Synthesis and Characterization of a New Aluminosilicate Molecular Sieve from Aluminosilica Perhydrate Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.3. Characterizations
3. Results and Discussion
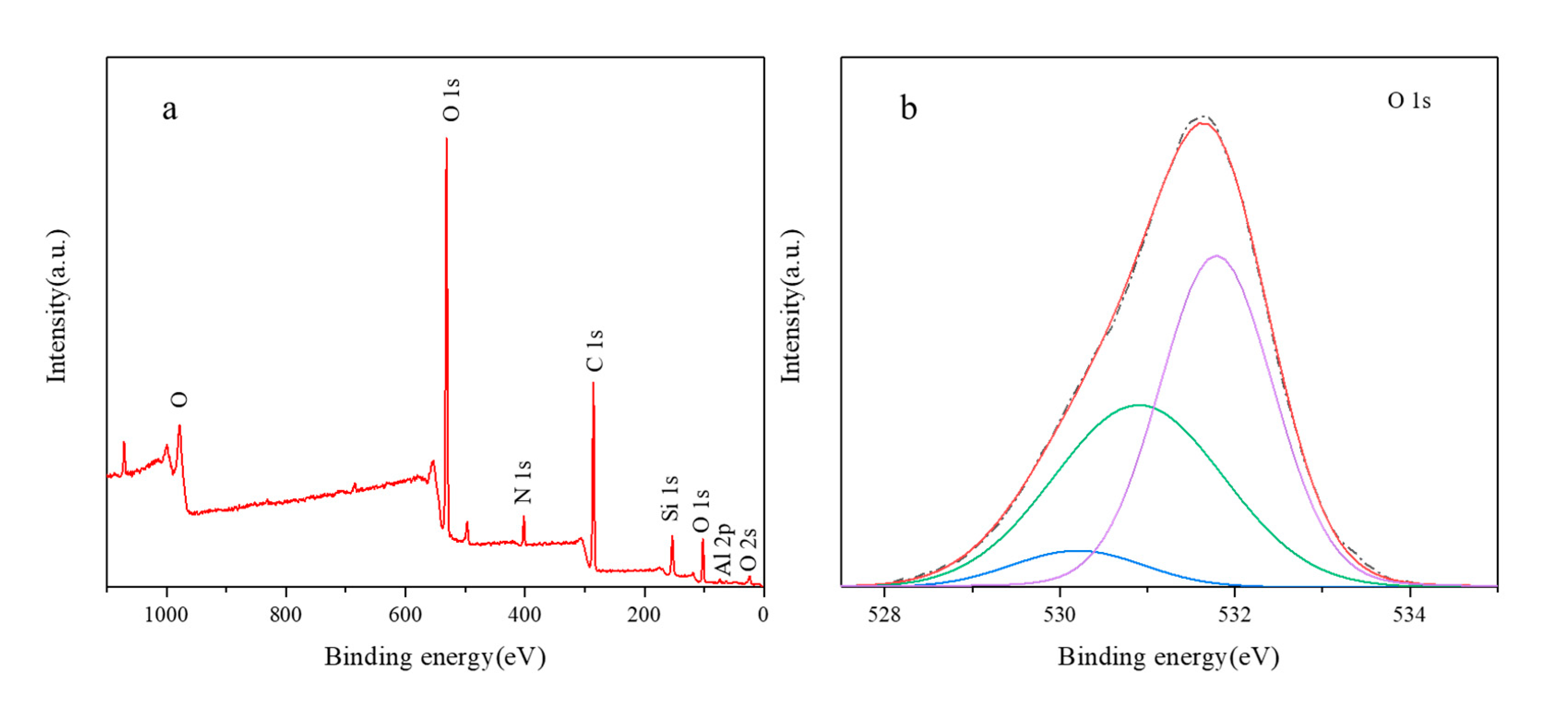
3.1. Characterization of Raw Materials
3.2. Optimized Synthesis and Characterization
3.3. Structural Analysis of BUCT-3 Molecular Sieves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rabo, J.A.; Kasai, P.H. Caging and electrolytic phenomena in zeolites. Prog. Solid State Chem. 1975, 9, 1–19. [Google Scholar] [CrossRef]
- Barthomeuf, D.A. General hypothesis on zeolites physicochemical properties. Applications to adsorption, acidity, catalysis, and electrochemistry. J. Phys. Chem. 1979, 83, 249–256. [Google Scholar] [CrossRef]
- Corma, A.; Davis, M.E. Issues in the synthesis of crystalline molecular sieves: Towards the crystallization of low framework-density structures. Chem. Phys. Chem. 2004, 5, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zones, S.I. Translating new materials discoveries in zeolite research to commercial manufacture. Microporous Mesoporous Mater. 2011, 144, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Rey, F.; Corma, A. Towards the rational design of efficient organic structure-directing agents for zeolite synthesis. Angew. Chemie Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef] [PubMed]
- Goepper, M.; Li, H.-X.; Davis, M.E. A possible role of alkali metal ions in the synthesis of pure-silica molecular sieves. J. Chem. Soc. Chem. Commun. 1992, 22, 1665–1666. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Xu, R. Progress in heteroatom-containing aluminophosphate molecular sieves. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 1955–1967. [Google Scholar] [CrossRef]
- Yi, T.; Xu, J.; Zi, G. Isomorphous substitution of metallic elements for zeolite Y in the aqueous solution. Chem. Res. Chin. Univ. 1990, 4, 332–340. [Google Scholar]
- Xu, Z.L.; Zhang, Y.Z.; Zheng, L.B. The secondary synthesis of zeolites via framework substitution for aluminum Ⅰ preparation and characterization of zeolite Y containing Ga. J. Mol. Catal. 1992, 6, 271–278. [Google Scholar]
- Dwyer, J.; Karim, K. The incorporation of heteroatoms into faujastic framework by secondary synthesis using aqueous fluoride complexes. J. Chem. Soc. Chem. Commun. 1991, 14, 905–906. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Cejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef] [PubMed]
- Díaz, U.; Corma, A. Layered zeolitic materials: An approach to designing versatile functional solids. Dalton Trans. 2014, 43, 10292–10316. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; CRC press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Dai, F.Y.; Suzuki, M.; Takahashi, H.; Saito, Y. Zeolite Synthesis; Occelli, M.L., Robson, H.E., Eds.; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Ryozi, H.; Yoshiro, M. Effects of changes of mother-liquor composition on crystallizatiolu process. Mem. Fac. Sci. Shimane Univ. 1994, 28, 47–58. [Google Scholar]
- Warzywoda, J.; Dixon, A.G.; Thompson, R.W.; Sacco Jr, A.; Suib, S.L. The role of the dissolution of silicic acid powders in aluminosilicate synthesis mixtures in the crystallization of large mordenite crystals. Zeolites 1996, 16, 125–137. [Google Scholar] [CrossRef]
- Warzywoda, J.; Dixon, A.G.; Thompson, R.W.; Sacco, A. Synthesis and control of the size of large mordenite crystals using porous silica substrates. J. Mater. Chem. 1995, 5, 1019–1025. [Google Scholar] [CrossRef][Green Version]
- Zhu, G.; Qiu, S.; Yu, J.; Gao, F.; Xiao, F.; Xu, R.; Sakamoto, Y.; Terasaki, O. Synthesis of Zeolite LTA Single Crystals of Macro-to Nanometer Size; Materials Research Society: Warrendale, PA, USA, 1999; pp. 1863–1870. [Google Scholar]
