Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results
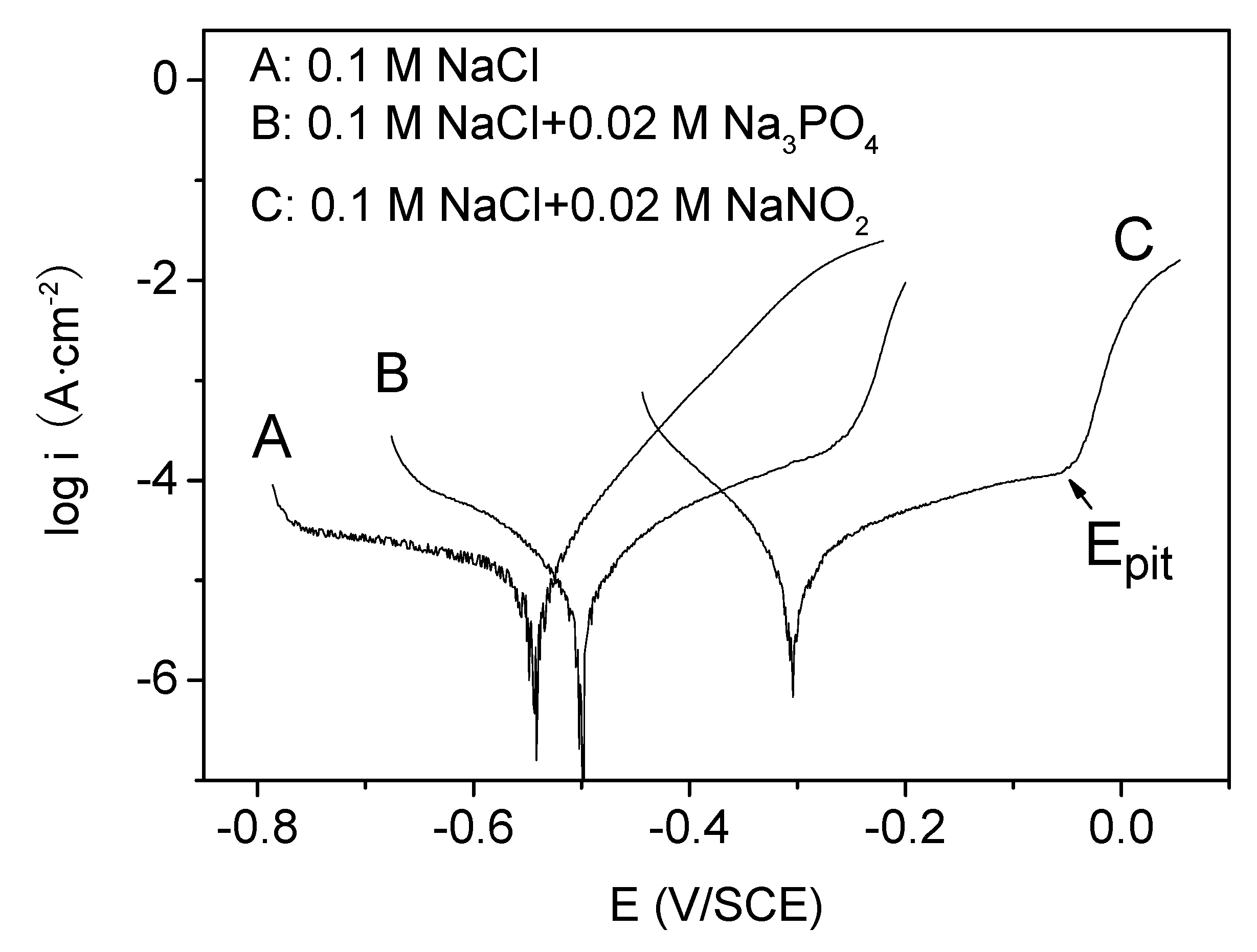
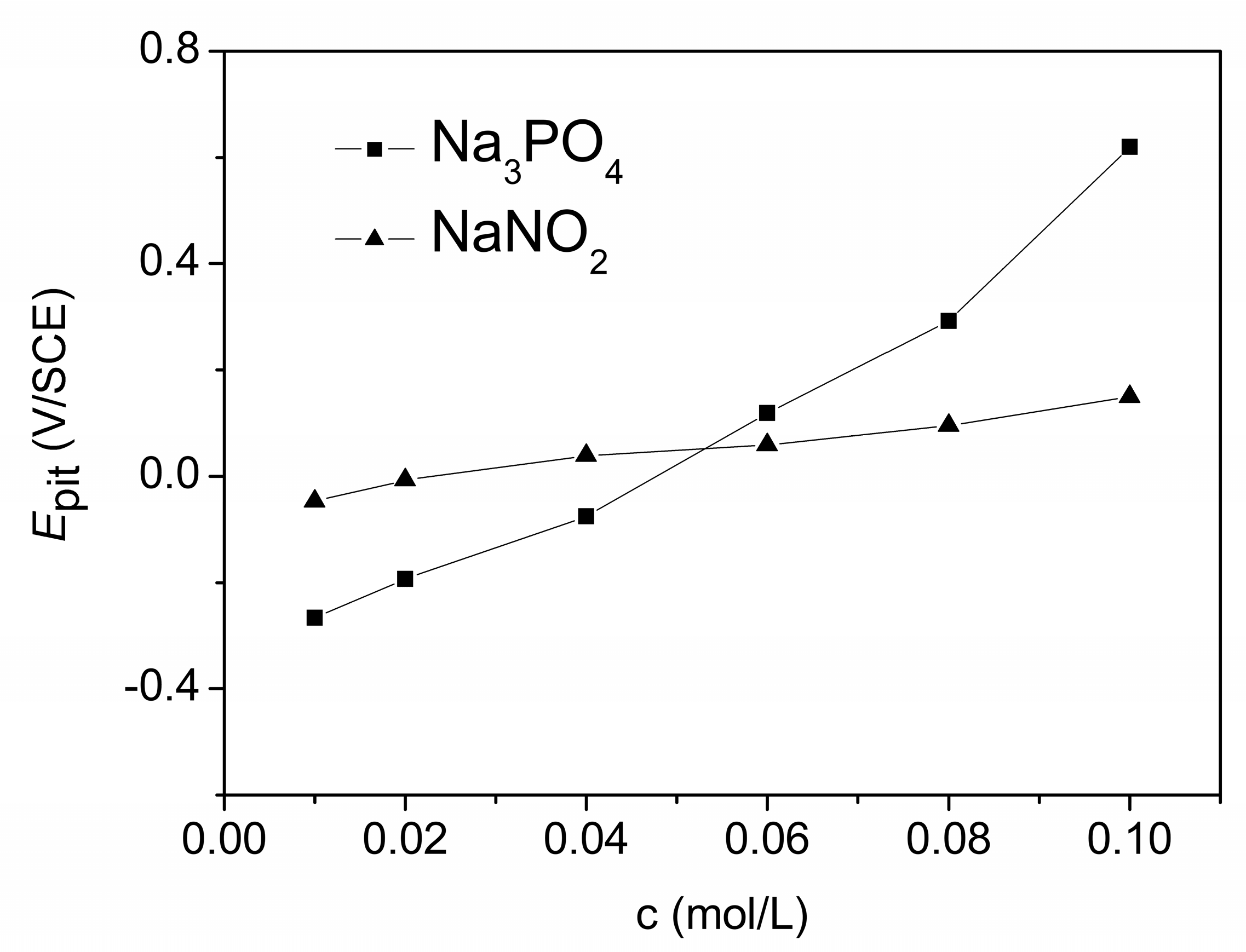
3.1. The Polarization Curves in the NaCl Solution without and with Na3PO4 and NaNO2
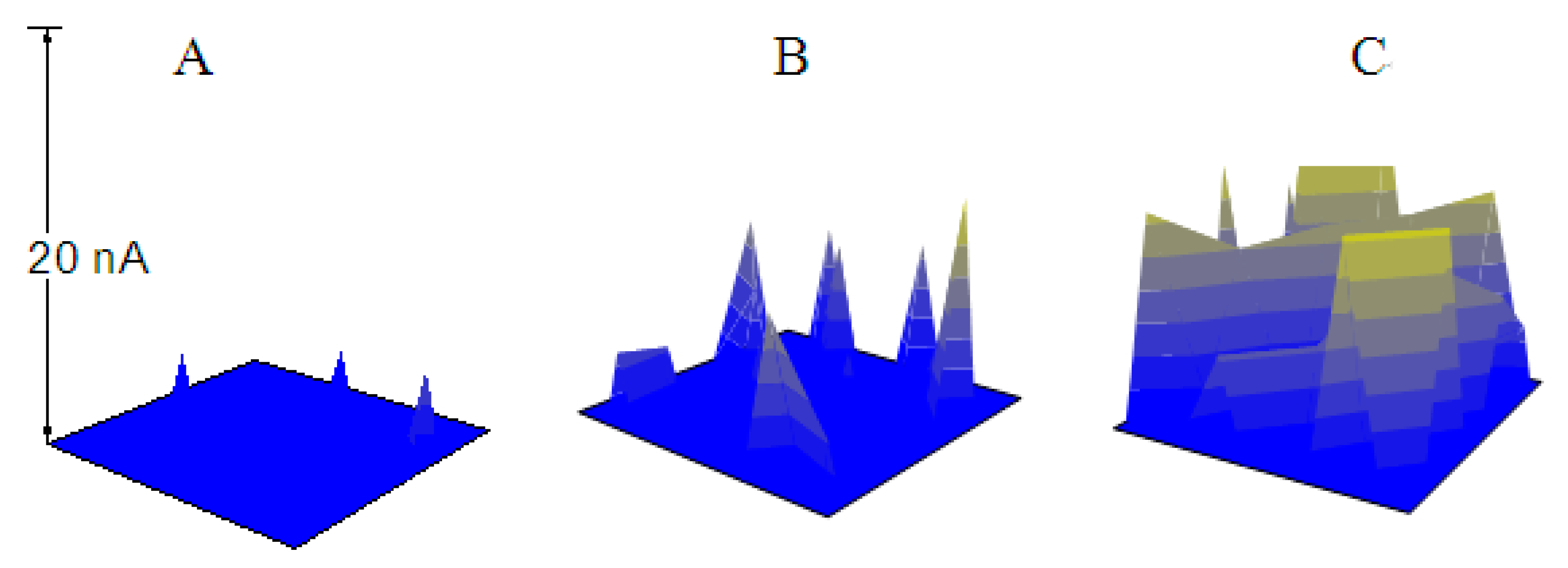
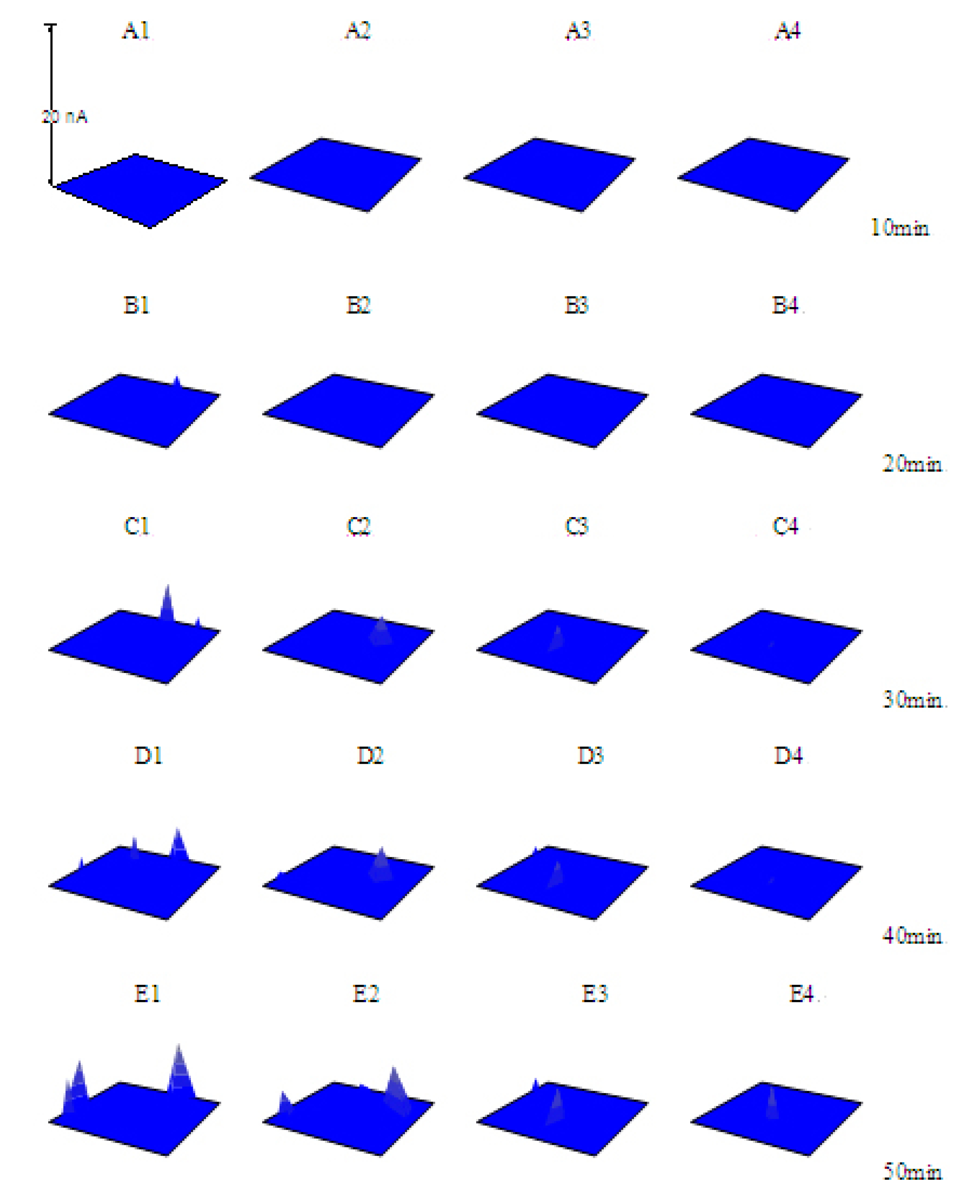
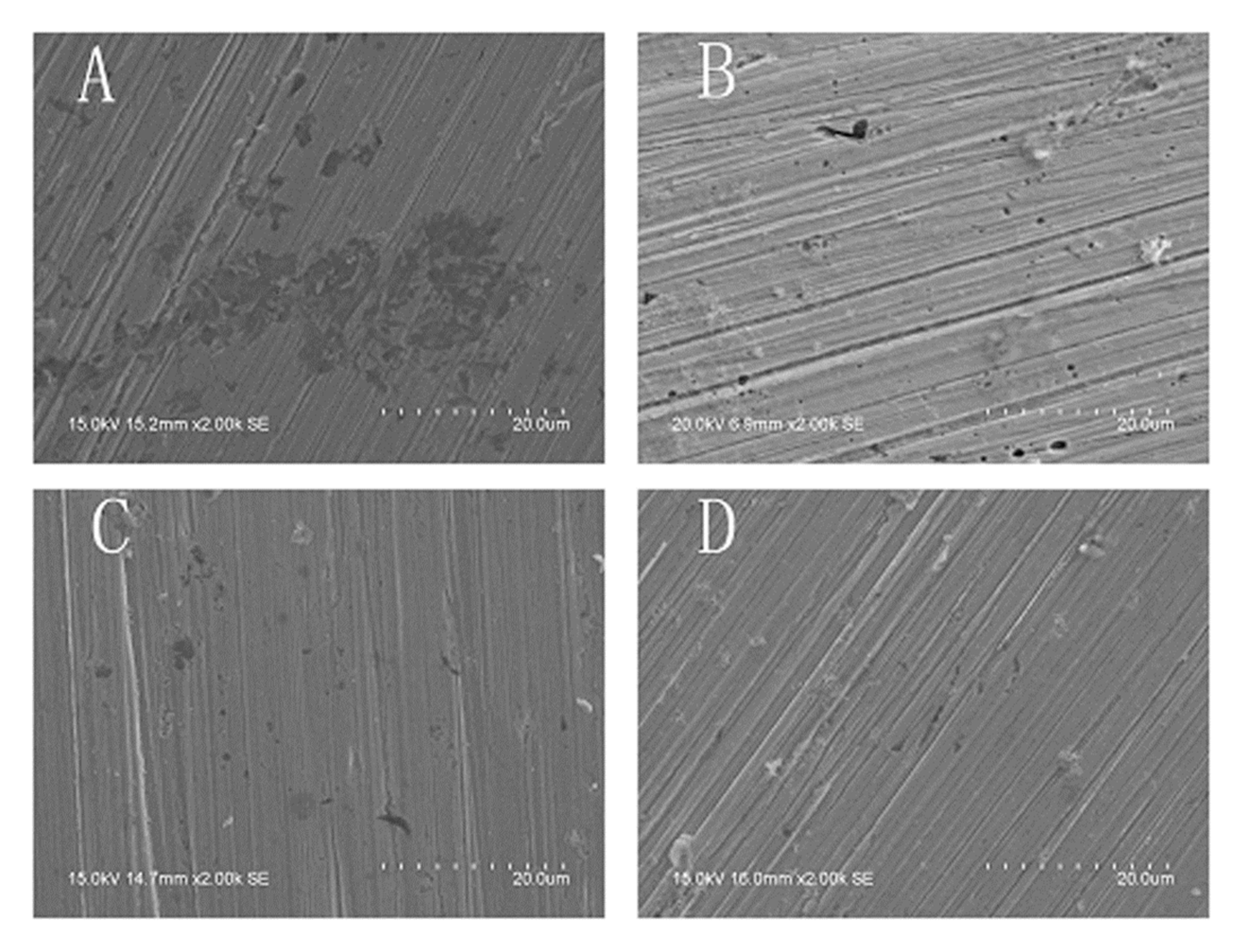
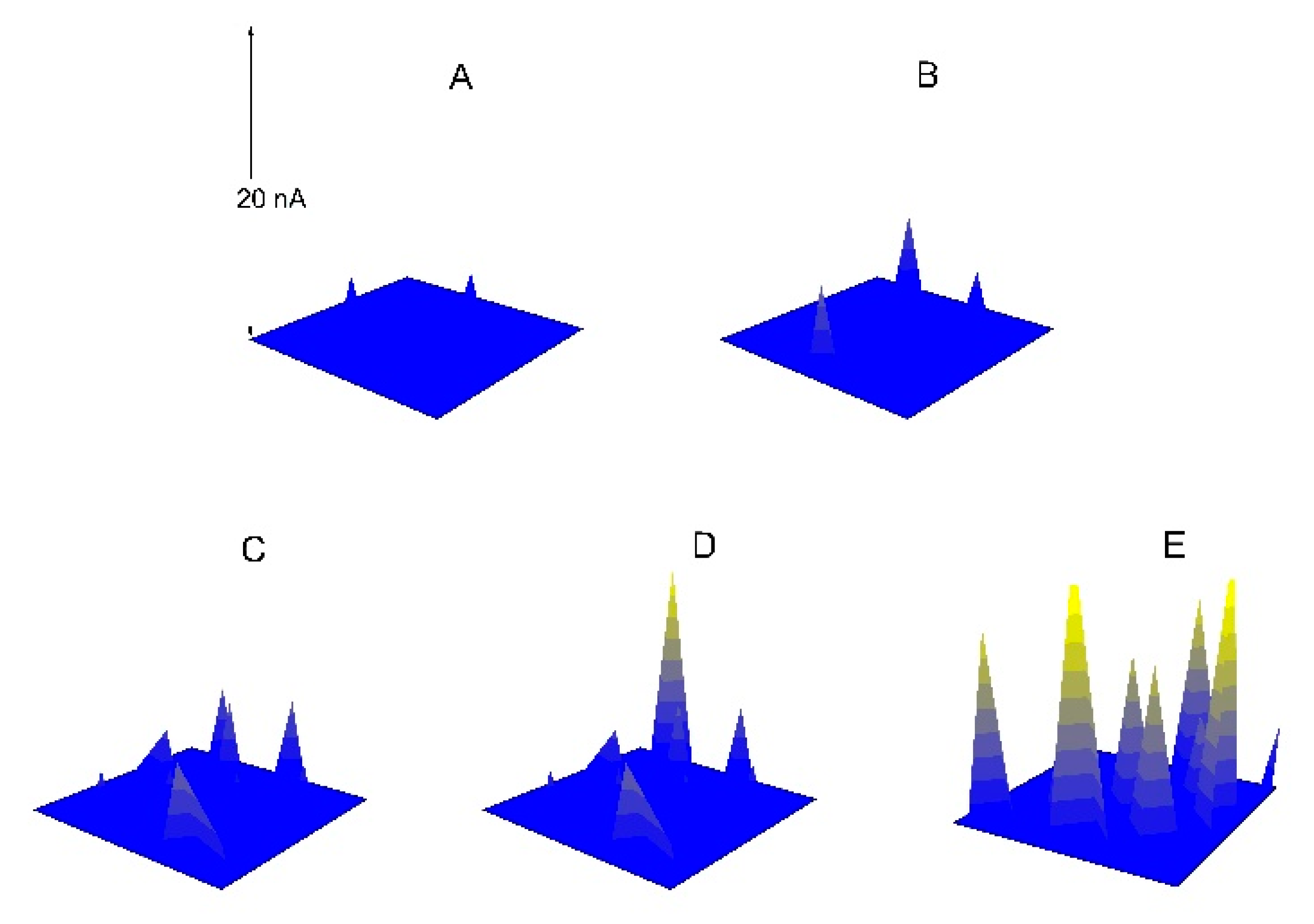
3.2. The Pitting Corrosion of X70 Carbon Steel in the NaCl Solution
3.3. The Pitting Corrosion of X70 Carbon Steel in the NaCl Solution with Na3PO4
3.4. The Pitting Corrosion of X70 Carbon Steel in the NaCl Solution with NaNO2
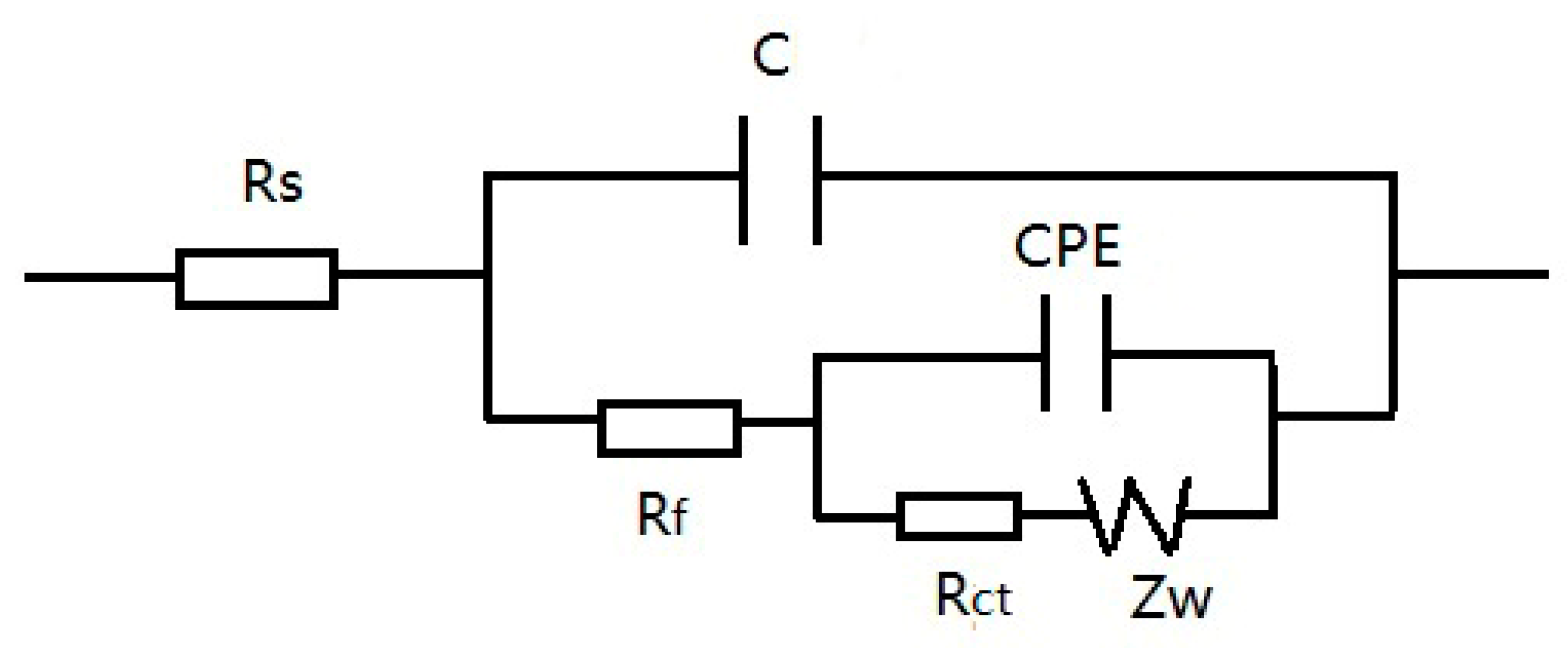
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Štefec, R.; Franz, F. A study of the pitting corrosion of cold-worked stainless steel. Corros. Sci. 1978, 18, 161–168. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Galvele, J.R. The mechanism of pitting of high purity iron in NaCl solutions. Corros. Sci. 1984, 24, 27–48. [Google Scholar] [CrossRef]
