Manipulation of TiO2 Nanoparticle/Polymer Coatings Wettability and Friction in Different Environments
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
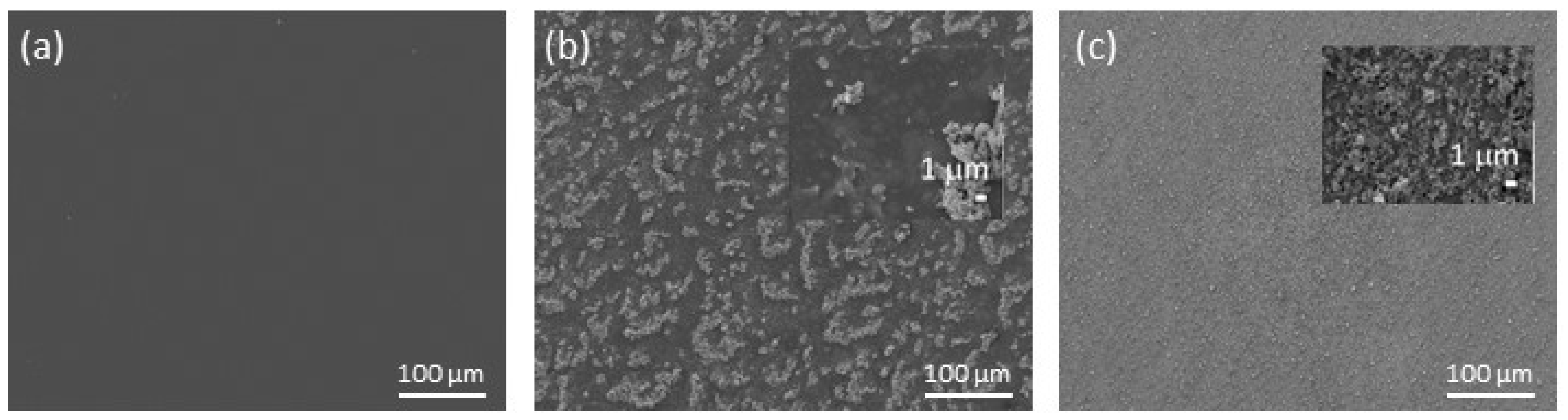
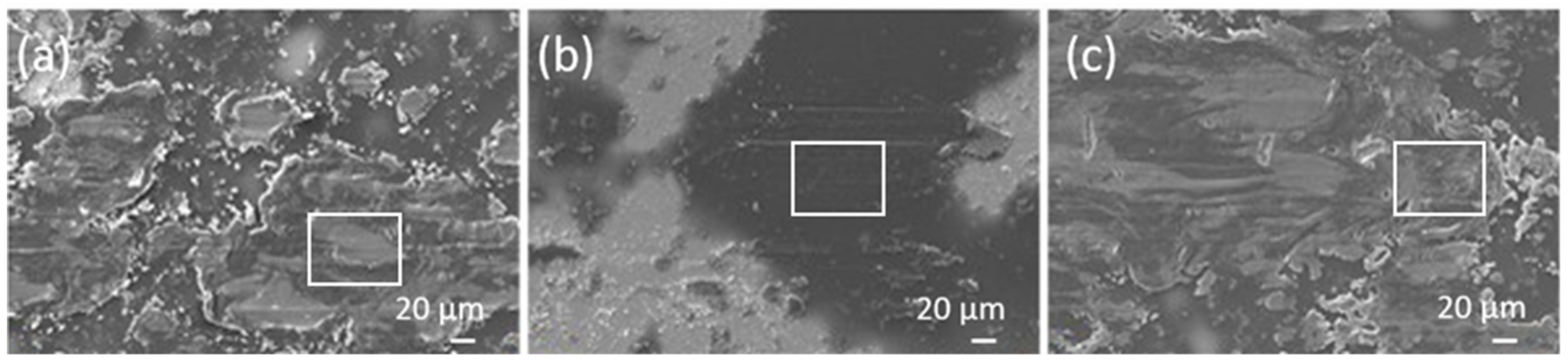
3.1. Surface Morphology
3.2. Wetting Properties
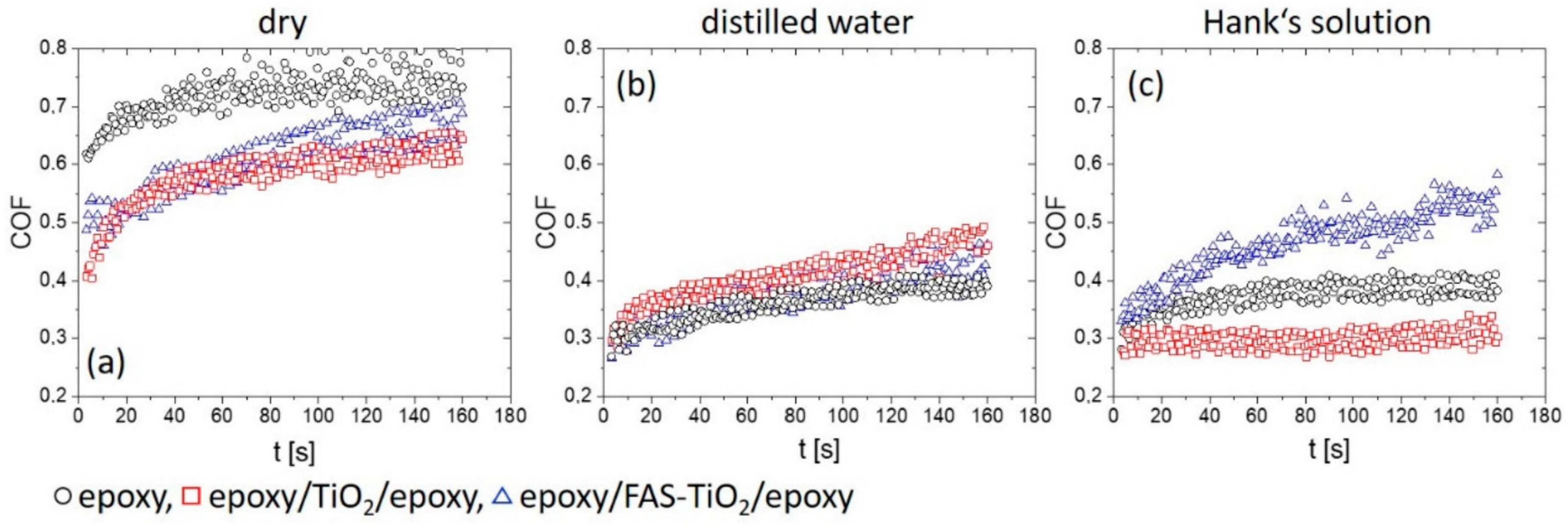
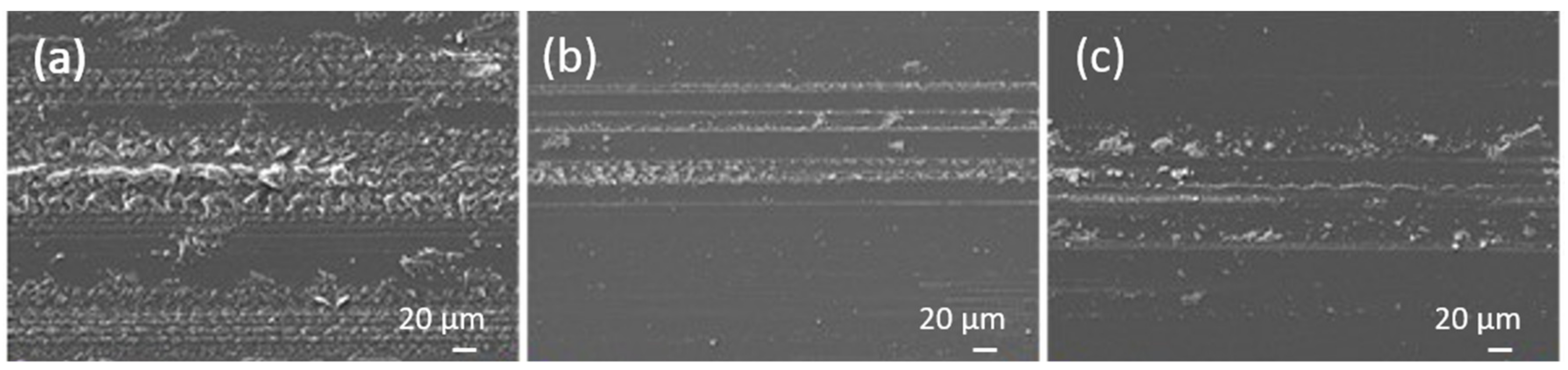
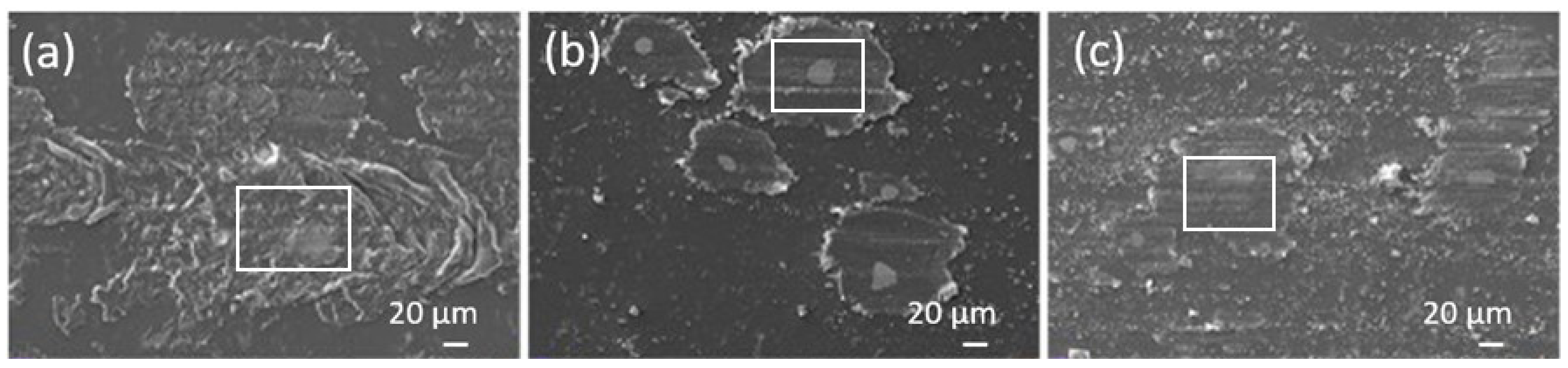
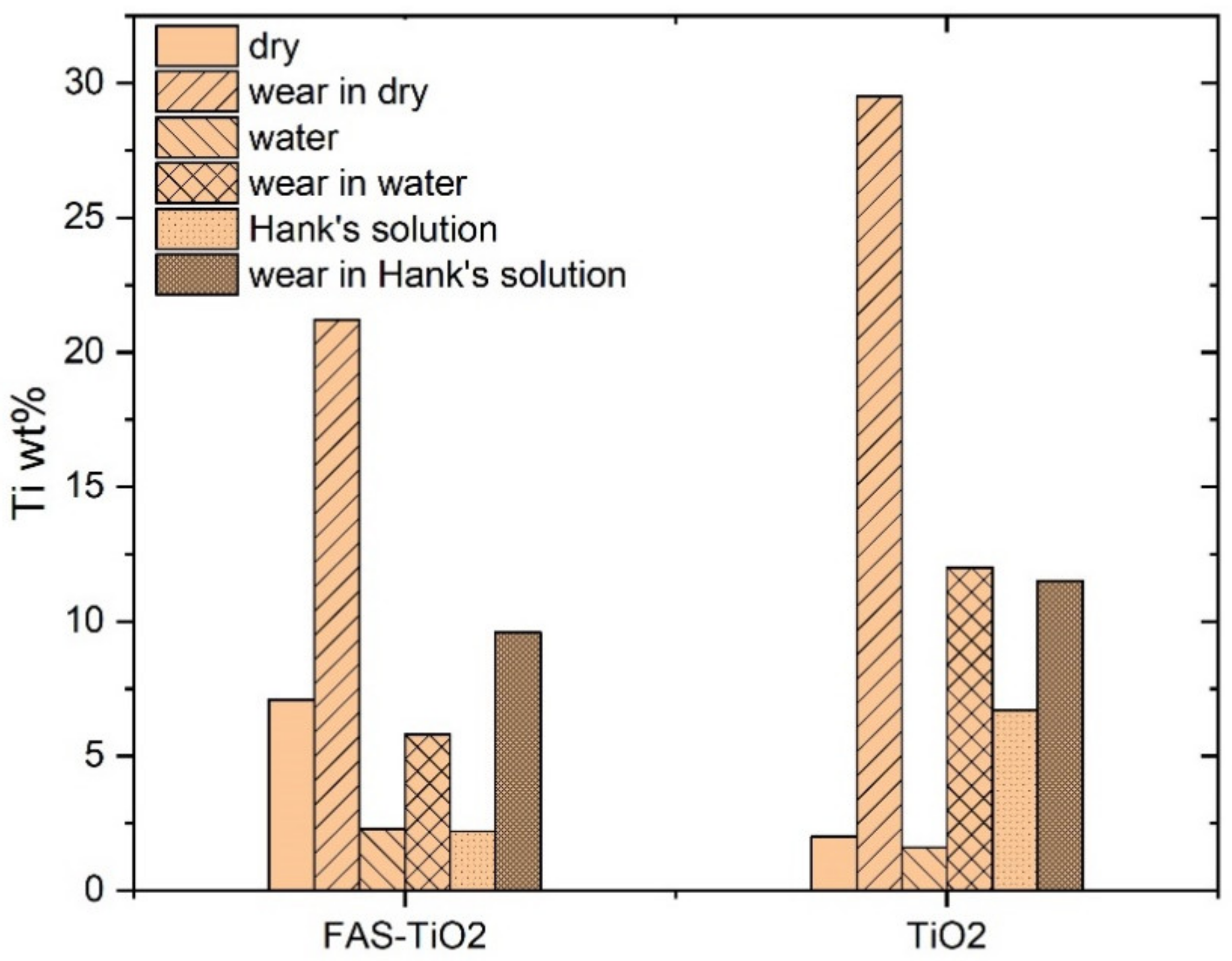
3.3. Tribological Behaviour
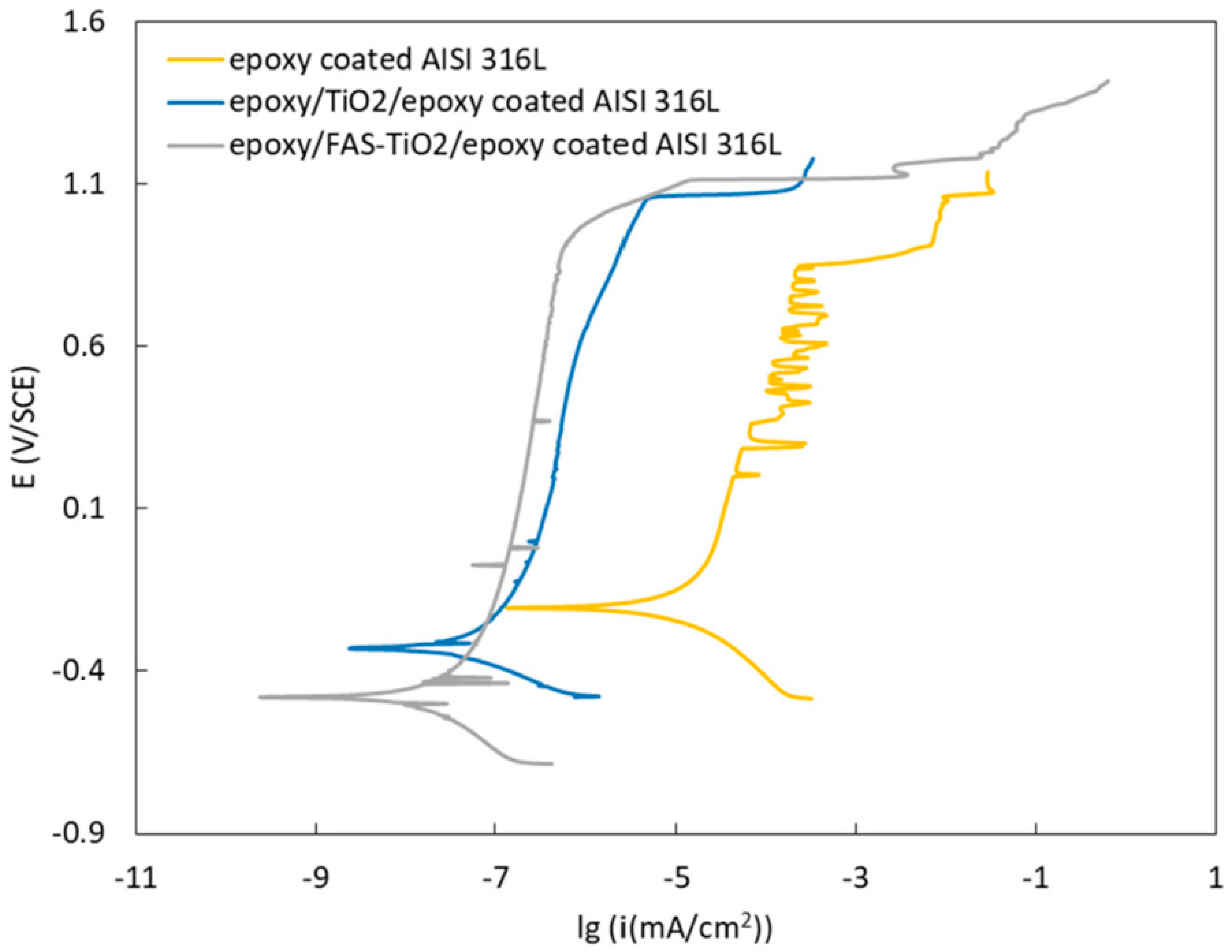
3.4. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galliano, F.; Landolt, D. Evaluation of corrosion protection properties of additives for waterborne epoxy coatings on steel. Prog. Org. Coat. 2002, 44, 217–225. [Google Scholar] [CrossRef]
- Talo, A.; Forsen, O.; Ylasaari, S. Corrosion protective polyaniline epoxy blend coatings on mild steel. Synth. Met. 1999, 102, 1394–1395. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Nishimoto, S.; Saito, H.; Murakami, T.; Fujishima, A. Preparation and photocatalytic wettability conversion of TiO2-based superhydrophobic surfaces. Langmuir 2006, 22, 9477–9479. