Inventive Microstructural and Durability Investigation of Cementitious Composites Involving Crystalline Waterproofing Admixtures and Portland Limestone Cement
Abstract
1. Introduction
2. Experimental Methodology
2.1. Phase I
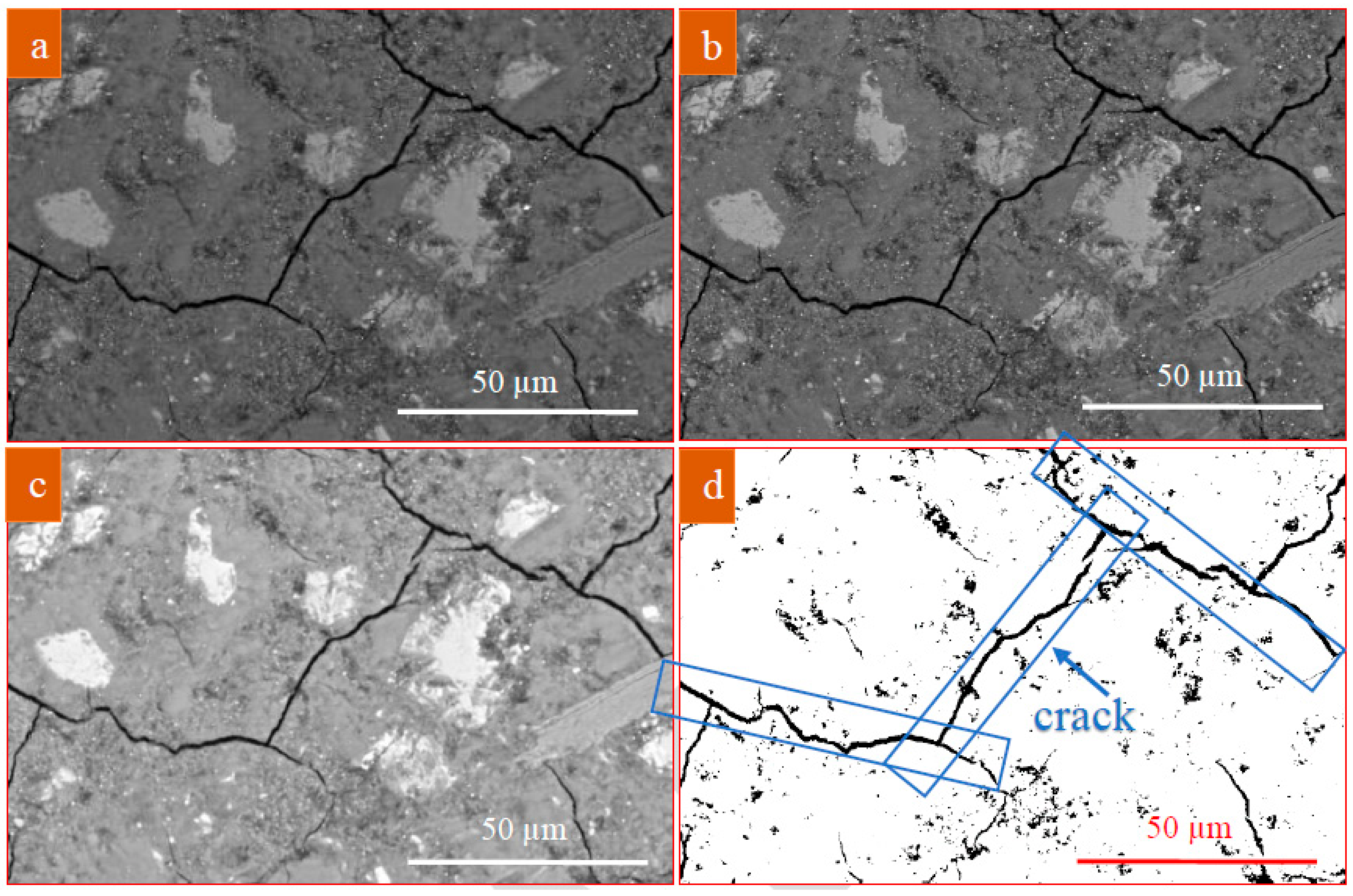
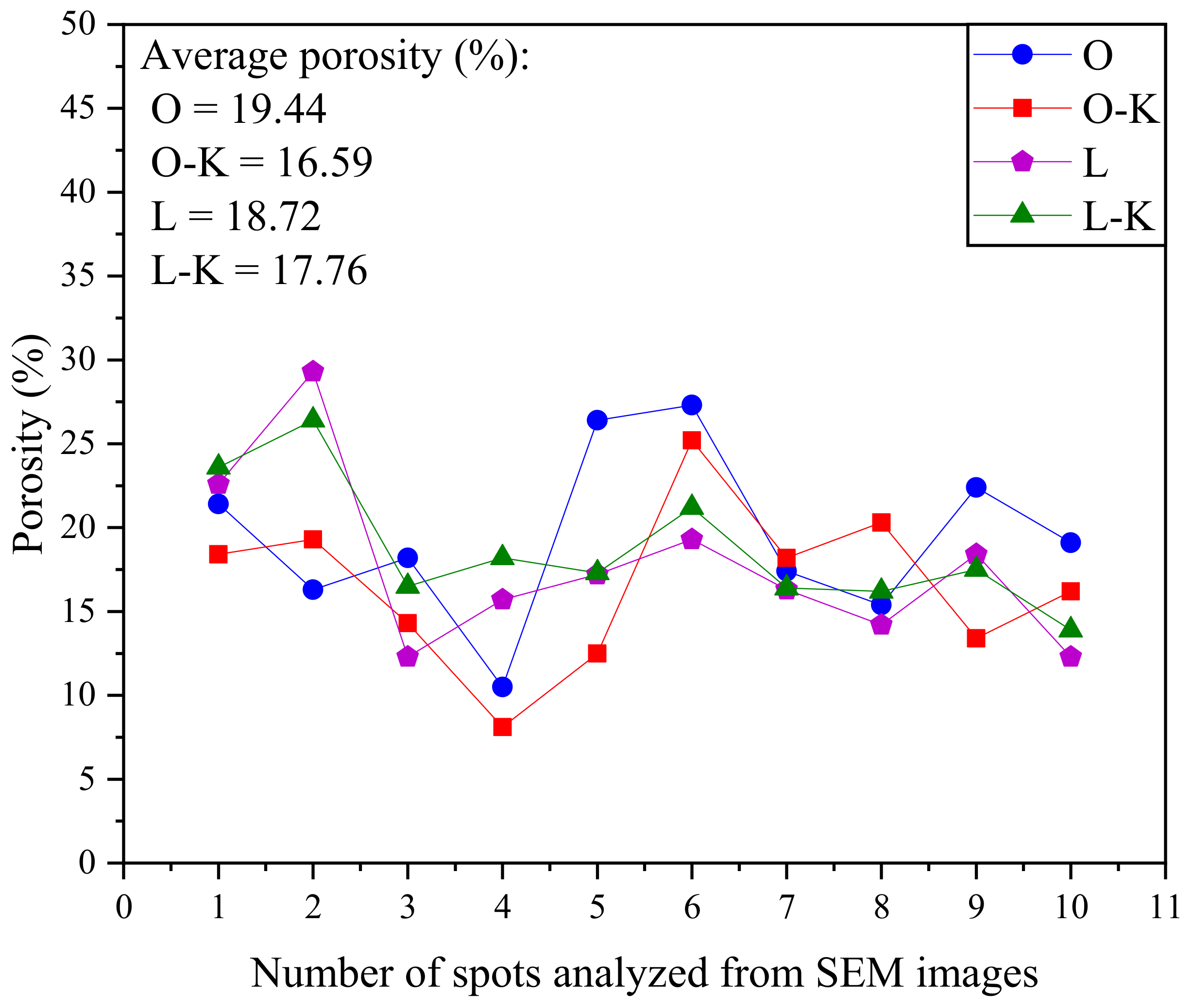
Image Analysis of Polished Sections
2.2. Phase II
2.3. Phase III
3. Results and Discussion
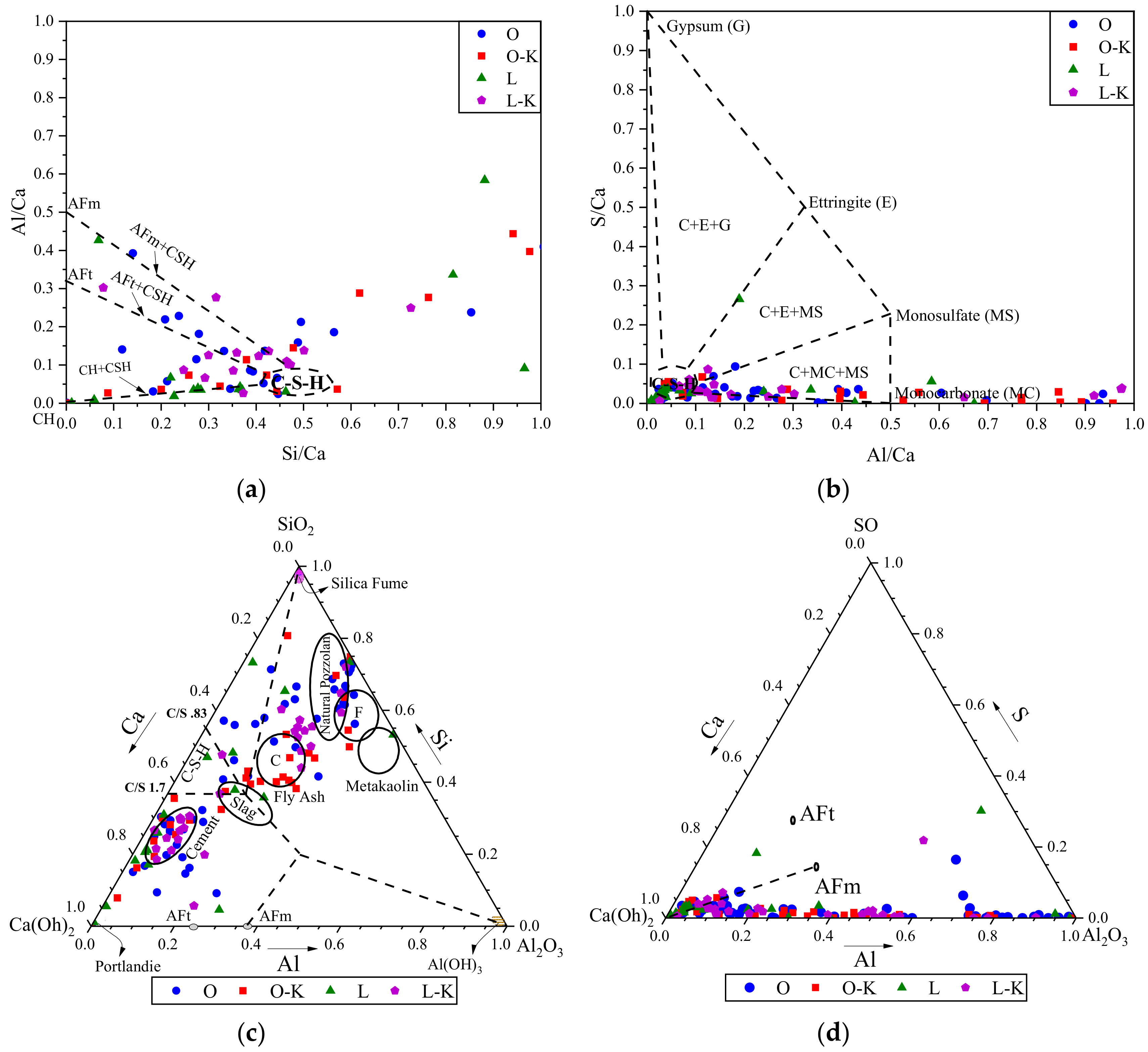
3.1. Phase I
3.2. Phase II
3.3. Phase III
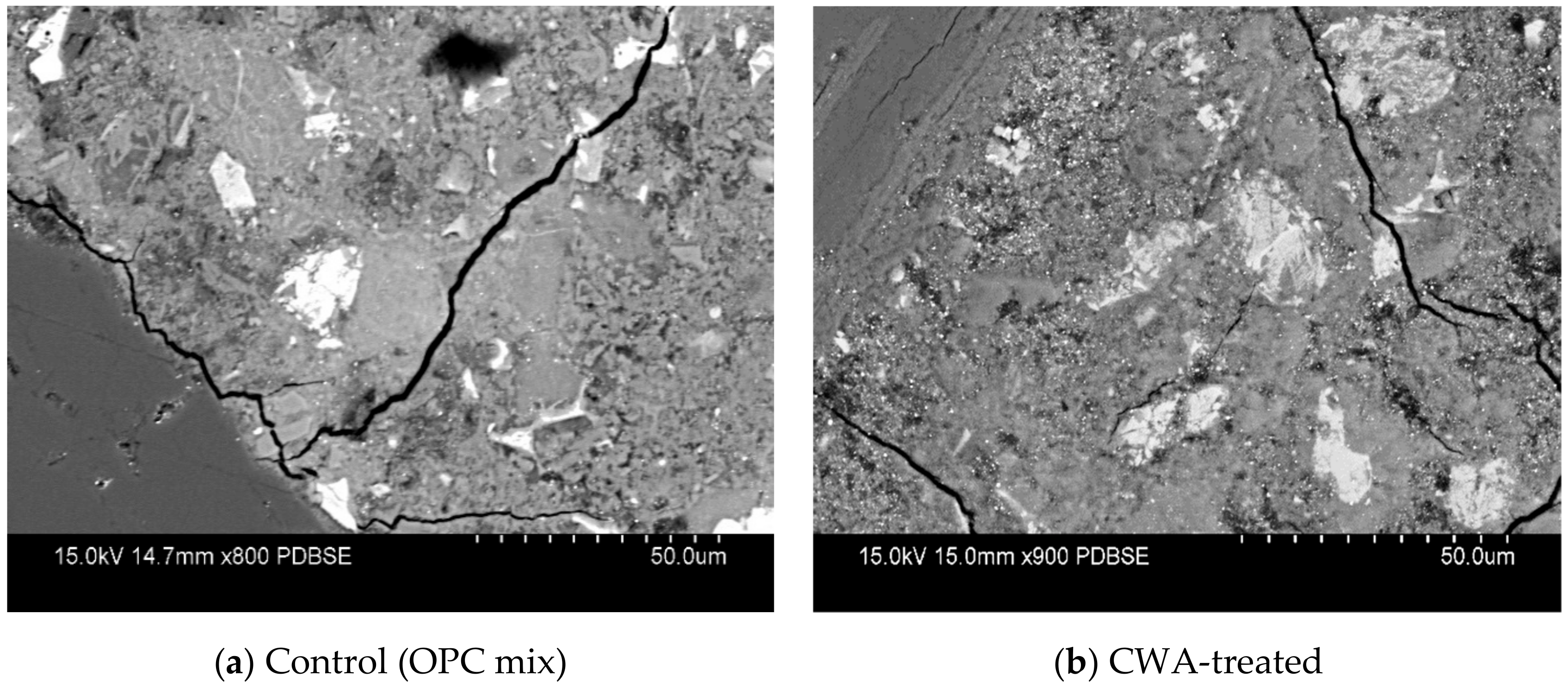
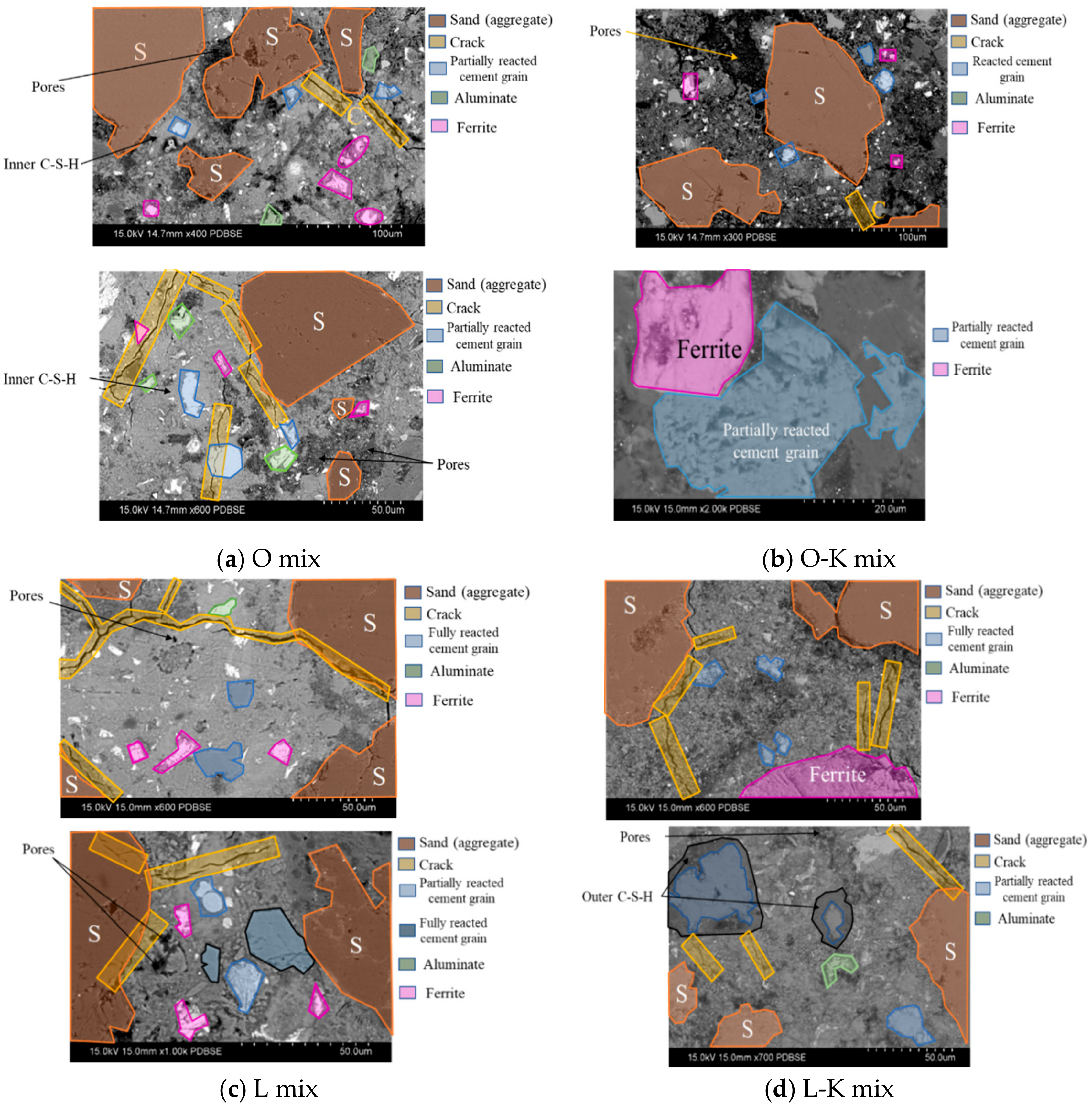
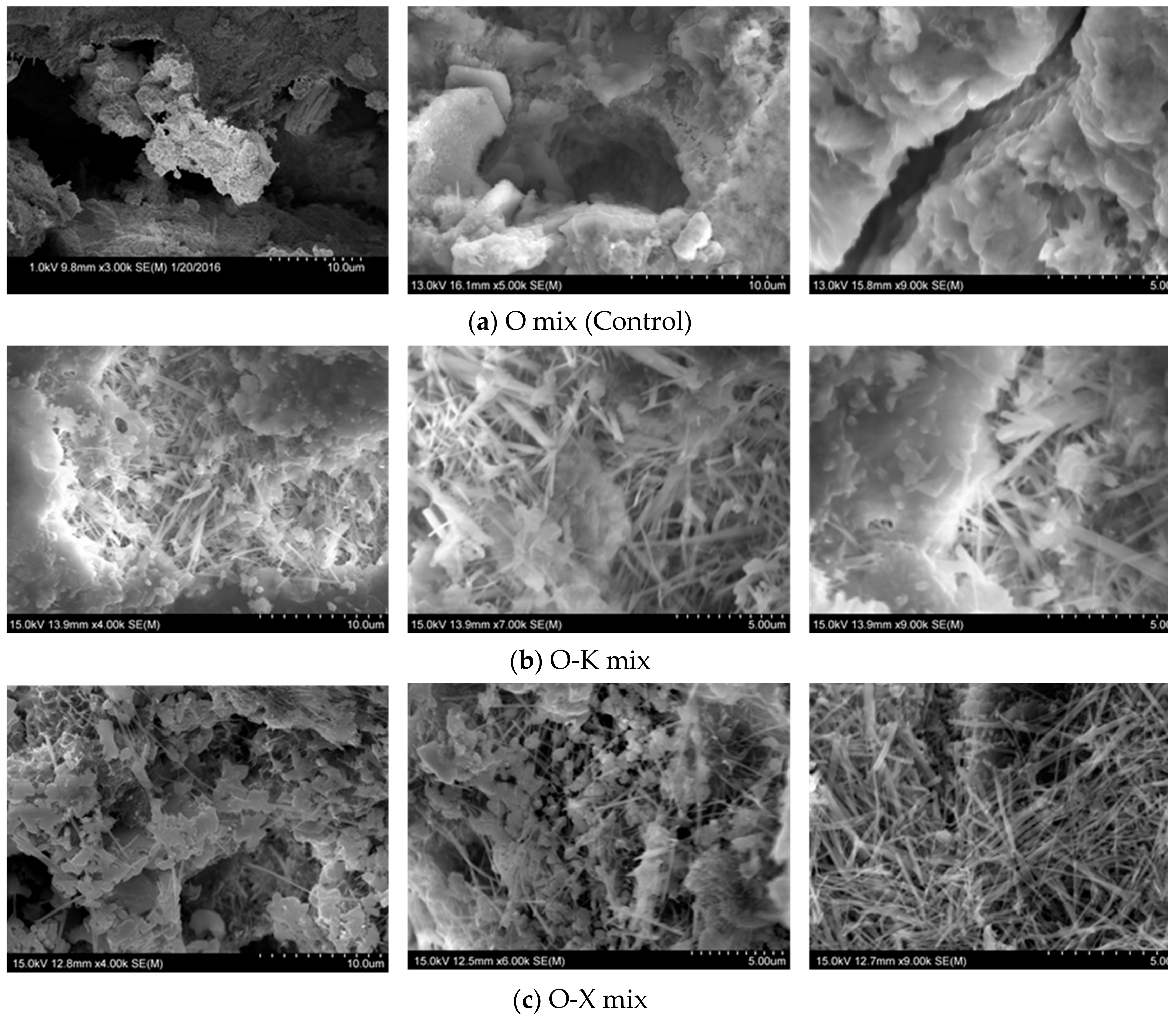
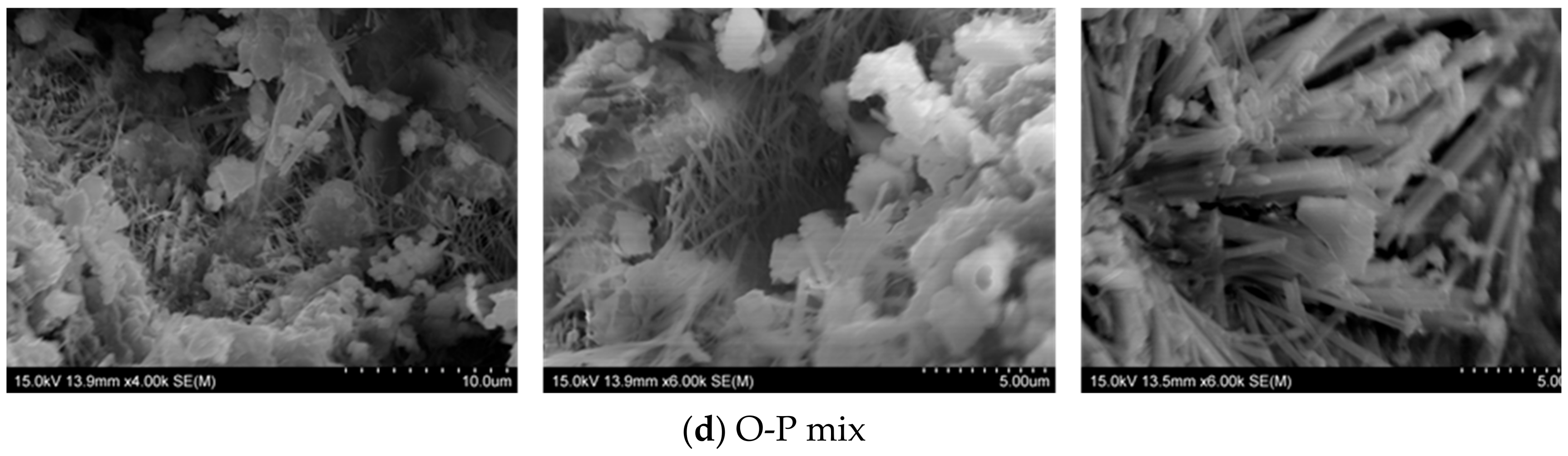
3.3.1. Micrographs and Morphological Aspects
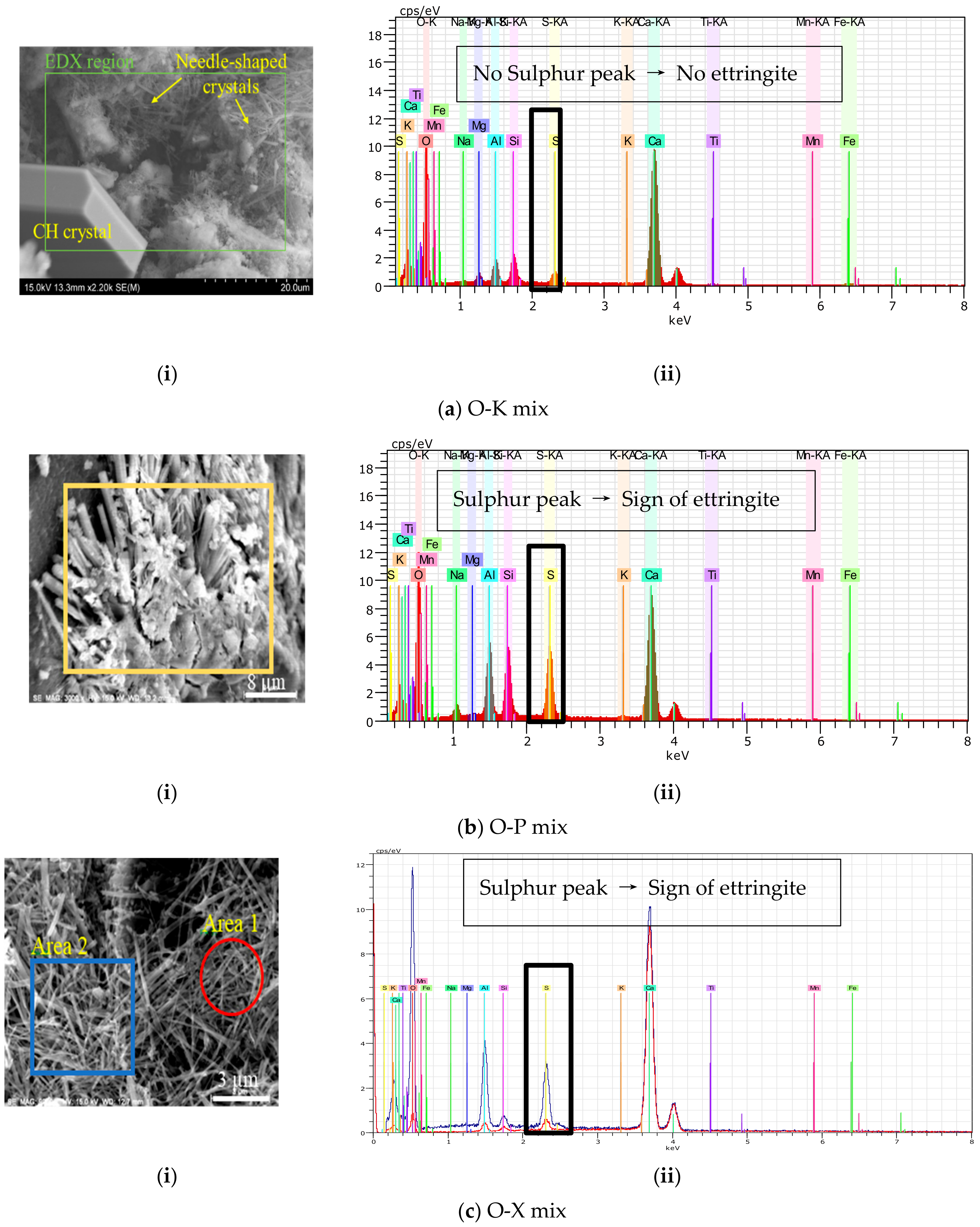
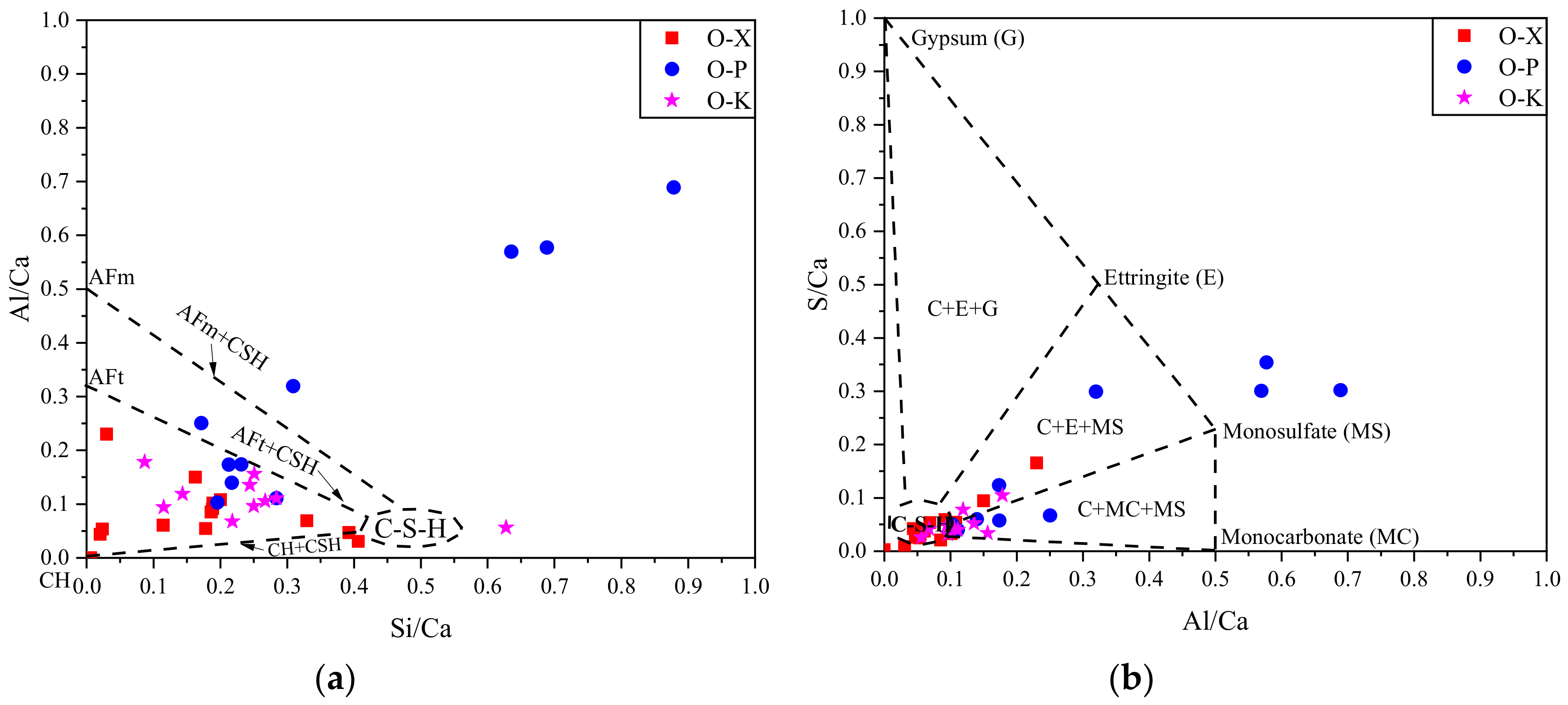
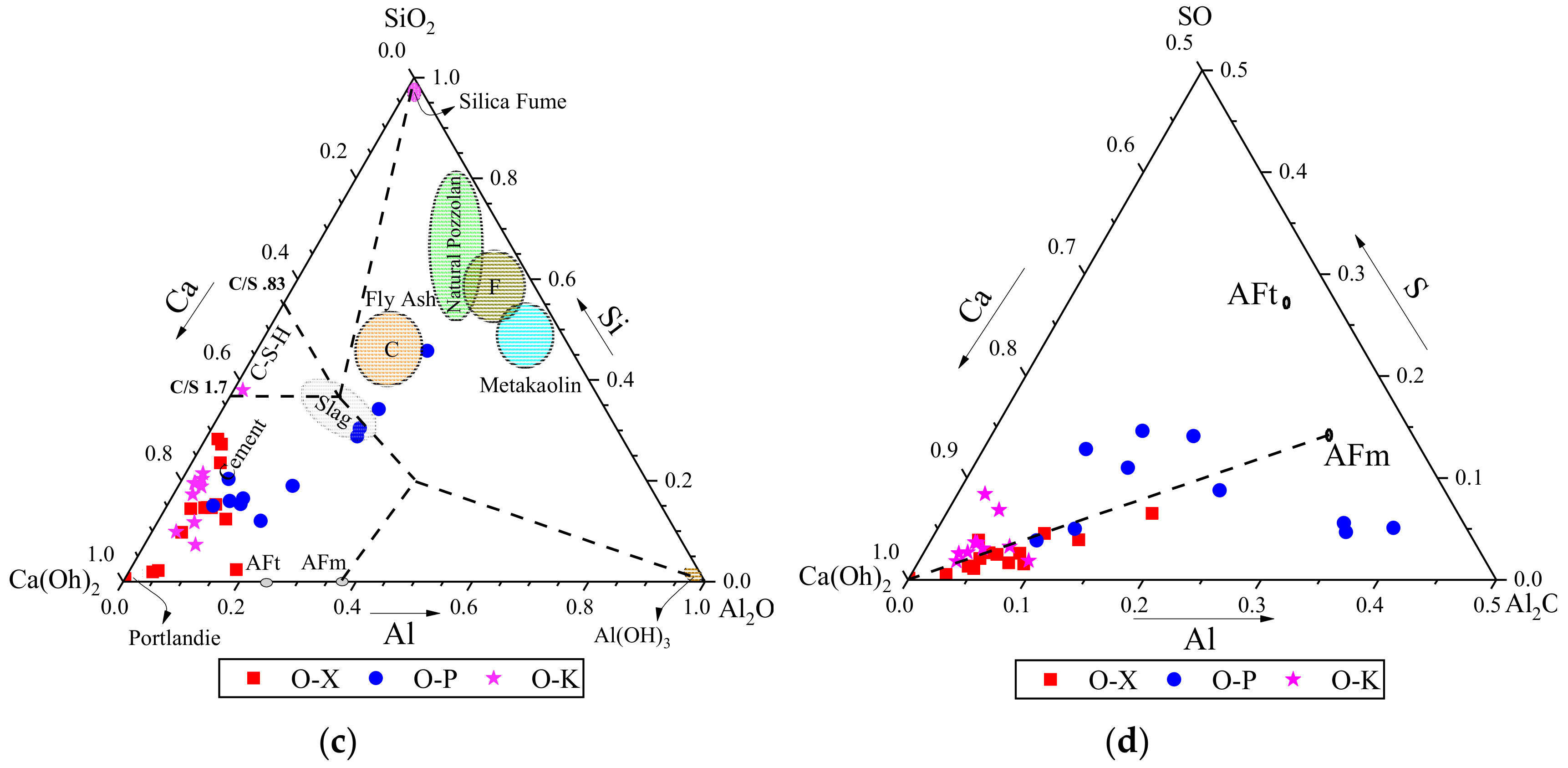
3.3.2. EDS results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, J.; Roberts, L.; Liu, J. Admixtures and sustainability. Cement Concr. Res. 2018, 114, 79–89. [Google Scholar] [CrossRef]
- Zhan, P.; He, Z. Application of shrinkage reducing admixture in concrete: A review. Constr. Build. Mater. 2019, 201, 676–690. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W.; Sam, A.R.M. Sustainability of nanomaterials based self-healing concrete: An all-inclusive insight. J. Build. Eng. 2019, 23, 155–171. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cement Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- ACI Committee 212—American Concrete Institute. Report on Chemical Admixtures for Concrete; American Concrete Institute (ACI): Michigan, MI, USA, 2016; ISBN 978-1-942727-80-4. [Google Scholar]
- Jaroenratanapirom, D.; Sahamitmongkol, R. Self-Crack Closing Ability of Mortar with Different Additives. J. Met. Mater. Miner. 2011, 21, 9–17. [Google Scholar]
- Jaroenratanapirom, D.; Sahamitmongkol, R. Effects of Different Mineral Additives and Cracking Ages on Self-Healing Performance of Mortar. In Proceedings of the 6th Annual Concrete Conference, Petchaburi, Thailand, 20–22 October 2010. [Google Scholar]
- Sisomphon, K.; Copuroglu, O.; Koenders, E.A.B. Self-healing of surface cracks in mortars with expansive additive and crystalline additive. Cement Concr. Compos. 2012, 34, 566–574. [Google Scholar] [CrossRef]
- Sisomphon, K.; Copuroglu, O.; Koenders, E.A.B. Effect of exposure conditions on self-healing behavior of strain hardening cementitious composites incorporating various cementitious materials. Constr. Build. Mater. 2013, 42, 217–224. [Google Scholar] [CrossRef]
- Ferrara, L.; Krelani, V.; Carsana, M. A “fracture testing” based approach to assess crack healing of concrete with and without crystalline admixtures. Constr. Build. Mater. 2014, 68, 535–551. [Google Scholar] [CrossRef]
