1. Introduction
Medical metallic biomaterials are made of titanium (titanium Grade 4) or its alloy, Ti-6Al-4V. The surface of implants dedicated to bone is usually mechanically treated, machined, polished, sand-blasted or etched [
1,
2]. The formation of one nanostructured layer or porous layer on the implants influences interactions between the surface and bone tissue. The nanostructured surface of titanium alloys is formed by etching or alkali treatment of the implant at various temperatures and with various alkalis (e.g., NaOH and KOH) or acidic solutions (e.g., HCl, HF, H
3PO
4 and CH
3COOH) [
3,
4]. Thin layers with open or closed pores (usually open) and irregular flakes may induce faster protein and water molecule adsorption and, thus, osteoblast cell adhesion. Thicker coatings with the included chemical compounds are formed using an electrochemical technique, such as anodization, including plasma electrolytic oxidation, electrophoretic deposition, electroreduction or electrodeposition. A good, adhesive layer on the substrate exhibits the oxides formed during plasma electrolytic oxidation (PEO) [
5]. Additionally, during anodization, chemical compounds from electrolytes might be incorporated when forming the porous oxide layer [
6]. The PEO process has limitations, such as the incorporation of ceramic particles into the layer or controlling the process to form an agglomeration of ceramic particles on the top layer. These particles at the top of the coatings play a key role in the interactions with proteins and osteoblast cells, which participate in the osseointegration process [
7,
8].
The formation of phosphate-based coatings on titanium alloys is well established. However, the final effect of the physico-chemical properties of the coatings and cytocompatibility of the surface vary. Hydroxyapatite (Ca
10(PO
4)
6(OH)
2), which is a material that influences osseointegration and osseoinduction, is often blended with other phosphates—e.g., Ca
3(PO
4)
2 (TCP). Calcium phosphates are present in two polymorphic forms: the α-phase Ca
3(PO
4)
2 is less soluble, and the β-phase Ca
3(PO
4)
2 is more commonly used in biomaterials [
9]. Biphasic ceramic materials comprising hydroxyapatite and tricalcium phosphate are formed as grains of various sizes, including nanoparticles or grains that might be deposited on the metal surface or mixed with polymer (synthetic) or biopolymer (natural) materials. The main goal of the biphasic HA/TCP is bioactivity, with favorable properties to form various shapes of the materials and the ability of resorption [
10]. HA/TCP biphase material might be deposited using the plasma spraying technique on a Ti-6Al-4V surface [
11] or on a Ti surface [
12]; the material might also be blended with another chemical compound, where ions such as Zn
2+, Ag
+ and Cu
2+ [
13,
14] also exhibit antimicrobial properties. Coating formation using the sol-gel technique allows the design of a surface with desirable chemical and phase compositions. This technique is used to form the hybrid layer and enrich the top, previously anodized titanium alloys. Titanium surface treatment may be provided by various techniques, such as magnetron sputtering and electrochemical deposition (formation of hydroxyapatite on Ti–Ta–Zr alloys) [
15], pulsed laser deposition (F/Mg HA doped on Ti implant) [
16] or via electron beam evaporations to form a hydroxyapatite layer on a β-phase Ti alloy [
17]. Previously anodized surfaces of Ti alloys can also be modified by using the electrophoretic technique to enrich the top part of the layer in selected compounds, such as chitosan [
18], graphene oxide (rGO)—bioglass [
19] or apatite/biopolymer [
20].
Another advantage of ceramic coatings is the easy adsorption of proteins, such as collagen type I or collagen type II, and lactoferrin, which stimulate the osseointegration process. Bone is a porous structure with various densities, depending on the type and the considered part of bone. The adsorption of biomolecular coatings comprising protein can stimulate peri-implant bone due to the formation of a microstructure favorable for the adsorption of water molecules and osteoblast cells [
21]. The formation of extracellular matrix (ECM) supports cell proliferation and cell migration and the final results of bone-to-implant contact (BIC). It was reported that ECM comprises almost 90% of collagen type I, which induces osteoid and mineralization processes, and the mRNA expression of cellular proteins promotes bone healing formation [
22]. Lactoferrin is a glycoprotein that is also used for surface treatment to promote the behavior of MG-63 osteoblast-like cells [
23]. Lactoferrin promotes the osteogenic differentiation of SAOS-2 cells to enhance bone regeneration in vivo [
24,
25]. The final biological response of the cells depends on several factors, not only the chemical composition. Thus, the evaluation of new coatings and materials should be examined and characterized in depth. In the scientific literature, information about favorable surfaces for bone regeneration is available, usually in some ranges. A Ca/P ratio such as 1.67 is recognized as a ratio in hydroxyapatite that promotes the osseointegration process [
26]. However, various materials comprising various concentrations of calcium phosphates or mixtures of apatite without the Ca/P ratio equaling 1.67 also promote the formation of new bone tissue [
27,
28].
The aim of this study is to form hybrid ceramic coatings to improve bioactive properties of the vanadium-free Ti-6Al-7Nb surface. The first step of surface treatment was the formation of a porous oxide layer with incorporated tricalcium phosphate (Ca3(PO4)2) on the Ti alloy using the plasma electrolytic oxidation process. Next, an additional coating was deposited via the sol-gel technique from a solution with precursors to form hydroxyapatite particles. The hybrid coatings were characterized in detail, particularly the surface morphology, cross-section and chemical and phase composition. To enhance adhesion and proliferation of the MG-63 osteoblast-like cells, the surfaces were immersed in protein solutions (collagen type I or lactoferrin).
3. Results
The surface of the Ti-6Al-7Nb sample (TAN) was anodized (TAN-A) in calcium hypophosphite solution with the addition of ceramic particles (Ca
3(PO
4)
2). Next, the samples were coated with an additional ceramic layer using the dip coating method to form additional bioactive particles (TAN-SG sample). The sample labels and process of the surface treatment are collected in
Table 1.
Figure 1 presents the surface morphology of the TAN-A sample (A), where a typical porous microstructure is observed.
The pore size and distribution depended on the parameters during the plasma electrolytic oxidation process, such as voltage, current density and the composition of the anodizing bath. The pores were distributed on the top of the layer but also through the layer as a result of the plasma electrolytic oxidation process. On the surface, single ceramic particles were observed due to the incorporation of Ca
3(PO
4)
2 particles from the bath. It is possible that some ceramic particles were synthesized and built in the coating from the calcium hypophosphite anodizing bath (as discussed in the XRD results).
