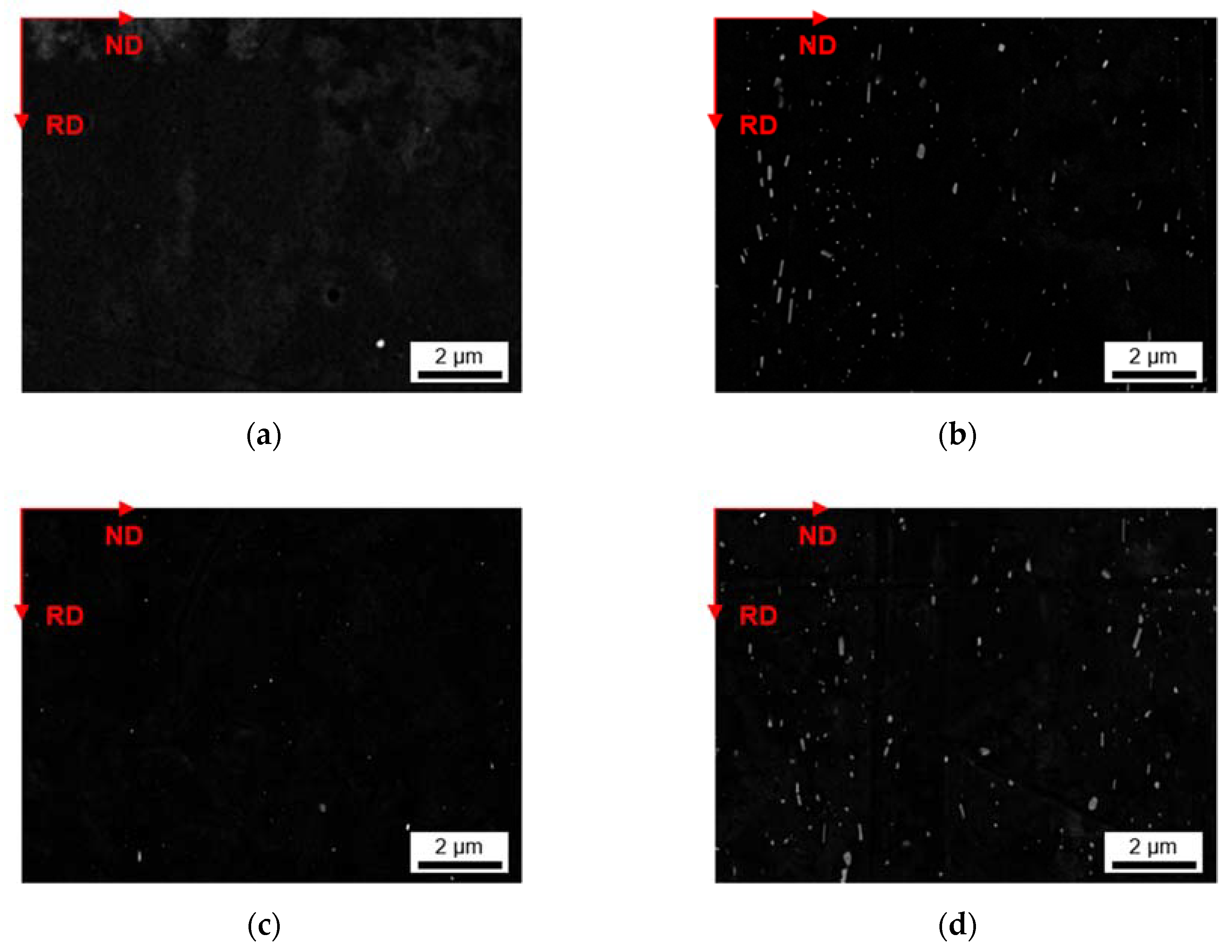
3.2.3. Phase Characterization Using TEM
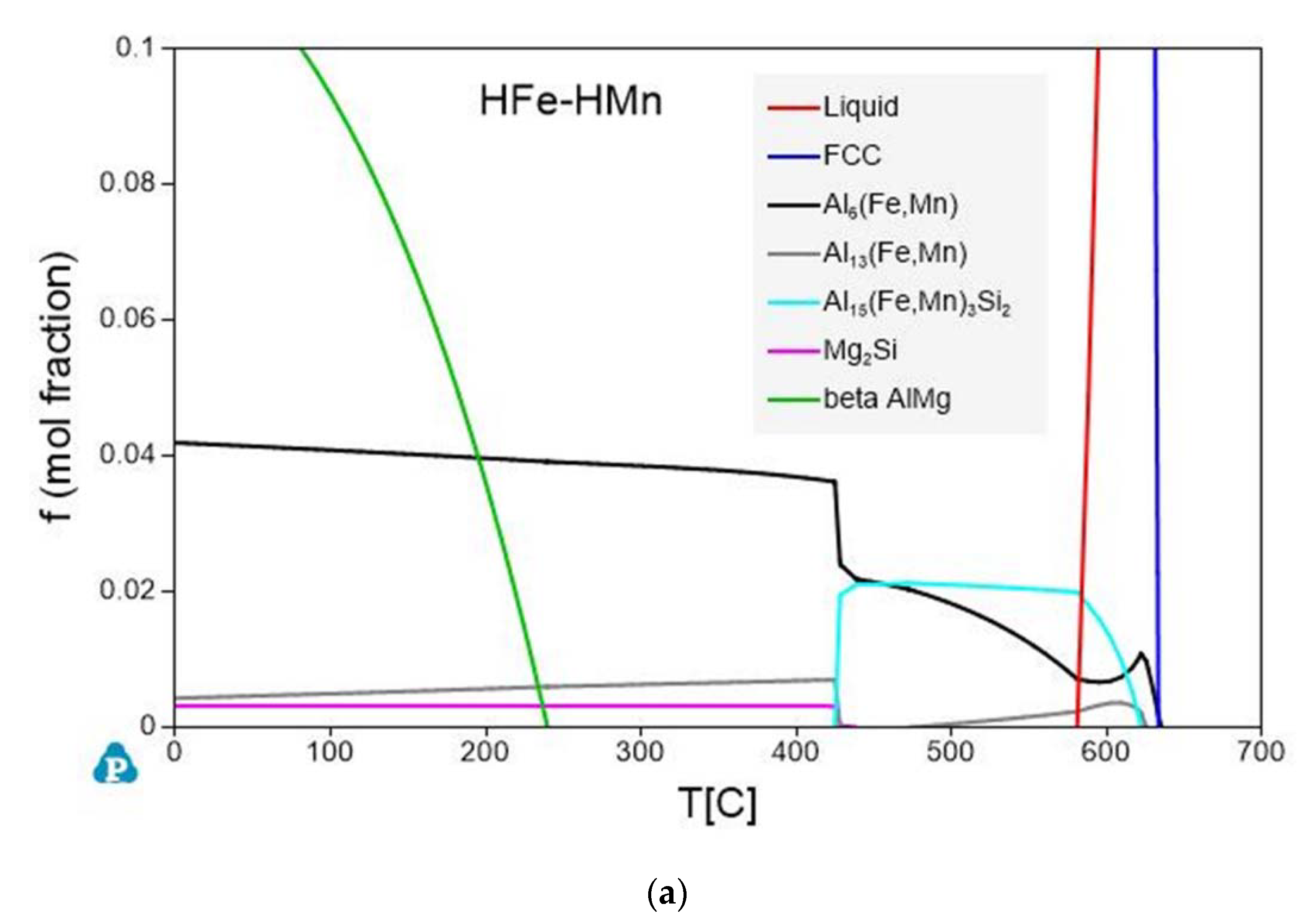
3.3. Thermodynamic Calculations
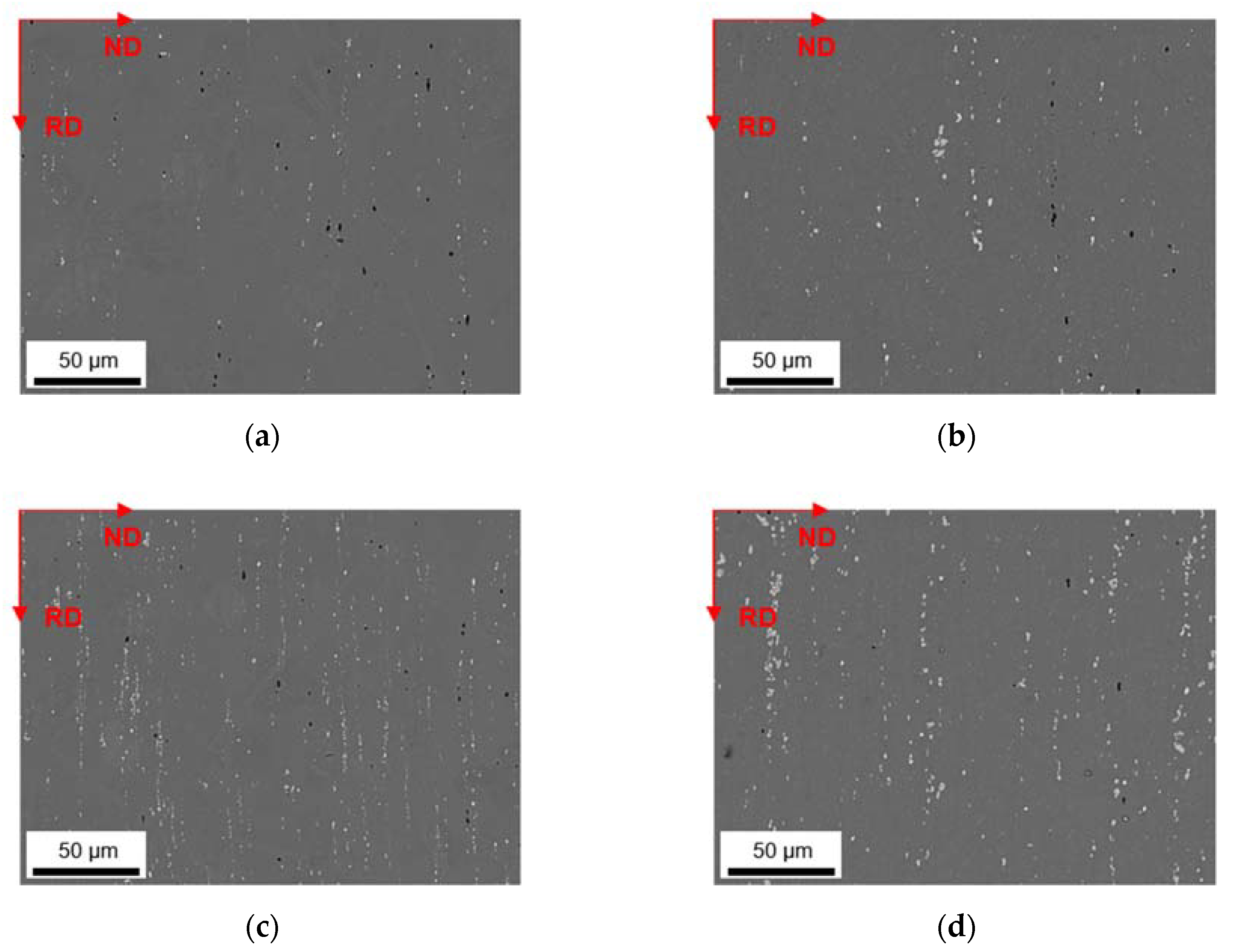
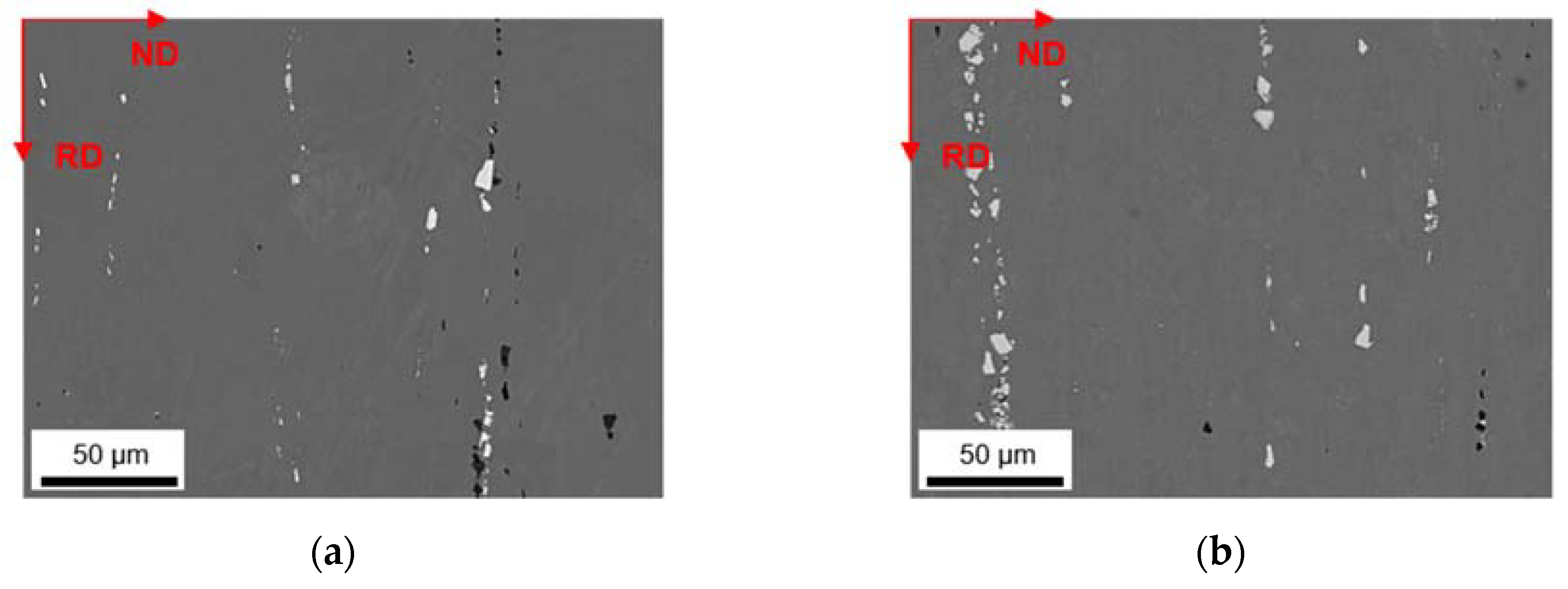
3.4. Soft Annealed State
4. Discussion
5. Conclusions
- High Fe/Mn ratios in the alloy contents favor the precipitation of Al3(Fe,Mn) primary phase in a characteristic needle-like shape. By increasing the Mn level, the primary phase coarsens and more likely forms Al6(Fe,Mn) or Al15(Fe,Mn)3Si2, depending on the dissolved Si solute content and the primary precipitation of Mg2Si, respectively.
- With near rapid cooling conditions in the casting process, the casting grain- and primary phase size are significantly reduced. The formation of different primary phase types in comparison to slow cooling conditions can be observed.
- The volume fractions of the primary phases considerably increase with the alloying contents, foremost by the Fe additions. The homogenization does not change the primary phases volume fractions nor compositions considerably.