- Mizuno, T. In-situ analysis of chloride ion concentration within pits during pitting of iron. Corros. Sci. 1990, 31, 497–502. [Google Scholar] [CrossRef]
- Newman, R.C.; Sieradzki, K. Metallic Corrosion. Science 1994, 263, 1708–1709. [Google Scholar] [CrossRef] [PubMed]
- Punckt, C.; Bölscher, M.; Rotermund, H.H.; Mikhailov, A.S.; Organ, L.; Budiansky, N.; Scully, J.R.; Hudson, J.L. Sudden onset of pitting corrosion on stainless steel as a critical phenomenon. Science 2004, 305, 1133–1136. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Wilmott, M.; Luo, J.L. The role of chloride ions in pitting of carbon steel studied by the statistical analysis of electrochemical noise. Appl. Surf. Sci. 1999, 152, 161–168. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Yuan, B.Y.; Chen, S.H. Numerical reconstruction of digital holograms for the study of pitting dynamic processes of the X70 carbon steel in NaCl solution. Electrochem. Commun. 2008, 10, 103–107. [Google Scholar] [CrossRef]
- Tang, Y.W.; Dai, N.W.; Wu, J.; Jiang, Y.M.; Li, J. Effect of surface roughness on pitting corrosion of 2205 duplex stainless steel investigated by electrochemical noise measurements. Materials 2019, 12, 738. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Qiao, L.J.; Volinsky, A.A.; Su, Y.S. A statistical study on the effect of hydrostatic pressure on metastable pitting corrosion of X70 pipeline steel. Materials 2017, 10, 1307. [Google Scholar] [CrossRef]
- Refaey, S.A.M.; Abd El-Rehim, S.S.; Taha, F.; Saleh, M.B.; Ahmed, R.A. Inhibition of chloride localized corrosion of mild steel by PO43−, CrO42−, MoO42−, and NO2− anions. Appl. Surf. Sci. 2000, 158, 190–196. [Google Scholar] [CrossRef]
- Refaey, S.A.M. Inhibition of steel pitting corrosion in HCl by some inorganic anions. Appl. Surf. Sci. 2005, 240, 396–404. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.T.; Zhao, J.M.; Xiong, J.P. The effects of some anions on metastable pitting of 316L stainless steel. Corros. Sci. 2002, 44, 