- Roig-Flores, M.; Moscato, S.; Serna, P.; Ferrara, L. Self-healing capability of concrete with crystalline admixtures in different environments. Constr. Build. Mater. 2015, 86, 1–11. [Google Scholar] [CrossRef]
- Roig-Flores, M.; Pirritano, F.; Serna, P.; Ferrara, L. Effect of crystalline admixtures on the self-healing capability of early-age concrete studied by means of permeability and crack closing tests. Constr. Build. Mater. 2016, 114, 447–457. [Google Scholar] [CrossRef]
- Ferrara, L.; Krelani, V.; Moretti, F. On the use of crystalline admixtures in cement based construction materials: From porosity reducers to promoters of self-healing. Smart Mater. Struct. 2016, 25, 084002. [Google Scholar] [CrossRef]
- Pazderka, J.; Hájková, E. Crystalline Admixtures and their Effect on Selected Properties of Concrete. Acta Polytech. 2016, 56, 306–311. [Google Scholar] [CrossRef]
- Drochytka, R.; Ledl, M.; Bydzovsky, J.; Zizkova, N.; Bester, J. Use of Secondary Crystallization and Fly Ash in Waterproofing Materials to Increase Concrete Resistance to Aggressive Gases and Liquids. Adv. Civil Eng. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Žižková, N.; Nevřivová, L.; Lédl, M. Effect of Humidity on the Properties of Cement Mortars with a Crystalline Additive. Solid State Phenom. 2018, 276, 53–59. [Google Scholar] [CrossRef]
- Žižková, N.; Nevřivová, L.; Lédl, M. Durability of Cement Based Mortars Containing Crystalline Additives. Defect Diffus. Forum 2018, 382, 246–253. [Google Scholar] [CrossRef]
