Effect of Jute Fibres on the Process of MICP and Properties of Biocemented Sand
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Testing of Properties
2.2. Fibre Preparation
Pre-Treatment of Jute Fibres
2.3. Bacteria Culture
2.4. Preparation of Cemention Medium
2.5. Urease Activity and Batch Test
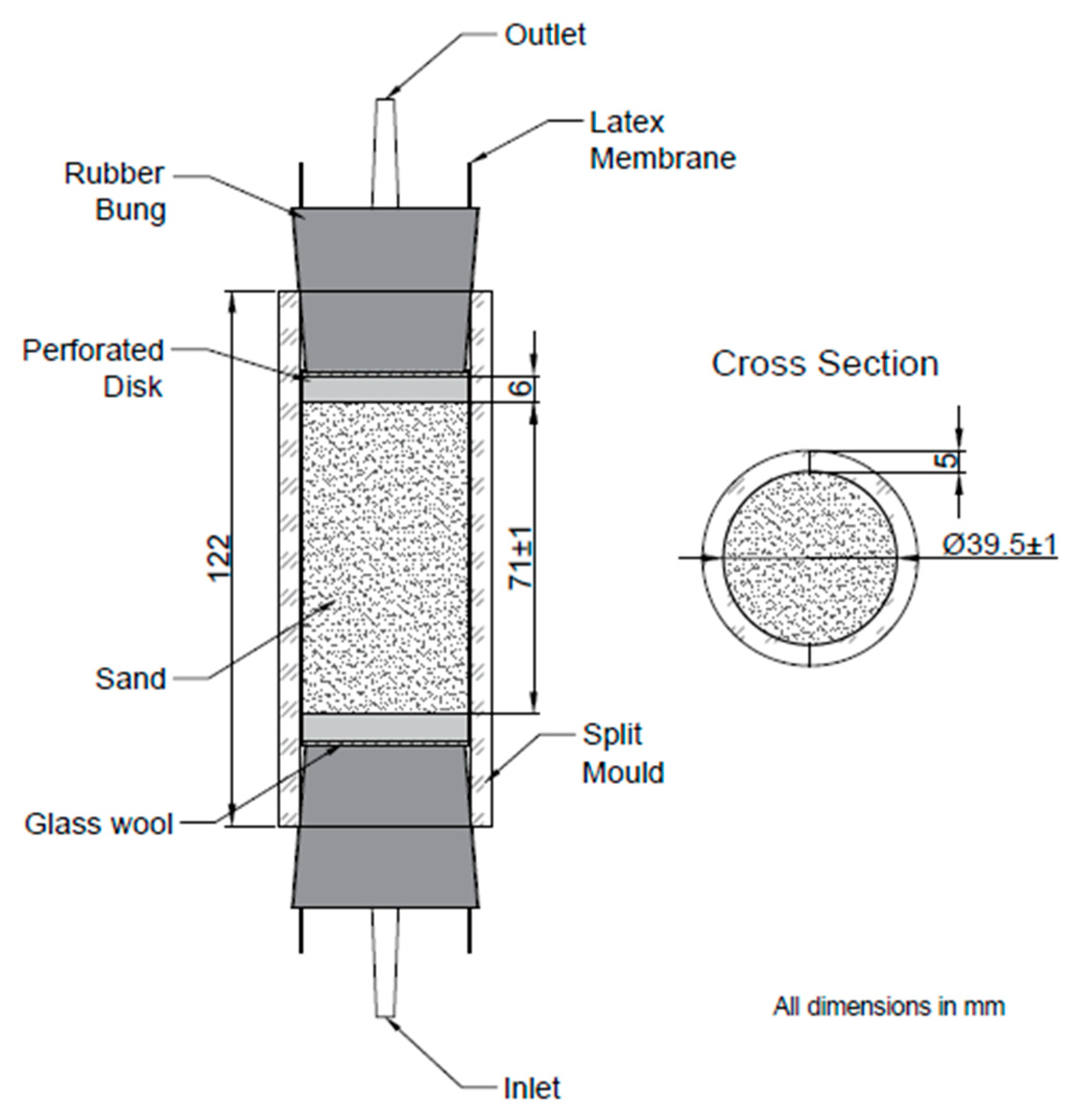
2.6. Preparation of Columns
2.7. Bacteria Fixing and Biocementation
