Dynamic Observation of Interfacial IMC Evolution and Fracture Mechanism of Sn2.5Ag0.7Cu0.1RE/Cu Lead-Free Solder Joints during Isothermal Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Soldering
2.2. Isothermal Aging
2.3. Characterization Methods
3. Results and Discussion
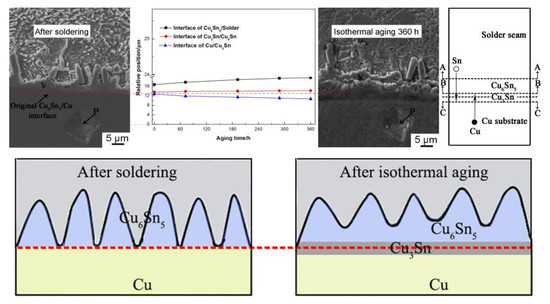
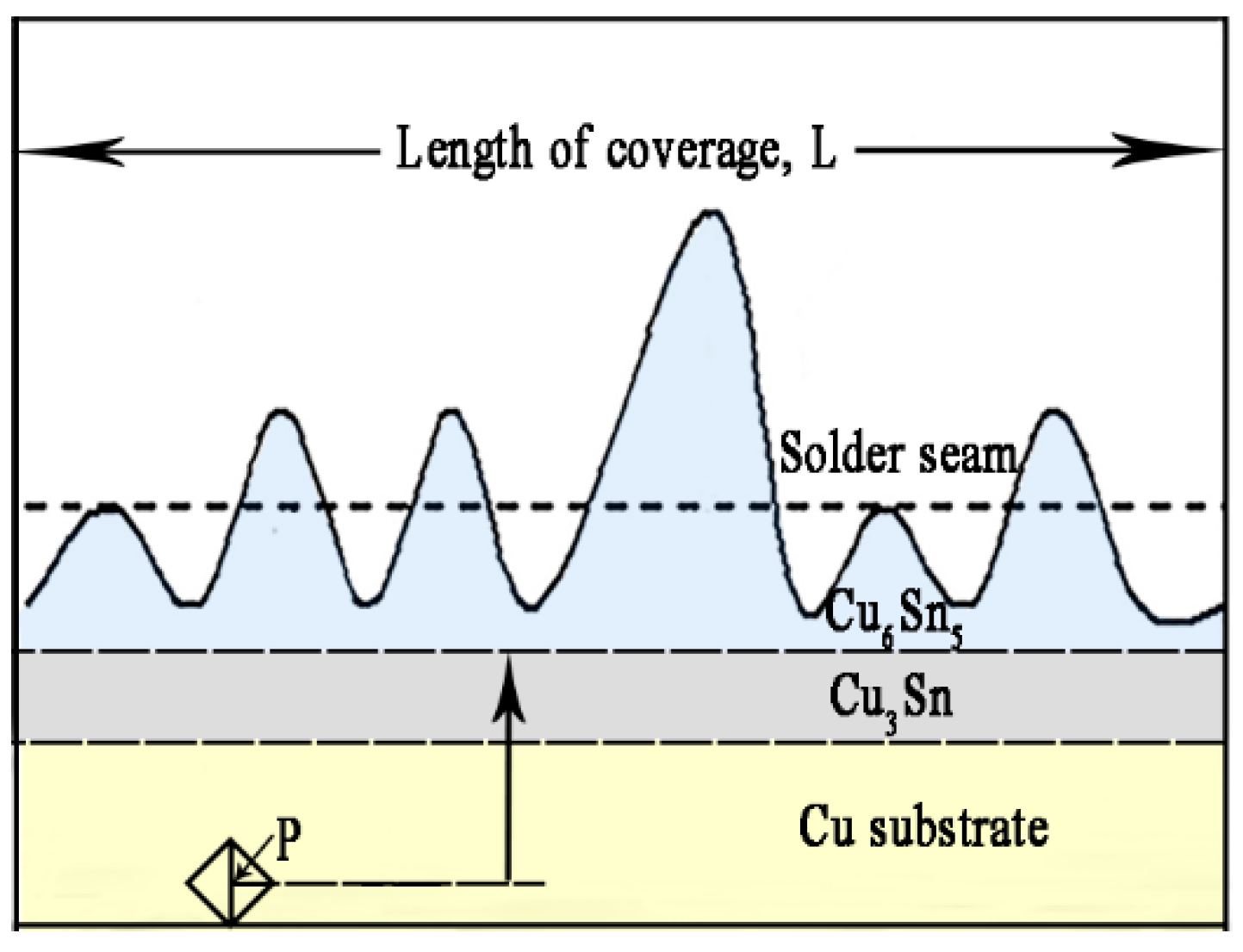
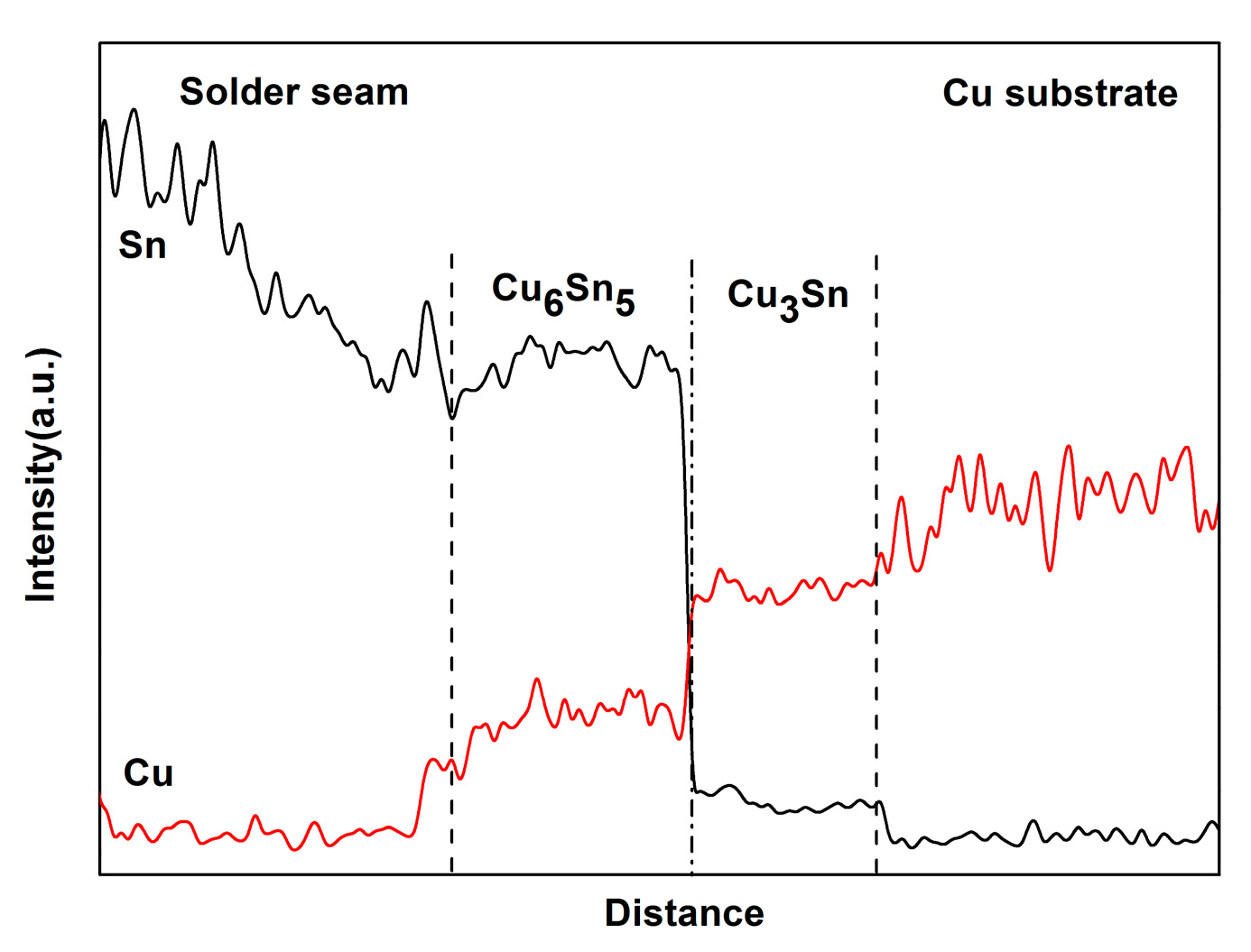
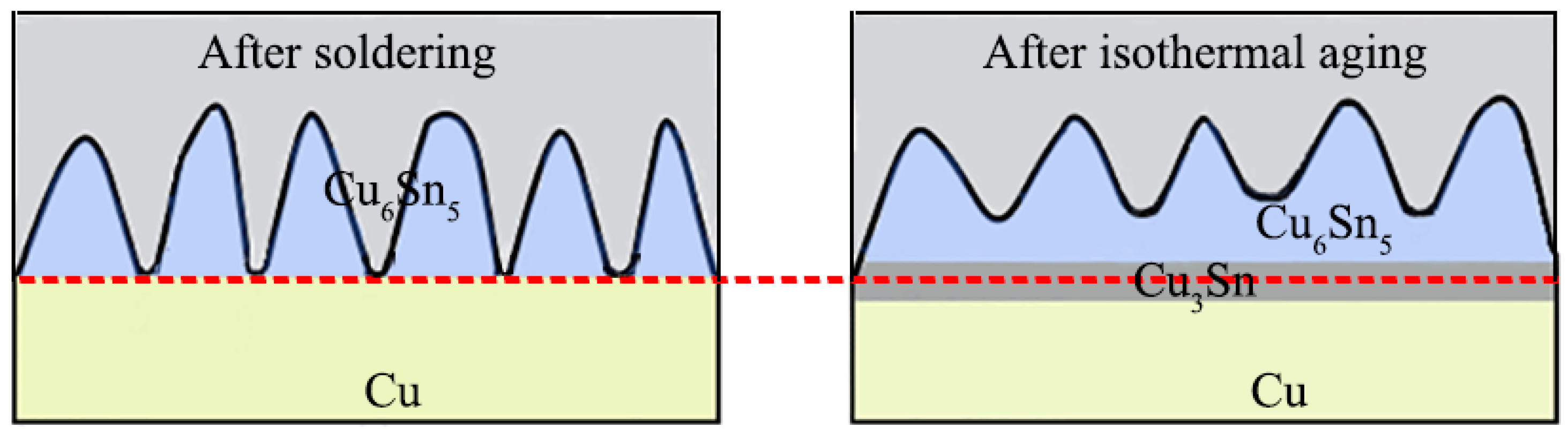
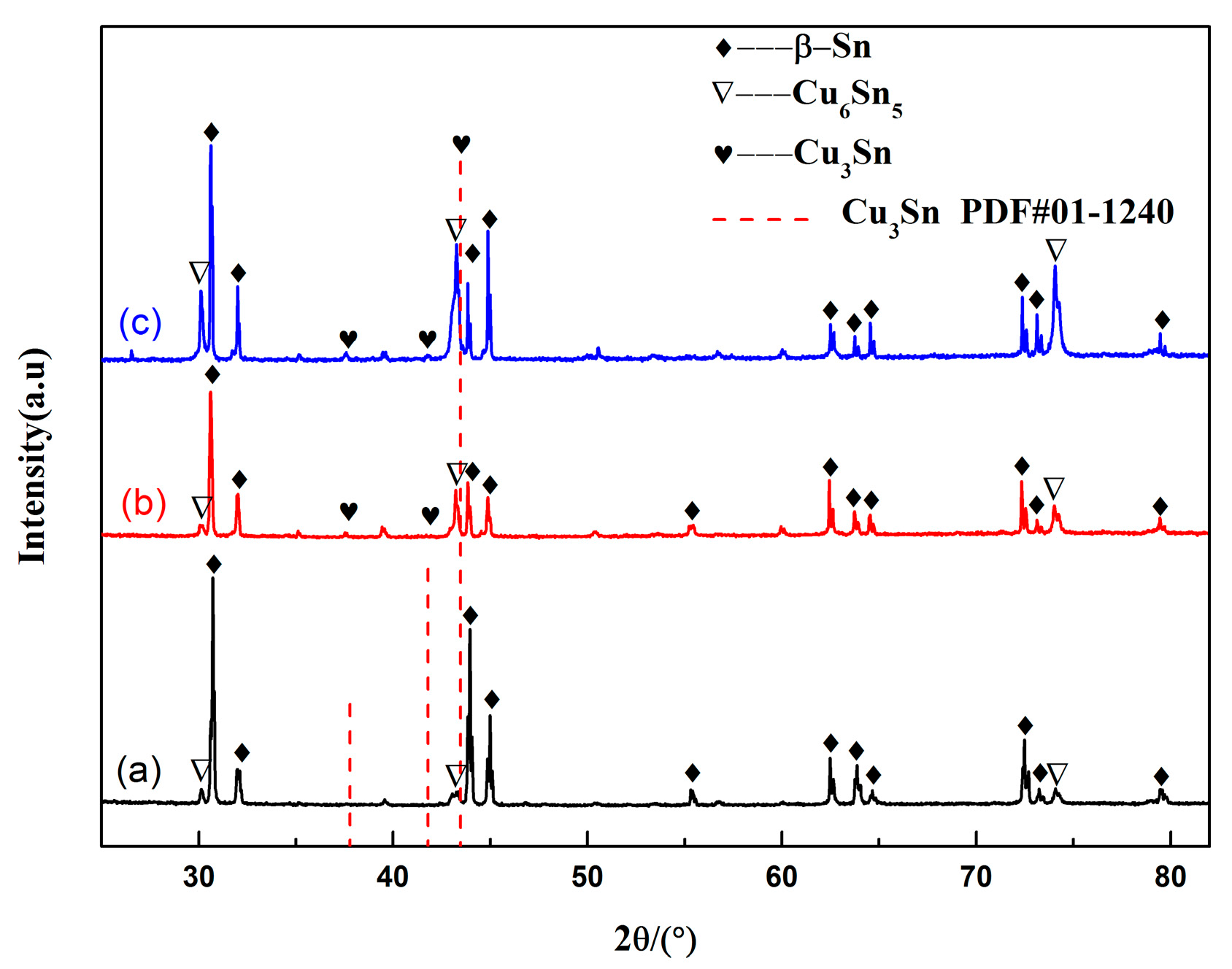
3.1. Interfacial IMC Evolution of the Sn2.5Ag0.7Cu0.1RE/Cu Solder Joints during Isothermal Aging
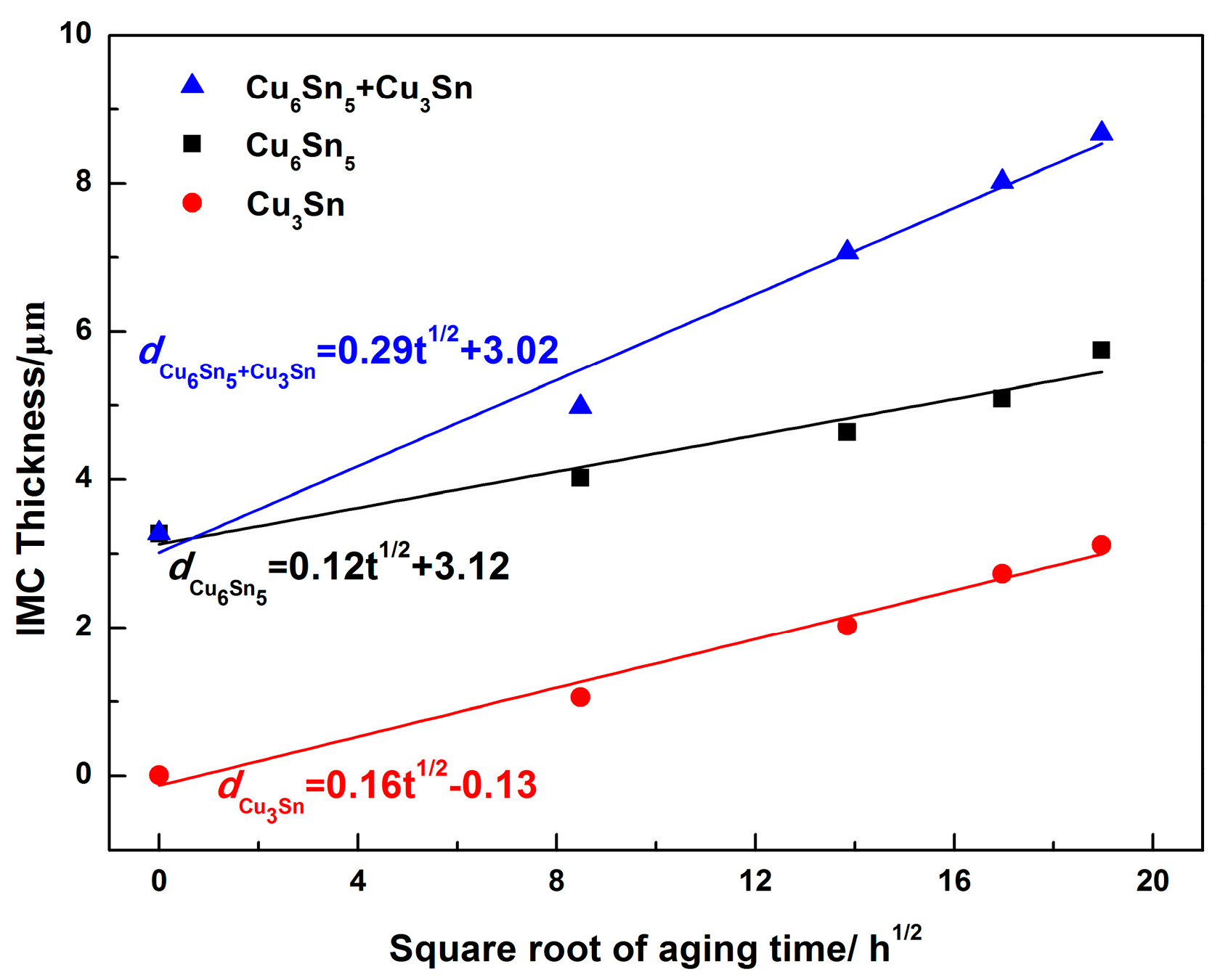
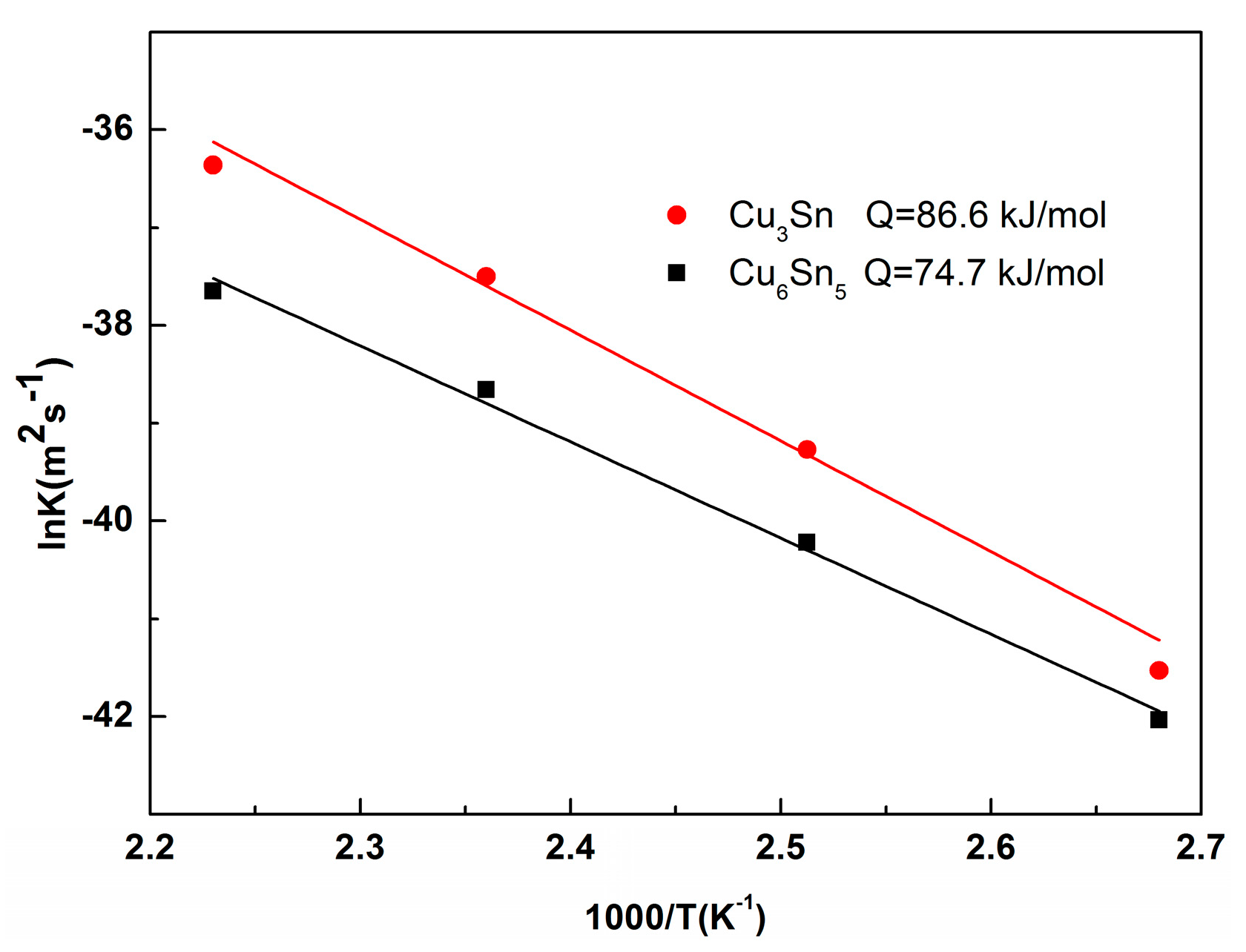
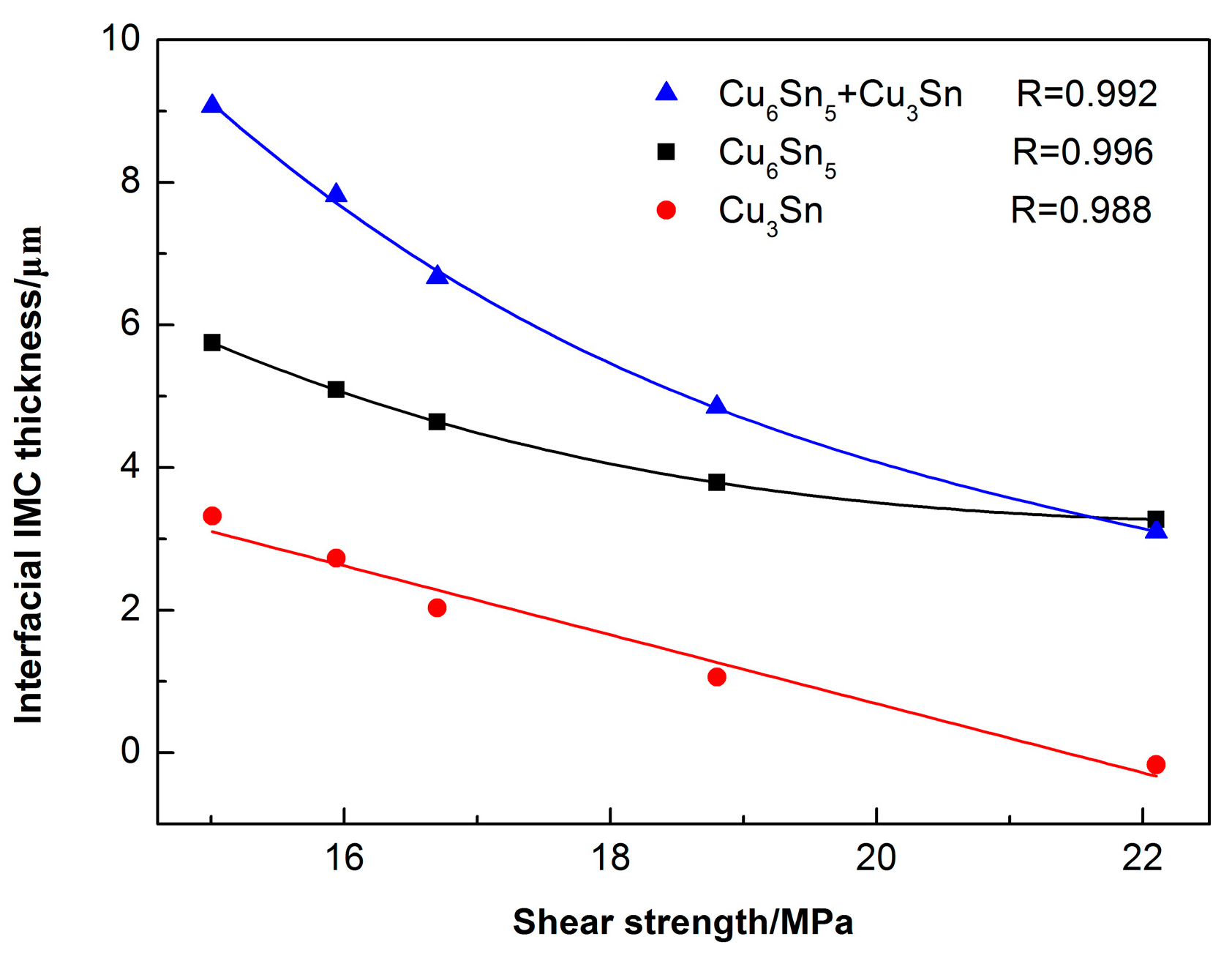
3.2. Interfacial IMC Growth Kinetics of the Sn2.5Ag0.7Cu0.1RE/Cu Solder Joints during Isothermal Aging
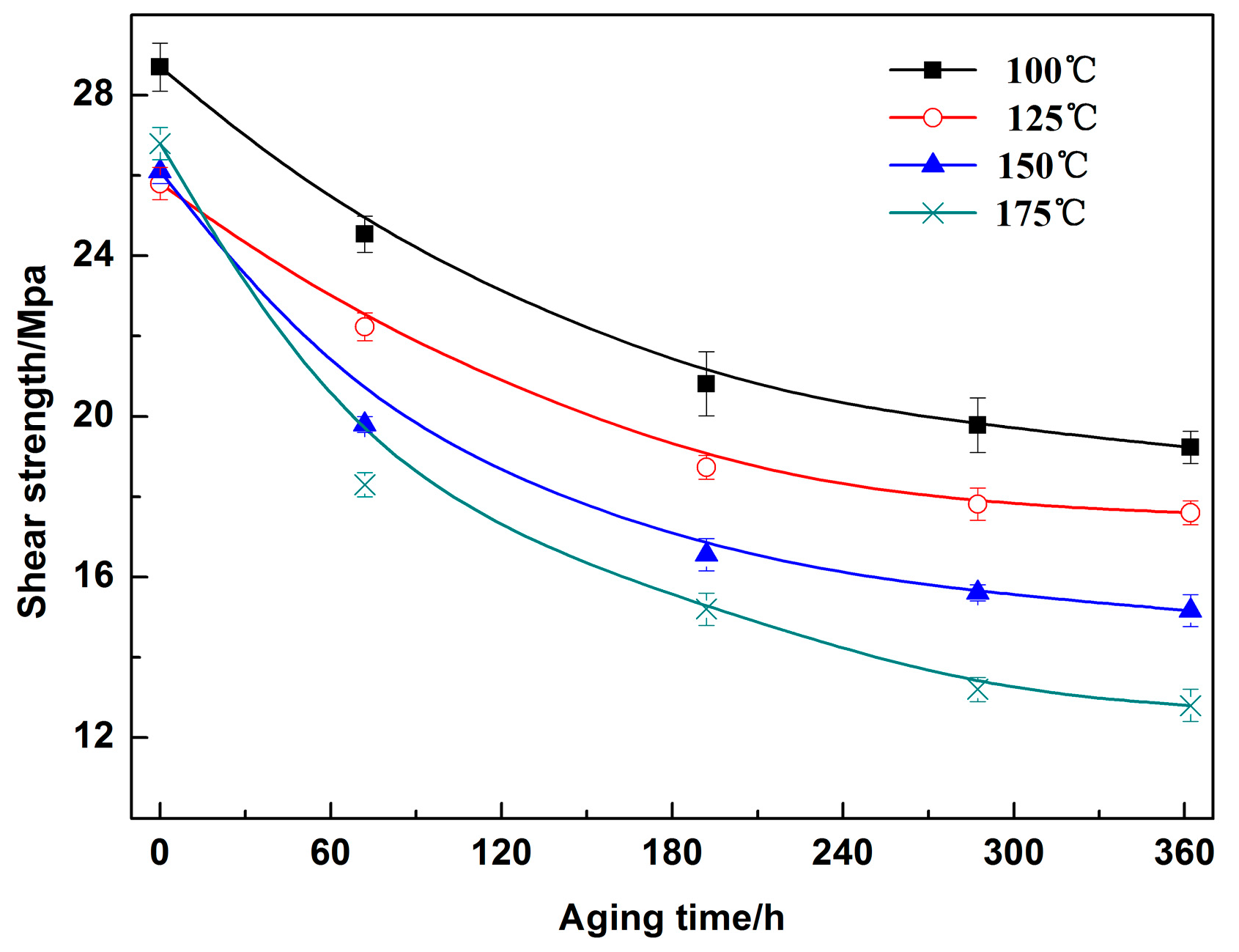
3.3. Mechanical Properties of the Sn2.5Ag0.7Cu0.1RE/Cu Solder Joints during Isothermal Aging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teo, J.W.R.; Sun, Y.F. Spalling behavior of interfacial intermetallic compounds in Pb-free solder joints subjected to temperature cycling loading. Acta Mater. 2008, 56, 242–249. [Google Scholar] [CrossRef]
- Hu, X.; Li, C.; Li, Q.; Yi, G. Insights on interfacial IMCs growth and mechanical strength of asymmetrical Cu/SAC305/Cu-Co system. Vacuum 2019, 167, 77–89. [Google Scholar] [CrossRef]