Figure 1B,C show the morphology of the anodized sample post-treatment via the sol-gel technique (TAN-SG sample). Agglomerations of the ceramic particles formed due to the post-treatment technique are indicated with red arrows. The ceramic particles filled some of the pores, partly covering the oxide layer. Agglomeration of the ceramic particles was observed on the SEM image at a higher magnification (
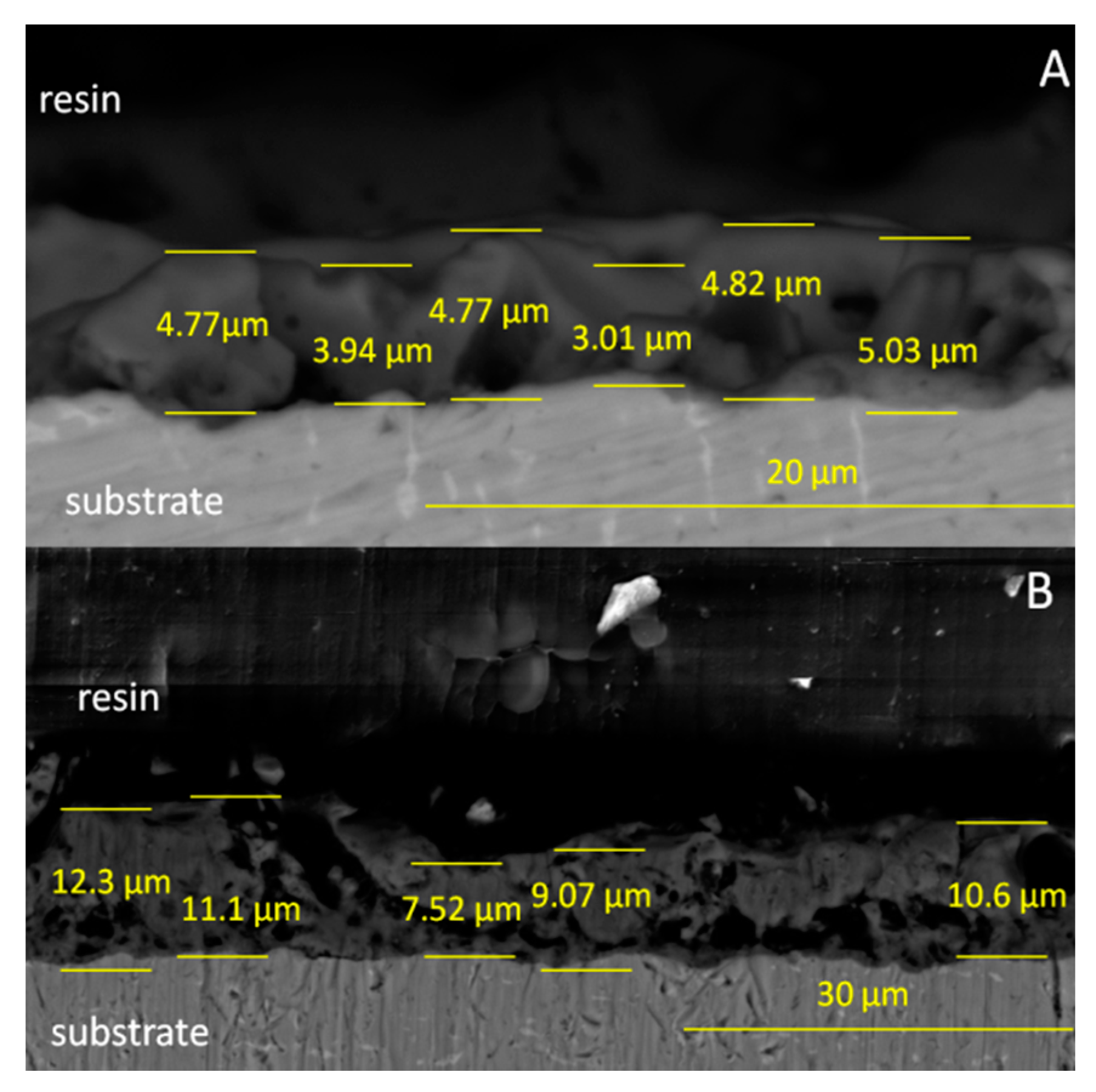
Figure 1C). The particles presented various distributions and did not cover the characteristic porous structure of the oxide layer. The thickness of the hybrid coatings formed on the Ti-6Al-7Nb surface was analyzed using SEM, and images of the cross-section of this sample are presented in
Figure 2.
The thickness of the oxide layer (TAN-A sample) was found in the range of 3.01–5.03 µm. The irregular shape of the oxide with open and closed pores is typical for the anodized Ti-6Al-7Nb alloy. The thickness of the hybrid ceramic layer was between 7.52 and 12.30 µm. The formation of the additional ceramic particles indicated that the total coating thickness was significantly increased. The morphology of the coatings also changed, and it is clearly visible that the coating is less porous and the sol-gel penetrated the pores of the oxide layer during the dip coating process.
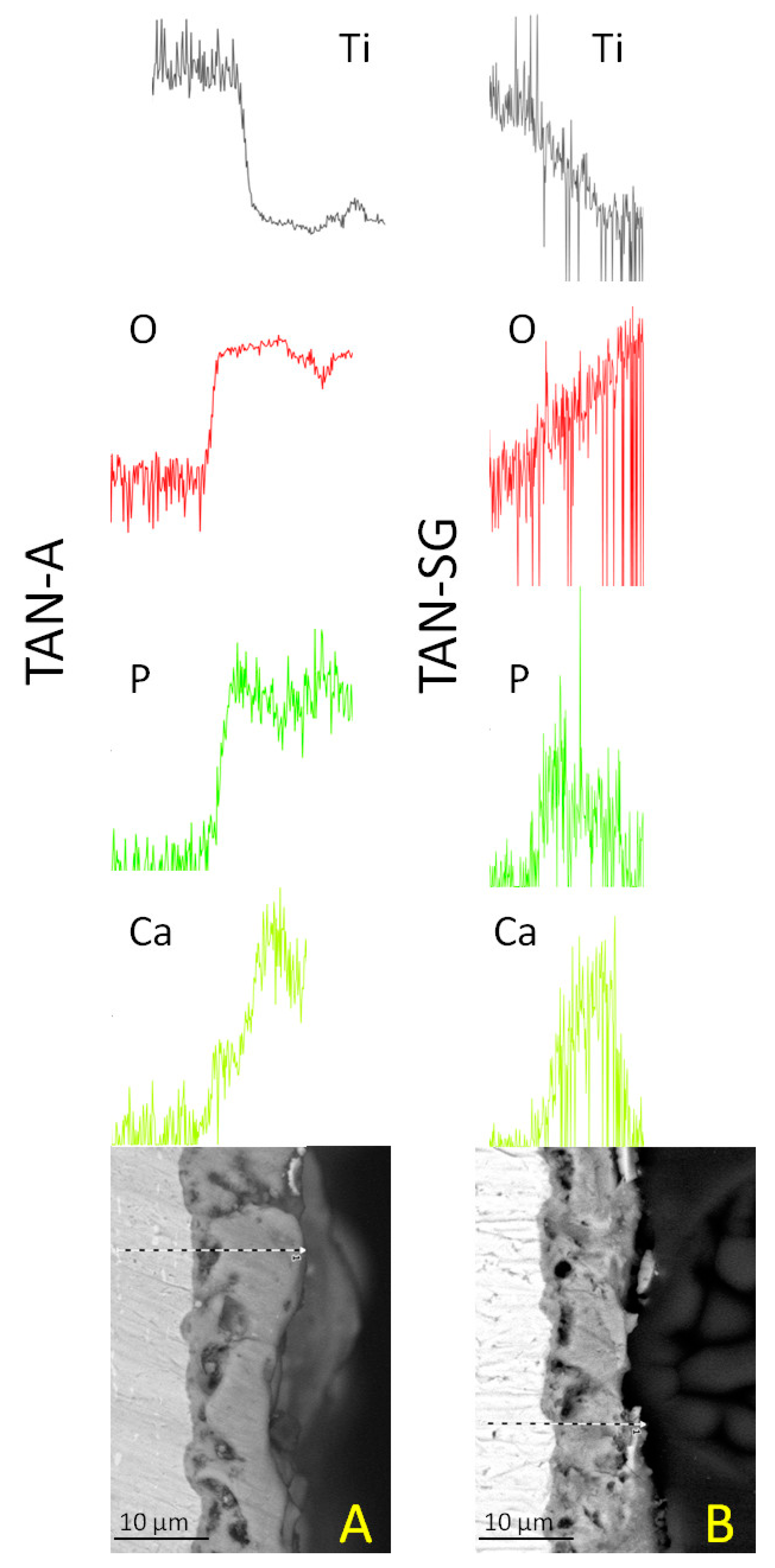
Figure 3 presents the cross-section and EDX analysis of the coatings.