13–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, Y. The inhibitive mechanisms of nitrite and molybdate anions on initiation and propagation of pitting corrosion for mild steel in chloride solution. Appl. Surf. Sci. 2015, 353, 924–932. [Google Scholar] [CrossRef]
- Eyu, G.D.; Will, G.; Dekkers, W.; Macleod, J. The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution. Materials 2016, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Y.; Wang, X.; He, L.; Huang, Y. Preferential Corrosion Behavior of Carbon Steel Weld in Simulation Pore Solution and the Inhibition Performance of Nitrite. Mater. Rep. 2017, 31, 119–124. [Google Scholar]
- Fujioka, E.; Nishihara, H.; Aramaki, K. The inhibition of pit nucleation and growth on the passive surface of iron in a borate buffer solution containing Cl- by oxidizing inhibitors. Corros. Sci. 1996, 38, 1915–1933. [Google Scholar] [CrossRef]
- Refaey, S.A.M.; Abd El Rehim, S.S. Inhibition of chloride pitting corrosion of tin in alkaline and near neutral medium by some inorganic anions. Electrochim. Acta 1996, 42, 667–674. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Jeannin, M.; Berziou, C.; Refait, P. Effects of NO2− ions on localised corrosion of steel in NaHCO3 + NaCl electrolytes. Electrochim. Acta 2007, 52, 7599–7606. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Jeannin, M.; Berziou, C.; Refait, P. Effects of phosphate species on localised corrosion of steel in NaHCO3 + NaCl electrolytes. Electrochim. Acta 2009, 54, 4389–4396. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Rairdan, B.R.; Luo, J.L. Features of electrochemical noise generated during pitting of inhibited A516-70 carbon steel in chloride solutions. J. Appl. Electrochem. 1998, 28, 1371–1375. [Google Scholar] [CrossRef]
- Dong, Z.H.; Guo, X.P.; Zheng, J.X.; Xu, L.M. Investigation on inhibition of CrO42− and MoO42− ions on carbon steel pitting corrosion by electrochemical noise analysis. J. Appl. Electrochem. 2002, 32, 395–400. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Passivity and pitting of carbon steel in chromate solutions. Electrochim. Acta 1999, 44, 4795–4804. [Google Scholar] [CrossRef]