- Zizkova, N.; Nevrivova, L.; Ledl, M.; Keprdova, S. Mortars with crystalline additive in aggressive environments. IOP Conf. Ser. Mater. Sci. Eng. 2018, 385, 012066. [Google Scholar] [CrossRef]
- Cuenca, E.; Tejedor, A.; Ferrara, L. A methodology to assess crack-sealing effectiveness of crystalline admixtures under repeated cracking-healing cycles. Constr. Build. Mater. 2018, 179, 619–632. [Google Scholar] [CrossRef]
- Nevřivová, L.; Žižková, N.; Lédl, M. Influence of a Crystalline Additive on the Porosity of Cement Mortars. Defect Diffus. Forum 2018, 382, 254–260. [Google Scholar] [CrossRef]
- Abro, F.U.R.; Buller, A.S.; Lee, K.-M.; Jang, S.Y. Using the Steady-State Chloride Migration Test to Evaluate the Self-Healing Capacity of Cracked Mortars Containing Crystalline, Expansive, and Swelling Admixtures. Materials 2019, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, B.; Chen, X.; Fu, B.; Wei, H.; Xiang, X. Synergetic effect of superabsorbent polymer (SAP) and crystalline admixture (CA) on mortar macro-crack healing. Constr. Build. Mater. 2020, 247, 118521. [Google Scholar] [CrossRef]
- Elsalamawy, M.; Mohamed, A.R.; Abosen, A.E. Performance of crystalline forming additive materials in concrete. Constr. Build. Mater. 2020, 230, 117056. [Google Scholar] [CrossRef]