- Wang, F.J.; Chen, H.; Huang, Y.; Liu, L.T.; Zhang, Z.J. Recent progress on the development of Sn-Bi based low-temperature Pb-free solders. J. Mater. Sci.-Mater. Electron. 2019, 30, 3222–3243. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Zhang, M. Fabrication and properties of Ni-modified graphene nanosheets reinforced Sn-Ag-Cu composite solder. J. Alloys Compd. 2019, 781, 761–772. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Quadir, M.Z. Thermal aging effects on microstructures and mechanical properties of an environmentally friendly eutectic tin-copper solder alloy. Mater. Des. 2016, 110, 275–283. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.K.; Huo, F.P.; Wang, H.G.; Wang, Y. Microstructures and Properties of Sn2.5Ag0.7Cu0.1RE Composite Solders Reinforced with Cu-Coated Graphene Nanosheets Synthesized by Pyrolysis. Materials 2019, 12, 289. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.B.; Wang, J.W.; Liu, S.; Wang, L.J. Recent progress of Sn-Ag-Cu lead-free solders bearing alloy elements and nanoparticles in electronic packaging. J. Mater. Sci. Mater. Electron. 2016, 27, 1–35. [Google Scholar] [CrossRef]
- Ma, C.L.; Xue, S.B.; Wang, B.; Long, W.M.; Zhong, S.J. Effects of Ga and Ce on the Microstructure and Properties of Cadmium-free Silver Filler Metals. Rare Metal. Mat. Eng. 2019, 48, 91–96. [Google Scholar]
- Wang, B.; Xue, S.B.; Wang, J.X.; Long, W.M.; Zhang, Q.K. Effect of Rare Earth Pr on Creep Behavior of Sn-0.3Ag-0.7Cu-0.5Ga Low-Ag Solder Alloys. Rare Met. Mater. Eng. 2018, 47, 2657–2662. [Google Scholar]
