Effect of Cooling Rate at the Eutectoid Transformation Temperature on the Corrosion Resistance of Zn-4Al Alloy
Abstract
1. Introduction
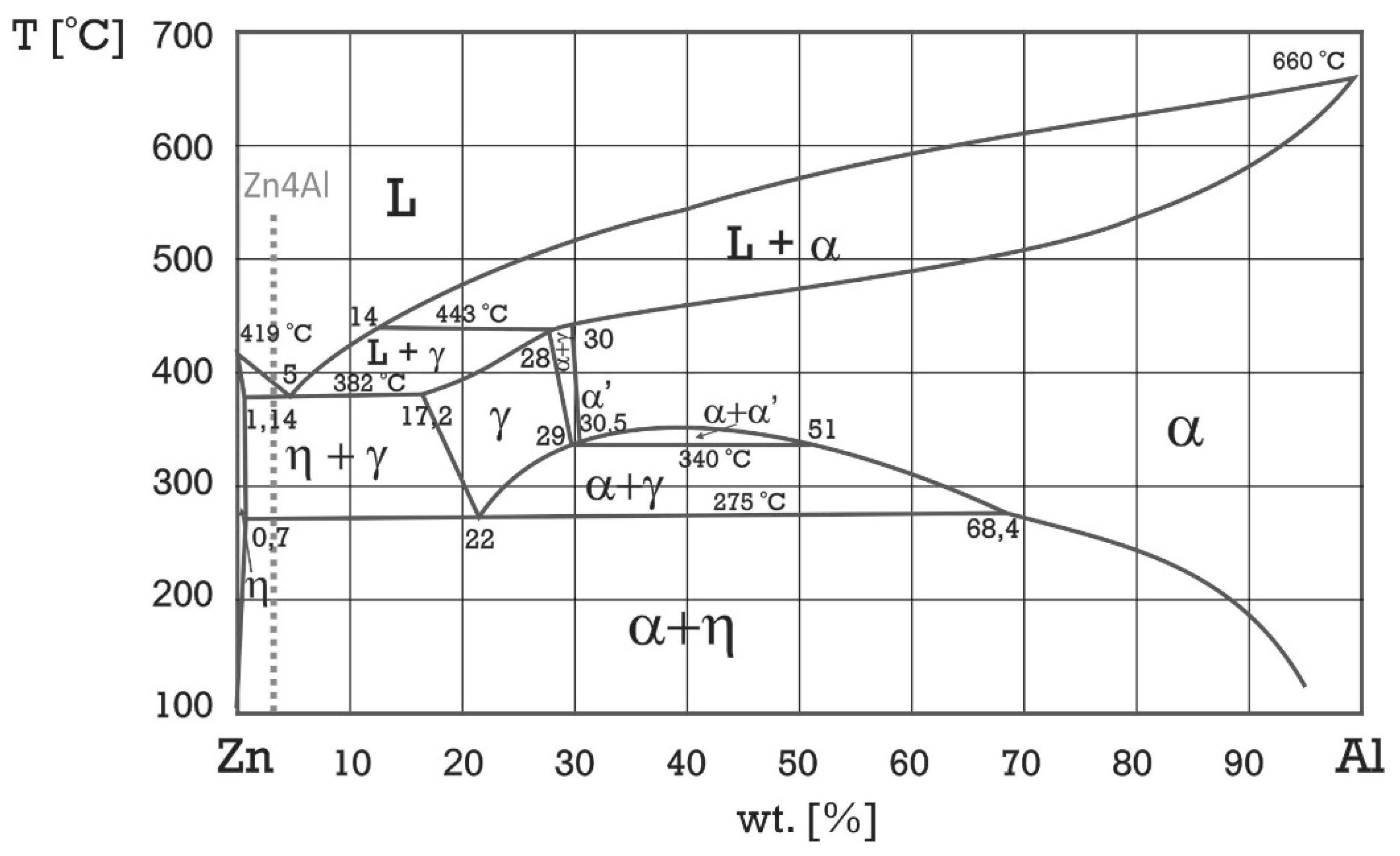
2. Materials and Methods
3. Results
3.1. Hardness Measurements
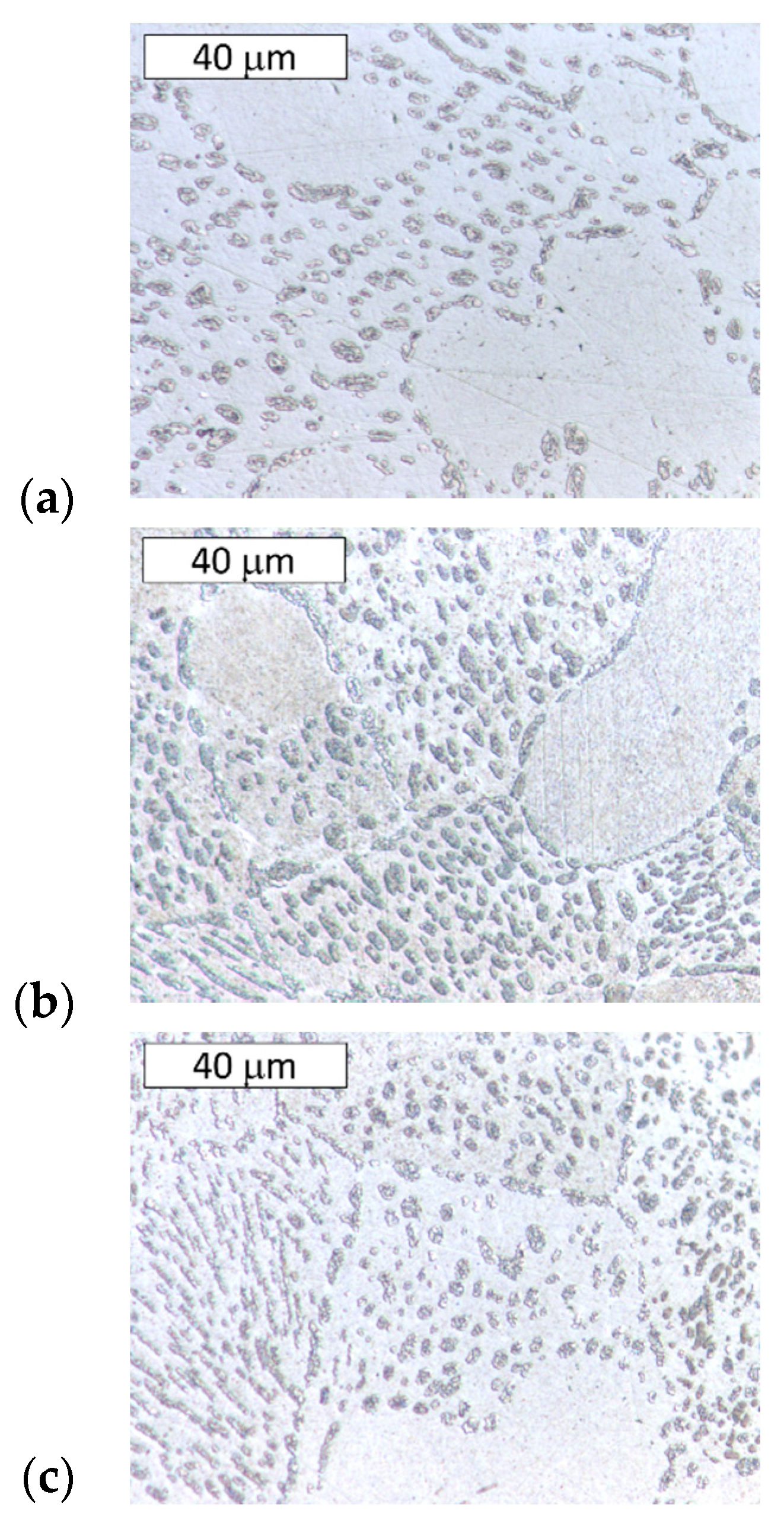