The EDX lines and element distributions of calcium, phosphorous and oxygen confirmed the formation of ceramic coatings on the TAN substrate. For the TAN-A sample, the calcium and phosphorous are distributed more uniformly than for the, TAN-SG sample. For the sample after the sol-gel process, Ca and P compounds are more concentrated in the middle of the coatings and in some parts on the top of the coating. Detection of Ti and O elements indicated that TiO2 was formed on both samples. EDX lines of these elements show that in the layers, the content of Ti decreased whereas content of oxygen increased.
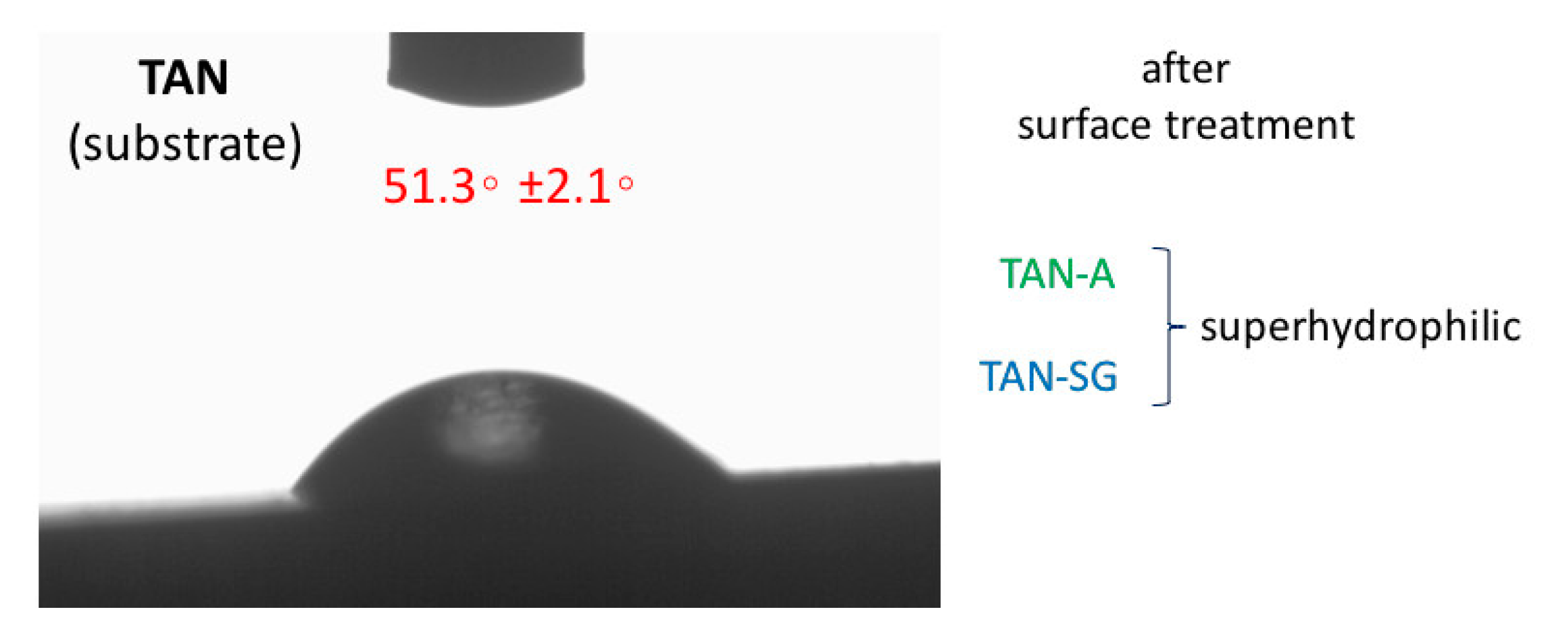
Figure 4 presents the results of water contact angle measurements of the drop placed on top of the investigated samples. The wettability of the substrate (Ti-6Al-7Nb) sample was 51.3 ± 2.1°. Surface treatment of the TAN sample showed that the wettability was changed and the surface was superhydrophilic. The contact angle could not be determined due to fast adsorption of the water drop into the porous structure of the layers (for both samples, TAN-A and TAN-SG).
The change in surface wettability also depends on the surface roughness.
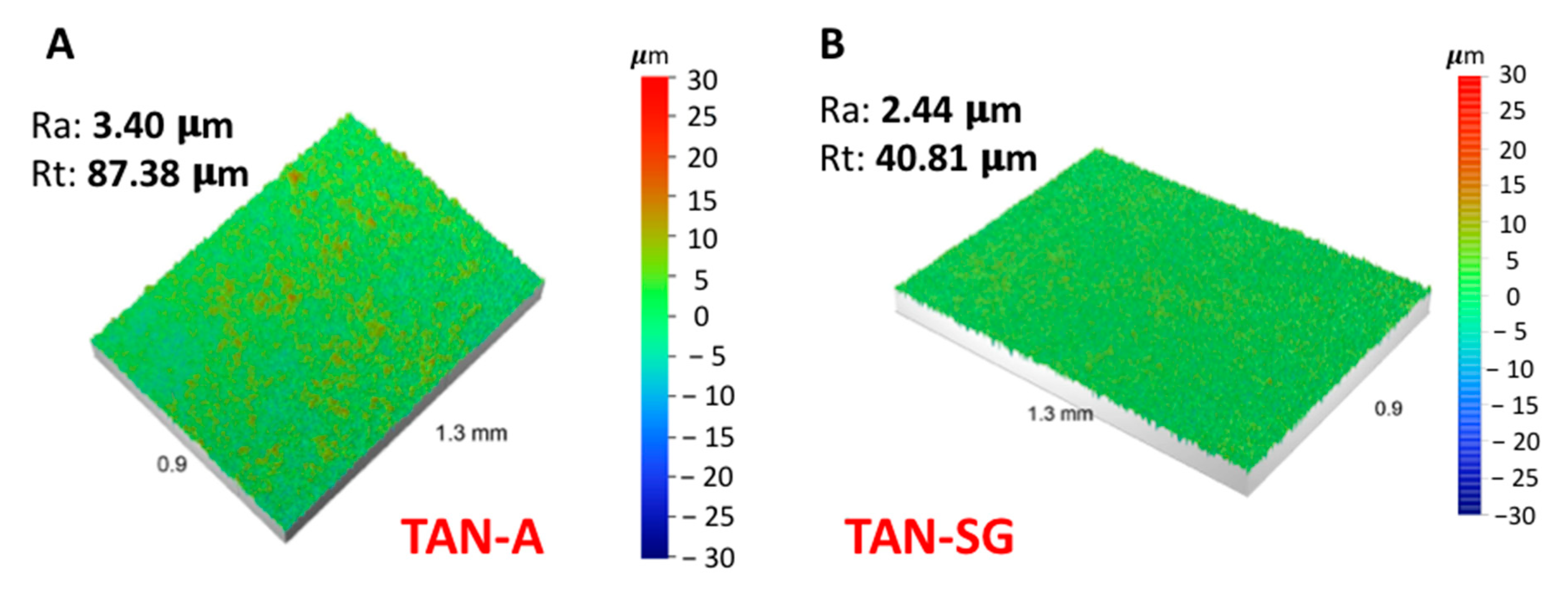
Figure 5 presents the results of the surface roughness analysis using a noncontact optical profilometer.