- Kalipcilar, H.; Culfaz, A. Influence of nature of silica source on template-free synthesis of ZSM-5. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2001, 36, 1197–1207. [Google Scholar] [CrossRef]
- Kalipcilar, H.; Culfaz, A. Synthesis of submicron silicalite-1 crystals from clear solutions. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2000, 35, 933–942. [Google Scholar] [CrossRef]
- Li, Q.; Mihailova, B.; Creaser, D.; Sterte, J. Aging effects on the nucleation and crystallization kinetics of colloidal TPA-silicalite-1. Microporous Mesoporous Mater. 2001, 43, 51–59. [Google Scholar] [CrossRef]
- Ko, Y.S.; Chang, S.H.; Ahn, W.S. 02-P-19-effects of synthesis parameters on zeolite l crystallization. Stud. Surf. Sci. Catal. 2001, 135, 189. [Google Scholar]
- Schmidt, W.; Toktarev, A.V.; Schueth, F.; Ione, K.G.; Unger, K. 02-P-23-the influence of different silica sources on the crystallization kinetics of zeolite beta. Stud. Surf. Sci. Catal. 2001, 135, 190. [Google Scholar]
- Hamidi, F.; Pamba, M.; Bengueddach, A.; Di Renzo, F.; Fajula, F. 03-P-19-factors affecting composition and morphology of mordenite. Stud. Surf. Sci. Catal. 2001, 135, 334. [Google Scholar]
- Lowe, B.M.; MacGilp, N.A.; Whittam, T.V. Active silicates and their role in zeolite synthesis. In Proceedings of the 5th International Conference on Zeolites, Naples, Italy, 2–6 June 1980. [Google Scholar]
- Rabo, J.A.; Schoonover, M.W. Early discoveries in zeolite chemistry and catalysis at union carbide, and follow-up in industrial catalysis. Appl. Catal. A Gen. 2001, 222, 261–275. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aly, H.M.; El-shahat, M.F.; Ibrahim, I.A. Effect of the silica sources on the crystallinity of nanosized ZSM-5 zeolite. Microporous Mesoporous Mater. 2005, 79, 7–12. [Google Scholar] [CrossRef]
- Elkin, P.B.; Shull, C.G.; Roess, L.C. Silica-alumina, gels. Ind. Eng. Chem. 1945, 37, 327–331. [Google Scholar] [CrossRef]
- Plank, C.J.; Drake, L.C. Differences between silica and silica-alumina gels. Factors affecting the porous structure of these gels. J. Colloid Sci. 1947, 2, 399–412. [Google Scholar] [CrossRef]
- Zegliński, J.; Piotrowski, G.P.; Piekoś, R. A study of interaction between hydrogen peroxide and silica gel by FTIR spectroscopy and quantum chemistry. J. Mol. Struct. 2006, 794, 83–91. [Google Scholar] [CrossRef]
- Wolanov, Y.; Shurki, A.; Prikhodchenko, P.V.; Tripoľskaya, T.A.; Novotortsev, V.M.; Pedahzur, R.; Lev, O. Aqueous stability of alumina and silica perhydrate hydrogels: Experiments and computations. Dalton Trans. 2014, 43, 16614–16625. [Google Scholar] [CrossRef]
- Szulbinski, W.S. ESR and resonance raman spectroscopic studies of peroxide intermediates derived from iron diazaoxacyclononane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2117–2124. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Rao, C.N.R. A Combined Eels-Xps study of molecularly chemisorbed oxygen on silver surfaces: Evidence for superoxo and peroxo species. Surf. Sci. 1987, 186, 575–580. [Google Scholar] [CrossRef]
- Scholes, F.H.; Hughes, A.E.; Hardin, S.G.; Lynch, P.; Miller, P.R. Influence of hydrogen peroxide in the preparation of nanocrystalline ceria. Chem. Mater. 2007, 19, 2321–2328. [Google Scholar] [CrossRef]
- Scholle, K.F.M.G.J.; Veeman, W.S.; Frenken, P.; Velden, G.P.M.V.D. Characterization of intermediate TPA-ZSM-5 type structures during crystallization. Appl. Catal. 1985, 17, 233–259. [Google Scholar] [CrossRef]
- Koningsveld, H.V.; Bekkum, H.V.; Jansen, J.C. On the location and disorder of the tetrapropylammonium (TPA) ion in zeolite ZSM-5 with improved framework accuracy. Acta Crystallogr. 1987, 43, 127–132. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: https://asia.iza-structure.org/IZA-SC/pow_pat_user-data.php (accessed on 15 May 2018).