- The homogenization heat treatment causes formation of secondary phases (Al6(Fe,Mn) or Al15(Fe,Mn)3Si2), especially in the high Mn containing alloys. The influence of the cooling rate during casting is marginally visible. Higher homogenization temperatures and higher Fe contents clearly result in coarsening of the plate-like dispersoids.
- Thermodynamic calculations highly affirm the experimental results. Besides the good conformity in the observed primary and secondary phase intermetallic types, the trends of the computed volume fractions accord with evaluated data from the micrographs.
- The multitude of primary and secondary phase states created by this experimental work highly impacts the final aluminum sheets microstructure and properties, which will be discussed in part two of the present study.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample State | Alloy | fbright [%] | Fe/Mn | SF | Sample State | fbright [%] | Fe/Mn | SF |
|---|---|---|---|---|---|---|---|---|
| S-C | LFe-LMn | 0.42 ± 0.24 | 5.0 ± 1.0 | 1.78 ± 1.24 | NR-C | 0.39 ± 0.02 | 6.1 ± 2.5 | 1.57 ± 1.01 |
| LFe-HMn | 0.96 ± 0.33 | 0.6 ± 0.4 | 3.06 ± 2.81 | 0.73 ± 0.14 | 0.7 ± 0.3 | 1.54 ± 1.06 | ||
| HFe-LMn | 1.00 ± 0.34 | 7.9 ± 2.4 | 2.08 ± 1.37 | 1.89 ± 0.29 | 8.6 ± 3.5 | 2.30 ± 2.42 | ||
| HFe-HMn | 3.49 ± 0.93 | 0.8 ± 0.5 | 3.37 ± 2.80 | 1.90 ± 0.18 | 1.3 ± 0.3 | 2.56 ± 2.17 | ||
| S-C, 500 °C | LFe-LMn | 0.30 ± 0.03 | 5.7 ± 2.0 | 2.10 ± 1.73 | NR-C, 500 °C | 0.36 ± 0.12 | 4.5 ± 1.7 | 1.88 ± 1.17 |
| LFe-HMn | 0.79 ± 0.33 | 0.6 ± 0.3 | 2.80 ± 2.84 | 0.71 ± 0.37 | 0.6 ± 0.2 | 1.51 ± 0.75 | ||
| HFe-LMn | 0.87 ± 0.27 | 8.5 ± 1.0 | 1.93 ± 1.40 | 1.30 ± 0.30 | 8.6 ± 2.7 | 2.00 ± 1.12 | ||
| HFe-HMn | 2.25 ± 0.20 | 0.8 ± 0.2 | 2.40 ± 1.88 | 2.39 ± 0.52 | 1.1 ± 0.3 | 2.27 ± 1.69 | ||
| S-C, 550 °C | LFe-LMn | 0.28 ± 0.05 | 4.3 ± 1.8 | 1.83 ± 1.38 | NR-C, 550 °C | 0.43 ± 0.18 | 3.1 ± 1.8 | 1.45 ± 0.67 |
| LFe-HMn | 0.90 ± 0.01 | 0.3 ± 0.2 | 1.84 ± 1.57 | 0.86 ± 0.09 | 0.3 ± 0.2 | 1.42 ± 0.73 | ||
| HFe-LMn | 1.00 ± 0.14 | 7.9 ± 1.3 | 1.71 ± 1.46 | 1.37 ± 0.41 | 7.1 ± 2.9 | 1.81 ± 1.17 | ||
| HFe-HMn | 3.22 ± 0.20 | 0.8 ± 0.2 | 2.80 ± 2.68 | 2.90 ± 0.58 | 0.5 ± 0.2 | 1.76 ± 1.15 |
| Sample State | Alloy | fD [vol.%] | r [nm] | AR | Sample State | fD [vol.%] | r [nm] | AR |
|---|---|---|---|---|---|---|---|---|
| S-C, 500 °C | LFe-LMn | 0.021 | 59 | 0.65 | NR-C, 500 °C | 0.061 | 87 | 0.57 |
| LFe-HMn | 0.756 | 87 | 0.57 | 0.620 | 71 | 0.58 | ||
| HFe-LMn | 0.024 | 74 | 0.55 | 0.117 | 74 | 0.65 | ||
| HFe-HMn | 1.440 | 84 | 0.57 | 2.341 | 86 | 0.57 | ||
| S-C, 550 °C | LFe-LMn | 0.047 | 144 | 0.56 | NR-C, 550 °C | 0.062 | 108 | 0.60 |
| LFe-HMn | 1.036 | 124 | 0.55 | 0.741 | 105 | 0.54 | ||
| HFe-LMn | 0.108 | 74 | 0.58 | 0.217 | 126 | 0.59 | ||
| HFe-HMn | 2.252 | 123 | 0.57 | 2.293 | 158 | 0.51 |






References
- Ostermann, F. Anwendungstechnologie Aluminium, 3rd ed.; Springer: Berlin, Germany, 2014; ISBN 9783662438077. [Google Scholar]
- Ebenberger, P.; Uggowitzer, P.J.; Kirnstötter, S.; Gerold, B.; Zaefferer, S.; Pogatscher, S. Processing-controlled suppression of Lüders elongation in AlMgMn alloys. Scr. Mater. 2019, 166, 64–67. [Google Scholar] [CrossRef]
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; de Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Burger, G.B.; Gupta, A.K.; Jeffrey, P.W.; Lloyd, D.J. Microstructural Control of Aluminum Sheet Used in Automotive Applications. Mater. Charact. 1998, 35, 23–39. [Google Scholar] [CrossRef]