The anodization process of the titanium alloy (TAN sample) at 350 V indicated that the surface roughness Ra (average arithmetical parameter) increased from 0.30 to 3.40 µm. Next, the formation of the additional ceramic layer decreased the Ra parameter to 2.44 µm. The profile of the measured coating also decreased from 87.38 to 40.81 µm. Sol-gel treatment of the previously anodized Ti alloy indicated that some pores were filled by ceramic particles and the final measurement of the average surface roughness (Ra) decreased.
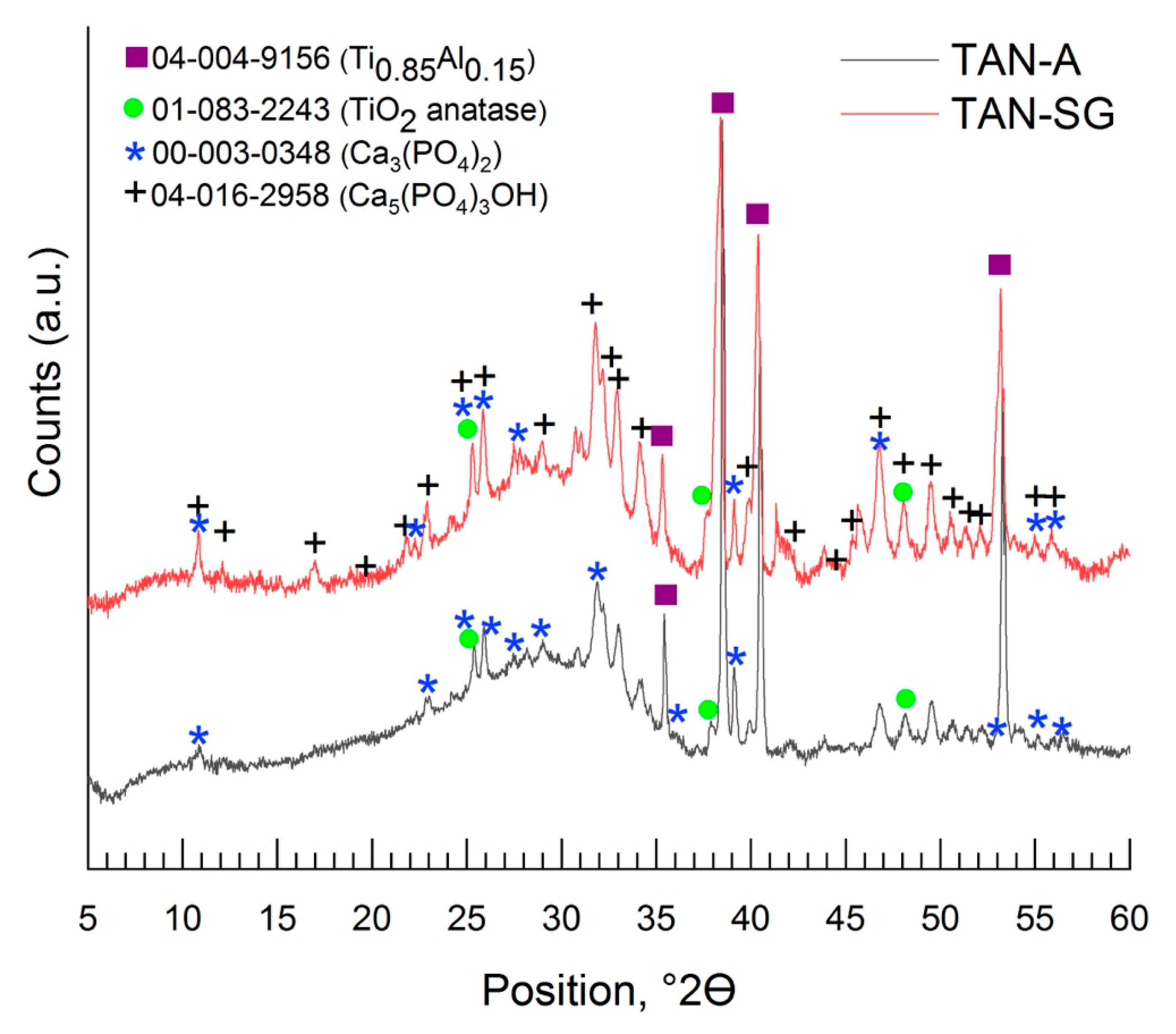
Figure 6 and
Figure 7 show the XRD patterns of the coatings formed on the Ti-6Al-7Nb alloy. The phase composition of the layers formed on the Ti alloy was characterized step by step; it means that besides the substrate measurements, the coating formed in Ca(H
2PO
2)
2 without ceramic particles was evaluated, then the coating formed in solution with Ca
3(PO
4)
2 (TAN-A) and then the last one, the hybrid ceramic coating, which was formed due to anodization in solution with Ca
3(PO
4)
2 particles and then coated with an addition layer via the sol-gel method (TAN-SG). For the Ti-6Al-7Nb titanium alloy, two phases were found: the phase of titanium and that of aluminum alloy (PDF #04-004-9156), where the main peak positions on the XRD pattern were detected at 35.40°, 38.47°,40.45°, 53.94°, 63.34°, 70.87°, 74.88°, 76.74°, 78.03°, 82.60°, 92.91° and 103.05° of 2θ. Anodization of the Ti-6Al-7Nb alloy in 0.1 M Ca(H
2PO
2)
2 solution caused the formation of the titanium oxide—TiO
2 anatase (PDF #01-083-2243) [
32] (
Figure 6). The main peaks for the titanium oxide were registered at 25.36°, 38.47°, 39.01°, 53.24°, 55.12°, 70.87° and 82.34° of 2θ.