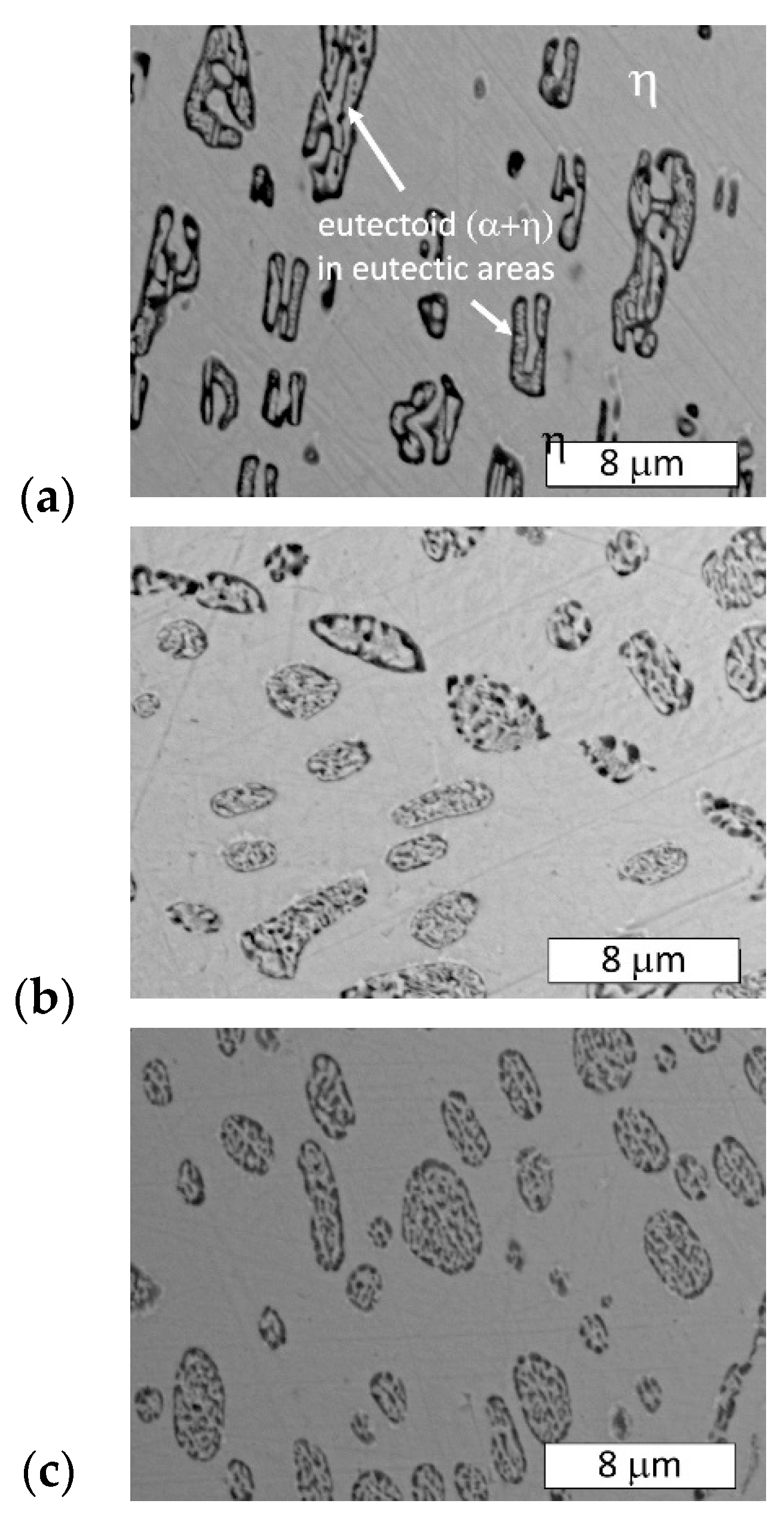
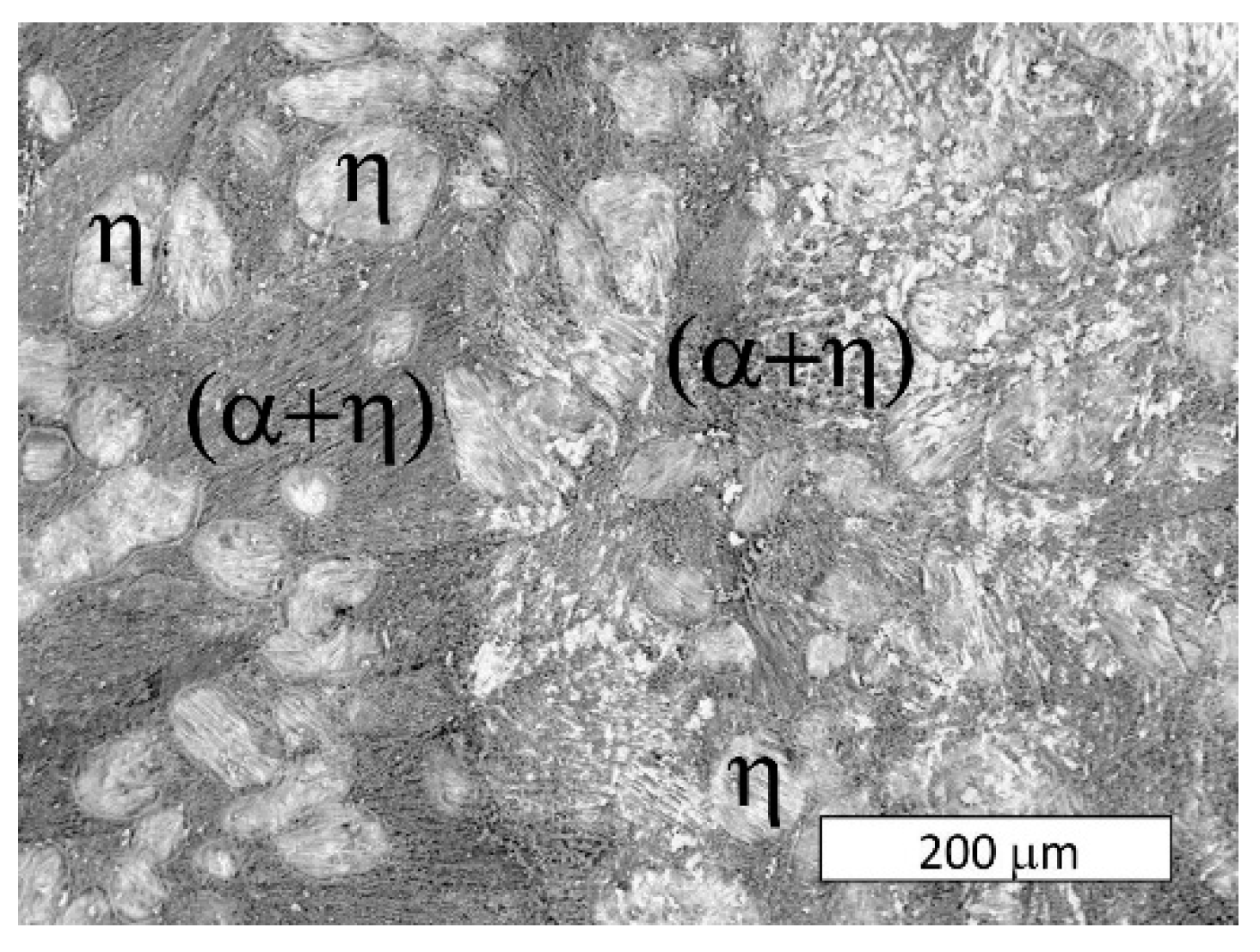
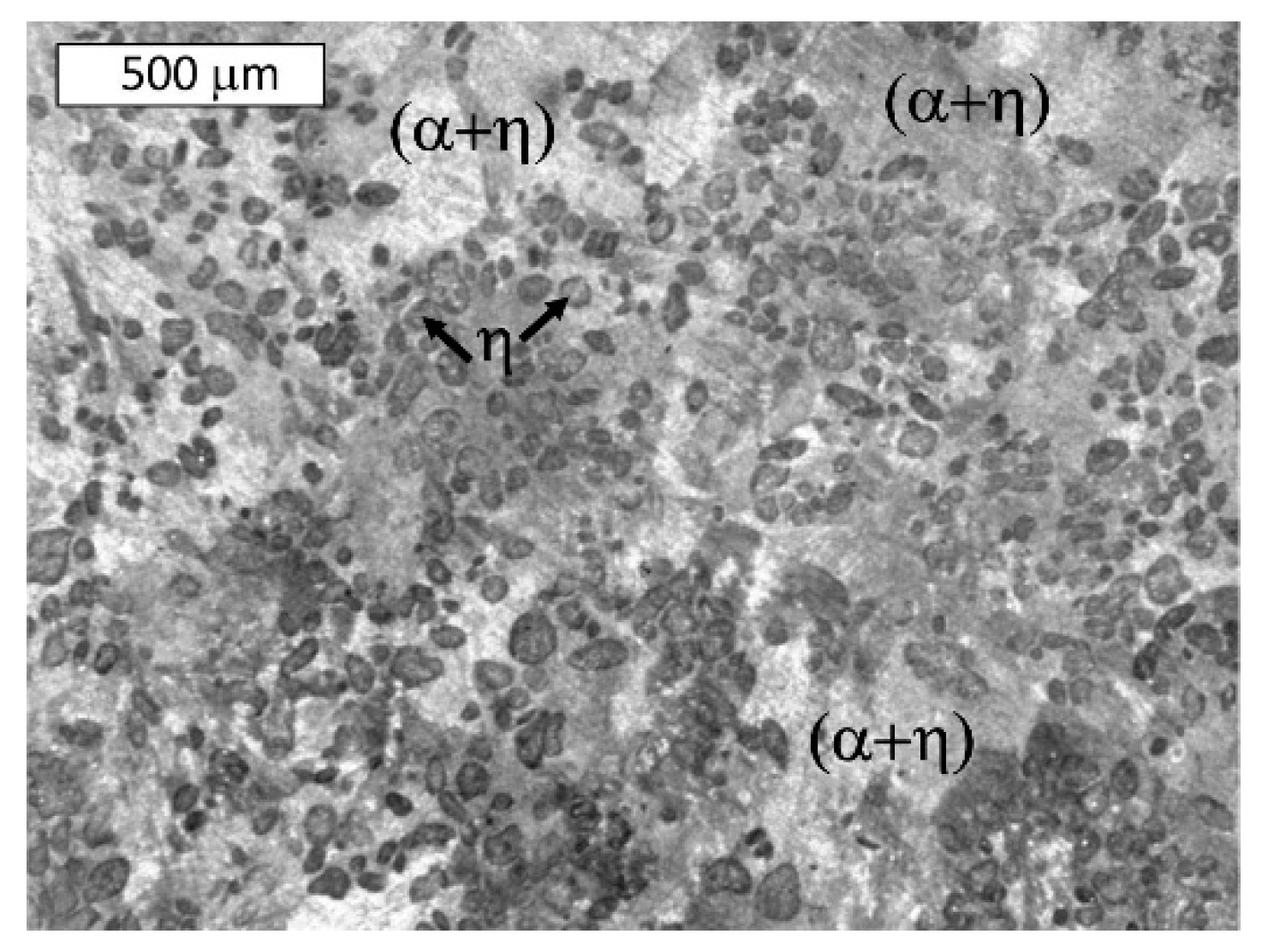
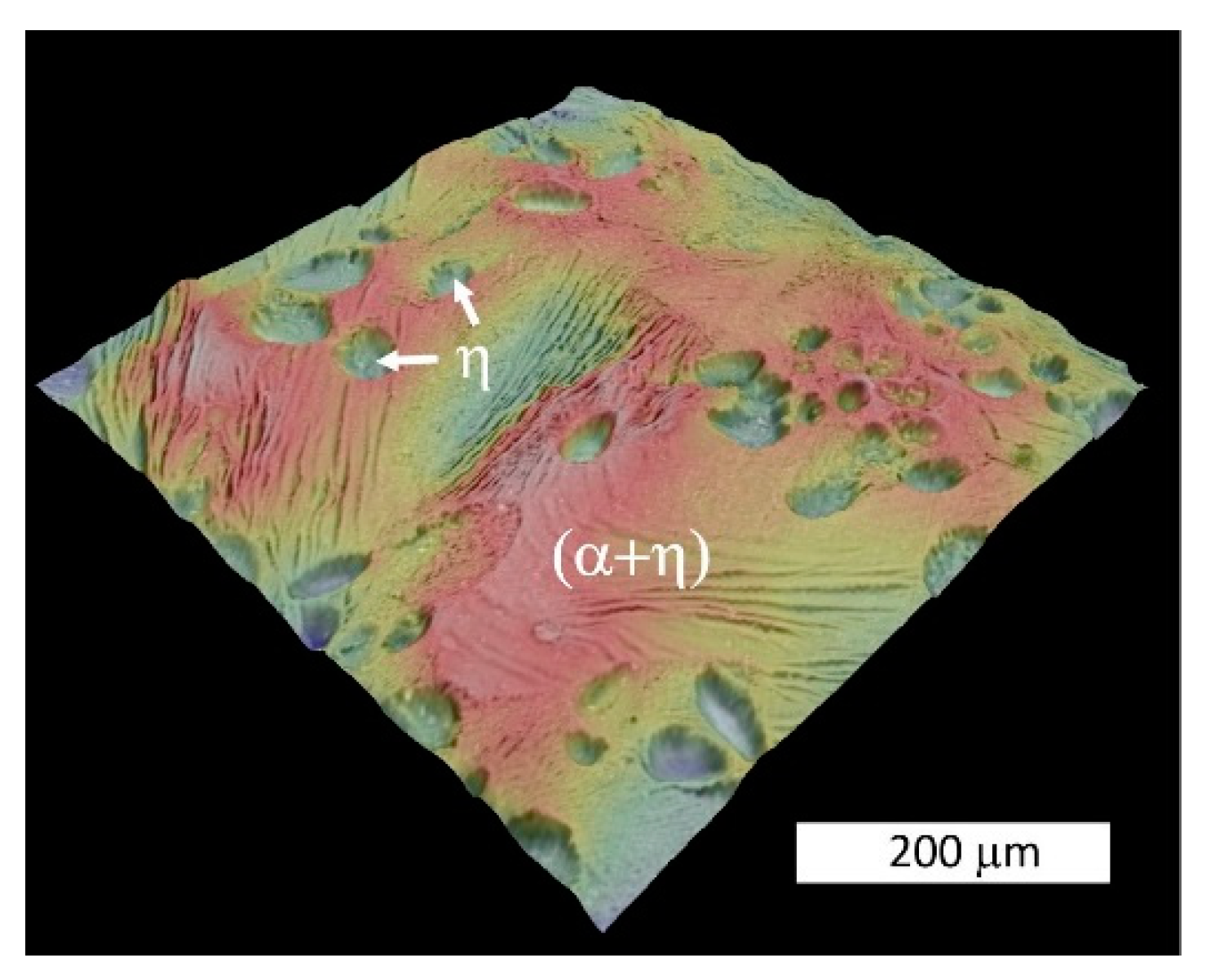
3.2. Microstructural Examination
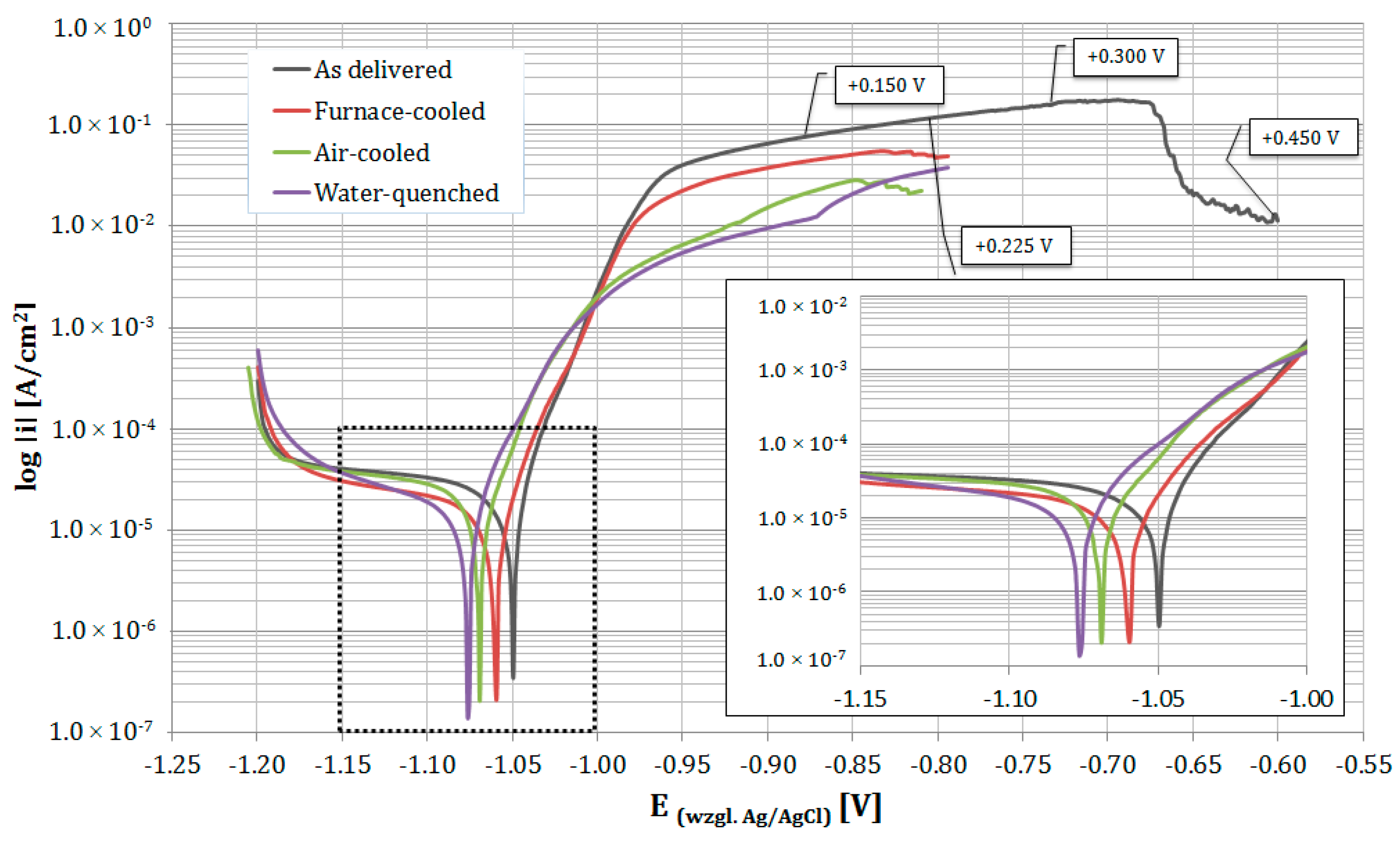
3.3. Electrochemical Examinations
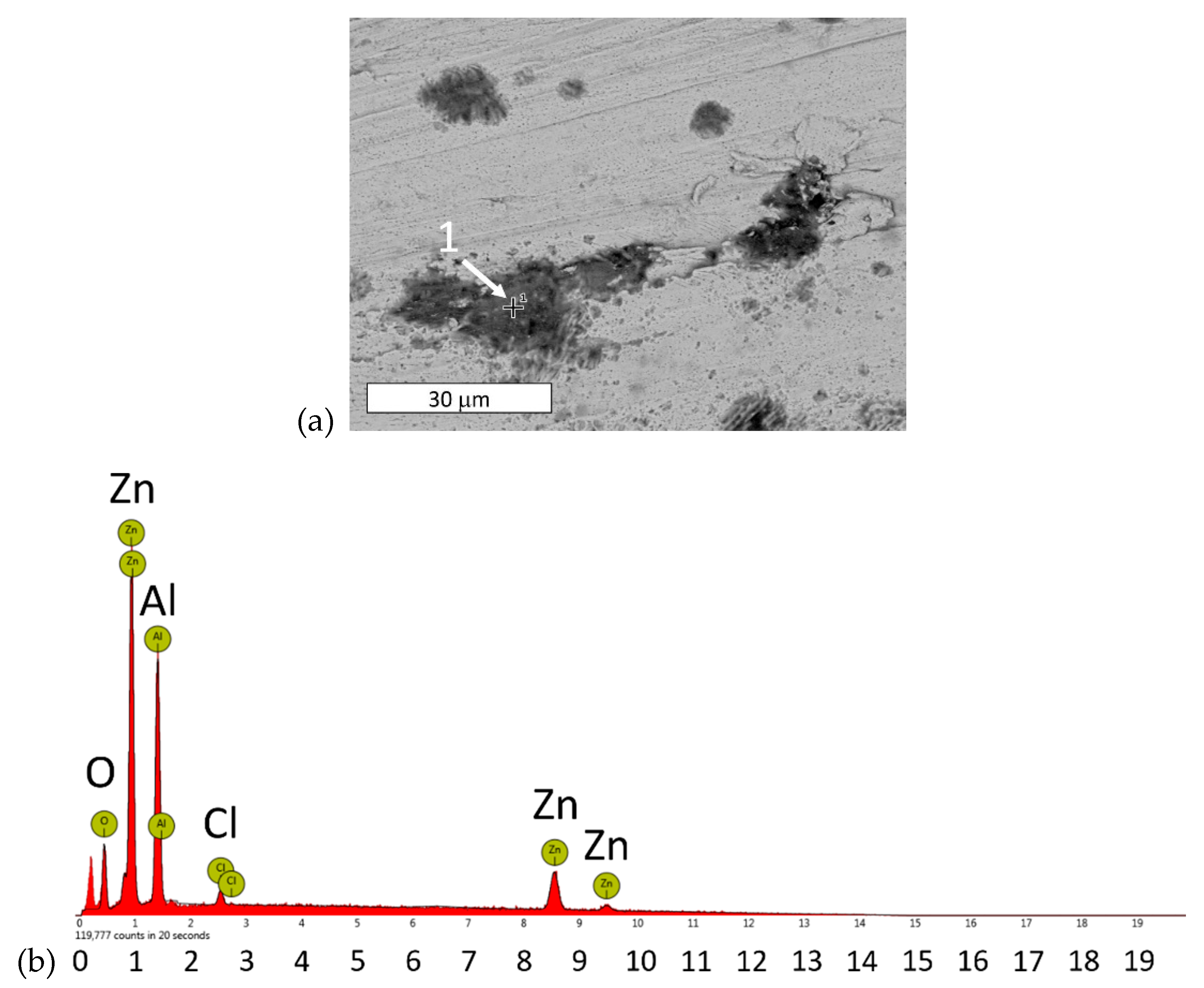