- Han, Y.; Xue, S.; Yang, J.; Long, W.; Zhang, Q. Effects of trace amount praseodymium and neodymium on microstructure and mechanical properties of Sn−0.3Ag−0.7Cu−0.5Ga solder. J. Mater. Sci. Mater. Electron. 2016, 27, 351–358. [Google Scholar] [CrossRef]
- Dudek, M.A.; Chawla, N. Effect of Rare-Earth (La, Ce, and Y) Additions on the Microstructure and Mechanical Behavior of Sn−3.9Ag−0.7Cu Solder Alloy. Metall. Mater. Trans. A 2010, 41, 610–620. [Google Scholar] [CrossRef]
- Law, C.M.T.; Wu, C.M.L.; Yu, D.Q.; Wang, L.; Lai, J.K.L. Microstructure, solderability, and growth of intermetallic compounds of Sn-Ag-Cu-RE lead-free solder alloys. J. Electron. Mater. 2006, 35, 89–93. [Google Scholar] [CrossRef]
- Yu, D.Q.; Zhao, J.; Wang, L. Improvement on the microstructure stability, mechanical and wetting properties of Sn-Ag-Cu lead-free solder with the addition of rare earth elements. J. Alloys Compd. 2004, 376, 170–175. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Yu, Y.; Wang, Y.; Fan, Y.; Cheng, G.; Han, L. Wetting match performance of SnAgCuRE lead-free solder for surface mount component. Chin. J. Nonferrous Met. 2006, 16, 1908–1912. [Google Scholar]
- Zhang, K.; Wang, Y.; Fan, Y.; Zhu, Y.; Zhang, X. Effect of Ce-La Mixed Rare Earth Content and Environment Conditions on the Creep Rupture Life of SnAgCu Solder Joints. Rare Metal. Mat. Eng. 2007, 36, 1473–1476. [Google Scholar]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Harsh service environment effects on the microstructure and mechanical properties of Sn-Ag-Cu-1 wt% nano-Al solder alloy. J. Mater. Sci. Mater. Electron. 2016, 27, 11273–11283. [Google Scholar] [CrossRef]