- Gupta, R.; Biparva, A. Do crystalline water proofing admixtures affect restrained plastic shrinkage behavior of concrete? Revista ALCONPAT 2017, 7, 15. [Google Scholar] [CrossRef]
- Gupta, R.; Biparva, A. Innovative Test Technique to Evaluate “Self-Sealing” of Concrete. J. Test. Eval. 2015, 43, 20130285. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R.; Biparva, A. Assessment of self-healing and durability parameters of concretes incorporating crystalline admixtures and Portland Limestone Cement. Cement Concr. Compos. 2019, 99, 17–31. [Google Scholar] [CrossRef]
- Azarsa, P. Multi-Scale Investigation and Resistivity-Based Durability Modeling of EShC Containing Crystalline Admixtures. Ph.D. Thesis, University of Victoria, Victoria, BC, USA, 2018. [Google Scholar]
- Azarsa, P.; Gupta, R. Specimen preparation for nano-scale investigation of cementitious repair material. Micron 2018, 107, 43–54. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Electrical Resistivity of Concrete for Durability Evaluation: A Review. Adv. Mater. Sci. Eng. 2017, 2017, 1–30. [Google Scholar] [CrossRef]
- El-Newihy, A.; Azarsa, P.; Gupta, R.; Biparva, A. Effect of Polypropylene Fibers on Self-Healing and Dynamic Modulus of Elasticity Recovery of Fiber Reinforced Concrete. Fibers 2018, 6, 9. [Google Scholar] [CrossRef]
- Gupta, R.; Biparva, A.; Azarsa, P. Concrete International; American Concrete Institute (ACI): Michigan, MI, USA, 2019; pp. 31–34. [Google Scholar]