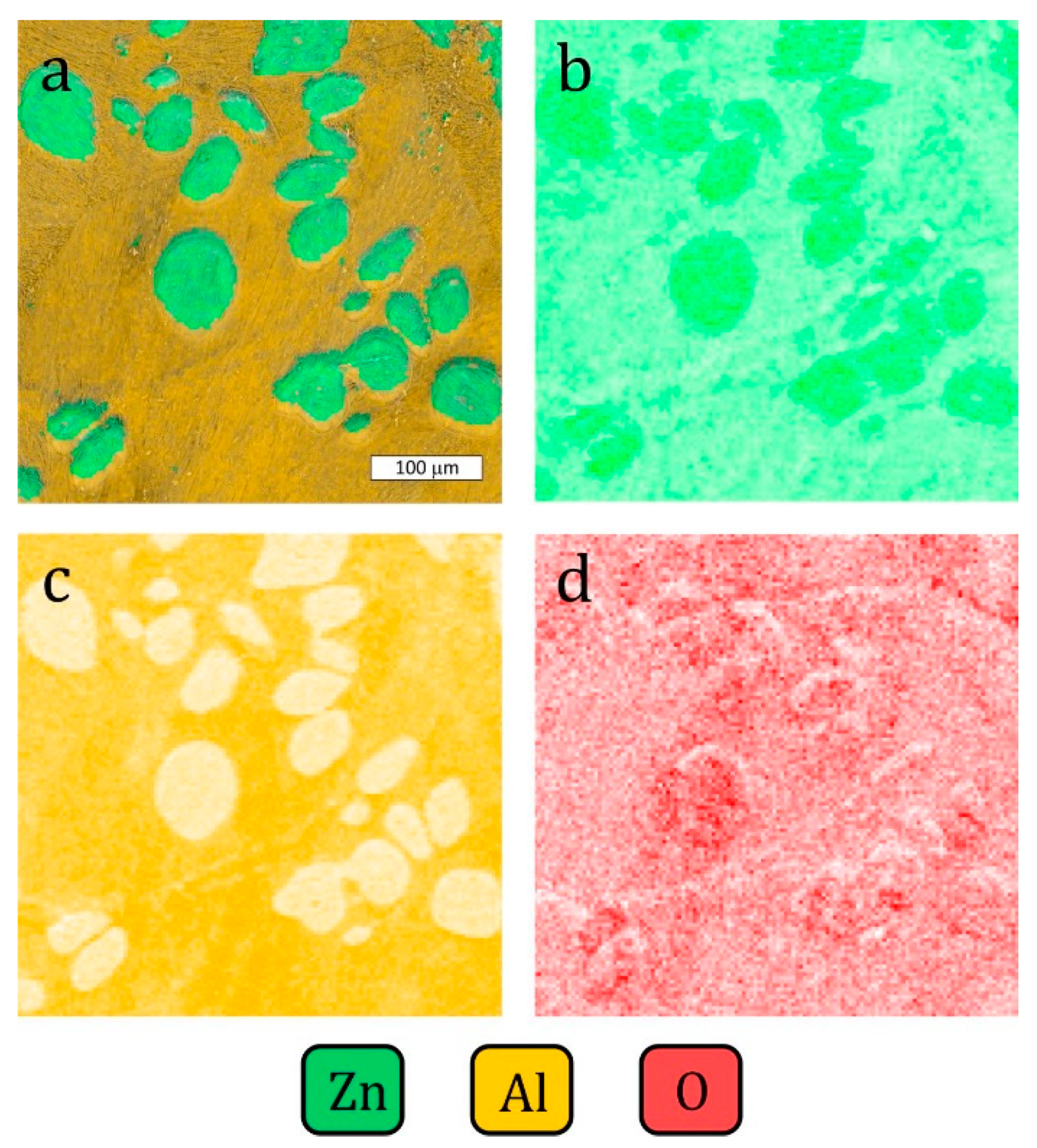
3.4. SEM Surface Evaluation after Corrosion Tests
4. Discussion
- 1)
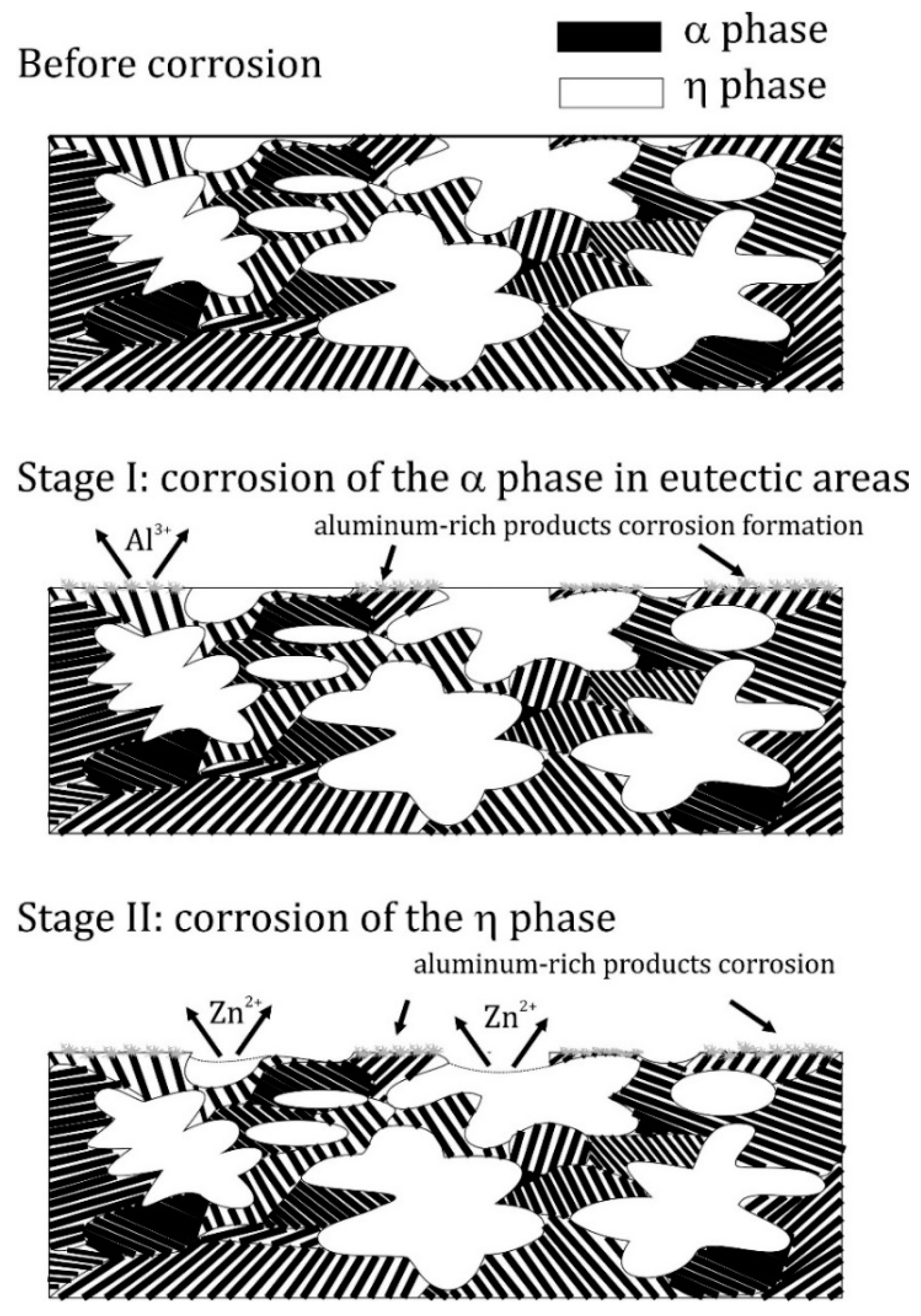
- The microstructure of the material was typical for hypoeutectic cast Zn-Al alloys and was composed of a dendritic η-phase: Zn(Al) solid solution and lamellar (α + η) eutectoid. It contained the products of the eutectoid reaction which transformed the γ phase to (α + η) at 275 °C. Increasing the susceptibility to corrosion by increasing the aluminum content in Zn-Al alloys has been reported in the previous literature [5,19] which may be associated with an increased volumetric fraction of the (α + η) eutectic. Upon progression of the corrosion process, the (α + η) eutectoid structure in eutectic areas was attacked first and subjected to intense corrosion. Therefore, increasing the eutectic volumetric fraction should deteriorate the corrosion resistance of Zn-Al alloys. The high corrosion tendency of eutectic areas may induce intergranular corrosion [10,22,23]. In the case of two cooperating details, the accompanying pulverisation promotes the penetration of material fragments and the corrosion products into the friction area [22,23].