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter. 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, N.; Xu, J. Fabrication and application of superhydrophilic surfaces: A review. J. Adhes. Sci. Technol. 2014, 28, 769–790. [Google Scholar] [CrossRef]
- Meng, Z.; Li, Y.; Yang, Y.; Xu, Z.Q.; Shi, B.; Zhao, S.L. Effect of a nanoparticulate anti-friction coating on galling resistance of threaded oil-casing couplings. J. Pet. Sci. Eng. 2015, 128, 140–144. [Google Scholar] [CrossRef]
- Wetzel, B.; Haupert, F.; Zhang, M.Q. Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 2003, 63, 2055–2067. [Google Scholar] [CrossRef]
- Yamini, S.; Young, R.J. Stability of crack-propagation in epoxy-resins. Polymer 1977, 18, 1075–1080. [Google Scholar] [CrossRef]
- Spanoudakis, J.; Young, R.J. Crack-propagation in a glass particle-filled epoxy-resin 2. Effect of particle matrix adhesion. J. Mater. Sci. 1984, 19, 487–496. [Google Scholar] [CrossRef]
- Moloney, A.C.; Kausch, H.H.; Stieger, H.R. The fracture of particulate filled epoxide-resins. J. Mater. Sci. 1984, 19, 1125–1130. [Google Scholar] [CrossRef]
- Hussain, M.; Oku, Y.; Nakahira, A.; Niihara, K. Effects of wet ball-milling on particle dispersion and mechanical properties of particulate epoxy composites. Mater. Lett. 1996, 26, 177–184. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. Appl. Organomet. Chem. 1998, 12, 675–680. [Google Scholar] [CrossRef]
- Zerda, A.S.; Lesser, A.J. Intercalated clay nanocomposites: Morphology, mechanics, and fracture behavior. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1137–1146. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Maistros, G.; Gomez, C.M.; Partridge, I.K. Elastomers and thermoplastics as modifiers for thermosetting resins. Makromol. Chem. Macromol. Symp. 1993, 70, 255–264. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Yong, K. Overcoming the Water Vulnerability of Electronic Devices: A Highly Water-Resistant ZnO Nanodevice with Multifunctionality. Adv. Mater. 2011, 23, 4398–4402. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Sun, Y.; Zheng, Y.; Wang, X.; Shang, Y.; Wang, L.; Liu, C. Facile fabrication of superhydrophobic surfaces with corrosion resistance by nanocomposite coating of TiO2 and polydimethylsiloxane. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 471–477. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Kumar, R. Superhydrophilic TiO2 thin film by nanometer scale surface roughness and dangling bonds. Appl. Surf. Sci. 2016, 364, 51–60. [Google Scholar] [CrossRef]
- De Falco, G.; Ciardiello, R.; Commodo, M.; del Gaudio, P.; Minutolo, P.; Porta, A.; D’Anna, A. TiO2 nanoparticle coatings with advanced antibacterial and hydrophilic properties prepared by flame aerosol synthesis and thermophoretic deposition. Surf. Coat. Technol. 2018, 349, 830–837. [Google Scholar] [CrossRef]
- Nakata, K.; Sakai, M.; Ochiai, T.; Murakami, T.; Takagi, K.; Fujishima, A. Antireflection and Self-Cleaning Properties of a Moth-Eye-Like Surface Coated with TiO2 Particles. Langmuir 2011, 27, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Conradi, M.; Kocijan, A. Fine-tuning of surface properties of dual-size TiO2 nanoparticle coatings. Surf. Coat. Technol. 