- Azarsa, P.; Gupta, R.; Biparva, A. Crystalline Waterproofing Admixtures Effects on Self-healing and Permeability of Concrete. In Proceedings of the International Conference on New Horizons in Green Civil Engineering, University of Victoria, Victoria, BC, Canada, 25–27 April 2018. [Google Scholar]
- Azarsa, P.; Gupta, R. Detection and Characterization of Surface Cracks and Defects in Concrete Structures Using Various NDTs; Canadian Society of Civil Engineering (CSCE): Vancouver, BC, USA, 2017. [Google Scholar]
- Biparva, A.; Gupta, R.; Azarsa, P. Advancements in Evaluation of the Self-Sealing Properties of Permeability Reducing Admixtures; ACI: Detroit, MI, USA, 2017. [Google Scholar]
- Cappellesso, V.G.; dos Santos Petry, N.; Dal Molin, D.C.C.; Masuero, A.B. Use of crystalline waterproofing to reduce capillary porosity in concrete. J. Build. Pathol. Rehabil. 2016, 1, 9. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Novel approach to microscopic characterization of cryo formation in air voids of concrete. Micron 2019, 122, 21–27. [Google Scholar] [CrossRef]
- DIN 1048. Testing Concrete: Testing of Hardened Concrete (Specimens Prepared in Mould); German Institute for Standardisation (Deutscher Ausschuß für Stahlbeton of the Normenausschuß Bauwesen): Berlin, Germany, 1991. [Google Scholar]
