Laser Fabrication of Anti-Icing Surfaces: A Review
Abstract
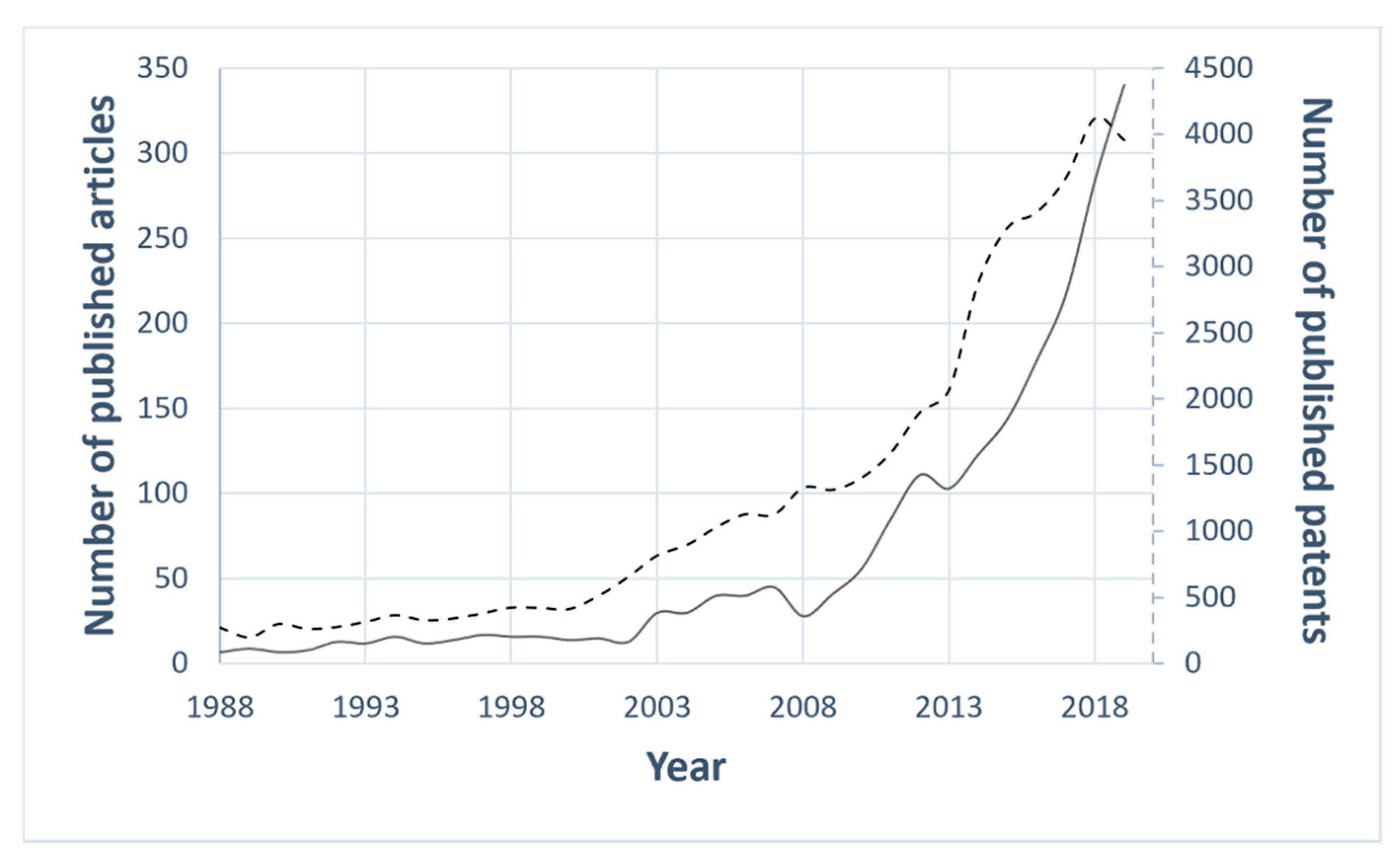
1. Introduction
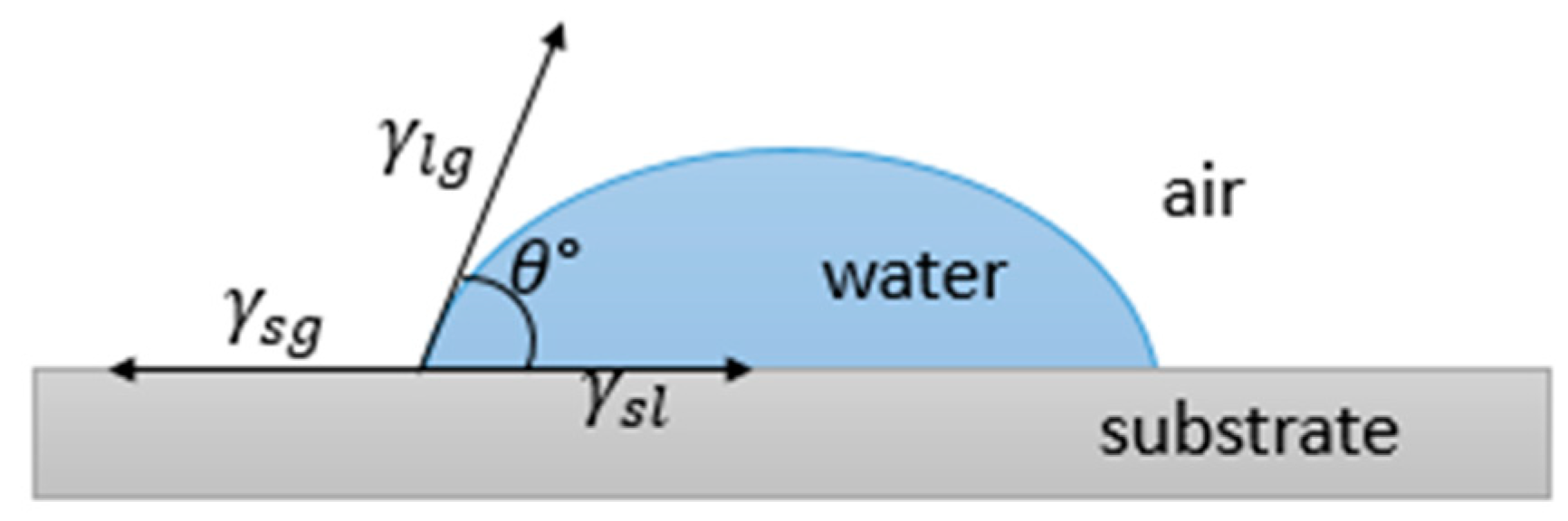
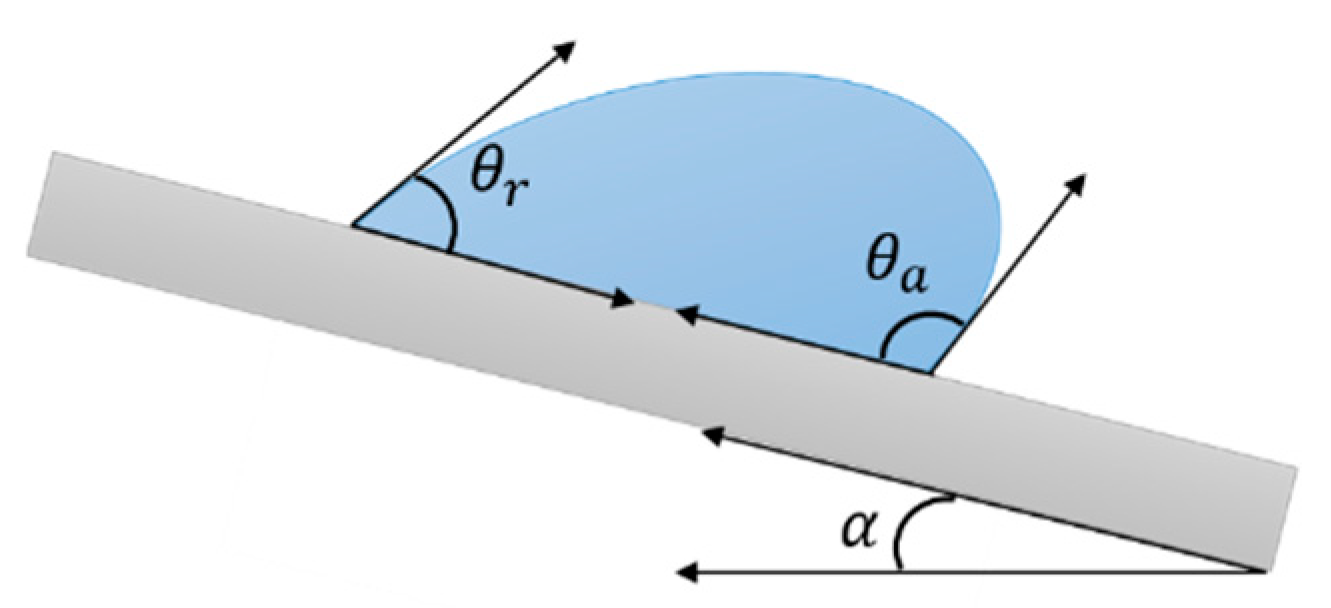
2. Surface Wettability
Key Concepts
3. Anti-Icing Properties
- icing delay, intended both as a time delay in the solidification of the water and as a reduction in the threshold temperature for freezing. A lower threshold for the formation of ice would, indeed, allow wider range of operating temperature conditions. Similarly, a postponement of freezing would increase the probability of water removal before solidification.
- interaction between water and the solid surface. That is, if before freezing occurs, water droplets were to roll off the surface, there would be no chance of ice being formed. This phenomenon is strictly correlated with the sliding angle.
- ice adhesion. Although ice is formed, its adhesion should be minimized, so that a small external force or, e.g., in case of a wind turbine, the centrifugal force of its own blades, is sufficient to remove the frozen layer, facilitating the de-icing process.
3.1. Icing Delay Time
3.2. Short Contact Time Interation
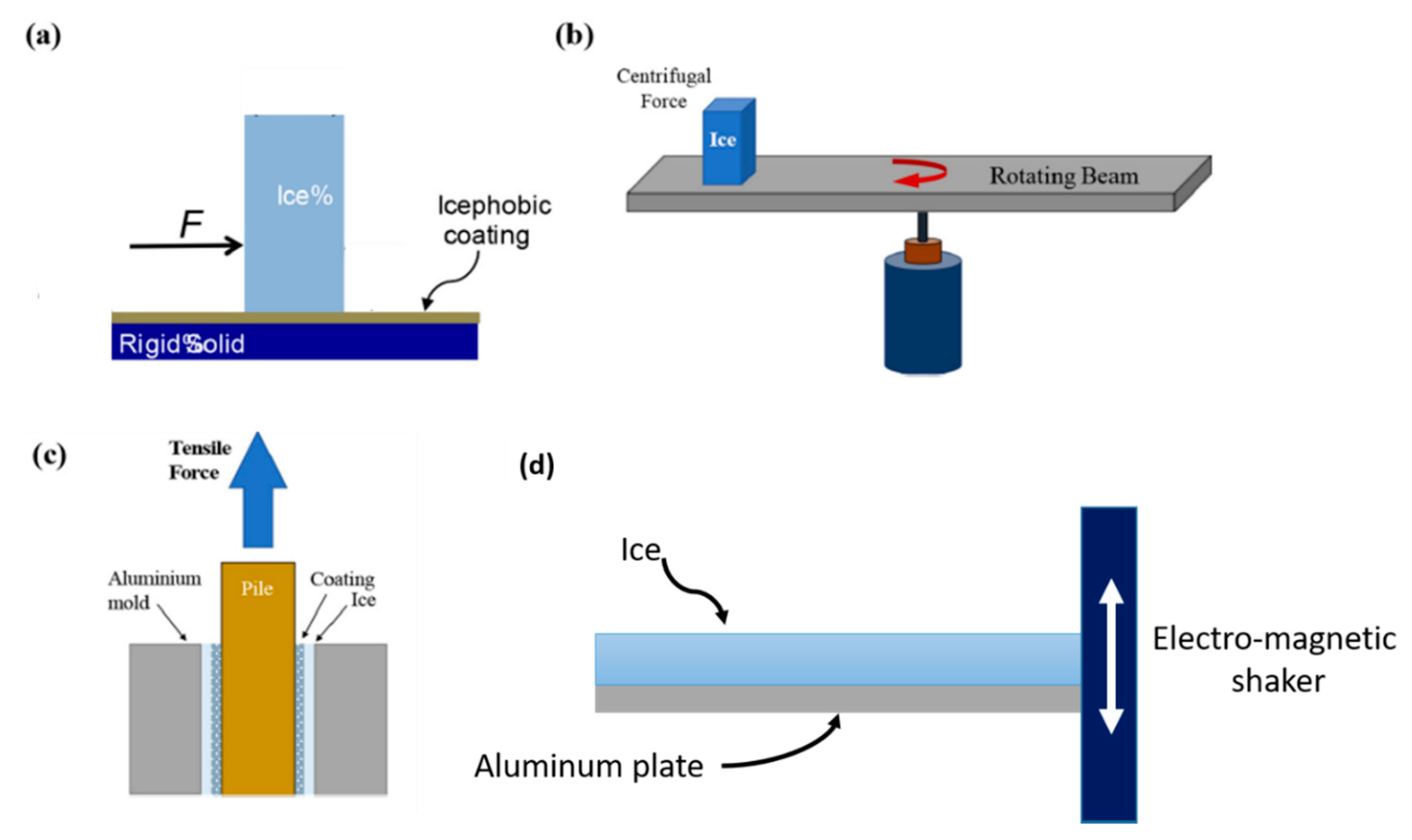
3.3. Ice Ahdesion on a Surface
4. Fabrication of Superhydrophobic Surfaces with Anti-Icing Properties
4.1. Non-Laser Methods
4.2. Laser Micro-/Nano-Structuring of Surfaces
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frankenstein, S.; Tuthill, A.M. Ice adhesion to locks and dams: Past work; future directions? J. Cold Reg. Eng. 2002, 16, 83–96. [Google Scholar] [CrossRef]
- Fillion, R.M.; Riahi, A.R.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Lynch, F.T.; Khodadoust, A. Effects of ice accretions on aircraft aerodynamics. Prog. Aerosp. Sci. 2001, 37, 669–767. [Google Scholar] [CrossRef]
- Vercillo, V.; Karpen, N.; Laroche, A.; Mayén Guillén, J.A.; Tonnicchia, S.; de Andrade Jorge, R.; Bonaccurso, E. Analysis and modelling of icing of air intake protection grids of aircraft engines. Cold Reg. Sci. Technol. 2019, 160, 265–272. [Google Scholar] [CrossRef]
- Song, M.; Dong, J.; Wu, C.; Jiang, Y.; Qu, M. Improving the frosting and defrosting performance of air source heat pump units: Review and outlook. HKIE Trans. Hong Kong Inst. Eng. 2017, 24, 88–98. [Google Scholar] [CrossRef]
- Laforte, J.L.; Allaire, M.A.; Laflamme, J. State-of-the-art on power line de-icing. Atmos. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Xiang, T.; Lv, Z.; Wei, F.; Liu, J.; Dong, W.; Li, C.; Zhao, Y.; Chen, D. Superhydrophobic civil engineering materials. Coatings 2019, 9, 753. [Google Scholar] [CrossRef]
- Ryerson, C.C. Ice protection of offshore platforms. Cold Reg. Sci. Technol. 2011, 65, 97–110. [Google Scholar] [CrossRef]
- Fang, Y.; Yong, J.; Chen, F.; Huo, J.; Yang, Q.; Zhang, J.; Hou, X. Bioinspired fabrication of Bi/Tridirectionally anisotropic sliding superhydrophobic PDMS surfaces by femtosecond laser. Adv. Mater. Interfaces 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3170. [Google Scholar] [CrossRef] [PubMed]
- Kotker, D. Training Recommendations and Background Information for De-Icing /Anti-Icing of Aeroplane on the Ground; Technical Report for AEA Publications; AEA Publications: Pittsburgh, PA, USA, August 2015. [Google Scholar]