No signals for the crystalline Ca-based phase were identified. The calcium phosphate was determined where the samples were anodized in the 0.1 M Ca(H
2PO
2)
2 solution with ceramic particles of calcium phosphate (Ca
3(PO
4)
2 (PDF #00-003-0348), and the XRD pattern is presented in
Figure 7 [
33].
The signals registered between 20° and 30° of 2θ indicated that the ceramic particles were incorporated. The peaks which correspond with the Ca
3(PO
4)
2 phase was detected mainly at 25.96°, 28.24°, 31.89° and 37.21° of 2θ. However, an amorphous phase was also observed. During the plasma electrolytic oxidation process, XRD showed that the ceramic particles were melted, as revealed by the amorphous scattering background. The XRD pattern of the hybrid coating TAN-SG sample (anodized sample coated by the sol-gel technique) is shown in
Figure 7. The signals from Ca-based compounds increased and new signals were determined. The new signals corresponded to calcium phosphate hydroxide (hydroxyapatite) (PDF #04-016-2958) [
34] and calcium phosphate Ca
3(PO
4)
2 (PDF #00-003-0348). For the hydroxyapatite, the main peaks are visible on the XRD pattern at 10.82°, 18.80°, 21.82°, 22.88°, 25.28°, 25.83°, 28.80°, 31.76°, 32.16°, 34.16°, 35.31°, 37.74°, 43.84°, 46.74°, 48.04°, 49.46°, 50.51° and 53.18° of 2θ. The signals of the anatase phase decreased due to formation of the additional ceramic layer, and the X-ray was mainly scattered by the layer formed by the sol-gel technique.
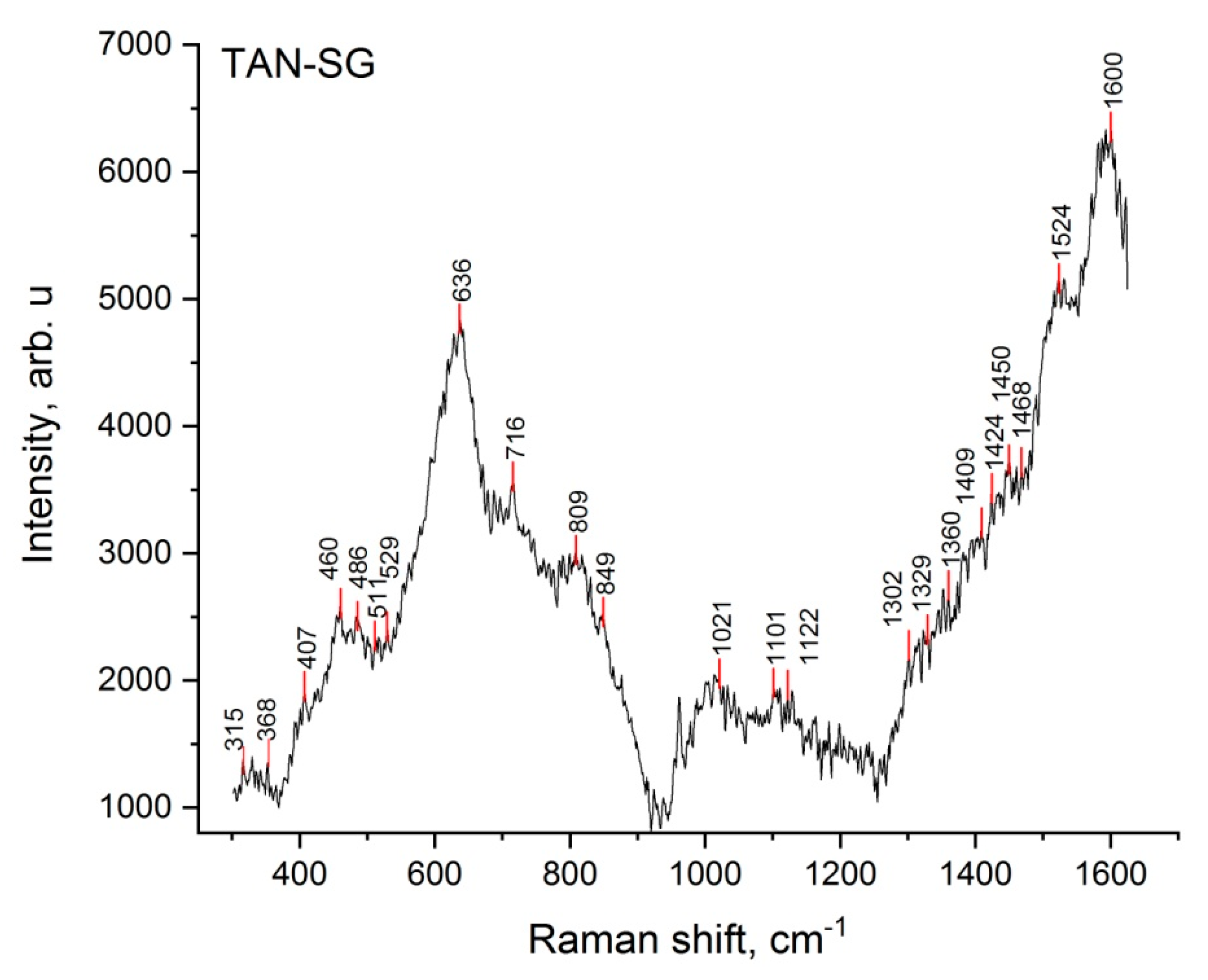
To better characterize the TAN-SG sample, Raman spectroscopy was applied to determine the chemical composition of the amorphous and crystal parts of the layers.
Figure 8 presents the Raman spectrum of the TAN-SG layer.
The most characteristic vibrational spectra were registered for PO
43− tetrahedra, which correspond to both ß-tricalcium phosphate (Ca
3(PO
4)
2) and hydroxyapatite (Ca
10(PO
4)
6(OH)
2) chemical compounds. The tetrahedron on phosphates in aqueous solutions corresponded to vibrations such as
v1 = 938 cm
−1,
v2 = 420 cm
−1 and
v3 = 1017 and 567 cm
−1 [
35]. In our study, the vibrations were detected between 403 and 412 cm
−1, with the maximum at 407 cm
−1, and then small peaks at 561 and 938 cm
−1. PO
43− exhibits vibrations that span frequency ranges of 400–490, 570–625 and 1020–1095 cm
−1. The clear visible signal at 962 cm
−1 corresponds to the nondegenerate
v1 mode of PO
43−, which corresponds only to the hydroxyapatite structure. The broad signals and small signals registered between 368 and 530 cm
−1 correspond to the
v2 modes for ß-tricalcium phosphate.