- 2)
- Different cooling rates affected the hardness of samples annealed at 300 °C. Water quenching promoted the creation of a finer (α + η) eutectoid structure from the γ phase in eutectic areas of the Zn-Al alloy and obtained higher hardness values. Slower cooling formed a coarser eutectic structure in the alloy, which translated into a lower hardness. After furnace cooling, a hardness similar to the as-cast material was obtained. Heat treatment at 250 °C showed no effect on the hardness of the Zn-4Al alloy.
- 3)
- A finer eutectoid structure decreased the corrosion current density Icorr compared with a coarse structure, which indicates that the short phase distances of eutectoid structures may contribute to the protection of the anode phase and reduce the corrosion rate. The corrosion potential Ecorr remained rather constant, although a slight decrease was observed.
- 4)
- In the initial corrosion stage, the α-phase Al-base solid solution served as the anode in a formed corrosion microcell in the examined corrosive environment. As corrosion further developed, it extended over the entire alloy surface. Thus, it can be stated that the dissolution of the η phase was the preferred corrosion mode due to anodic dissolution reactions. This phenomenon may have been related to the formation of an α-phase corrosion product film. The formation of this film can also explain the lower corrosion current density due to a decrease in the cathode activity due to a smaller distance between eutectoid components.
- 5)
- If there is an anode phase whose fragments are fine and homogeneously distributed within the grain, corrosion will lead to their dissolution and the material eventually becomes quasi-homogeneous. A very different situation takes place for large η phase dendrites which occurs in the microstructure of Zn-Al alloy. In this case, corrosion develops involving these structural elements, which decrease the cross-sections of components made of this material.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Savaşkan, T.; Pürçek, G.; Murphy, S. Sliding wear of cast zinc-based alloy bearings under static and dynamic loading conditions. Wear 2002, 252, 693–703. [Google Scholar] [CrossRef]
- Murphy, S.; Savaskan, T. Comparative wear behaviour of Zn-Al-based alloys in an automotive engine application. Wear 1984, 98, 151–161. [Google Scholar] [CrossRef]
- Calayag, T.S. Zinc alloys replace bronze in mining equipment bushings and bearings. Min. Eng. 1983, 35, 727–728. [Google Scholar]
- Babić, M.; Ninković, R. Zn-Al alloys as tribomaterials. Tribol. Ind. 2004, 26, 3–7. [Google Scholar]
- Ares, A.E.; Gassa, L.M. Corrosion susceptibility of Zn–Al alloys with different grains and dendritic microstructures in Nacl solutions. Corros. Sci. 2012, 59, 290–306. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Song, Y.; Liu, S.; Qi, Y.; Wang, X.; Wang, Q.; Cui, C. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Kannan, M.B.; Moore, C.; Saptarshi, S.; Somasundaram, S.; Rahuma, M.; Lopata, A.L. Biocompatibility and biodegradation studies of a commercial zinc alloy for temporary mini-implant applications. Sci. Rep. 2017, 7, 15605. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Bowen, P.K.; Seitz, J.-M.; Guillory, R.J.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of wrought Zn-Al alloys (1, 3, and 5 wt % Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef]
- Sullivan, J.; Penney, D.; Elvins, J.; Khan, K. The effect of ultrasonic irradiation on the microstructure and corrosion rate of a Zn–4.8wt.% Al galvanising alloy used in high performance construction coatings. Surf. Coat. Technol. 2016, 306, 480–489. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kallip, S.; Lisenkov, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Initial stages of localized corrosion at cut-edges of adhesively bonded Zn and Zn-Al-Mg galvanized steel. Electrochim. Acta 2016, 211, 126–141. [Google Scholar] [CrossRef]
- Salgueiro Azevedo, M.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg,Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Prosek, T.; Persson, D.; Stoulil, J.; Thierry, D. Composition of corrosion products formed on Zn–Mg, Zn–Al and Zn–Al–Mg coatings in model atmospheric conditions. Corros. Sci. 2014, 86, 231–238. [Google Scholar] [CrossRef]
- Vu, T.N.; Volovitch, P.; Ogle, K. The effect of pH on the selective dissolution of Zn and Al from Zn–Al coatings on steel. Corros. Sci. 2013, 67, 42–49. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The role of macrostructural morphology and grain size on the corrosion resistance of Zn and Al castings. Mater. Sci. Eng. A 2005, 402, 22–32. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The effect of the dendritic microstructure on the corrosion resistance of Zn–Al alloys. J. Alloys Compd. 2005, 397, 179–191. [Google Scholar] [CrossRef]
- Zhang, X.; Leygraf, C.; Odnevall Wallinder, I. Atmospheric corrosion of Galfan coatings on steel in chloride-rich environments. Corros. Sci. 2013, 73, 62–71. [Google Scholar] [CrossRef]
- Ares, A.E.E.; Gassa, L.M.M.; Schvezov, C.E.E.; Rosenberger, M.R.R. Corrosion and wear resistance of hypoeutectic Zn–Al alloys as a function of structural features. Mater. Chem. Phys. 2012, 136, 394–414. [Google Scholar] [CrossRef]
- Jasionowski, R.; Polkowski, W.; Zasada, D. Destruction Mechanism of ZnAl4 as Cast Alloy Subjected to Cavitational Erosion Using Different Laboratory Stands. Arch. Foundry Eng. 2016, 16, 19–24. [Google Scholar] [CrossRef][Green Version]
- Jasionowski, R.; Zasada, D.; Polkowski, W. Destruction Mechanism of Z10400 Zn-based Alloy Subjected to Cavitational Erosion. Arch. Foundry Eng. 2013, 13, 48–52. [Google Scholar]