- Thomas, S.K.; Cassoni, R.P.; MacArthur, C.D. Aircraft anti-icing and de-icing techniques and modeling. J. Aircr. 1996, 33, 841–854. [Google Scholar] [CrossRef]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F.; Wetterwald, M.; Lasagni, A.F.; Bonaccurso, E.; Kunze, T. Self-limited ice formation and efficient de-icing on superhydrophobic micro-structured airfoils through direct laser interference patterning. Adv. Mater. Interfaces 2020, 2001231, 1–10. [Google Scholar] [CrossRef]
- Available online: https://worldwide.espacenet.com/patent/ (accessed on 7 December 2020).
- Hejazi, V.; Sobolev, K.; Nosonovsky, M. From superhydrophobicity to icephobicity: Forces and interaction analysis. Sci. Rep. 2013, 3, 2194. [Google Scholar] [CrossRef] [PubMed]
- Vertuccio, L.; De Santis, F.; Pantani, R.; Lafdi, K.; Guadagno, L. Effective de-icing skin using graphene-based flexible heater. Compos. Part B Eng. 2019, 162, 600–610. [Google Scholar] [CrossRef]
- Farzaneh, M.; Volat, C.; Leblond, A. Anti-icing and de-icing techniques for overhead lines. Atmos. Icing Power Netw. 2008, 229–268. [Google Scholar] [CrossRef]
- Hille, J. Boeing deicing and anti-icing fluid residues. Boeing 2007, 25, 14–21. [Google Scholar]
- Water Spray and High Humidity Endurance Test Methods for AMS1424 and AMS1428 Aircraft Deicing/Anti-Icing Fluids AS5901D; Society of Automotive Engineers International: Warrendale, PA, USA, 2019. [CrossRef]
- Standard Test Method for Aerodynamic Acceptance of SAE AMS1424 and SAE AMS1428 Aircraft Deicing/Anti-icing Fluids; Society of Automotive Engineers International: Warrendale, PA, USA, 2016.
- Yong, J.S. Evaluation of the Environmental Impacts and Alternative Technologies of Deicing/Anti-Icing Operations at Airports. Ph.D. Thesis, University of Illinois at Urbana Champaign, Champaign, IL, USA, 2001. [Google Scholar]
- Goraj, Z. An overview of the deicing and antiicing technologies with prospects for the future. In Proceedings of the 24th International Congress of the Aeronautical Sciences, 29 August–3 September 2004; ICAS: Yokohama, Japan, 2004; pp. 1–11. [Google Scholar]
- Huang, X.; Tepylo, N.; Pommier-budinger, V.; Budinger, M.; Villedieu, P.; Bennani, L.; Huang, X.; Tepylo, N.; Pommier-budinger, V.; Budinger, M.; et al. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications To cite this version: HAL Id: Hal-02024879. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Raji, A.R.O.; Li, Y.; Sikkema, W.K.A.; Tour, J.M. Passive anti-icing and active deicing films. ACS Appl. Mater. Interfaces 2016, 8, 14169–14173. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 86501. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D. From extremelywater-repellent coatings to passive icing protection-principles, limitations and innovative application aspects. Coatings 2020, 10, 66. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. How wetting hysteresis influences ice adhesion strength on superhydrophobic surfaces. Langmuir 2009, 25, 8854–8856. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Paiè, P.; Ancona, A.; Osellame, R. Polymeric fully inertial lab-on-a-chip with enhanced-throughput sorting capabilities. Microfluid. Nanofluidics 2019, 23, 37. [Google Scholar] [CrossRef]