- Hancock, P.; Mayne, J.E.O. The inhibition of the corrosion of iron in the neutral and alkaline solutions. J. Appl. Chem. 1959, 9, 345–352. [Google Scholar] [CrossRef]
- Sakashita, M.; Sato, N. The effect of molybdate anion on the ion-selectivity of hydrous ferric oxide films in chloride solutions. Corros. Sci. 1977, 17, 473–486. [Google Scholar] [CrossRef]
- El Dahan, H.A. Pitting corrosion inhibition of 316 stainless steel in phosphoric acid-chloride solutions. J. Mater. Sci. 1999, 34, 851–857. [Google Scholar] [CrossRef]
- Virtanen, S.; Surber, B.; Nylund, P. Influence of MoO42− anion in the electrolyte on passivity breakdown of iron. Corros. Sci. 2001, 43, 1165–1177. [Google Scholar] [CrossRef]
- Zhao, J.M.; Zuo, Y. The effects of molybdate and dichromate anions on pit propagation of mild steel in bicarbonate solution containing Cl−. Corros. Sci. 2002, 44, 2119–2130. [Google Scholar] [CrossRef]
- MacCafferty, E. A competitive adsorption model for the inhibition of crevice corrosion and pitting. J. Electrochem. Soc. 1990, 137, 3731–3740. [Google Scholar] [CrossRef]
- Tang, Y.M.; Zuo, Y.; Zhao, H. The current fluctuations and accumulated pitting damage of mild steel in NaNO2-NaCl solution. Appl. Surf. Sci. 2005, 243, 82–88. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Wang, C.; Zhu, Y.Y. Study of the effects of hydrogen on the pitting processes of X70 carbon steel with SECM. Electrochem. Commun. 2010, 12, 1804–1807. [Google Scholar] [CrossRef]
- Simőes, A.M.; Bastos, A.C.; Ferreira, M.G.; González-García, Y.; González, S.; Souto, R.M. Use of SVET and SECM to study the galvanic corrosion of an iron–zinc cell. Corros. Sci. 2007, 49, 726–739. [Google Scholar] [CrossRef]
- Zhu, R.K.; Luo, J.L. Investigation of stress-enhanced surface reactivity on Alloy 800 using scanning electrochemical microscopy. Electrochem. Commun. 2010, 12, 1752–1755. [Google Scholar] [CrossRef]