- Cao, C.C.; Zhang, K.K.; Shi, B.J.; Wang, H.G.; Zhao, D.; Sun, M.M.; Zhang, C. The Interface Microstructure and Shear Strength of Sn2.5Ag0.7Cu0.1RExNi/Cu Solder Joints under Thermal-Cycle Loading. Metals 2019, 7, 518. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J.; He, C.; Guo, Y. Reliability behavior of lead-free solder joints in electronic components. J. Mater. Sci. Mater. Electron. 2013, 24, 172–190. [Google Scholar] [CrossRef]
- Xu, T.; Hu, X.; Li, Y.; Jiang, X. The growth behavior of interfacial intermetallic compound between Sn−3.5Ag−0.5Cu solder and Cu substrate under different thermal-aged conditions. J. Mater. Sci. Mater. Electron. 2017, 25, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, X.; He, C.; Guo, Y. Intermetallic compound layer growth between SnAgCu solder and Cu;substrate in electronic packaging. J. Mater. Sci. Mater. Electron. 2013, 24, 3249–3254. [Google Scholar] [CrossRef]
- Hu, X.; Xu, T.; Keer, L.M.; Li, Y.; Jiang, X. Microstructure evolution and shear fracture behavior of aged Sn3Ag0.5Cu/Cu solder joints. Mater. Sci. Eng. A 2016, 673, 167–177. [Google Scholar] [CrossRef]
- Hu, X.; Xu, T.; Keer, L.M.; Li, Y.; Jiang, X. Shear strength and fracture behavior of reflowed Sn3.0Ag0.5Cu/Cu solder joints under various strain rates. J. Alloys Compd. 2017, 690, 720–729. [Google Scholar] [CrossRef]