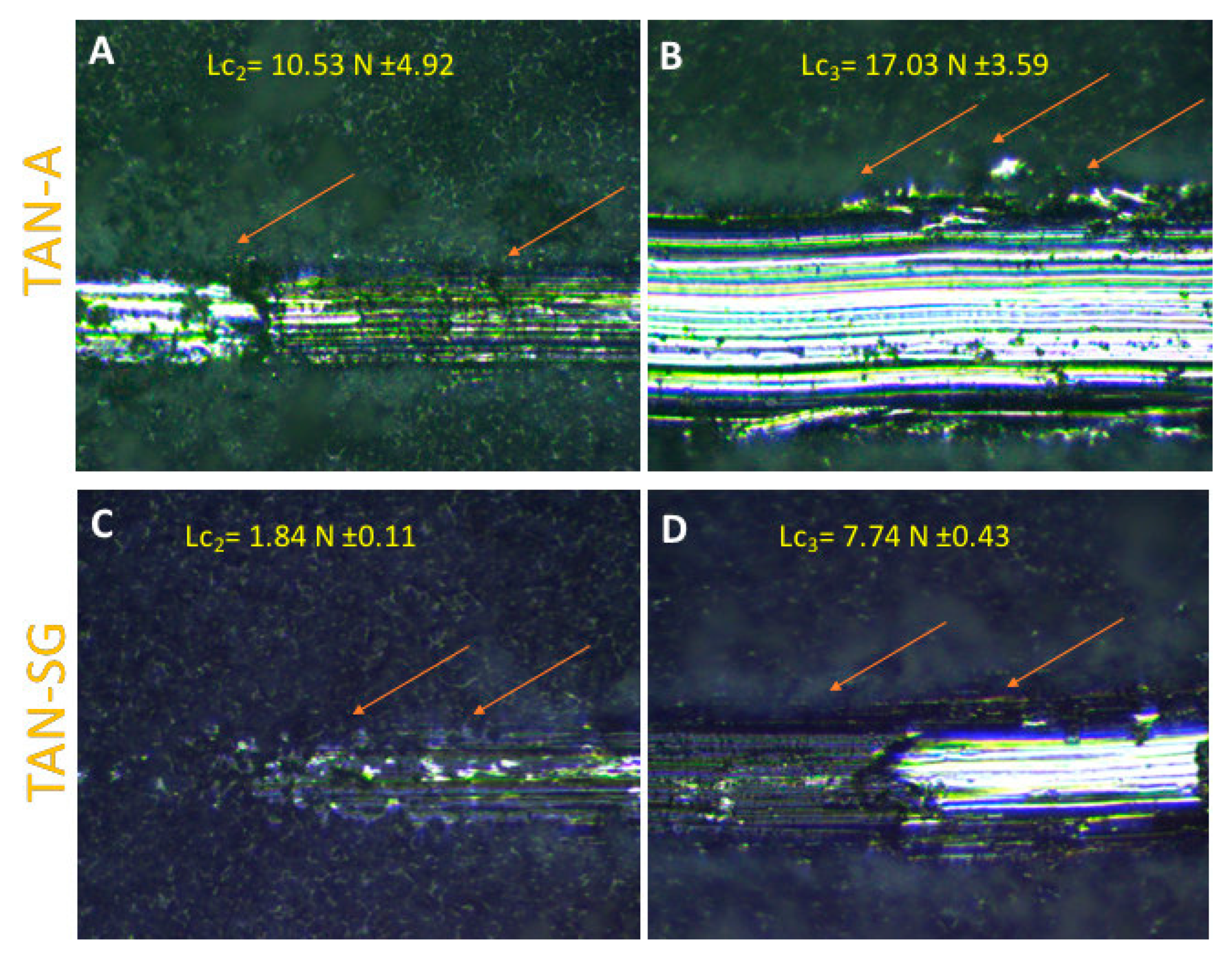
Figure 9 shows the evaluation of the ceramic coating adhesion to the substrate. The crack and layer during the first stage of the scratching of the coatings were not observed for both samples. According to the EN 1071-3:2005 standard [
36], Lc
1 is labeled when breakdown during the first stage of the intender penetrate is observed. Lc
2 (plastic deformation of the layer) was determined for both layers (
Figure 9A,C), occurring for the TAN-A sample when the friction force was 10.53 N ± 4.92 and for TAN-SG when the friction force was 1.84 N ± 4.0.11. For this layer, changes in the corner of the scratched part of the layer were observed (marked by red arrows). Lc
3 is determined where the substrate is observed and the coatings are delaminated. For the TAN-A sample, the Lc
2 and Lc
3 were close and it was difficult to identify the raw substrate; thus, the final value for Lc
3 was determined at 17.03 N ± 3.59. For the TAN-SG sample, Lc
3 was observed when the friction force was 7.74 N ± 0.43.
Because the TAN-SG sample is to be used in a final application as a bone implant, biological characterization was provided for this surface. The TAN-SG sample was immersed in PBS solution containing 0.200 mg/mL of two different proteins—collagen and lactoferrin. The possibility of the adsorption of the protein and biological response was evaluated using MG-63 cells, particularly because the cell response was different after 3 and 7 days of the MG-63 cell culture.
Figure 10 presents the viability of MG-63 cells cultured up to 7 days with the TAN-SG sample and the sample coated with collagen (TAN-SG-C) and protein (TAN-SG-L). The viability of the cells was also determined for the unmodified titanium alloy sample (TAN) and reference tissue culture polystyrene (TCPS).