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. In Molecular Sieve Zeolites; Advances in Chemistry: Washington, DC, USA, 1971; pp. 201–229. [Google Scholar]
- Liu, X.; Wang, Q.; Wang, C.; Xu, J.; Deng, F. Hydrogen-bond induced crystallization of silicalite-1 zeolite as revealed by solid-state NMR spectroscopy silicalite-1. Acta Phys. Sin. 2020, 36, 1–9. [Google Scholar]

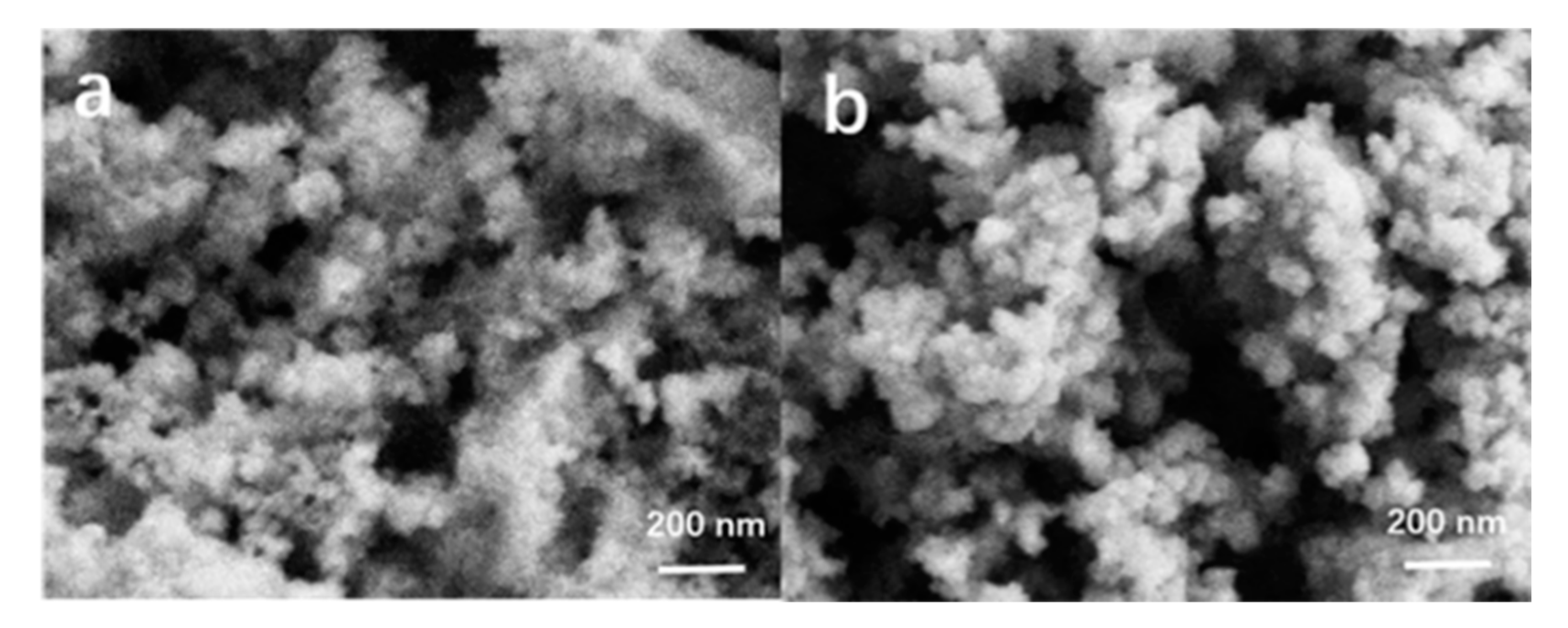
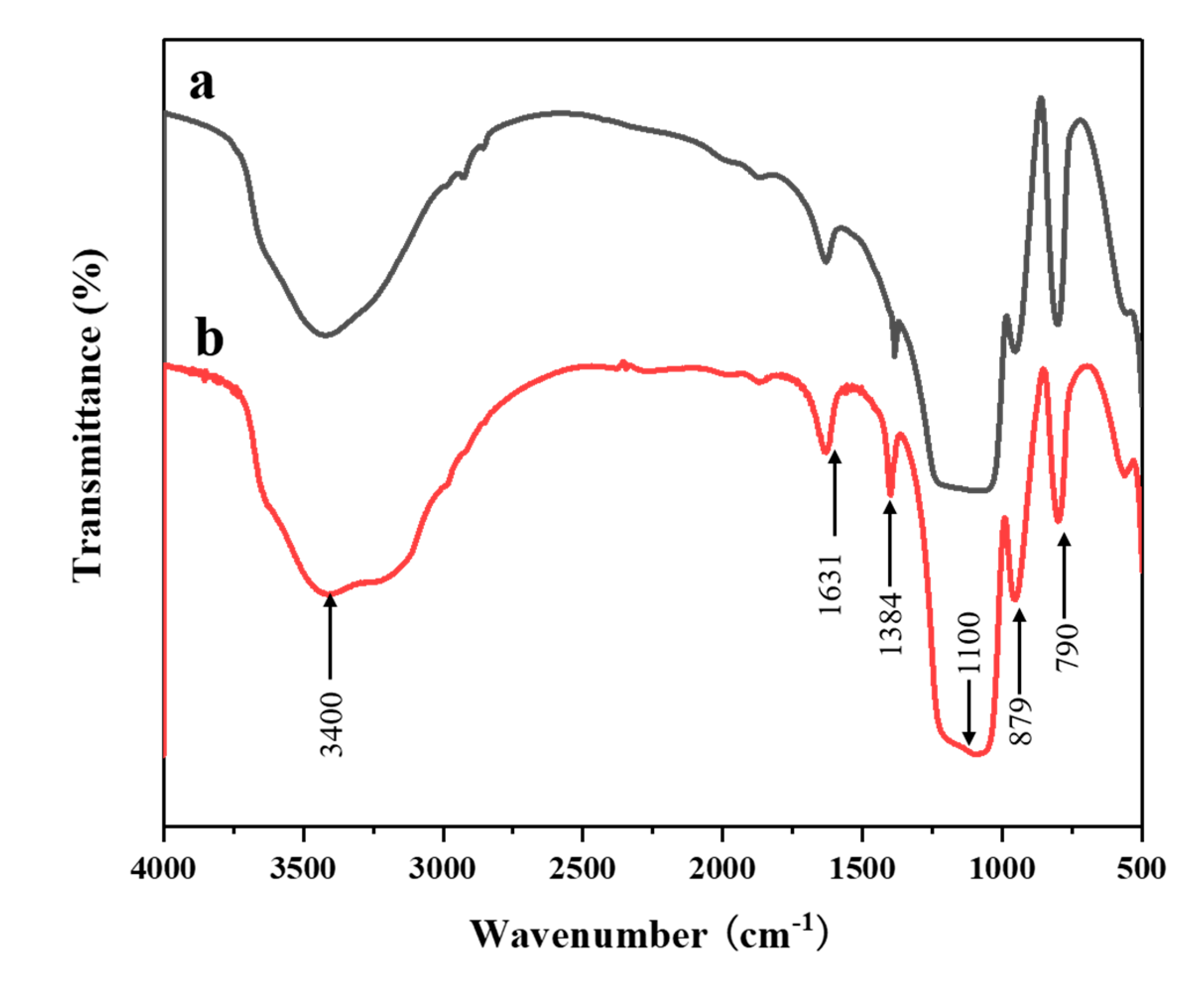
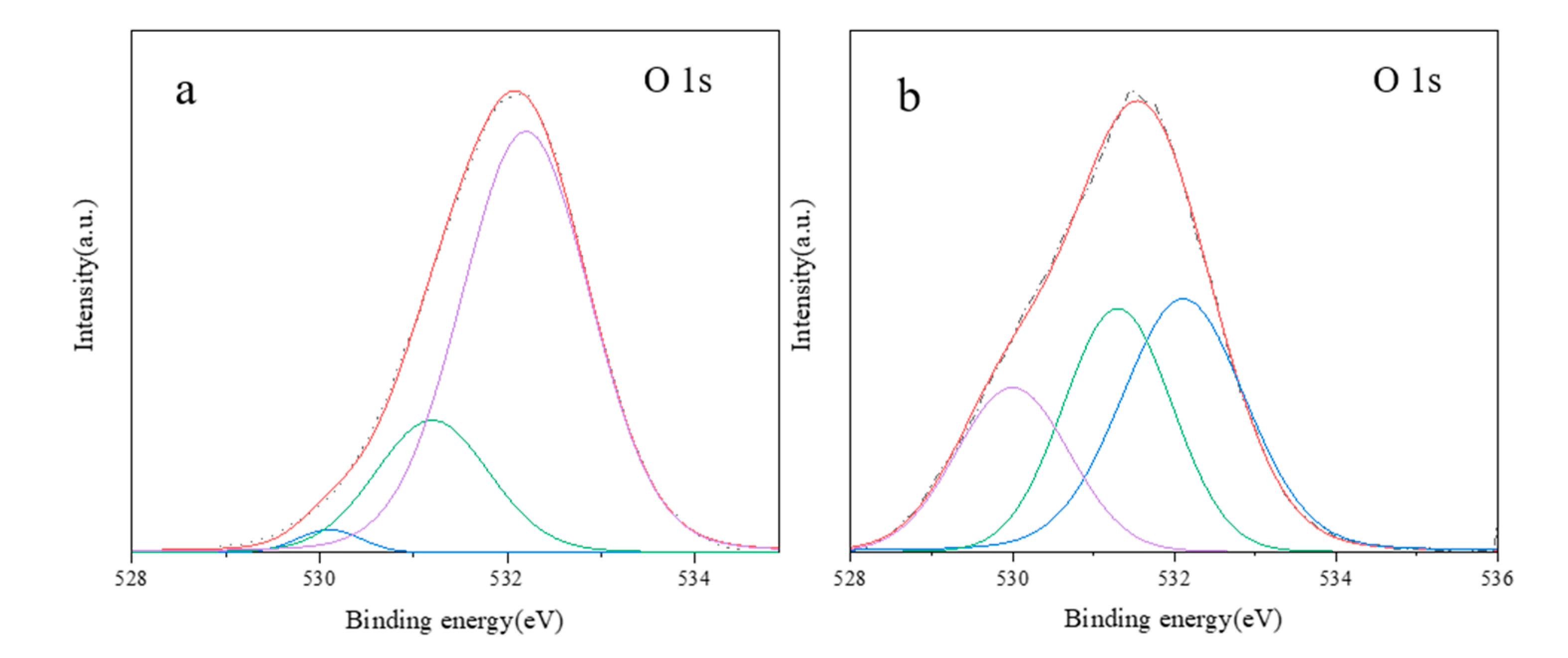