- Cole, G.S.; Sherman, A.M. Lightweight Materials for Automotive Applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Algendy, A.Y.; Liu, K.; Chen, X.-G. Formation of intermetallic phases during solidification in Al-Mg-Mn 5xxx alloys with various Mg levels. MATEC Web Conf. 2020, 326, 2002. [Google Scholar] [CrossRef]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E. Iron intermetallic phases in the Al corner of the Al-Si-Fe system. Metall. Mater. Trans. A 2003, 34, 807–825. [Google Scholar] [CrossRef]
- Belmares-Perales, S.; Zaldívar-Cadena, A.A. Addition of iron for the removal of the β-AlFeSi intermetallic by refining of α-AlFeSi phase in an Al-7.5Si-3.6Cu alloy. Mater. Sci. Eng. B 2010, 174, 191–195. [Google Scholar] [CrossRef]
- Li, Y.J.; Arnberg, L. Solidification structures and phase selection of iron-bearing eutectic particles in a DC-cast AA5182 alloy. Acta Mater. 2004, 52, 2673–2681. [Google Scholar] [CrossRef]
- Engler, O.; Kuhnke, K.; Hasenclever, J. Development of intermetallic particles during solidification and homogenization of two AA 5xxx series Al-Mg alloys with different Mg contents. J. Alloys Compd. 2017, 728, 669–681. [Google Scholar] [CrossRef]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of iron on the microstructure and mechanical property of Al-Mg-Si-Mn and Al–Mg–Si diecast alloys. Mater. Sci. Eng. A 2013, 564, 130–139. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-rich Intermetallics in Al–5Mg–0.8Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Ratchev, P.; Verlinden, B.; van Houtte, P. Effect of preheat temperature on the orientation relationship of (Mn,Fe)Al6 precipitates in an AA 5182 Aluminium—Magnesium alloy. Acta Metall. Mater. 1995, 43, 621–629. [Google Scholar] [CrossRef]
- Seifeddine, S.; Johansson, S.; Svensson, I.L. The influence of cooling rate and manganese content on the β-Al5FeSi phase formation and mechanical properties of Al–Si-based alloys. Mater. Sci. Eng. A 2008, 490, 385–390. [Google Scholar] [CrossRef]
- Sweet, L.; Zhu, S.M.; Gao, S.X.; Taylor, J.A.; Easton, M.A. The Effect of Iron Content on the Iron-Containing Intermetallic Phases in a Cast 6060 Aluminum Alloy. Metall. Mater. Trans. A 2011, 42, 1737–1749. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G.; Sun, Y.; Zhang, L.; Huang, Z.; Wang, J.; Liu, C. Effect of Mn and Fe on the Formation of Fe- and Mn-Rich Intermetallics in Al-5Mg-Mn Alloys Solidified Under Near-Rapid Cooling. Materials 2016, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Skjerpe, P. Intermetallic phases formed during DC-casting of an Al-0.25 Wt Pct Fe-0.13 Wt Pct Si alloy. MTA 1987, 18, 189–200. [Google Scholar] [CrossRef]
- Allen, C.M.; O’Reilly, K.; Cantor, B.; Evans, P.V. Intermetallic phase selection in 1XXX Al alloys. Prog. Mater. Sci. 1998, 43, 89–170. [Google Scholar] [CrossRef]
- Sreeja Kumari, S.S.; Pillai, R.M.; Rajan, T.; Pai, B.C. Effects of individual and combined additions of Be, Mn, Ca and Sr on the solidification behaviour, structure and mechanical properties of Al–7Si–0.3Mg–0.8Fe alloy. Mater. Sci. Eng. A 2007, 460–461, 561–573. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.-G. Tensile Properties of Al-Cu 206 Cast Alloys with Various Iron Contents. Metall. Mater. Trans. A 2014, 45, 2498–2507. [Google Scholar] [CrossRef]