- Lachowicz, M.M.; Lachowicz, M.B. Intergranular Corrosion of the as Cast Hypoeutectic Zinc-Aluminium Alloy. Arch. Foundry Eng. 2017, 17, 79–84. [Google Scholar] [CrossRef]
- Lachowicz, M.M. A study on the intergranular corrosion-fatigue failure of the Zn-Al alloy solenoid valve. Eng. Fail. Anal. 2019, 103, 184–194. [Google Scholar] [CrossRef]
- Prosek, T.; Thierry, D.; Hagström, J.; Persson, D.; Fuertes, N.; Lindberg, F.; Taxén, C.; Šerák, J. Relationship between corrosion performance and microstructure of Zn-AI and Zn-AI-Mg model alloys. In Proceedings of the European Corrosion Congress, EUROCORR 2015, Graz, Austria, 6–10 September 2015; Volume 358. [Google Scholar]
- Elvins, J.; Spittle, J.A.; Worsley, D.A. Relationship between microstructure and corrosion resistance in Zn–Al alloy coated galvanised steels. Corros. Eng. Sci. Technol. 2003, 38, 197–204. [Google Scholar] [CrossRef]
- Elvins, J.; Spittle, J.A.; Worsley, D.A. Microstructural changes in zinc aluminium alloy galvanising as a function of processing parameters and their influence on corrosion. Corros. Sci. 2005, 47, 2740–2759. [Google Scholar] [CrossRef]
- Krupińska, B.; Dobrzański, L.A.; Rdzawski, Z.M.; Labisz, K. Cooling rate influence on microstructure of the Zn-Al cast alloy. Arch. Mater. Sci. Eng. 2010, 43, 13–20. [Google Scholar]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Imshinetskiy, I.M.; Vyaliy, I.E.; Gnedenkov, S.V. Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy. Materials 2019, 12, 2615. [Google Scholar] [CrossRef]
- Gancarz, T.; Mech, K.; Guśpiel, J.; Berent, K. Corrosion studies of Li, Na and Si doped Zn-Al alloy immersed in NaCl solutions. J. Alloys Compd. 2018, 767, 1225–1237. [Google Scholar] [CrossRef]
- Prosek, T.; Hagström, J.; Persson, D.; Fuertes, N.; Lindberg, F.; Chocholatý, O.; Taxén, C.; Šerák, J.; Thierry, D. Effect of the microstructure of Zn-Al and Zn-Al-Mg model alloys on corrosion stability. Corros. Sci. 2016, 110, 71–81. [Google Scholar] [CrossRef]
- Snihirova, D.; Taryba, M.; Lamaka, S.V.; Montemor, M.F. Corrosioninhibition synergies on a model Al-Cu-Mg sample studied by localized scanning electrochemical techniques. Corros. Sci. 2016, 112, 408–417. [Google Scholar]
- Queiroz, F.M.; Donatus, U.; Prada Ramirez, O.M.; de Sousa Araujo, J.V.; Goncalves de Viveiros, B.V.; Lamaka, S.; Zheludkvich, M.; Masoumi, M.; Vivier, V.; Costa, I.; et al. Effect of unequal levels of deformation and fragmentation on the electrochemical response of friction stir welded AA2024-T3 alloy. Electrochim. Acta 2019, 313, 271–281. [Google Scholar] [CrossRef]
- Qiu, P.; Leygraf, C.; Odnevall Wallinder, I. Evolution of corrosion products and metal release from Galvalume coatings on steel during short and long-term atmospheric exposures. Mater. Chem. Phys. 2012, 133, 419–428. [Google Scholar] [CrossRef]
- Cao, Z.; Kong, G.; Che, C.; Wang, Y. Influence of Nd addition on the corrosion behavior of Zn-5%Al alloy in 3.5wt.% NaCl solution. Appl. Surf. Sci. 2017, 426, 67–76. [Google Scholar] [CrossRef]
- Neto, P.D.L.; Correia, A.N.; Colares, R.P.; Araujo, W.S. Corrosion study of electrodeposited Zn and Zn-Co coatings in chloride medium. J. Braz. Chem. Soc. 2007, 18, 1164–1175. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Z. EIS investigation and structural characterization of different hot-dipped zinc-based coatings in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2013, 8, 7753–7767. [Google Scholar]
- Tang, N.Y.; Liu, Y. Corrosion performance of aluminum-containing zinc coatings. ISIJ Int. 2010, 50, 455–462. [Google Scholar] [CrossRef][Green Version]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials 2018, 11, 2177. [Google Scholar] [CrossRef]





| Material | Methods | |
|---|---|---|
| As delivered | Hardness measurements, Microstructural examination, Electrochemical examination, SEM surface evaluation | |
| Heat treatment at 250 °C | Furnace cooled | Hardness measurements |
| Air cooled | ||
| Water quenched | ||
| Heat treatment at 300 °C | Furnace cooled | Hardness measurements, Microstructural examination, Electrochemical examination, SEM surface evaluation |
| Air cooled | ||
| Water quenched | ||
| Sample | Icorr (µA/cm2) | Ecorr (V) vs. Ag/AgCl | EOCP (V) |
|---|---|---|---|
| As delivered | 9.45 ± 0.36 | −1.05 ± 0.01 | −1.02 ± 0.01 |
| Furnace-cooled | 7.01 ± 0.23 | −1.06 ± 0.01 | −1.02 ± 0.01 |
| Air-cooled | 5.47 ± 0.9 | −1.06 ± 0.01 | −1.03 ± 0.01 |
| Water-quenched | 4.74 ± 0.20 | −1.07 ± 0.01 | −1.05 ± 0.02 |
| Element | Atomic % | Weight % |
|---|---|---|
| Zn | 22.15 | 45.88 |
| Al | 39.14 | 33.47 |
| O | 37.04 | 18.78 |
| Cl | 1.67 | 1.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, M.M.; Jasionowski, R. Effect of Cooling Rate at the Eutectoid Transformation Temperature on the Corrosion Resistance of Zn-4Al Alloy. Materials 2020, 13, 1703. https://doi.org/10.3390/ma13071703
Lachowicz MM, Jasionowski R. Effect of Cooling Rate at the Eutectoid Transformation Temperature on the Corrosion Resistance of Zn-4Al Alloy. Materials. 2020; 13(7):1703. https://doi.org/10.3390/ma13071703
Chicago/Turabian StyleLachowicz, Marzena M., and Robert Jasionowski. 2020. "Effect of Cooling Rate at the Eutectoid Transformation Temperature on the Corrosion Resistance of Zn-4Al Alloy" Materials 13, no. 7: 1703. https://doi.org/10.3390/ma13071703
APA StyleLachowicz, M. M., & Jasionowski, R. (2020). Effect of Cooling Rate at the Eutectoid Transformation Temperature on the Corrosion Resistance of Zn-4Al Alloy. Materials, 13(7), 1703. https://doi.org/10.3390/ma13071703