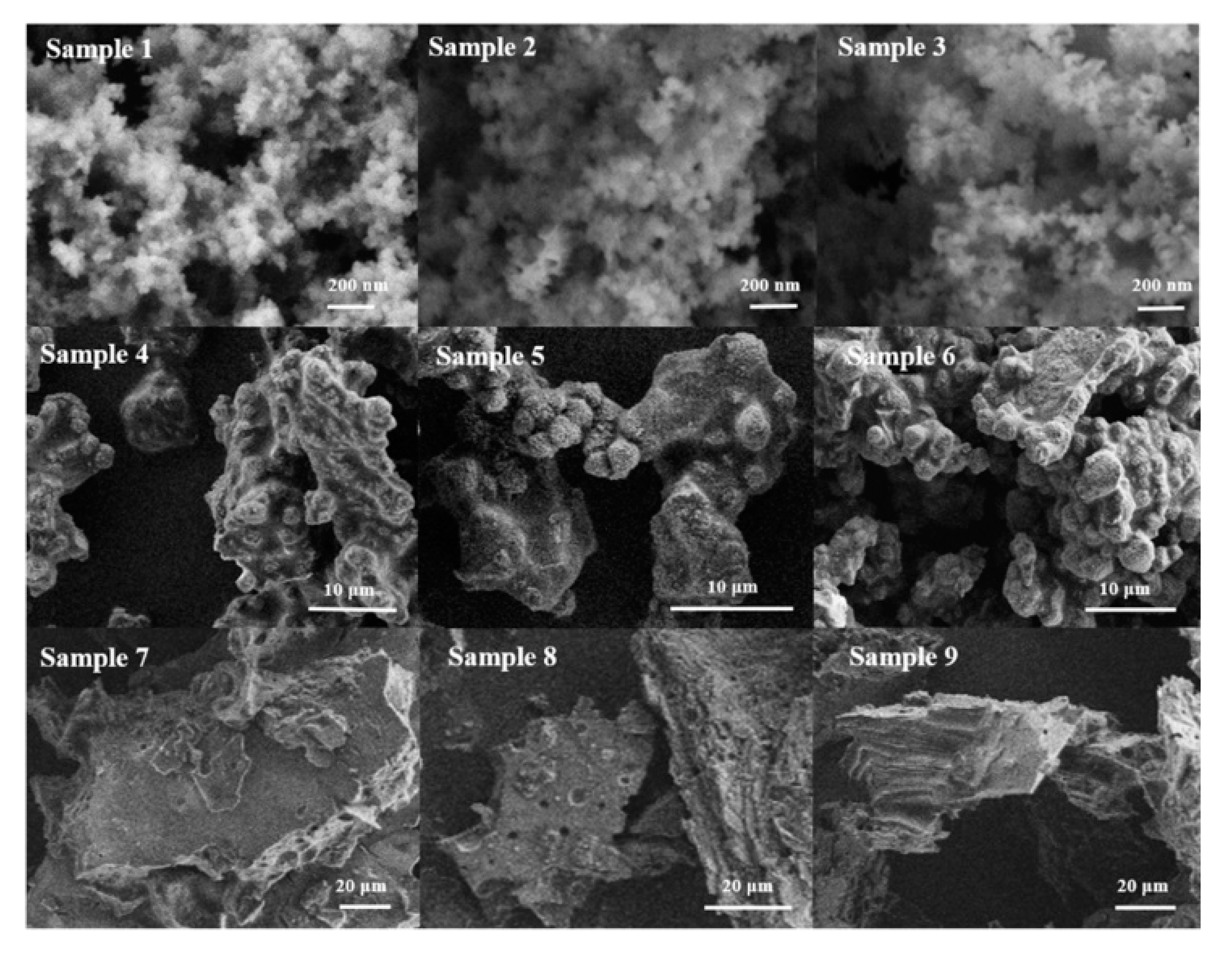
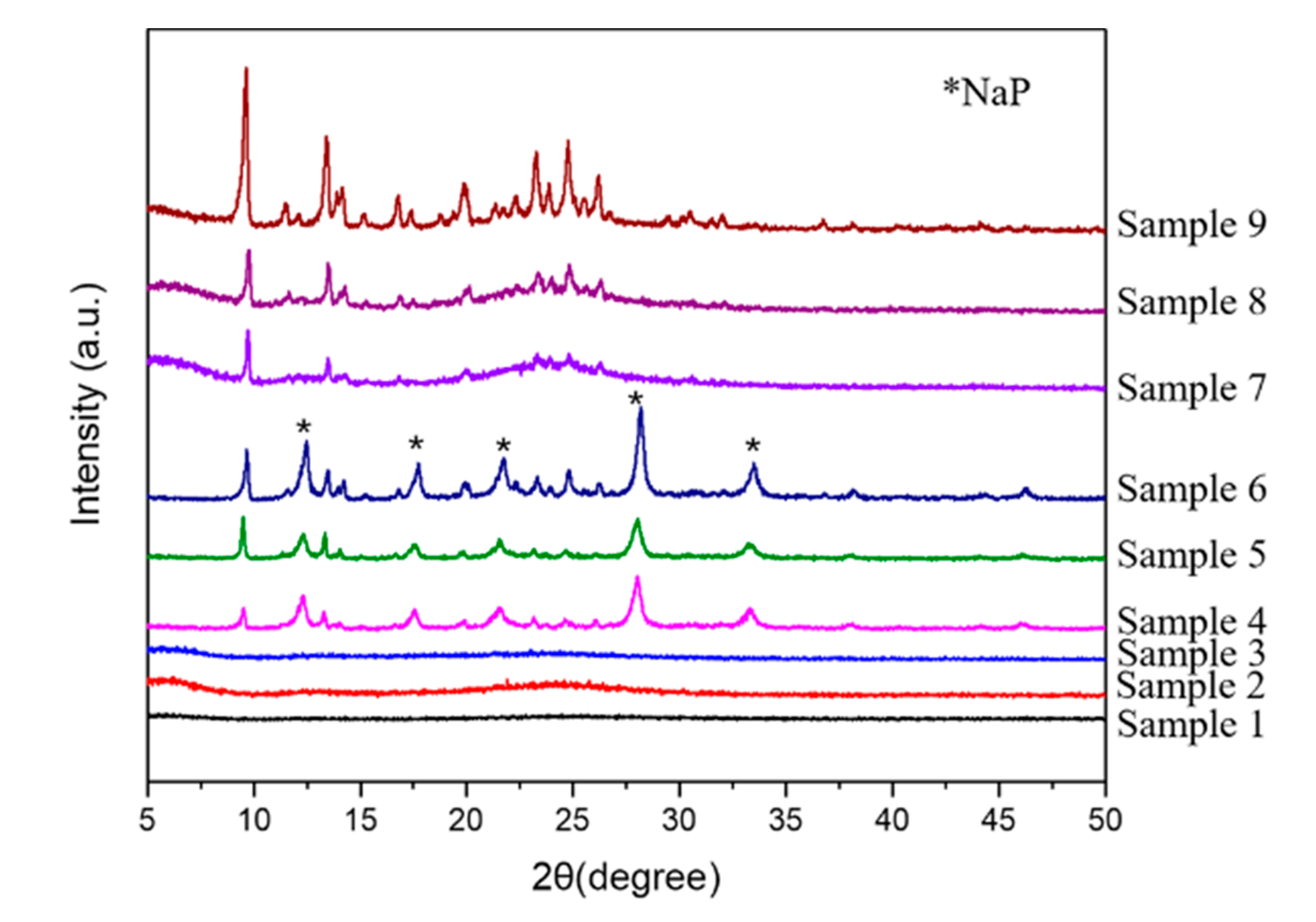
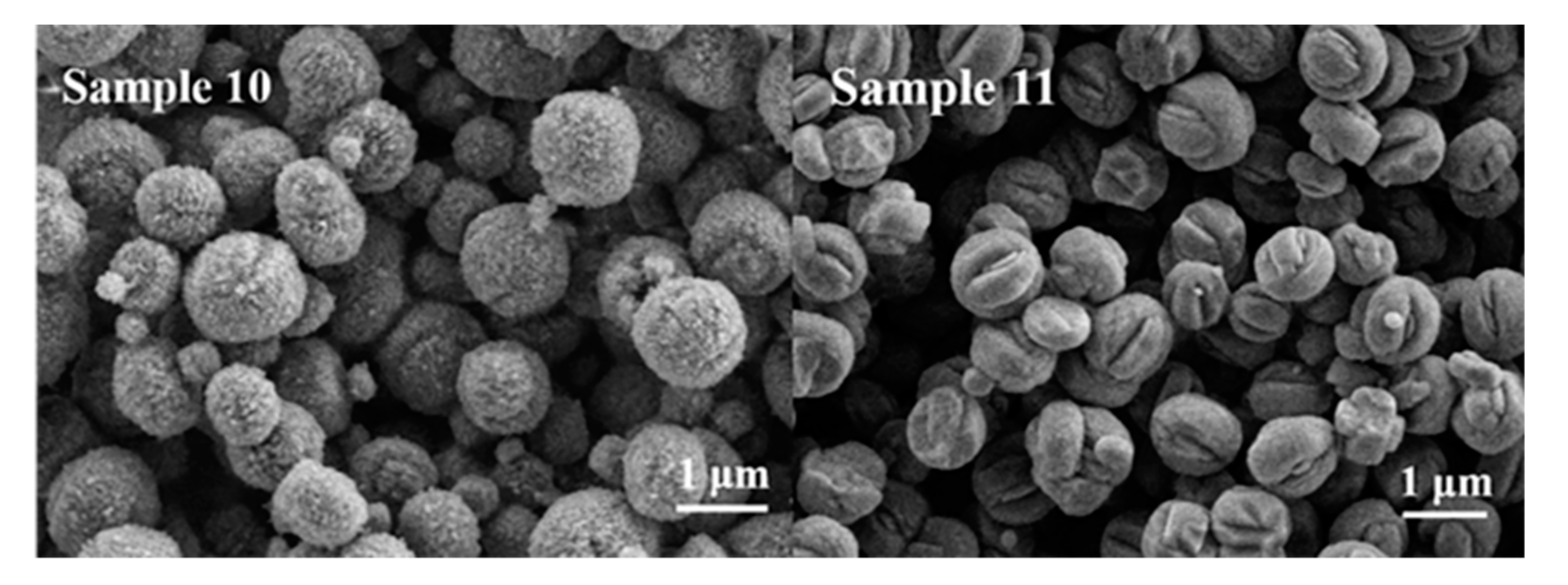
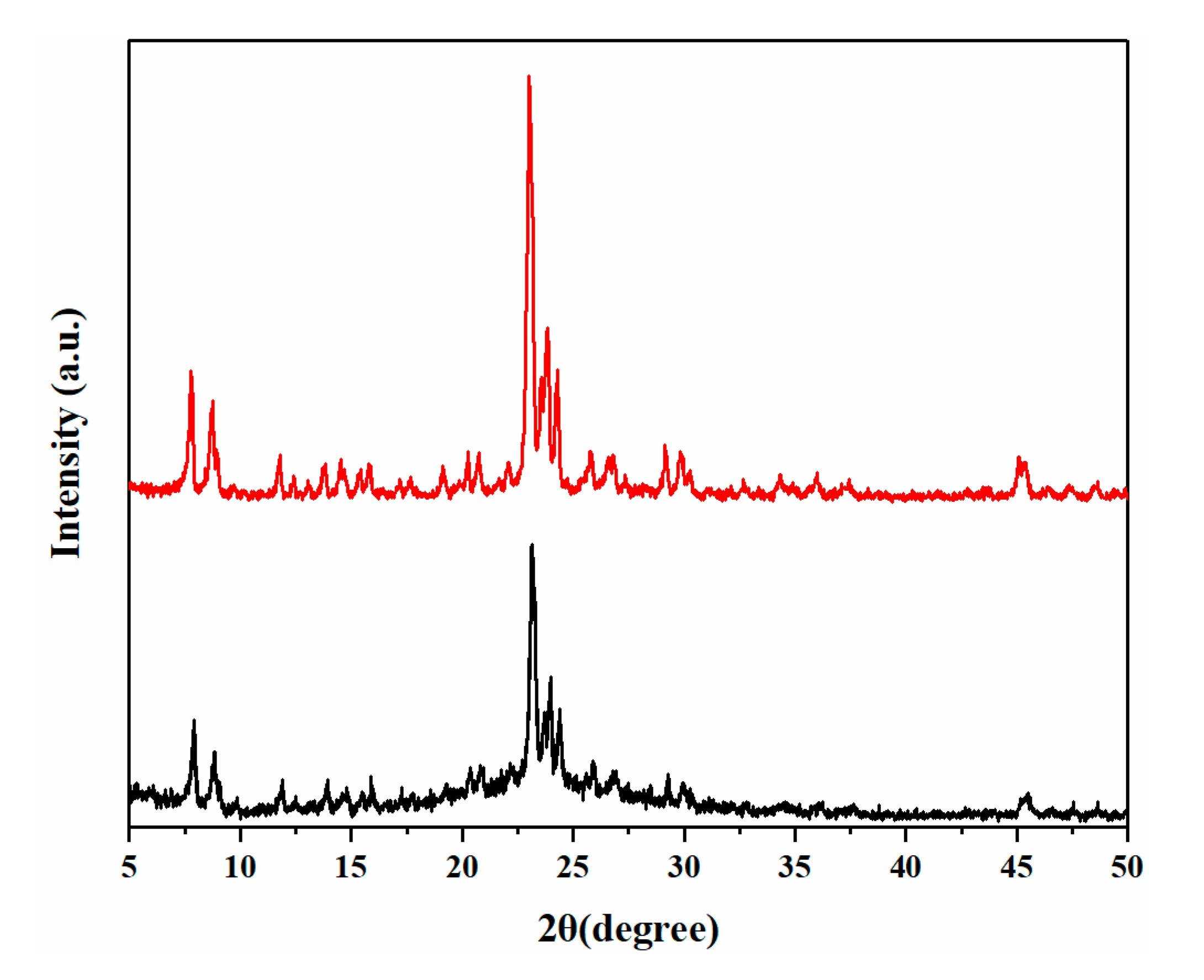
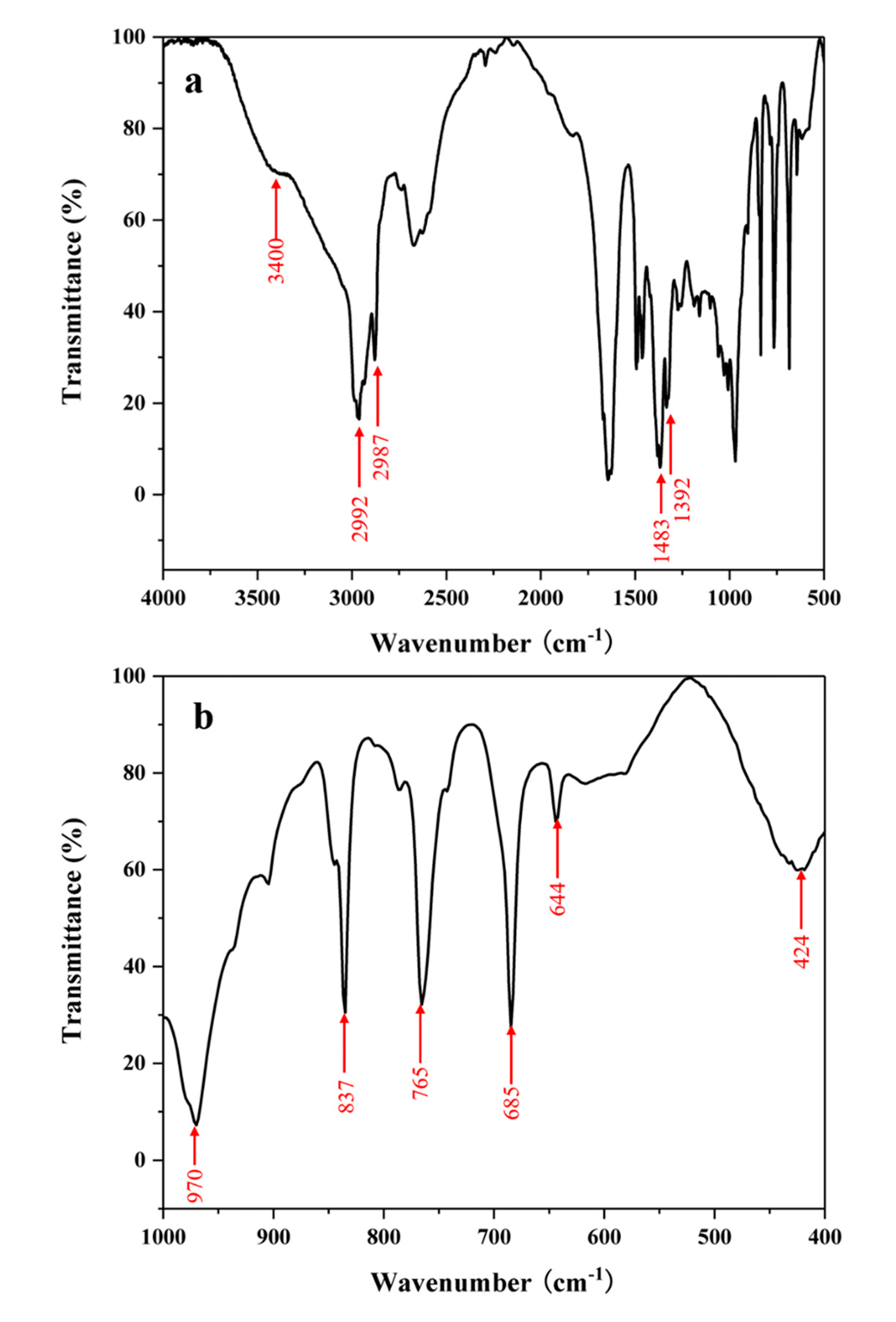

| Sample | O22− (%) | OH− (%) | Bulk O (%) |
|---|---|---|---|
| SiAl-1 | 2.1 | 21.6 | 76.1 |
| SiAl-2 | 23.4 | 33.2 | 42.3 |
| Sample Number | Silicon and Aluminum Source | Temperature (°C) | Time (d) | Phase |
|---|---|---|---|---|
| Sample 1 | SiO2 + NaAlO2 | 70 | 4 | amorphous |