- Li, Y.; Arnberg, L. A eutectoid phase transformation for the primary intermetallic particle from Alm(Fe,Mn) to Al3(Fe,Mn) in AA5182 alloy. Acta Mater. 2004, 52, 2945–2952. [Google Scholar] [CrossRef]
- Kuijpers, N.; Vermolen, F.J.; Vuik, C.; Koenis, P.; Nilsen, K.E.; van der Zwaag, S. The dependence of the β-AlFeSi to α-Al(FeMn)Si transformation kinetics in Al–Mg–Si alloys on the alloying elements. Mater. Sci. Eng. A 2005, 394, 9–19. [Google Scholar] [CrossRef]
- Narayanan, L.A.; Samuel, F.H.; Gruzleski, J.E. Crystallization behavior of iron-containing intermetallic compounds in 319 aluminum alloy. Metall. Mater. Trans. A 1994, 25, 1761–1773. [Google Scholar] [CrossRef]
- Engler, O.; Miller-Jupp, S. Control of second-phase particles in the Al-Mg-Mn alloy AA 5083. J. Alloys Compd. 2016, 689, 998–1010. [Google Scholar] [CrossRef]
- Osman, M.; Engler, O.; Karhausen, K.; Löchte, L.; McLaren, A.J. Effect of homogenisation conditions on recrystallisation in Al–Mg–Mn alloy AA 5454. Mater. Sci. Technol. 2007, 23, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Engler, O.; Liu, Z.; Kuhnke, K. Impact of homogenization on particles in the Al–Mg–Mn alloy AA 5454—Experiment and simulation. J. Alloys Compd. 2013, 560, 111–122. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chai, W.; Wang, Q.; Yang, Z.; Ye, L.; Tang, J. Effects of Combined Additions of Mn and Zr on Dispersoid Formation and Recrystallization Behavior in Al-Zn-Mg Alloys. Metall. Mater. Trans. A 2019, 50, 4877–4890. [Google Scholar] [CrossRef]
- Liu, Z.; Mohles, V.; Engler, O.; Gottstein, G. Thermodynamics based modelling of the precipitation kinetics in commercial aluminium alloys. Comput. Mater. Sci. 2014, 81, 410–417. [Google Scholar] [CrossRef]
- Raghunathan, N.; Zaidi, M.A.; Sheppard, T. Recrystallization kinetics of Al–Mg alloys AA 5056 and AA 5083 after hot deformation. Mater. Sci. Technol. 1986, 2, 938–945. [Google Scholar] [CrossRef]
- Grasserbauer, J.; Weißensteiner, I.; Falkinger, G.; Mitsche, S.; Uggowitzer, P.J.; Pogatscher, S. Evolution of Microstructure and Texture in Laboratory- and Industrial-Scaled Production of Automotive Al-Sheets. Materials 2020, 13, 469. [Google Scholar] [CrossRef] [Green Version]
- Schmid, F.; Stemper, L.; Ebner, T.; Leitner, W.; Pogatscher, S. Industry-oriented sample preparation of 6xxx and 5xxx aluminum alloys in laboratory scale. In Proceedings of the EMC 2019, Düsseldorf, Germany, 24–26 June 2019; pp. 639–652. [Google Scholar]
- Österreicher, J.A.; Grabner, F.; Schiffl, A.; Schwarz, S.; Bourret, G.R. Information depth in backscattered electron microscopy of nanoparticles within a solid matrix. Mater. Charact. 2018, 138, 145–153. [Google Scholar] [CrossRef]
- Scheil, E. Bemerkungen zur Schichtkristallbildung. Z. Metall. 1942, 34, 70–72. [Google Scholar]
- Sheppard, T.; Raghunathan, N. Modification of cast structures in Al–Mg alloys by thermal treatments. Mater. Sci. Technol. 1989, 5, 268–280. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.-G. Effects of Al(MnFe)Si dispersoids with different sizes and number densities on microstructure and ambient/elevated-temperature mechanical properties of extruded Al–Mg–Si AA6082 alloys with varying Mn content. J. Alloys Compd. 2021, 861, 157937. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation crystallography of plate-shaped Al6(Mn,Fe) dispersoids in AA5182 alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Rohrer, G.S.; Rollett, A.D. Recrystallization and Related Annealing Phenomena, 3rd ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780080982694. [Google Scholar]