- Ancona, A.; Joshi, G.S.; Volpe, A.; Scaraggi, M.; Lugarà, P.M.; Carbone, G. Non-uniform laser surface texturing of an un-tapered square pad for tribological applications. Lubricants 2017, 5, 41. [Google Scholar] [CrossRef]
- Ancona, A.; Carbone, G.; De Filippis, M.; Volpe, A.; Lugarà, P.M. Femtosecond laser full and partial texturing of steel surfaces to reduce friction in lubricated contact. Adv. Opt. Technol. 2014, 3. [Google Scholar] [CrossRef]
- Putignano, C.; Parente, G.; Profito, F.J.; Gaudiuso, C.; Ancona, A.; Carbone, G. Laser microtextured surfaces for friction reduction: Does the pattern matter? Materials 2020, 13, 4915. [Google Scholar] [CrossRef]
- Gaudiuso, C.; Volpe, A.; Ancona, A. One-step femtosecond laser stealth dicing of quartz. Micromachines 2020, 1, 327. [Google Scholar] [CrossRef]
- Putignano, C.; Scarati, D.; Gaudiuso, C.; Di Mundo, R.; Ancona, A.; Carbone, G. Soft matter laser micro-texturing for friction reduction: An experimental investigation. Tribol. Int. 2019, 136, 82–86. [Google Scholar] [CrossRef]
- Trotta, G.; Vázquez, R.M.; Volpe, A.; Modica, F.; Ancona, A.; Fassi, I.; Osellame, R. Disposable optical stretcher fabricated by microinjection moulding. Micromachines 2018, 9, 388. [Google Scholar] [CrossRef]
- Giannuzzi, G.; Gaudiuso, C.; Di Franco, C.; Scamarcio, G.; Lugarà, P.M.; Ancona, A. Large area laser-induced periodic surface structures on steel by bursts of femtosecond pulses with picosecond delays. Opt. Lasers Eng. 2019, 114, 15–21. [Google Scholar] [CrossRef]
- Butt, H.J.; Golovko, D.S.; Bonaccurso, E. On the derivation of young’s equation for sessile drops: Nonequilibrium effects due to evaporation. J. Phys. Chem. B 2007, 111, 5277–5283. [Google Scholar] [CrossRef] [PubMed]
- Menini, R.; Ghalmi, Z.; Farzaneh, M. Cold regions science and technology highly resistant icephobic coatings on aluminum alloys. Cold Reg. Sci. Technol. 2011, 65, 65–69. [Google Scholar] [CrossRef]
- Bormashenko, E. Physics of solid-liquid interfaces: From the young equation to the superhydrophobicity. Low Temp. Phys. 2016, 42, 622–635. [Google Scholar] [CrossRef]
- Jamil, M.I.; Ali, A.; Haq, F.; Zhang, Q.; Zhan, X.; Chen, F.; Jamil, M.I.; Ali, A.; Haq, F.; Zhang, Q.; et al. Icephobic strategies and materials with superwettability: Design principles and mechanism. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Hongru, A.; Xiangqin, L.; Shuyan, S.; Ying, Z.; Tianqing, L. Measurement of Wenzel roughness factor by laser scanning confocal microscopy. RSC Adv. 2017, 7, 7052–7059. [Google Scholar] [CrossRef]
- Marmur, A. The lotus effect: Superhydrophobicity and metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.J.; Dettre, R.H. Contact angle hysteresis: Study of an idealized rough surface. In Contact Angle, Wettability, and Adhesion; American Chemical Society: Washington, DC, USA, 1964; pp. 112–135. [Google Scholar]