- Nishikawa, H.; Iwata, N. Formation and growth of intermetallic compound layers at the interface during laser soldering using Sn-Ag Cu solder on a Cu Pad. J. Mater. Process. Technol. 2015, 215, 6–11. [Google Scholar] [CrossRef]
- Kim, D.G.; Jung, S.B. Interfacial reactions and growth kinetics for intermetallic compound layer between In-48Sn solder and bare Cu substrate. J. Alloys Compd. 2005, 386, 151–156. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.F.; Zhou, M.; Liu, Z.Q. The interfacial reaction between In-48Sn solder and polycrystalline Cu substrate during solid state aging. J. Alloys Compd. 2018, 740, 500–509. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Wang, X.; He, P. Microstructural evolution and joint strength of Sn-58Bi/Cu joints through minor Zn alloying substrate during isothermal aging. J. Alloys Compd. 2016, 688, 639–648. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Zhang, Z.; Yan, C. Interfacial Reaction and Mechanical Properties of Sn-Bi Solder joints. Materials 2017, 10, 920. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, K.; Cui, J.; Ma, N.; Pan, Y.; Yin, C. Effect of ultrasonic vibration on the interfacial IMC three-dimensional morphology and mechanical properties of Sn2.5Ag0.7Cu0.1RE0.05Ni/Cu halogen free solder joints. J. Mater. Sci. Mater. Electron. 2018, 29, 18828–18839. [Google Scholar] [CrossRef]