- ASTM C150. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- CSA. A23.1-14 Standard for Concrete Materials and Methods of Concrete Construction; Canadian Standards Association: Toronto, ON, Canada, 2014. [Google Scholar]
- ASTM C33/C33M. Standard Specification for Concrete Aggregates; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C128. Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM C305. Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Winter, N.B. Scanning Electron Microscopy of Cement and Concrete; WHD Microanalysis: Woodbridge, Canada, 2012; ISBN 978-0-9571045-1-8. [Google Scholar]
- National Institutes of Health. ImajeJ; National Institutes of Health: Bethesda, Maryland, MI, USA, 2018. [Google Scholar]
- Sahu, S.; Badger, S.; Thaulow, N.; Lee, R.J. Determination of water–cement ratio of hardened concrete by scanning electron microscopy. Cement Concr. Compos. 2004, 26, 987–992. [Google Scholar] [CrossRef]
- Wong, H.S.; Head, M.K.; Buenfeld, N.R. Pore segmentation of cement-based materials from backscattered electron images. Cement Concr. Res. 2006, 36, 1083–1090. [Google Scholar] [CrossRef]
- Ridler, T.W.; Calvard, S. Picture Thresholding Using an Iterative Selection Method. IEEE Trans. Syst. Man Cybern. 1978, 8, 630–632. [Google Scholar]
- Gabriel Landini ImageJ Software—Auto-Threshold. Available online: https://imagej.net/Auto_Threshold (accessed on 15 February 2017).