- Olsen, D.A.; Joyner, P.A.; Olson, M.D. The sliding of liquid drops on solid surfaces. J. Phys. Chem. 1962, 66, 883–886. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of surface structure on the hydrophobicity and sliding behavior of water droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Pierce, E.; Carmona, F.J.; Amirfazli, A. Understanding of sliding and contact angle results in tilted plate experiments. Colloids Surf. A Phys. Eng. Asp. 2008, 323, 73–82. [Google Scholar] [CrossRef]
- Fumridge, C.G.L. Studies at phase interfaces I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Quére, D.; Lafuma, A.; Bico, J. Slippy and sticky microtextured solids. Nanotechnology 2003, 14, 1109–1112. [Google Scholar] [CrossRef]
- Giannuzzi, G.; Gaudiuso, C.; Di Mundo, R.; Mirenghi, L.; Fraggelakis, F.; Kling, R.; Lugarà, P.M.; Ancona, A. Short and long term surface chemistry and wetting behaviour of stainless steel with 1D and 2D periodic structures induced by bursts of femtosecond laser pulses. Appl. Surf. Sci. 2019, 494, 1055–1065. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Su, J.; Li, H.; Zhao, Y.; Yuan, X. Improvement of anti-icing properties of low surface energy coatings by introducing phase-change microcapsules. Polym. Eng. Sci. 2018, 58, 973–979. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- He, M.; Li, H.; Wang, J.; Song, Y. Superhydrophobic surface at low surface temperature. Appl. Phys. Lett. 2011, 98, 2011–2014. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Y.; Jiang, L. Applications of bio-inspired special wettable surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Jagdheesh, R.; Diaz, M.; Oca, J.L. Bio inspired self-cleaning ultrahydrophobic aluminium surface by laser processing. RSC Adv. 2016, 6, 72933–72941. [Google Scholar] [CrossRef]
- Groenendijk, M. Fabrication of super hydrophobic surfaces by fs laser pulses. Laser Tech J. 2008, 5, 44–47. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224. [Google Scholar] [CrossRef]
- Tourkine, P.; Le Merrer, M.; Quéré, D. Delayed freezing on water repellent materials. Langmuir 2009, 25, 7214–7216. [Google Scholar] [CrossRef]
- Xing, W.; Li, Z.; Yang, H.; Li, X.; Wang, X.; Li, N. Anti-icing aluminum alloy surface with multi-level micro-nano textures constructed by picosecond laser. Mater. Des. 2019, 183, 108156. [Google Scholar] [CrossRef]
- Ng, Y.H.; Tay, S.W.; Hong, L. Formation of icephobic surface with micron-scaled hydrophobic heterogeneity on polyurethane aerospace. ACS Appl. Mater. Interfaces 2018, 10, 37517–37528. [Google Scholar] [CrossRef]
- Sojoudi, M.; Wang, N.D.; Boscher, G.H.M. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12. [Google Scholar] [CrossRef]
- Li, K.; Xu, S.; Shi, W.; He, M.; Li, H.; Li, S.; Zhou, X.; Wang, J.; Song, Y. Investigating the effects of solid surfaces on ice nucleation. Langmuir 2012, 28, 10749–10754. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, J.; Li, H.; Song, Y. Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 2011, 7, 3993–4000. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Allen, S.M.; Carter, W.C. Kinetics of Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9783540773405. [Google Scholar]
- Wood, G.R.; Walton, A.G. Homogeneous nucleation kinetics of ice from water. J. Appl. Phys. 1970, 41, 3027–3036. [Google Scholar] [CrossRef]