- Huo, F.; Zhang, K.; Zhang, M.; Wang, H. The interfacial intermetallic and shear strength of Ni nanoparticle-decorated reduced graphene oxide reinforced Sn2.5Ag0.5Cu lead-free composite soldering joints. Adv. Eng. Mater. 2018, 20, 1800147–1800155. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Desoky, W.M.; Elmosalami, T.A.; El-Shaarawy, M.G.; Abdraboh, A.M. Microstructural modifications and properties of SiC nanoparticles-reinforced Sn−3.0Ag−0.5Cu solder alloy. Mater. Des. 2015, 65, 1196–1204. [Google Scholar] [CrossRef]
- Cui, J.G.; Zhang, K.K.; Zhao, D.; Ma, N.; Cao, C.C.; Pan, Y.B. Microstructure and properties of Sn2.5Ag0.7Cu0.1RE0.05Ni/Cu solder joints obtained by external energy assisted soldering. Rare Metal. Mat. Eng. 2018, 47, 2800–2806. [Google Scholar]
- Peng, W.; Monlevade, E.; Marques, M.E. Effect of thermal aging on the interfacial structure of SnAgCu solder joints on Cu. Microelectron. Reliab. 2007, 47, 2161–2168. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Jiang, X.; Li, Y. Influences of Mono-Ni(P) and Dual-Cu/Ni(P) Plating on the Interfacial Microstructure Evolution of Solder Joints. Metall. Mater. Trans. A 2019, 50, 480–492. [Google Scholar] [CrossRef]
- Paul, A.; Laurila, T.; Vuorinen, V.; Divinski, S.V. Thermodynamics, Diffusion and the Kirkendall Effect in Solids; Springer: Berlin, Germany, 2014; pp. 115–139. [Google Scholar]