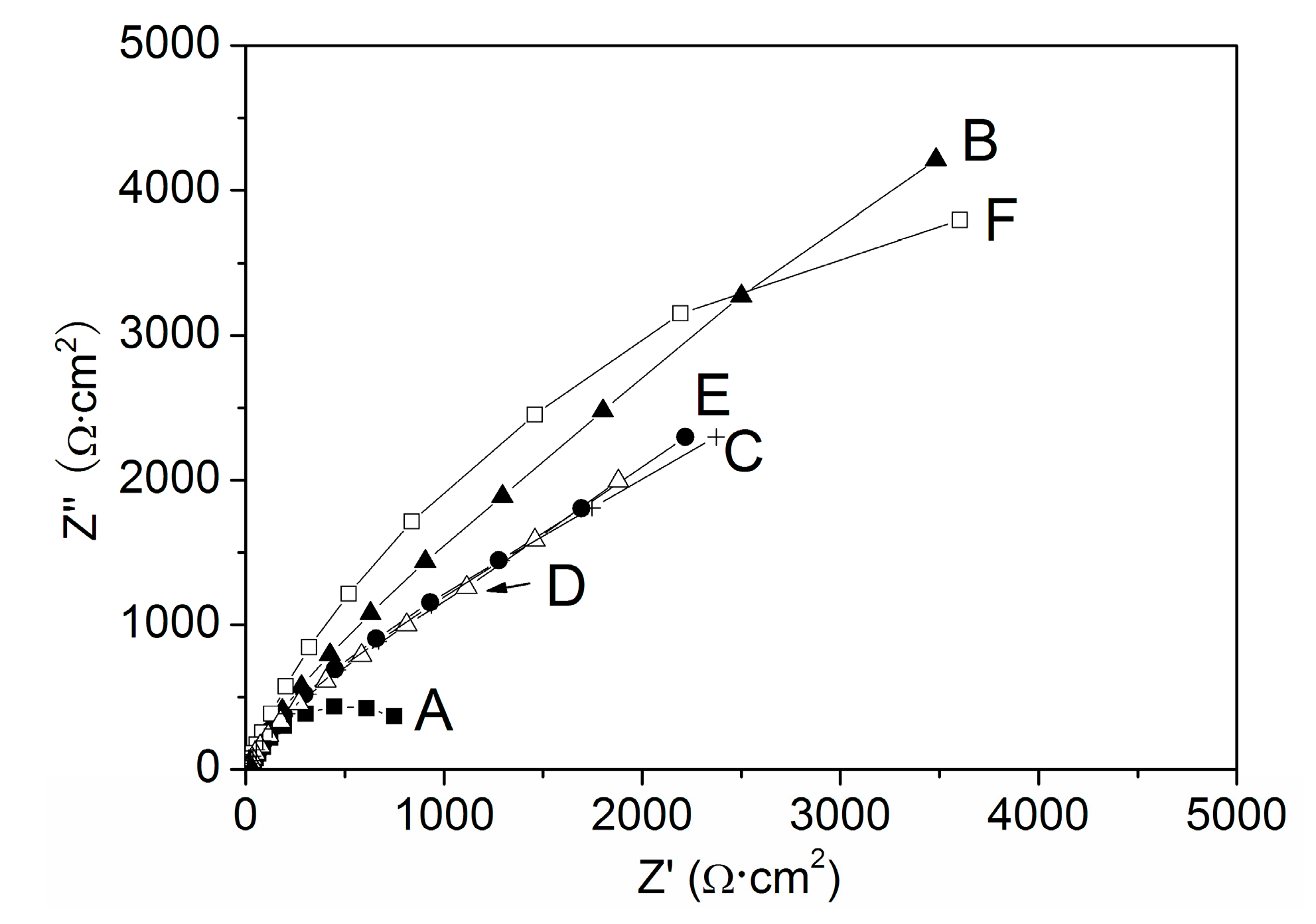
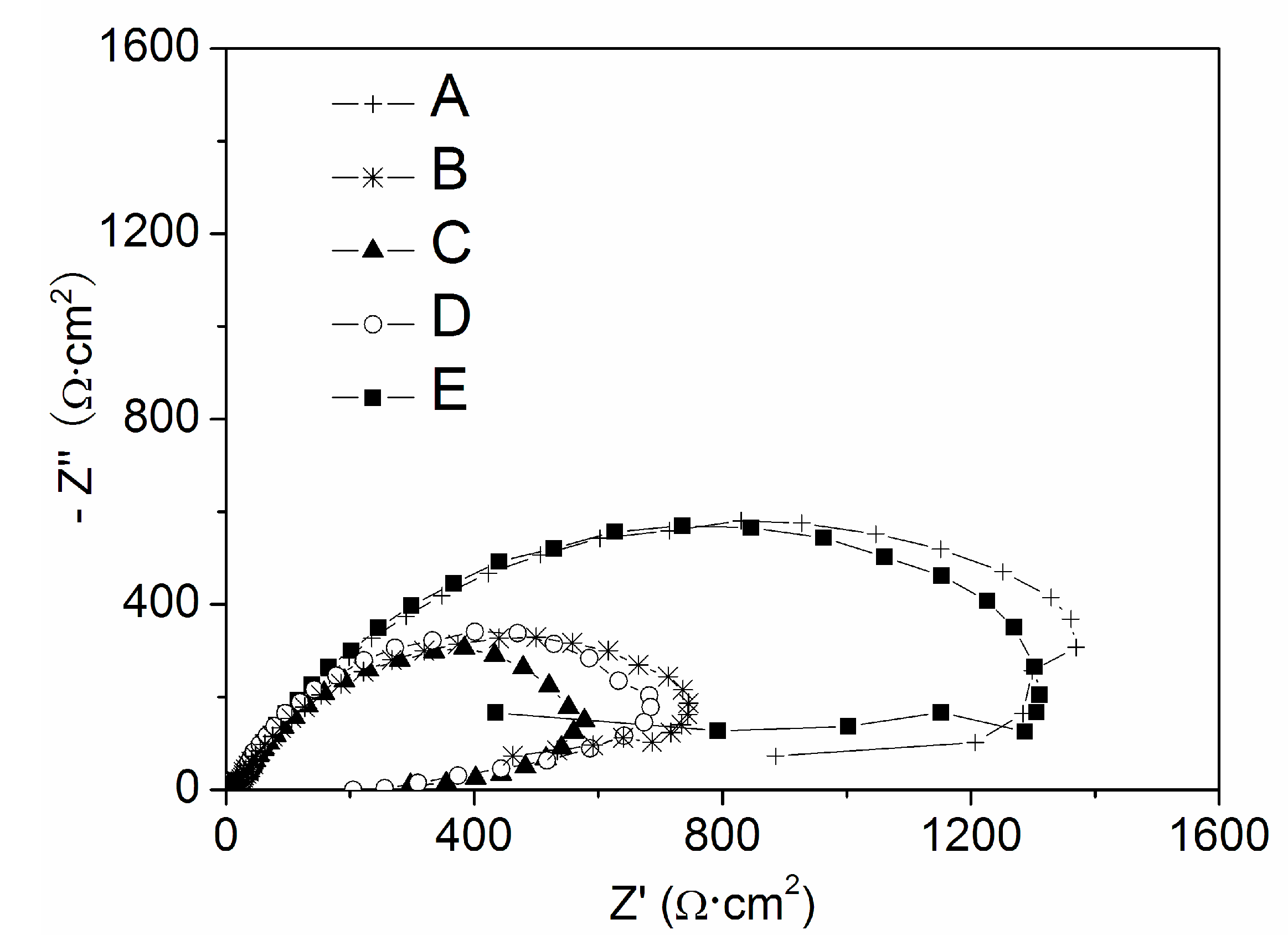
| Solution | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Rct/Ω·cm2 | 1064 | 3316 | 2172 | 1784 | 2109 | 3408 |
| P% | -- | 67.91 | 51.01 | 40.36 | 49.55 | 68.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Ding, J.; Zhang, J.; Li, L. Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution. Materials 2020, 13, 5392. https://doi.org/10.3390/ma13235392
Zhu Y, Ding J, Zhang J, Li L. Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution. Materials. 2020; 13(23):5392. https://doi.org/10.3390/ma13235392
Chicago/Turabian StyleZhu, Yongyan, Jiayi Ding, Jianli Zhang, and Liang Li. 2020. "Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution" Materials 13, no. 23: 5392. https://doi.org/10.3390/ma13235392
APA StyleZhu, Y., Ding, J., Zhang, J., & Li, L. (2020). Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution. Materials, 13(23), 5392. https://doi.org/10.3390/ma13235392