- Jiang, D.; Fan, P.; Gong, D.; Long, J.; Zhang, H.; Zhong, M. High-temperature imprinting and superhydrophobicity of micro/nano surface structures on metals using molds fabricated by ultrafast laser ablation. J. Mater. Process. Technol. 2016, 236, 56–63. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 1–7. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Bahadur, V.; Mishchenko, L.; Hatton, B.; Taylor, J.A.; Aizenberg, J.; Krupenkin, T. Predictive model for ice formation on superhydrophobic surfaces. Langmuir 2011, 27, 14143–14150. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically robust superhydrophobic steel surface with anti-icing, UV-durability, and corrosion resistance properties. Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef]
- Menini, R.; Farzaneh, M. Advanced icephobic coatings. J. Adhes. Sci. Technol. 2017, 4243, 971–992. [Google Scholar] [CrossRef]
- Irajizad, P.; Nazi, S.; Ghasemi, H. Icephobic surfaces: Definition and figures of merit. Adv. Colloid Interface Sci. 2019, 269, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Strobl, T.; Raps, D.; Hornung, M. Comparative evaluation of ice adhesion behavior. World Acad. Sci. Eng. Technol. 2012, 68, 1673–1678. [Google Scholar]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Farzaneh, M. Ice adhesion on super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 8153–8157. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J.; Audibert-Hayet, A.; Melot, D. Nano-micro structured superhydrophobic zinc coating on steel for prevention of corrosion and ice adhesion. J. Colloid Interface Sci. 2014, 447, 240–247. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9. [Google Scholar] [CrossRef]
- Yang, S.; Xia, Q.; Zhu, L.; Xue, J.; Wang, Q.; Chen, Q. Applied surface science research on the icephobic properties of fluoropolymer-based materials. Appl. Surf. Sci. 2011, 257, 4956–4962. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent progress in preparation and anti-icing applications of superhydrophobic coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef]
- Pan, S.; Wang, N.; Xiong, D.; Deng, Y.; Shi, Y. Fabrication of superhydrophobic coating via spraying method and its applications in anti-icing and anti-corrosion. Appl. Surf. Sci. 2016, 389, 547–553. [Google Scholar] [CrossRef]
- Sharifi, N.; Dolatabadi, A.; Pugh, M.; Moreau, C. Anti-icing performance and durability of suspension plasma sprayed TiO2 coatings. Cold Reg. Sci. Technol. 2019, 159, 1–12. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The Lowest Surface Free Energy Based on −CF3 Alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Chen, Y.; Zhang, H.; Han, Y.; Liu, J.; Huang, L.; Liu, X. Electrochemical fabrication of superhydrophobic passive films on aeronautic steel surface. Colloids Surf. A Phys. Eng. Asp. 2019, 572, 317–325. [Google Scholar] [CrossRef]
- Bhushan, B.; Her, E.K. Fabrication of superhydrophobic surfaces with high and low adhesion inspired from rose petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- He, Z.; Xiao, S.; Gao, H.; He, J.; Zhang, Z. Multiscale crack initiators promoted super-low ice adhesion surfaces. Soft Matter 2017, 13, 6562–6568. [Google Scholar] [CrossRef]