- Deng, X.; Piotrowski, G.; Williams, J.J.; Chawla, N. Influence of initial morphology and thickness of Cu6Sn5 and Cu3Sn intermetallics on growth and evolution during thermal aging of Sn-Ag solder/Cu joints. J. Electron. Mater. 2003, 32, 1403–1413. [Google Scholar] [CrossRef]
- Rizvi, M.J.; Chan, Y.C.; Bailey, C.; Lu, H.; Islam, M.N. Effect of adding 1wt% Bi into the Sn−2.8Ag−0.5Cu solder alloy on the intermetallic formations with Cu-substrate during soldering and isothermal aging. J. Alloys Compd. 2006, 407, 208–214. [Google Scholar] [CrossRef]
- Yu, D.Q.; Wang, L. The growth and roughness evolution of intermetallic compounds of Sn-Ag-Cu/Cu interface during soldering reaction. J. Alloys Compd. 2008, 458, 542–547. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, S.B.; Zeng, G.; Gao, L.L.; Ye, H. Interface reaction between SnAgCu/SnAgCuCe solders and Cu substrate subjected to thermal cycling and isothermal aging. J. Alloys Compd. 2012, 510, 38–45. [Google Scholar] [CrossRef]
- Kim, Y.M.; Roh, H.R.; Kim, S.; Kim, Y.H. Kinetics of Intermetallic Compound Formation at the Interface Between Sn−3.0Ag−0.5Cu Solder and Cu-Zn Alloy Substrates. J. Electron. Mater. 2010, 39, 2504–2512. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Tan, A.T.; Tan, A.W.; Yusof, F. Influence of nanoparticle addition on the formation and growth of intermetallic compounds (IMCs) in Cu/Sn–Ag–Cu/Cu solder joint during different thermal conditions. Sci. Technol. Adv. Mater. 2015, 16, 033505. [Google Scholar]