After 24 h of MG-63 cell culture, the highest number of cells was observed in the TAN-SG-L sample (besides reference TCPS). Surface modification, such as formation of a hybrid ceramic layer and immersion in proteins, influenced the differences in the number of cells compared with the unmodified TAN sample. After 3 days of culture, the cell number was similar for all the investigated samples. After 7 days, the highest cytocompatibility of the materials with MG-63 osteoblast-like cells was observed for the TAN-SG-L sample. Differences in the osteoblast-like MG-63 cells were observed after live/dead staining, and the optical images are presented in
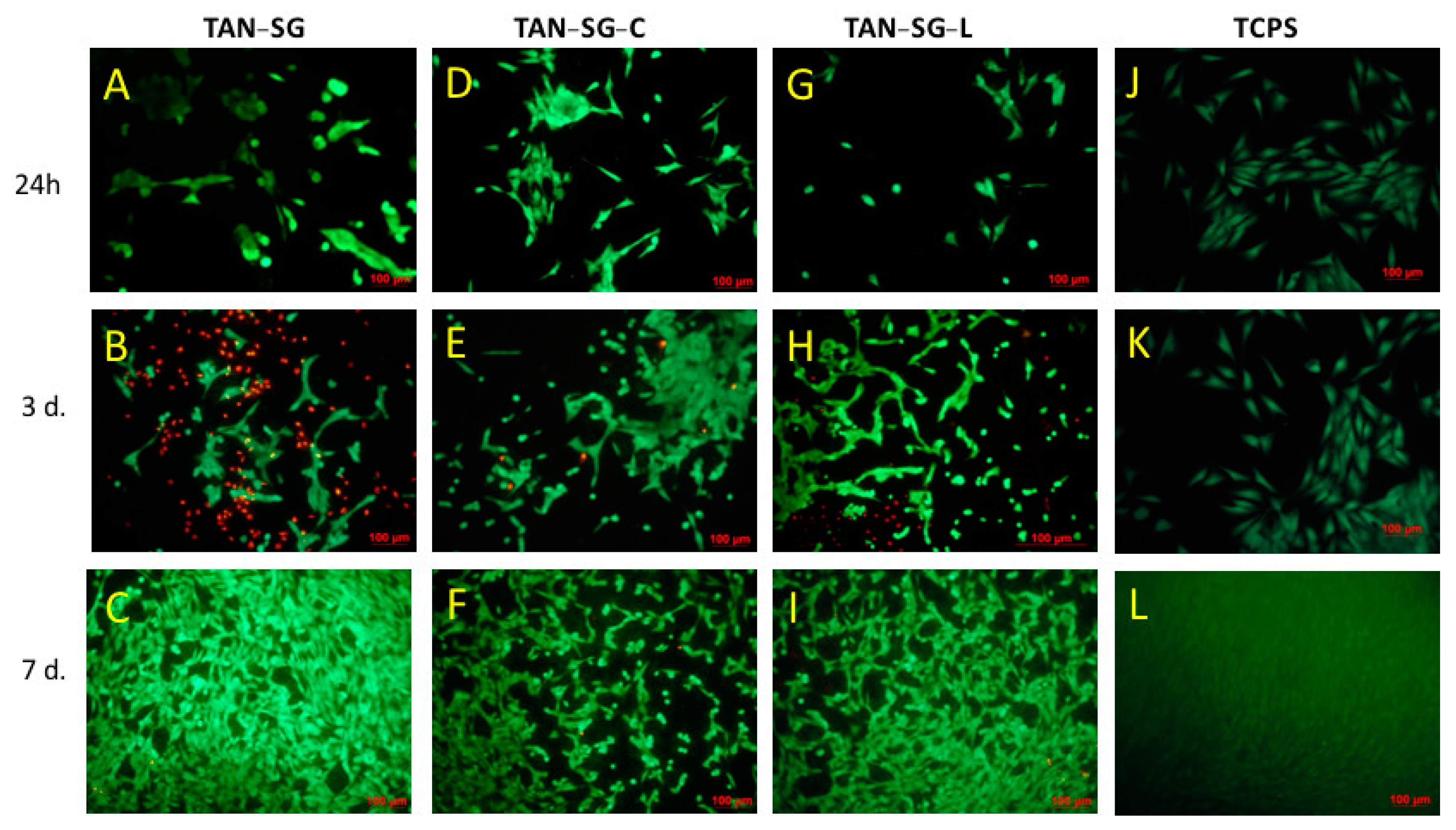
Figure 11. The live cells are visible in green and dead cells are visible in red.
Optical images after osteoblast-like MG-63 cell staining showed effects of the protein on the cell morphology and cell viability. After 24 h on TAN-SG, some cell agglomeration was visible. When the TAN-SG sample was immersed in collagen type I (TAN-SG-C) and lactoferrin (TAN-SG-L) solution, the MG-63 osteoblast-like cells were better adhered. Few dead cells were observed. After 3 days of culture, many dead cells (red cells) were observed in the TAN-SG sample, whereas only single dead cells were visible on the surfaces after protein immersion. On all the surfaces, osteoblast-like MG-63 cells well adhered and their cell number increased with culture time. The highest number of cells was observed on the surfaces after 7 days of culture.
4. Discussion
Anodization is a well-known method to modify valve metal surfaces. This phenomenon was mentioned in many studies, where the mechanism of the plasma electrolytic oxidation process is presented in detail [
37,
38]. Titanium alloys without vanadium, such as Ti-6Al-7Nb, not only enhance biocompatibility but also exhibit better (more favorable for bone tissue) mechanical properties compared to Ti Grade 4. The surface of long-term titanium implants for bone is modified to enrich the surface material with bioactivity. In our case, we focused on post-treatment of the previously anodized Ti-6Al-7Nb alloy to obtain a hybrid, bioactive ceramic layer. The SEM images show that on the porous layer, additional ceramic particles were formed after sol-gel treatment. Agglomeration of the ceramic particles occurred mainly in pores and on the top of the surface. The post-treatment analysis showed that the total layer thickness on the TAN-SG surface significantly increased. The EDX line analysis indicated that the sol penetrated the pores of the oxide layer and formed particles inside the oxide layer and on the top as well. For both samples, TAN-A and TAN-SG, the EDX line of oxygen and titanium indicated formation of the TiO
2. A similar effect was observed for the alloying elements (Al, Nb) which are also anodized during the PEO process, but their content on the oxides in the total layer is low. Bioactivity is recognized as a process to enhance the formation of apatite comprising calcium phosphates with various ratios of Ca and P ions. It remains debatable whether hydroxyapatite presents higher bioactivity or tricalcium phosphate or a mixture of these materials and formation of biphasic ceramic material. Thus, post-treatment of the anodized surface is often performed to add specific functions of the materials—e.g., bioactivity or antibacterial properties [
39,
40,
41].
Surface treatment of the T-6Al-7Nb alloy caused the surface roughness to increase, which is favorable for the implant to join bone tissue [
42,
43]. The results of the reference sample (TAN) surface roughness measurements were presented in our previous paper [
29], where Ra was 0.30 µm and Rt was 0.15 µm. High surface roughness may lead to markedly decreased cytocompatibility of the material. Post-treatment of the anodized Ti alloys reduced their surface roughness from 3.40 to 2.44 µm. Hydrophilicity is a parameter that also positively affects the adsorption of proteins and then osteoblast cells and the osseointegration process [
44]. For both surfaces, the wettability was highly hydrophilic and the contact angle could not be determined, but the surface of the TAN-SG sample was favorable for protein adsorption.
Based on previous studies, we hypothesized that calcium-based compounds (various, not only hydroxyapatite) are favorable for the osseointegration process. Calcium phosphate might be formed in crystalline or amorphous phase on top of the oxide layer; thus, Raman spectroscopy and the XRD technique were used for analyzing the coatings in detail. The broad shapes of the peaks observed in the Raman spectrum might be due to the high porosity of the coatings and formation of many OH
− groups. The signals at 638, 823 and between 315 and 330 cm
−1 correspond to TiO
2 anatase due to the anodization of the Ti alloy and were discussed in detail in our previous paper [
45]. To understand the mechanism of formation the ceramic layer and results of cytocompatibility, the phase composition of the layers was analyzed in detail, step by step. First, the substrate was measured, where the phase of Ti.