- Nes, E.; Ryum, N.; Hunderi, O. On the Zener drag. Acta Metall. 1985, 33, 11–22. [Google Scholar] [CrossRef]






| Alloy | Mg | Si | Fe | Mn |
|---|---|---|---|---|
| LFe-LMn | 4.51 | 0.12 | 0.10 | 0.22 |
| LFe-HMn | 4.40 | 0.12 | 0.11 | 0.94 |
| HFe-LMn | 4.48 | 0.11 | 0.40 | 0.22 |
| HFe-HMn | 4.56 | 0.11 | 0.39 | 1.04 |
| ftotal [%] | fbright [%] | fdark [%] | Fe/Mn | SF | Al3(Fe,Mn) | Al6(Fe,Mn) | Al15(Fe,Mn)3Si2 | |
|---|---|---|---|---|---|---|---|---|
| LFe-LMn | 0.90 ± 0.12 | 0.42 ± 0.24 | 0.48 ± 0.12 | 5.0 ± 1.0 | 1.78 ± 1.24 | yes | no | no |
| LFe-HMn | 1.48 ± 0.26 | 0.96 ± 0.33 | 0.52 ± 0.07 | 0.6 ± 0.4 | 3.06 ± 2.81 | little | little | yes |
| HFe-LMn | 1.46 ± 0.43 | 1.00 ± 0.34 | 0.46 ± 0.09 | 7.9 ± 2.4 | 2.08 ± 1.37 | yes | no | no |
| HFe-HMn | 4.09 ± 0.78 | 3.49 ± 0.93 | 0.60 ± 0.15 | 0.8 ± 0.5 | 3.37 ± 2.80 | little | yes | no |
| ftotal [%] | fbright [%] | fdark [%] | Fe/Mn | SF | Al3(Fe,Mn) | Al6(Fe,Mn) | Al15(Fe,Mn)3Si2 | |
|---|---|---|---|---|---|---|---|---|
| LFe-LMn | 0.83 ± 0.16 | 0.39 ± 0.02 | 0.44 ± 0.14 | 6.1 ± 2.5 | 1.57 ± 1.01 | yes | no | no |
| LFe-HMn | 1.23 ± 0.21 | 0.73 ± 0.14 | 0.50 ± 0.07 | 0.7 ± 0.3 | 1.54 ± 1.06 | no | little | yes |
| HFe-LMn | 2.24 ± 0.42 | 1.89 ± 0.29 | 0.35 ± 0.13 | 8.6 ± 3.5 | 2.30 ± 2.42 | yes | no | no |
| HFe-HMn | 2.31 ± 0.13 | 1.90 ± 0.18 | 0.41 ± 0.05 | 1.3 ± 0.3 | 2.56 ± 2.17 | no | yes | yes |
| ftotal [%] | fbright [%] | fdark [%] | Fe/Mn | SF | ||
|---|---|---|---|---|---|---|
| S-C cast | LFe-LMn | 0.71 ± 0.11 | 0.30 ± 0.03 | 0.40 ± 0.08 | 5.7 ± 2.0 | 2.10 ± 1.73 |
| LFe-HMn | 1.16 ± 0.36 | 0.79 ± 0.33 | 0.37 ± 0.03 | 0.6 ± 0.3 | 2.80 ± 2.84 | |
| HFe-LMn | 1.22 ± 0.08 | 0.87 ± 0.27 | 0.35 ± 0.19 | 8.5 ± 1.0 | 1.93 ± 1.40 | |
| HFe-HMn | 2.67 ± 0.27 | 2.25 ± 0.20 | 0.42 ± 0.07 | 0.8 ± 0.2 | 2.40 ± 1.88 | |
| NR-C cast | LFe-LMn | 0.74 ± 0.15 | 0.36 ± 0.12 | 0.38 ± 0.03 | 4.5 ± 1.7 | 1.88 ± 1.17 |
| LFe-HMn | 0.91 ± 0.47 | 0.71 ± 0.37 | 0.20 ± 0.10 | 0.6 ± 0.2 | 1.51 ± 0.75 | |
| HFe-LMn | 1.53 ± 0.38 | 1.30 ± 0.30 | 0.33 ± 0.09 | 8.6 ± 2.7 | 2.00 ± 1.12 | |
| HFe-HMn | 2.82 ± 0.62 | 2.39 ± 0.52 | 0.42 ± 0.10 | 1.1 ± 0.3 | 2.27 ± 1.69 | |
| fD [vol.%] | r [nm] | AR | ||
|---|---|---|---|---|
| S-C cast | LFe-LMn | 0.021 | 59 | 0.65 |
| LFe-HMn | 0.756 | 87 | 0.57 | |
| HFe-LMn | 0.024 | 74 | 0.55 | |
| HFe-HMn | 1.440 | 84 | 0.57 | |
| NR-C cast | LFe-LMn | 0.061 | 87 | 0.57 |
| LFe-HMn | 0.620 | 71 | 0.58 | |
| HFe-LMn | 0.117 | 74 | 0.65 | |
| HFe-HMn | 2.341 | 86 | 0.57 | |
| ftotal [%] | fbright [%] | fdark [%] | Fe/Mn | SF | ||
|---|---|---|---|---|---|---|
| S-C cast | LFe-LMn | 0.51 ± 0.14 | 0.28 ± 0.05 | 0.23 ± 0.09 | 4.3 ± 1.8 | 1.83 ± 1.38 |
| LFe-HMn | 1.06 ± 0.05 | 0.90 ± 0.01 | 0.16 ± 0.04 | 0.3 ± 0.2 | 1.84 ± 1.57 | |
| HFe-LMn | 1.29 ± 0.21 | 1.00 ± 0.14 | 0.29 ± 0.07 | 7.9 ± 1.3 | 1.71 ± 1.46 | |
| HFe-HMn | 3.54 ± 0.32 | 3.22 ± 0.20 | 0.32 ± 0.12 | 0.8 ± 0.2 | 2.80 ± 2.68 | |
| NR-C cast | LFe-LMn | 0.73 ± 0.20 | 0.43 ± 0.18 | 0.30 ± 0.02 | 3.1 ± 1.8 | 1.45 ± 0.67 |
| LFe-HMn | 0.95 ± 0.05 | 0.86 ± 0.09 | 0.09 ± 0.04 | 0.3 ± 0.2 | 1.42 ± 0.73 | |