- Kong, D.; Huang, S.; Corr, D.; Yang, Y.; Shah, S.P. Whether do nano-particles act as nucleation sites for C-S-H gel growth during cement hydration? Cement Concr. Compos. 2018, 87, 98–109. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Ghiasvand, E.; Nickseresht, I.; Mahdikhani, M.; Moodi, F. Influence of various amounts of limestone powder on performance of Portland limestone cement concretes. Cement Concr. Compos. 2009, 31, 715–720. [Google Scholar] [CrossRef]
- Hassett, D.J.; McCarthy, G.J.; Kumarathasan, P.; Pflughoeft-Hassett, D. Synthesis and characterization of selenate and sulfate-selenate ettringite structure phases. Mater. Res. Bull. 1990, 25, 1347–1354. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Sánchez Moreno, M.; Carballosa, P.; Pedrosa, F.; Tavares, F. Improvement of the Concrete Permeability by Using Hydrophilic Blended Additive. Materials 2019, 12, 2384. [Google Scholar] [CrossRef]
- Newman, J.; Choo, B.S. (Eds.) Advanced Concrete Technology; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands; Boston, MA, USA, 2003; ISBN 978-0-7506-5103-5. [Google Scholar]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.W.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cement Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003; ISBN 978-0-13-064632-3. [Google Scholar]
- Le Saoût, G.; Lothenbach, B.; Hori, A.; Higuchi, T.; Winnefeld, F. Hydration of Portland cement with additions of calcium sulfoaluminates. Cement Concr. Res. 2013, 43, 81–94. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cement Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Brown, P.; Taylor, H. Materials Science of Concrete: Sulfate Attack Mechanisms; Marchand, J., Skalny, J.P., Eds.; American Ceramic Society: Westerville, OH, USA, 1999; pp. 73–97. [Google Scholar]
- Clifton, J.; Pommersheim, J. Sulfate Attack of Cementitious Materials: Volumetric Relations and Expansions; NIST Interagency Report 5390; National Institute Standards and Technology: Gaithersburg, MD, USA, 1994. [Google Scholar]
- Gollop, R.; Taylor, H. Microstructural and microanalytical studies of sulfate attack. I. Ordinary Portland cement paste. Cement Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Baur, I.; Keller, P.; Mavrocordatos, D.; Wehrli, B.; Johnson, C.A. Dissolution-precipitation behaviour of ettringite, monosulfate, and calcium silicate hydrate. Cement Concr. Res. 2004, 34, 341–348. [Google Scholar] [CrossRef]
| Physical Properties | ||||
|---|---|---|---|---|
| K Admixture | P Admixture | X Admixture | Cement | |
| Color | Gray | Gray | Gray | Gray to gray-brown |
| Texture | Powder | Powder | Powder | Granular Solid |
| Particle size | 40–150 µm | NR | NR | NR |
| Specific Gravity | 2.6–3.0 | NR | 2.0–2.8 | 3.15 |
| pH | 12–14 | 10–13 | 9.1–9.8 | 13 |
| Solids | 100% | 100% | 100% | 100% |
| Chemical Properties | ||||
| Portland Cement | 28%–40% | 65%–80% | 35%–60% | - |
| Silica Sand | 30%–40% | - | 30%–40% | - |
| Alkaline Earth Compounds (Calcium dihydroxide) | - | - | 5%–20% | - |
| Calcium magnesium hydroxide | - | 1.5%–6% | - | - |
| Calcium magnesium hydroxide oxide | - | 1.5%–6% | - | - |
| Calcium hydroxide | 5%–20% | 1%–2% | - | - |
| Proprietary Portion | NR | 15%–40% | NR | - |
| Phase | Mix ID | Materials | Curing Age (Days) | ||
|---|---|---|---|---|---|
| Mix Proportions (kg/m3) | |||||
| Cement | Fine Aggregate | Water | |||
| Phase I and II | O (OPC) | 736 | 2207 | 368 | 28 |
| O-K | |||||
| L (PLC) | |||||
| L-K | |||||
| Phase III | O (Control) | 56 | |||
| O-K | |||||
| O-P | |||||
| O-X | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarsa, P.; Gupta, R.; Biparva, A. Inventive Microstructural and Durability Investigation of Cementitious Composites Involving Crystalline Waterproofing Admixtures and Portland Limestone Cement. Materials 2020, 13, 1425. https://doi.org/10.3390/ma13061425
Azarsa P, Gupta R, Biparva A. Inventive Microstructural and Durability Investigation of Cementitious Composites Involving Crystalline Waterproofing Admixtures and Portland Limestone Cement. Materials. 2020; 13(6):1425. https://doi.org/10.3390/ma13061425
Chicago/Turabian StyleAzarsa, Pejman, Rishi Gupta, and Alireza Biparva. 2020. "Inventive Microstructural and Durability Investigation of Cementitious Composites Involving Crystalline Waterproofing Admixtures and Portland Limestone Cement" Materials 13, no. 6: 1425. https://doi.org/10.3390/ma13061425
APA StyleAzarsa, P., Gupta, R., & Biparva, A. (2020). Inventive Microstructural and Durability Investigation of Cementitious Composites Involving Crystalline Waterproofing Admixtures and Portland Limestone Cement. Materials, 13(6), 1425. https://doi.org/10.3390/ma13061425