2016, 304. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Zorko, M.; Verpoest, I. Damage resistance and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Prog. Org. Coat. 2015, 80. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A. Epoxy powder coatings hot mixed with nanoparticles to improve their abrasive wear. Wear 2020, 448–449. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204. [Google Scholar] [CrossRef]
- Camilleri, J. Tricalcium silicate cements with resins and alternative radiopacifiers. J. Endod. 2014. [Google Scholar] [CrossRef]
- ASTM International. ASTM G102-89(2015)e1, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
| Substrate | θW (o) | θHank (o) | Sa (nm) |
|---|---|---|---|
| Epoxy | 74.5 ± 1.6 | 77.7 ± 2.0 | 67.4 ± 6.0 |
| Epoxy/FAS-TiO2/epoxy | 121.4 ± 1.8 | 120.2 ± 2.1 | 702.8 ± 12.0 |
| Epoxy/TiO2/epoxy | 76.0 ± 1.0 | 73.8 ± 1.4 | 512.6 ± 10.0 |
| Material | E (I = 0) (mV) | icorr (nA/cm2) | vcorr (nm/year) |
|---|---|---|---|
| Epoxy on AISI 316L | −204.4 | 20.000 | 226.5 |
| Epoxy/TiO2/epoxy on AISI 316L | −329.2 | 0.069 | 0. 8 |
| Epoxy/FAS-TiO2/epoxy on AISI 316L | −479.3 | 0.032 | 0. 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conradi, M.; Kocijan, A.; Kosec, T.; Podgornik, B. Manipulation of TiO2 Nanoparticle/Polymer Coatings Wettability and Friction in Different Environments. Materials 2020, 13, 1702. https://doi.org/10.3390/ma13071702
Conradi M, Kocijan A, Kosec T, Podgornik B. Manipulation of TiO2 Nanoparticle/Polymer Coatings Wettability and Friction in Different Environments. Materials. 2020; 13(7):1702. https://doi.org/10.3390/ma13071702
Chicago/Turabian StyleConradi, Marjetka, Aleksandra Kocijan, Tadeja Kosec, and Bojan Podgornik. 2020. "Manipulation of TiO2 Nanoparticle/Polymer Coatings Wettability and Friction in Different Environments" Materials 13, no. 7: 1702. https://doi.org/10.3390/ma13071702
APA StyleConradi, M., Kocijan, A., Kosec, T., & Podgornik, B. (2020). Manipulation of TiO2 Nanoparticle/Polymer Coatings Wettability and Friction in Different Environments. Materials, 13(7), 1702. https://doi.org/10.3390/ma13071702