| HFe-LMn | 1.63 ± 0.50 | 1.37 ± 0.41 | 0.26 ± 0.10 | 7.1 ± 2.9 | 1.81 ± 1.17 | |
| HFe-HMn | 3.16 ± 0.68 | 2.90 ± 0.58 | 0.27 ± 0.11 | 0.5 ± 0.2 | 1.76 ± 1.15 | |
| fD [vol.%] | r [nm] | AR | ||
|---|---|---|---|---|
| S-C cast | LFe-LMn | 0.047 | 144 | 0.56 |
| LFe-HMn | 1.036 | 124 | 0.55 | |
| HFe-LMn | 0.108 | 74 | 0.58 | |
| HFe-HMn | 2.252 | 123 | 0.57 | |
| NR-C cast | LFe-LMn | 0.062 | 108 | 0.60 |
| LFe-HMn | 0.741 | 105 | 0.54 | |
| HFe-LMn | 0.217 | 126 | 0.59 | |
| HFe-HMn | 2.293 | 158 | 0.51 | |
| Fe in Fcc ×10−3 [wt.%] Nonequi./Equi. | Mn in Fcc [wt.%] Nonequi./Equi. | Al13(Fe,Mn)4 [vol.%] Nonequi./Equi. | Al15(Fe,Mn)3Si2 [vol.%] Nonequi./Equi. | Al6(Fe,Mn) [vol.%] Nonequi./Equi. | |
|---|---|---|---|---|---|
| LFe-LMn | 7.6/4.7 | 0.195/0.092 | 0.20/- | - | -/0.54 |
| LFe-HMn | 7.0/1.0 | 0.700/0.257 | 0.08/- | 0.31/0.90 | 0.87/1.57 |
| HFe-LMn | 17.0/6.7 | 0.165/0.066 | 0.77/0.56 | -/0.51 | - |
| HFe-HMn | 15.0/5.2 | 0.651/0.233 | 0.67/0.20 | 0.40/1.62 | 0.78/1.28 |
| Sample State | Alloy | fD [vol.%] | r [nm] | AR | |
|---|---|---|---|---|---|
| Homogenization | CRD | ||||
| 500 °C | 35% | LFe-LMn | 0.01 | 78 | 0.60 |
| LFe-HMn | 0.96 | 81 | 0.50 | ||
| HFe-LMn | 0.01 | 45 | 0.57 | ||
| HFe-HMn | 0.64 | 62 | 0.55 | ||
| 63% | LFe-LMn | 0.04 | 43 | 0.65 | |
| LFe-HMn | 0.75 | 61 | 0.51 | ||
| HFe-LMn | 0.02 | 39 | 0.60 | ||
| HFe-HMn | 0.63 | 58 | 0.55 | ||
| 550 °C | 35% | LFe-LMn | 0.03 | 75 | 0.55 |
| LFe-HMn | 0.64 | 82 | 0.53 | ||
| HFe-LMn | 0.01 | 72 | 0.59 | ||
| HFe-HMn | 1.35 | 78 | 0.52 | ||
| 63% | LFe-LMn | 0.01 | 72 | 0.66 | |
| LFe-HMn | 1.27 | 91 | 0.51 | ||
| HFe-LMn | 0.03 | 45 | 0.65 | ||
| HFe-HMn | 1.63 | 92 | 0.47 | ||
| Sample State | Alloy | fD [vol.%] | r [nm] | AR | |
|---|---|---|---|---|---|
| Homogenization | CRD | ||||
| 500 °C | 35% | LFe-LMn | 0.02 | 54 | 0.68 |
| LFe-HMn | 1.11 | 74 | 0.48 | ||
| HFe-LMn | 0.05 | 62 | 0.49 | ||
| HFe-HMn | 0.90 | 61 | 0.49 | ||
| 63% | LFe-LMn | 0.01 | 50 | 0.65 | |
| LFe-HMn | 1.01 | 73 | 0.47 | ||
| HFe-LMn | 0.08 | 67 | 0.51 | ||
| HFe-HMn | 1.07 | 63 | 0.48 | ||
| 550 °C | 35% | LFe-LMn | 0.01 | 58 | 0.65 |
| LFe-HMn | 0.82 | 71 | 0.52 | ||
| HFe-LMn | 0.03 | 77 | 0.58 | ||
| HFe-HMn | 1.20 | 88 | 0.50 | ||
| 63% | LFe-LMn | 0.04 | 65 | 0.62 | |
| LFe-HMn | 0.58 | 81 | 0.47 | ||
| HFe-LMn | 0.06 | 76 | 0.61 | ||
| HFe-HMn | 1.00 | 79 | 0.52 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasserbauer, J.; Weißensteiner, I.; Falkinger, G.; Kremmer, T.M.; Uggowitzer, P.J.; Pogatscher, S. Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases. Materials 2021, 14, 3204. https://doi.org/10.3390/ma14123204
Grasserbauer J, Weißensteiner I, Falkinger G, Kremmer TM, Uggowitzer PJ, Pogatscher S. Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases. Materials. 2021; 14(12):3204. https://doi.org/10.3390/ma14123204
Chicago/Turabian StyleGrasserbauer, Jakob, Irmgard Weißensteiner, Georg Falkinger, Thomas M. Kremmer, Peter J. Uggowitzer, and Stefan Pogatscher. 2021. "Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases" Materials 14, no. 12: 3204. https://doi.org/10.3390/ma14123204
APA StyleGrasserbauer, J., Weißensteiner, I., Falkinger, G., Kremmer, T. M., Uggowitzer, P. J., & Pogatscher, S. (2021). Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases. Materials, 14(12), 3204. https://doi.org/10.3390/ma14123204