085Al
0.15 was found, with peaks close to that of the Ti phase. However, the maximum peaks of the recorded XRD patterns much better correspond to the phase with titanium and a small content of aluminum, such as the chemical composition of our Ti-6Al-7Nb alloys. The anodization process, only in Ca(H
2PO
2)
2 solution, caused the formation of the oxide layer; however, some ions of calcium and phosphorous compounds were incorporated into the layer. The main part of the oxide layer comprises TiO
2; in our case, the peaks correspond to the anatase phase. It is possible to obtain the layer with TiO
2 in another polymorphic form, such as brookite or rutile, which was reported previously [
46]. The differences in the polymorphic forms and the forms in coatings on the Ti alloy by the PEO process have been previously described [
47]. The shape of the XRD pattern indicates that some amorphous parts were formed in the layer. No peaks from the crystalline calcium phosphate compounds were found. The peaks that corresponded to tricalcium phosphate (Ca
3(PO
4)
2) were reordered when the Ti alloy was anodized in the solution (suspensions) with the ceramic particles of Ca
3(PO
4)
2. In our case, some of the ceramic particles likely melted and were recorded by XRD as an amorphous-crystalline phase.
Figure 7 shows that in our case, the amorphous parts on the XRD pattern increased. Some parts of ceramic particles were likely melted but existed inside the crystal and could be detected by XRD. Another explanation is the possibility to form calcium phosphates from the anodizing bath due to spark discharges. The temperature of the spark discharges could be different than those that occur during anodization in solution without ceramic particles. The formation of the additional ceramic layer by sol-gel revealed the hydroxyapatite and tricalcium phosphate phases (
Figure 7). The peaks corresponding to the Ca
3(PO
4)
2 phase was slightly shifted and less visible than those observed for the TAN-A sample. However, the peaks corresponding to the hydroxyapatite particles were well-defined at 2θ, indicating that this phase was formed during the post-treatment process (formation of the layer by the sol-gel technique). The chemical composition of the TAN-SG sample was also analyzed by Raman spectroscopy, which confirmed that the mixture of Ca
3(PO
4)
2 and Ca
10(PO
4)
6(OH
2) was formed during the formation of the hybrid, ceramic coatings and formation of the calcium phosphate compounds from the anodizing bath. Formation of the compounds may be explained using the following reactions:
After the anodization process, Ca
2+, HPO
42− and OH
− probably exist in the layer. Thus, the formation of the additional ceramic layer cannot only explain the formation of the hydroxyapatite layer. The mechanism might be explained by reactions reported in a study [
48].
In addition to the bioactivity of the surface, the mechanical properties of long-term implants play a crucial role in medicine. One method to evaluate the adhesion of the coating to the substrate is the scratch-test method. Oxide layers formed on a Ti alloy by the anodization process usually present good adhesion. This type of ceramic coating and the layer formed by the PEO process may be brittle. It is not obvious that all of the layers will be favorable for implant manufacturing due to their delamination during the implantation procedure. Takemoto et al. [
49] reported that implanted implants with formed ceramic coatings (formed by the plasma-spray method) with a porosity of 40% showed a larger index of bone–implant contact with the bone tissue of rabbits than the non-treated surface of Ti. The differences in the affinity index were significant, and the final effect suggests that the layer was biocompatible and promising for clinical application. In our case, the layers were also porous. In our case, for all of the coatings, no characteristic signal for their brittleness was recorded (no acoustic emission signal). However, significant differences in their values of Lc
2 and Lc
3 were recorded. For the only-anodized surface (TAN-A) sample, the Lc
2 and Lc
3 values were higher, which indicates that the adhesion of the layer was much better than for the hybrid layer (TAN-SG). However, measurement of the porous coatings with higher surface roughness was less repeatable, and the highest standard deviation was calculated for the results of measurement of the TAN-A sample. Formation of the additional ceramic layer by the sol-gel process caused the thickness of the total layer to increase. However, the hybrid coating was not brittle and no cracks were observed during the scratch-testing. Surface roughness for this layer was lower, and repetition of the measurements showed better reproducibility. Beside the lower values of Lc
2 and Lc
3 for the TAN-SG sample, the results indicate that the layer was still well adhered. It is difficult to compare our results with others published in the literature due to differences in parameters applied during the scratching, applying different intenders and the thickness of the coatings, which play a key role in the coatings analysis [
50]. The decrease in the adhesion of the TAN-SG sample could be caused by the heat treatment process, during which the porosity of the layer could change and moisture or other gases present inside the oxide layer might also evolve.
Adsorption of proteins influences the adhesion and proliferation of osteoblast-like cells. More than 28 types of collagen were recognized. For bone tissue treatment, one of them, collagen type I, is widely used as a commercial product, used to fill the bone hole as they are injected around the bones. Collagen types III, V and XXIV have been classified as fibril-forming, which contributes, with collagen type I, for example, in embryonic tissues and in cornea or plays a role in selective expression in developing cornea and bone [
51]. Collagen type I exists mainly in the organic part of bone matrix. It participates in the remodeling process of bone tissue and it is used to modify the surface of ceramic materials, such as scaffolds. Collagen type I enhances osteoconductive properties and increases the surface area for osteoblast cell adhesion [
52]. Different mechanisms of cell interaction with lactoferrin (LF) were recognized as well. It was found that LF promotes in vitro and in vivo osteoblast proliferation and survival and inhibits osteoclastogenesis as well [
53]. It was reported that LF induces ERK (erk-kinase) phosphorylation in osteoblast cells.
The main purpose of surface treatment with ceramic materials is to enhance the bioactivity of the surface. Immersion of the samples in the solution with the proteins (collagen or lactoferrin) influenced the osteoblast-like MG-63 cell adhesion and proliferation (see
Figure 10 and
Figure 11). It was clearly seen that after immersion of the TAN-SG sample in the lactoferrin solution, the cells were faster and much better adhered, so their proliferation could be observed. These results were obtained after 3 and 7 days of cell culture, meaning that the response is from the surface of the sample, not from the solution and culture medium, which could be slightly modified during the first 24 h of the cell culture (the proteins could diffuse from the surface to the culture medium). The fluorescence image indicates that on the TAN-SG sample surface, a number of dead cells (red cells) are observed after 3 days of the culture. However, the number of live cells is slightly lower (on the fluorescence images) than the number of cells observed on the TAN-SG sample after immersion in the solution of collagen type I or in the solution of lactoferrin. After 7 days of the culture, for all the surfaces, the cells were well adhered and only single dead cells were observed, which are usually detected during such in vitro experiments. Thus, we have concluded that lactoferrin supports the proliferation of osteoblast-like MG-63 cells. The ability of this protein to adsorb on ceramic coatings is a factor which potentially might be used to form the materials with LF to induce synthesis of growth factors and cytokines by osteoblasts. It was reported that hLF (human lactoferrin) exhibits anti-apoptotic properties towards MC3T3 pre-osteoblast cells [
54]. The sample immersed in collagen type I solution (TAN-SG-C) did not present much better cytocompatibility with the cells; however, the surface was not cytotoxic. The TAN-SG sample without protein was also cytocompatible with osteoblast-like MG-63 cells up to 7 days of their culture. The surfaces were completely covered with the cells and only single dead cells occurred, likely resulting from the in vitro conditions and limited space for cell proliferation. Significant differences were observed between the surfaces up to 3 days of culture, in which immersion of the TAN-SG sample in lactoferrin solution positively influenced cell behavior, such as adhesion and proliferation. Application of osteoblast cell growth factors on the bone implants is one of the ways to promote osseointegration and to promote adhesion of osteoblast cells on the implant surface faster than, for example avoid septic inflammation which may occur after the surgery.