| Sample 2 | SiO2 + NaAlO2 | 90 | 2 | amorphous |
| Sample 3 | SiO2 + NaAlO2 | 90 | 4 | amorphous |
| Sample 4 | SiAl-1 | 70 | 4 | NaP + BUCT-3 |
| Sample 5 | SiAl-1 | 90 | 2 | NaP + BUCT-3 |
| Sample 6 | SiAl-1 | 90 | 4 | NaP + BUCT-3 |
| Sample 7 | SiAl-2 | 70 | 4 | BUCT-3 (L) |
| Sample 8 | SiAl-2 | 90 | 2 | BUCT-3 |
| Sample 9 | SiAl-2 | 90 | 4 | BUCT-3 |
| Sample Number | Silicon and Aluminum Source | Temperature (°C) | Time(d) | Phase |
|---|---|---|---|---|
| Sample 10 | SiO2 + NaAlO2 | 120 | 2 | ZSM-5 |
| Sample 11 | SiAl-2 | 120 | 2 | ZSM-5 |
| Unit Cell Parameters | BUCT-3 |
|---|---|
| a (Å) | 8.9645 |
| Standard deviation of a | 0.0034 |
| b (Å) | 15.2727 |
| Standard deviation of b | 0.0067 |
| c (Å) | 11.3907 |
| Standard deviation of c | 0.0034 |
| α (°) | 90 |
| β (°) | 93.858 |
| γ (°) | 90 |
| Crystal system | Monoclinic |
| Weight Percentage (%) | Atom Ratio | ||||
|---|---|---|---|---|---|
| Element | N | C | H | O | C/N |
| BUCT-3 | 4.62 | 50.94 | 9.88 | 34.56 | 12.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Jiao, K.; Xu, X.; Song, J. Synthesis and Characterization of a New Aluminosilicate Molecular Sieve from Aluminosilica Perhydrate Hydrogel. Materials 2020, 13, 5469. https://doi.org/10.3390/ma13235469
Ma H, Jiao K, Xu X, Song J. Synthesis and Characterization of a New Aluminosilicate Molecular Sieve from Aluminosilica Perhydrate Hydrogel. Materials. 2020; 13(23):5469. https://doi.org/10.3390/ma13235469
Chicago/Turabian StyleMa, Haiqiang, Kun Jiao, Xiangyu Xu, and Jiaqing Song. 2020. "Synthesis and Characterization of a New Aluminosilicate Molecular Sieve from Aluminosilica Perhydrate Hydrogel" Materials 13, no. 23: 5469. https://doi.org/10.3390/ma13235469
APA StyleMa, H., Jiao, K., Xu, X., & Song, J. (2020). Synthesis and Characterization of a New Aluminosilicate Molecular Sieve from Aluminosilica Perhydrate Hydrogel. Materials, 13(23), 5469. https://doi.org/10.3390/ma13235469