- Hou, W.; Shen, Y.; Tao, J.; Xu, Y.; Jiang, J.; Chen, H.; Jia, Z. Anti-icing performance of the superhydrophobic surface with micro-cubic array structures fabricated by plasma etching. Colloids Surf. A Phys. Eng. Asp. 2020, 586, 124180. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally stable silver nanoparticles synthesized by laser ablation in alcoholic organic solvent. Colloids Surf. A Phys. Eng. Asp. 2018, 559. [Google Scholar] [CrossRef]
- Vázquez, R.M.; Trotta, G.; Volpe, A.; Paturzo, M.; Modica, F.; Bianco, V.; Coppola, S.; Ancona, A.; Ferraro, P.; Fassi, I.; et al. Plastic Lab.-on-Chip for the Optical Manipulation of Single Cells; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783319943589. [Google Scholar]
- Volpe, A.; Trotta, G.; Krishnan, U.; Ancona, A. Prediction model of the depth of the femtosecond laser micro-milling of PMMA. Opt. Laser Technol. 2019, 120, 105713. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, C.; Wei, X.; Hu, W.; Ren, Q. Laser machining of transparent brittle materials: From machining strategies to applications. Opto-Electron. Adv. 2019, 1–13. [Google Scholar] [CrossRef]
- Jagdheesh, R. Fabrication of a superhydrophobic Al2 O3 surface using picosecond laser pulses. Langmuir 2014, 30, 12067–12073. [Google Scholar] [CrossRef]
- Arnaldo Del Cerro, D.; Römer, G.R.B.E.; Veld, A.J. Ultra short laser pulse generation of anti-ice surfaces. J. Laser Appl. 2010, 1356. [Google Scholar] [CrossRef]
- Rode, E.G.G.A.V.; Tikhonchuk, V.T. Ablation of solids by femtosecond lasers: Ablation mechanism and ablation thresholds for metals and dielectrics. Phys. Plasmas 2001, 9, 1–27. [Google Scholar]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Nanostructuring of laser textured surface to achieve superhydrophobicity on engineering metal surface. J. Laser Appl. 2019, 31, 022515. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, F.; Yang, Q.; Yong, J.; Bian, H.; Ou, Y.; Si, J.; Meng, X.; Hou, X. A simple way to achieve pattern-dependent tunable adhesion in superhydrophobic surfaces by a femtosecond laser. ACS Appl. Mater. Interfaces 2012, 4, 4905–4912. [Google Scholar] [CrossRef]
- Trotta, G.; Volpe, A.; Ancona, A.; Fassi, I. Flexible micro manufacturing platform for the fabrication of PMMA microfluidic devices. J. Manuf. Process. 2018, 35, 107–117. [Google Scholar] [CrossRef]
- Sataeva, N.E.; Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. Laser-assisted processing of aluminum alloy for the fabrication of superhydrophobic coatings withstanding multiple degradation factors. Surf. Coat. Technol. 2020, 397, 125993. [Google Scholar] [CrossRef]
- Römer, G.; del Cerro, D.A.; Sipkema, R.C.J.; Groenendijk, M.N.W.; in ‘t Veld, A.J.H. Ultra short pulse laser generated surface textures for anti-ice apllications in aviation. In Proceedings of the ICALEO® 2009 28th International Congress on Laser Materials Processing, Laser Microprocessing and Nanomanufacturing, Orlando, FL, USA, 2–5 November 2009; pp. 30–37. [Google Scholar]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic surfaces fabricated by femtosecond laser with tunable water adhesion: From lotus leaf to rose petal. Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Rambabu, P.; Eswara, P.N.; Kutumbarao, V.V. Aluminium alloys for aerospace applications. In Aerospace Materials and Material Technologies; Springer: Singapore, 2017. [Google Scholar]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-step fabrication of robust superhydrophobic steel surfaces with mechanical durability, thermal stability, and anti-icing function. ACS Appl. Mater. Interfaces 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Kietzig, A.M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Volpe, A.; Gaudiuso, C.; Di Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A.; Interateneo, D.; Amendola, V.G. Direct femtosecond laser fabrication of superhydrophobic aluminum alloy surfaces with anti-icing properties. Coatings 2020, 587. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Vazirinasa, E.; Maghsoudi, K.; Jafari, R.; Momen, G. A Comparative Study of the Icephobic and Self-Cleaning Properties of Teflon Materials Having Different Surface Morphologies Journal. J. Mater. Process. Technol. 2019, 116415. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Li, H.; Hu, L.Y.; Yu, Z.L.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloids Surf. A Phys. Eng. Asp. 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Chen, L.; Ping, H.; Yang, T.; Hu, T.; Bennett, P.; Zheng, Z.; Yang, Q. Icing performance of superhydrophobic silicone rubber surfaces by laser texturing. Mater. Res. Express 2019, 6, 1250e2. [Google Scholar] [CrossRef]
- Lie, C.; Xing, W.; Tao, Y.; Heng, P.; Peter, B.; Zhong, Q.Y. Superhydrophobic micro-nano structures on silicone rubber by nanosecond laser processing. J. Phys. D. Appl. Phys. 2018, 51, 445301. [Google Scholar]
- Römer, G.R.B.E.; Skolski, J.Z.P.; Oboňa, J.V.; Huis In ’t Veld, A.J. Finite-difference time-domain modeling of laser-induced periodic surface structures. Phys. Procedia 2014, 56, 1325–1333. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Li, J.; Ye, X.; Li, G.; Cai, L. Superhydrophobic surfaces fabricated by microstructuring of stainless steel using a femtosecond laser. Appl. Surf. Sci. 2009, 256, 61–66. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.C. Wetting behaviour of femtosecond laser textured Ti-6Al-4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef]
- Martìnez-Calderon, M.; Rodìıguez, A.; Dias, A.; Morant-Minana, M.C.; Gòmez-Aranzadi, S.M. Femtosecond laser fabrication of highly hydrophobic stainless steel surface with hierarchical structures fabricated by combining ordered microstructures and LIPSS. Appl. Surf. Sci. 2015, 374, 81–89. [Google Scholar] [CrossRef]
- Indrisiunas, S.; Voisiat, B.; Žukauskas, A.; Račiukaitis, G. Direct laser beam interference patterning technique for fast high aspect ratio surface structuring. In Laser Applications in Microelectronic and Optoelectronic Manufacturing; International Society for Optics and Photonics: San Francisco, CA, USA, 2015. [Google Scholar] [CrossRef]
- Cardoso, J.T.; Aguilar-morales, A.I.; Alamri, S.; Huerta-murillo, D.; Cordovilla, F. Superhydrophobicity on hierarchical periodic surface structures fabricated via direct laser writing and direct laser interference patterning on an aluminium alloy. Opt. Lasers Eng. 2018, 111, 193–200. [Google Scholar] [CrossRef]