- Shen, J.; Zhao, M.; He, P.; Pu, Y. Growth behaviors of intermetallic compounds at Sn−3Ag−0.5Cu/Cu interface during isothermal and non-isothermal aging. J. Alloys Compd. 2013, 574, 451–458. [Google Scholar] [CrossRef]

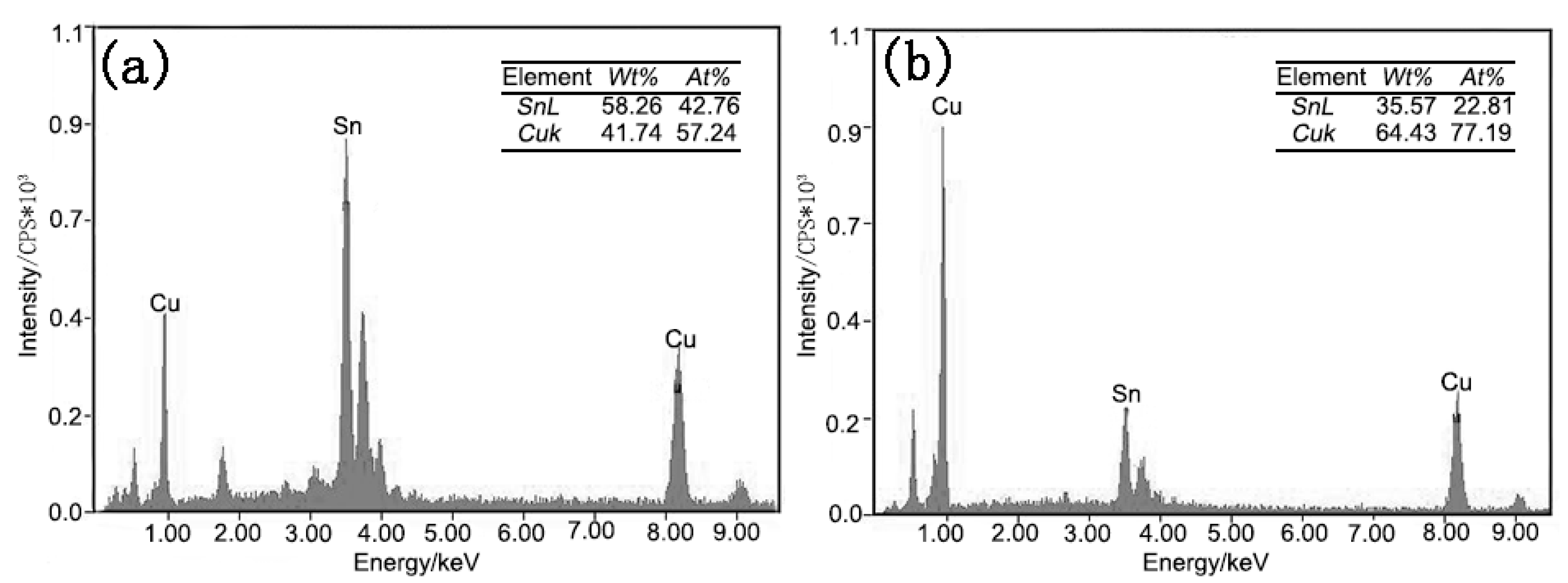
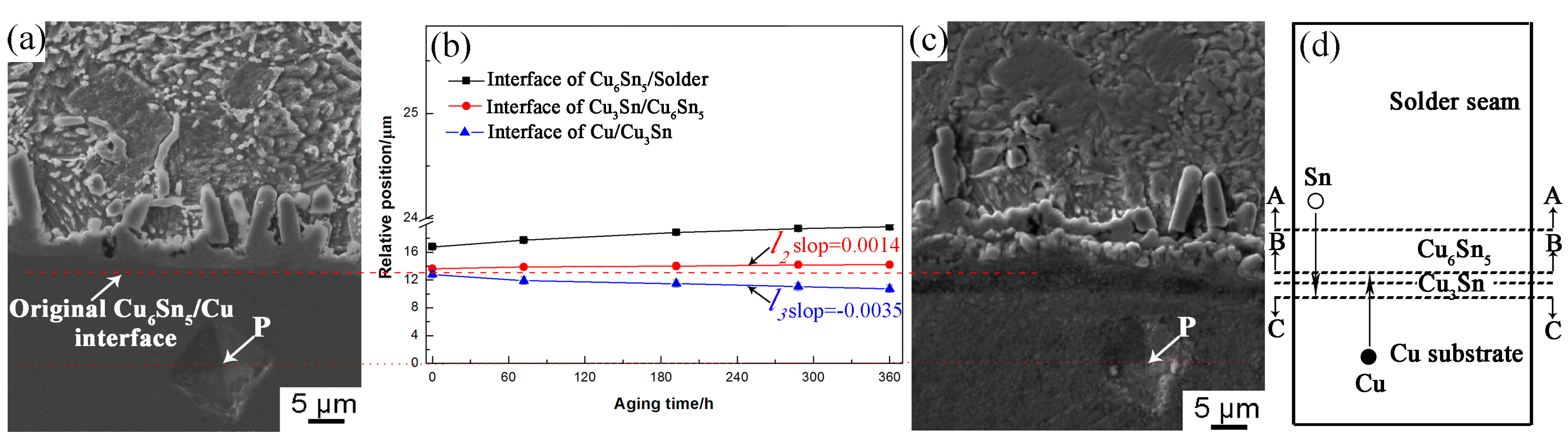



| Area | Mole Fraction/% | ||
|---|---|---|---|
| Sn | Cu | Ag | |
| A | 93.49 | 5.34 | 1.18 |
| B | 24.72 | 75.28 | - |
| C | 46.64 | 53.36 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zhang, K.; Ma, N.; Li, S.; Yin, C.; Huo, F. Dynamic Observation of Interfacial IMC Evolution and Fracture Mechanism of Sn2.5Ag0.7Cu0.1RE/Cu Lead-Free Solder Joints during Isothermal Aging. Materials 2020, 13, 831. https://doi.org/10.3390/ma13040831
Zhao D, Zhang K, Ma N, Li S, Yin C, Huo F. Dynamic Observation of Interfacial IMC Evolution and Fracture Mechanism of Sn2.5Ag0.7Cu0.1RE/Cu Lead-Free Solder Joints during Isothermal Aging. Materials. 2020; 13(4):831. https://doi.org/10.3390/ma13040831
Chicago/Turabian StyleZhao, Di, Keke Zhang, Ning Ma, Shijie Li, Chenxiang Yin, and Fupeng Huo. 2020. "Dynamic Observation of Interfacial IMC Evolution and Fracture Mechanism of Sn2.5Ag0.7Cu0.1RE/Cu Lead-Free Solder Joints during Isothermal Aging" Materials 13, no. 4: 831. https://doi.org/10.3390/ma13040831
APA StyleZhao, D., Zhang, K., Ma, N., Li, S., Yin, C., & Huo, F. (2020). Dynamic Observation of Interfacial IMC Evolution and Fracture Mechanism of Sn2.5Ag0.7Cu0.1RE/Cu Lead-Free Solder Joints during Isothermal Aging. Materials, 13(4), 831. https://doi.org/10.3390/ma13040831