- Rodriguez, A.; Echeverría, M.; Ellman, M.; Perez, N.; Verevkin, Y.K.; Peng, C.S.; Berthou, T.; Wang, Z.; Ayerdi, I.; Savall, J.; et al. Laser interference lithography for nanoscale structuring of materials: From laboratory to industry. Microelectron. Eng. 2009, 86, 937–940. [Google Scholar] [CrossRef]
- Milles, S.; Soldera, M.; Voisiat, B.; Lasagni, A.F. Fabrication of superhydrophobic and ice-repellent surfaces on pure aluminium using single and multiscaled periodic textures. Sci. Rep. 2019, 9, 13944. [Google Scholar] [CrossRef] [PubMed]
- Milles, S.; Voisiat, B.; Nitschke, M.; Lasagni, A.F. Influence of roughness achieved by periodic structures on the wettability of aluminum using direct laser writing and direct laser interference patterning technology. J. Mater. Process. Technol. 2019, 270, 142–151. [Google Scholar] [CrossRef]
- Karmouch, R.; Ross, G.G. Experimental study on the evolution of contact angles with temperature near the freezing point. J. Phys. Chem. C 2010, 114, 4063–4066. [Google Scholar] [CrossRef]
- Vercillo, V.; Tonnicchia, S.; Romano, J.; García-girón, A.; Aguilar-morales, A.I.; Alamri, S.; Dimov, S.S.; Kunze, T. Design Rules for Laser-Treated Icephobic Metallic Surfaces for Aeronautic Applications. Adv. Funct. Mater. 2020, 30, 1910268. [Google Scholar] [CrossRef]
- Hauk, T.; Strobl, D.R. Implementation and calibration of the icing and contamination research facility (iCORE). In Proceedings of the ILASS-Europe 25th European Conference on Liquid, Chania, Greece, 1–4 September 2013. [Google Scholar]
- Vercillo, V.; Tiago, J.; Huerta-Murillo, D.; Tonnicchia, S.; Laroche, A.; Alejandro, J.; Guillén, M.; Luis, J.; Fabián, A. Materials Letters: X Durability of superhydrophobic laser-treated metal surfaces under icing conditions. Mater. Lett. X 2019, 3, 100021. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, K.; Suh, J.; Lee, J. Laser scanner stage on-the-fly method for ultrafast and wide area fabrication. Phys. Procedia 2011, 12, 452–458. [Google Scholar] [CrossRef]
- Gebhardt, M.; Hänel, J.; Allenstein, F.; Scholz, C.; Clair, M. Laser structuring of flexible organic solar cells. Laser Tech. J. 2013, 10, 25–28. [Google Scholar] [CrossRef]



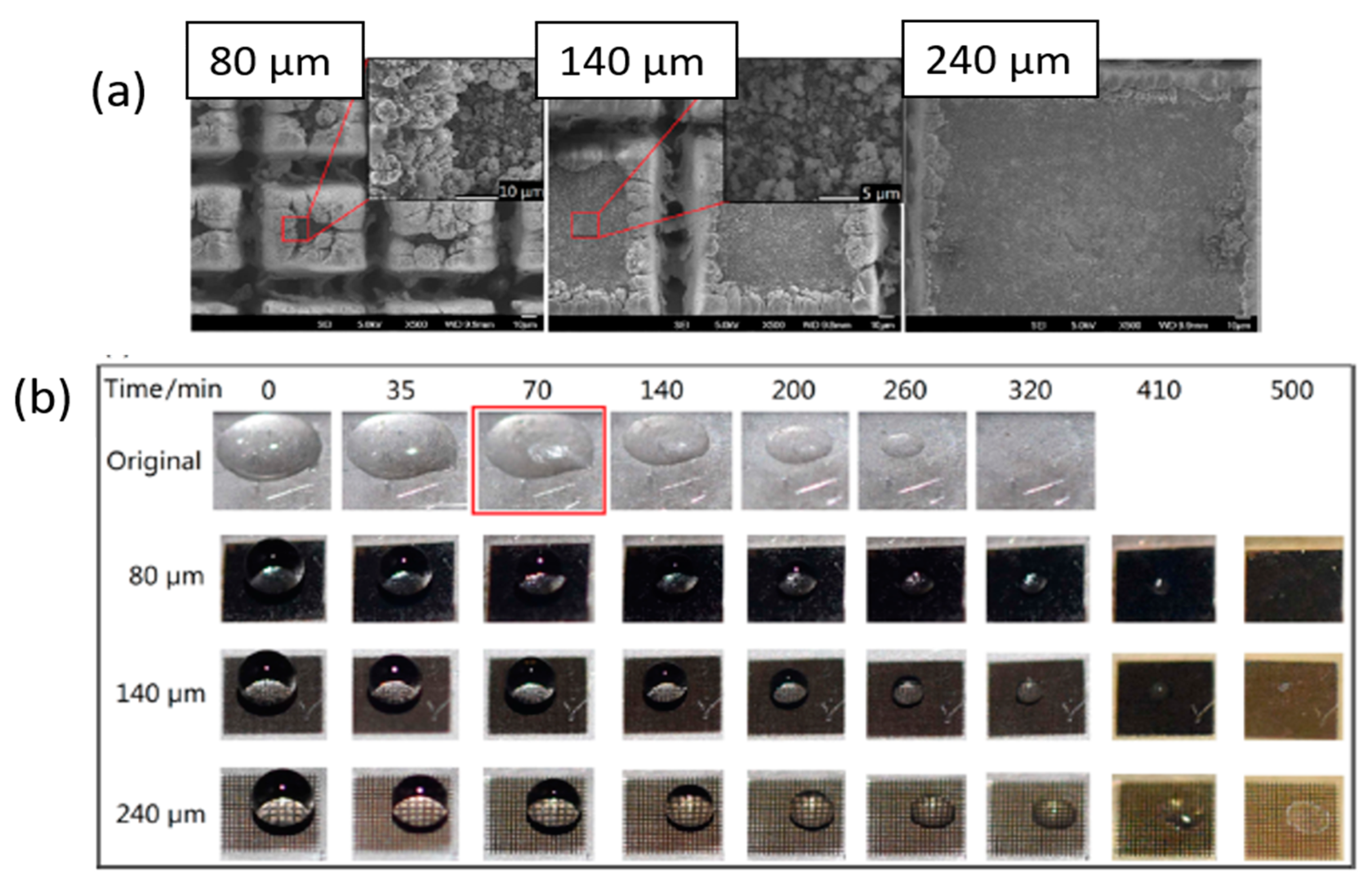

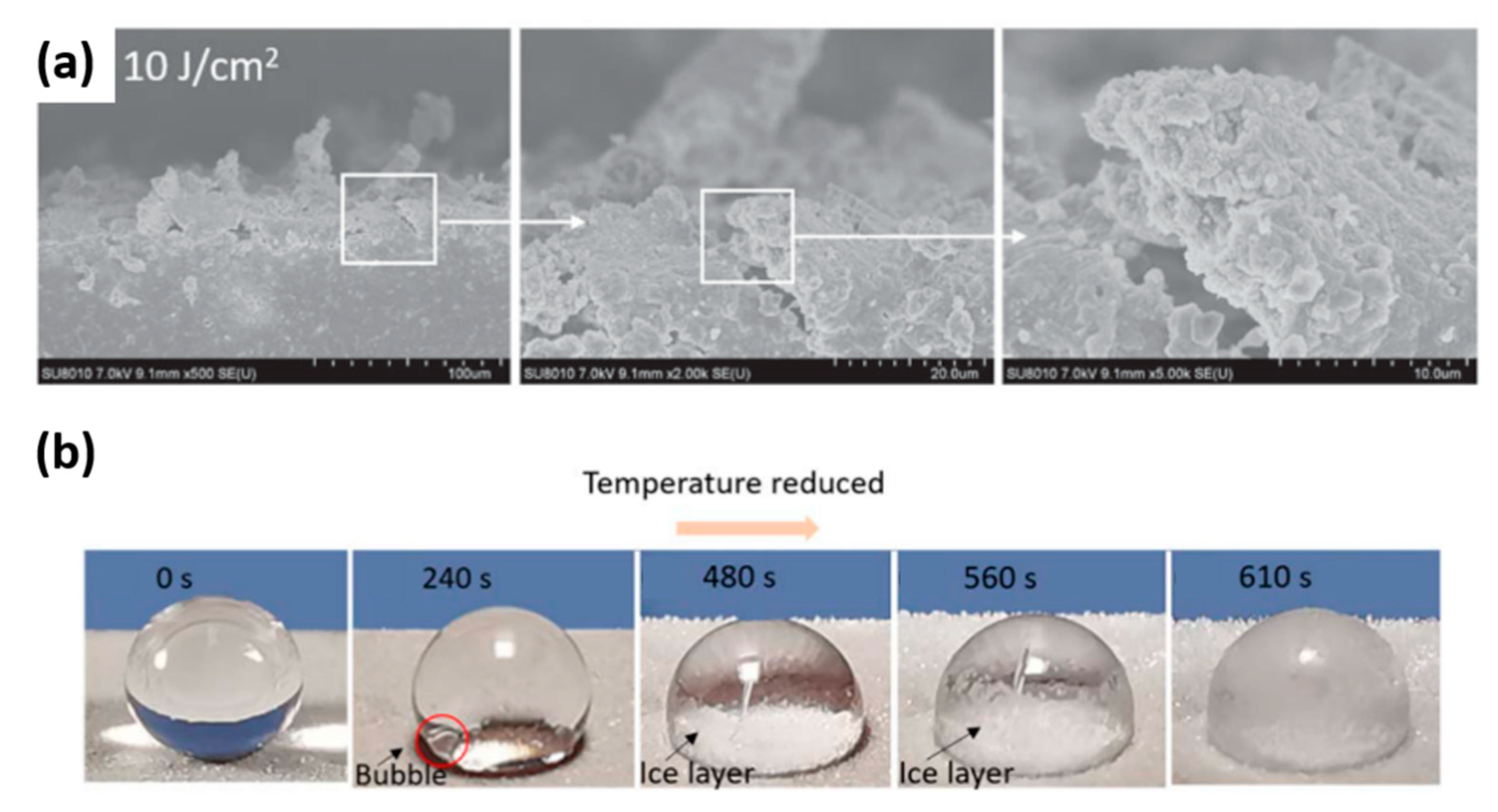
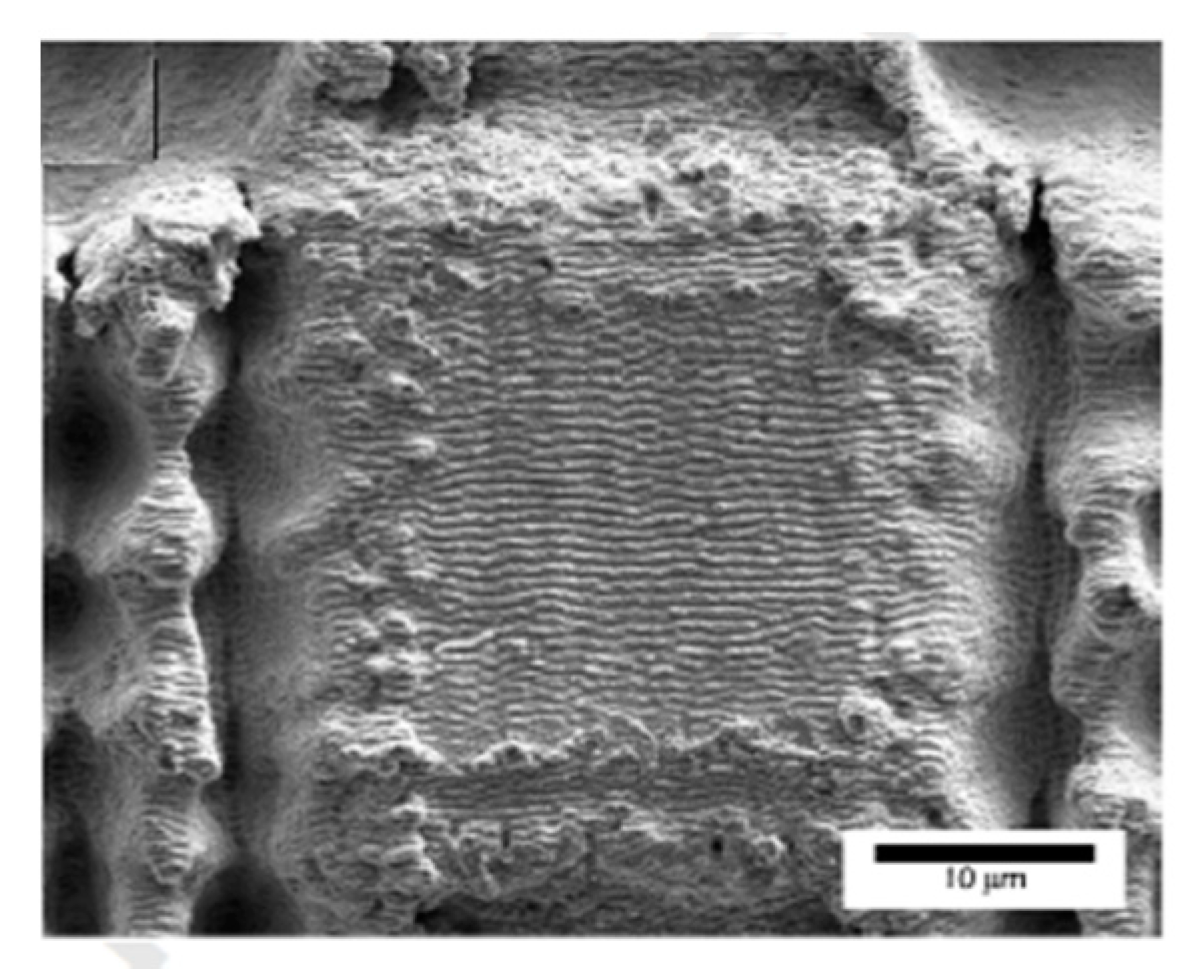
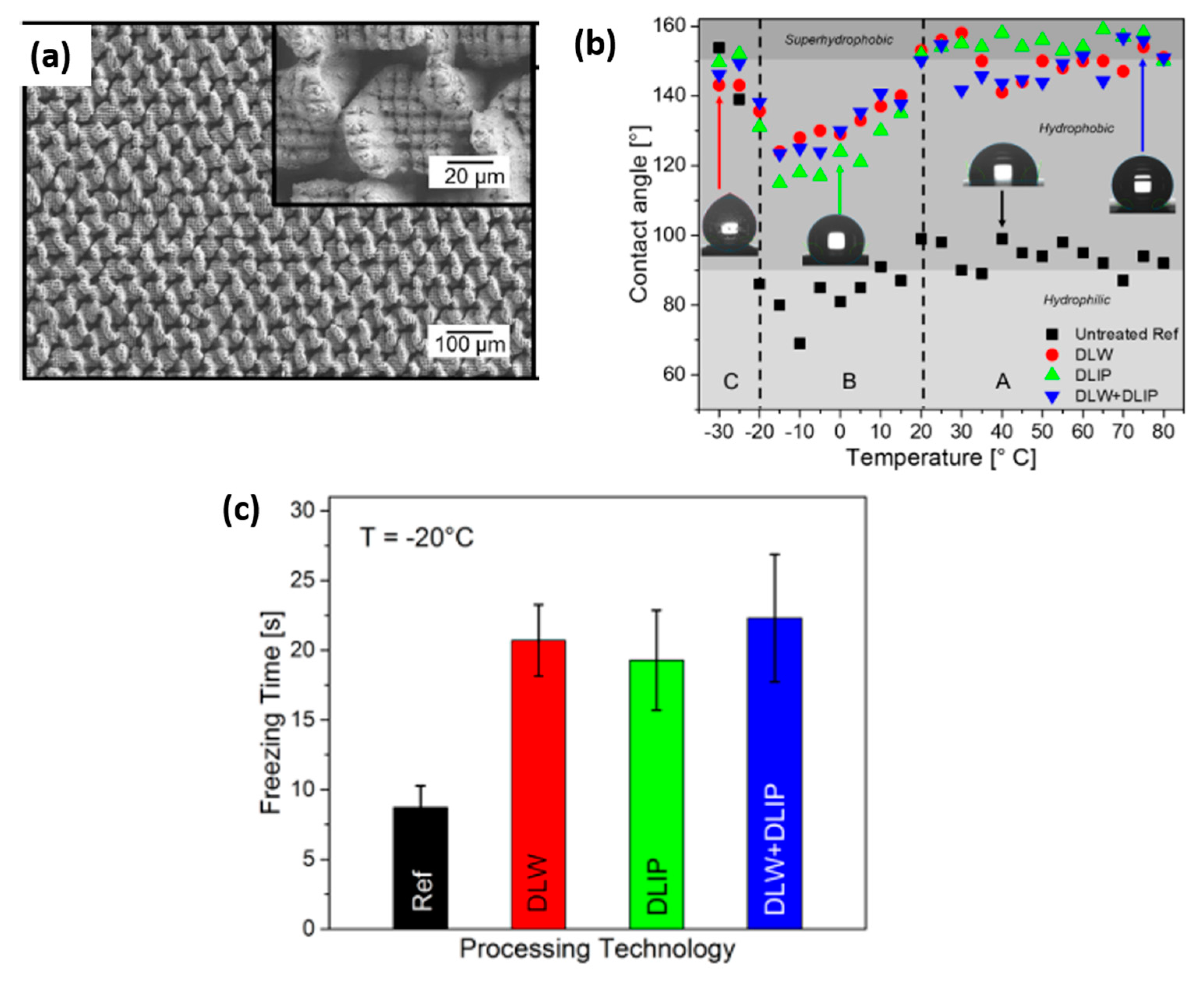
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, A.; Gaudiuso, C.; Ancona, A. Laser Fabrication of Anti-Icing Surfaces: A Review. Materials 2020, 13, 5692. https://doi.org/10.3390/ma13245692
Volpe A, Gaudiuso C, Ancona A. Laser Fabrication of Anti-Icing Surfaces: A Review. Materials. 2020; 13(24):5692. https://doi.org/10.3390/ma13245692
Chicago/Turabian StyleVolpe, Annalisa, Caterina Gaudiuso, and Antonio Ancona. 2020. "Laser Fabrication of Anti-Icing Surfaces: A Review" Materials 13, no. 24: 5692. https://doi.org/10.3390/ma13245692
APA StyleVolpe, A., Gaudiuso, C., & Ancona, A. (2020). Laser Fabrication of Anti-Icing Surfaces: A Review. Materials, 13(24), 5692. https://doi.org/10.3390/ma13245692