2.8. Measurement of Electrical Conductivity, pH and Evaluation of Bacterial Fixing
2.9. Geochemical Analysis
2.10. Quantification of Calcium Carbonate Precipitate
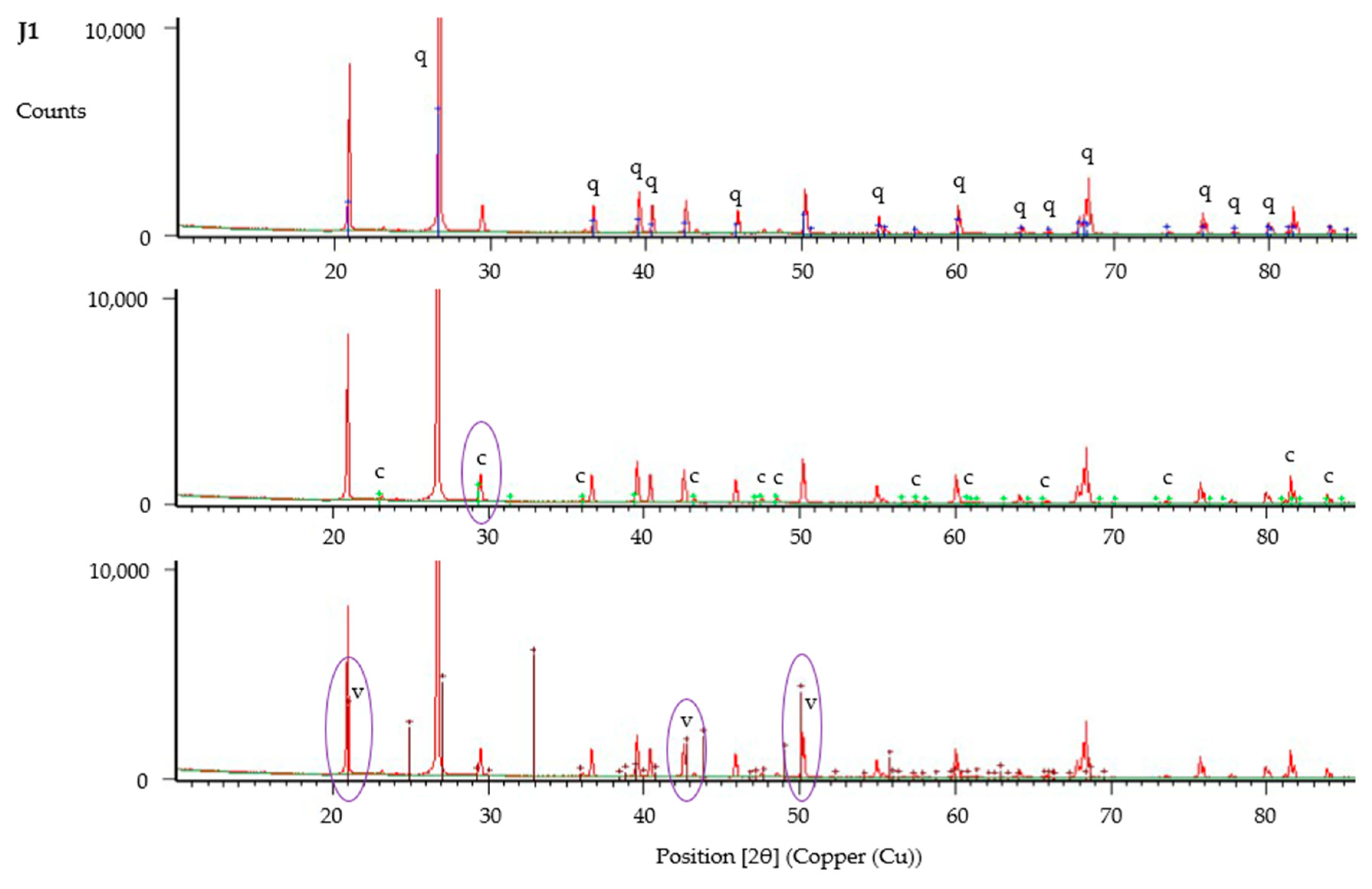
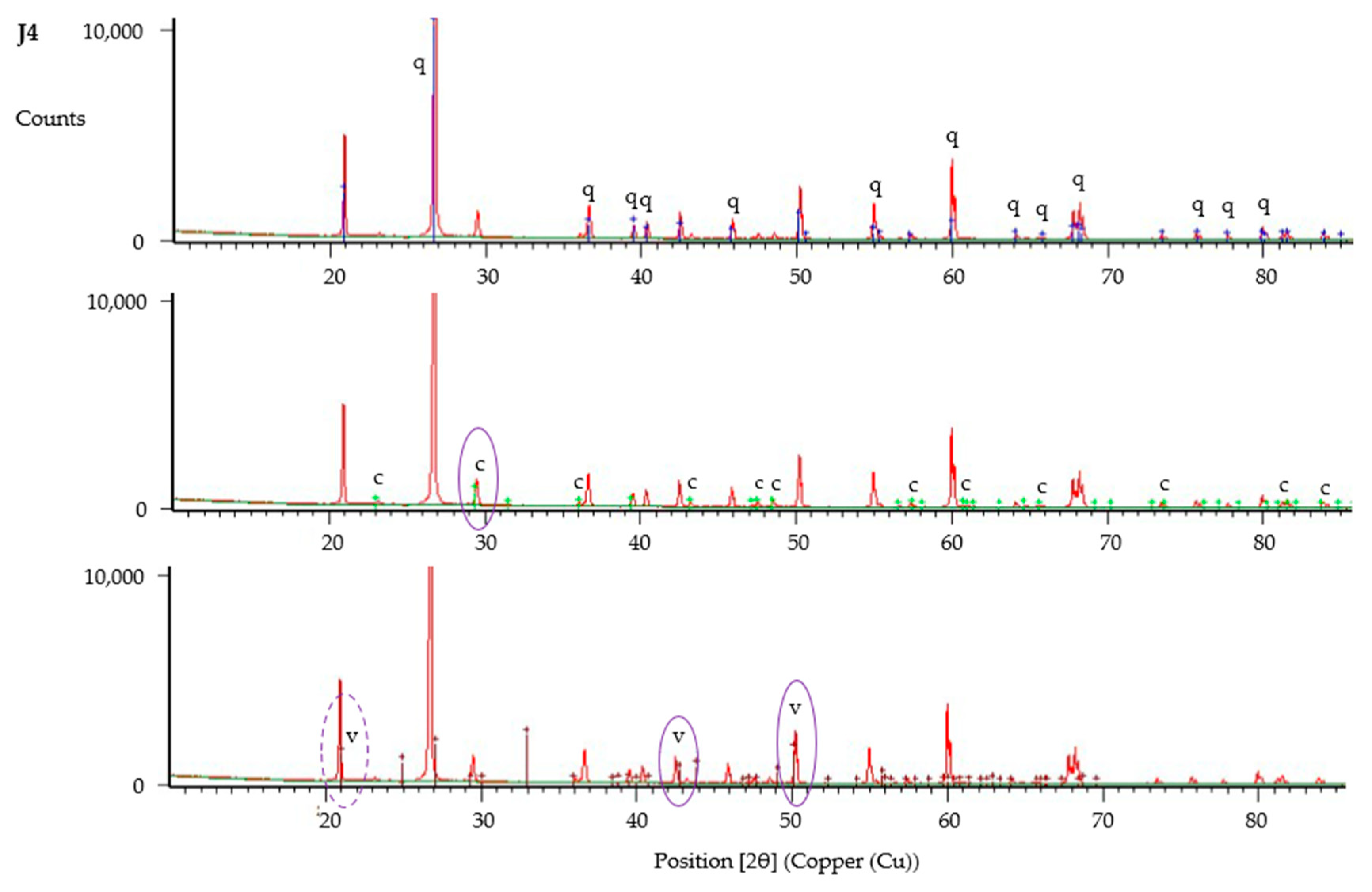
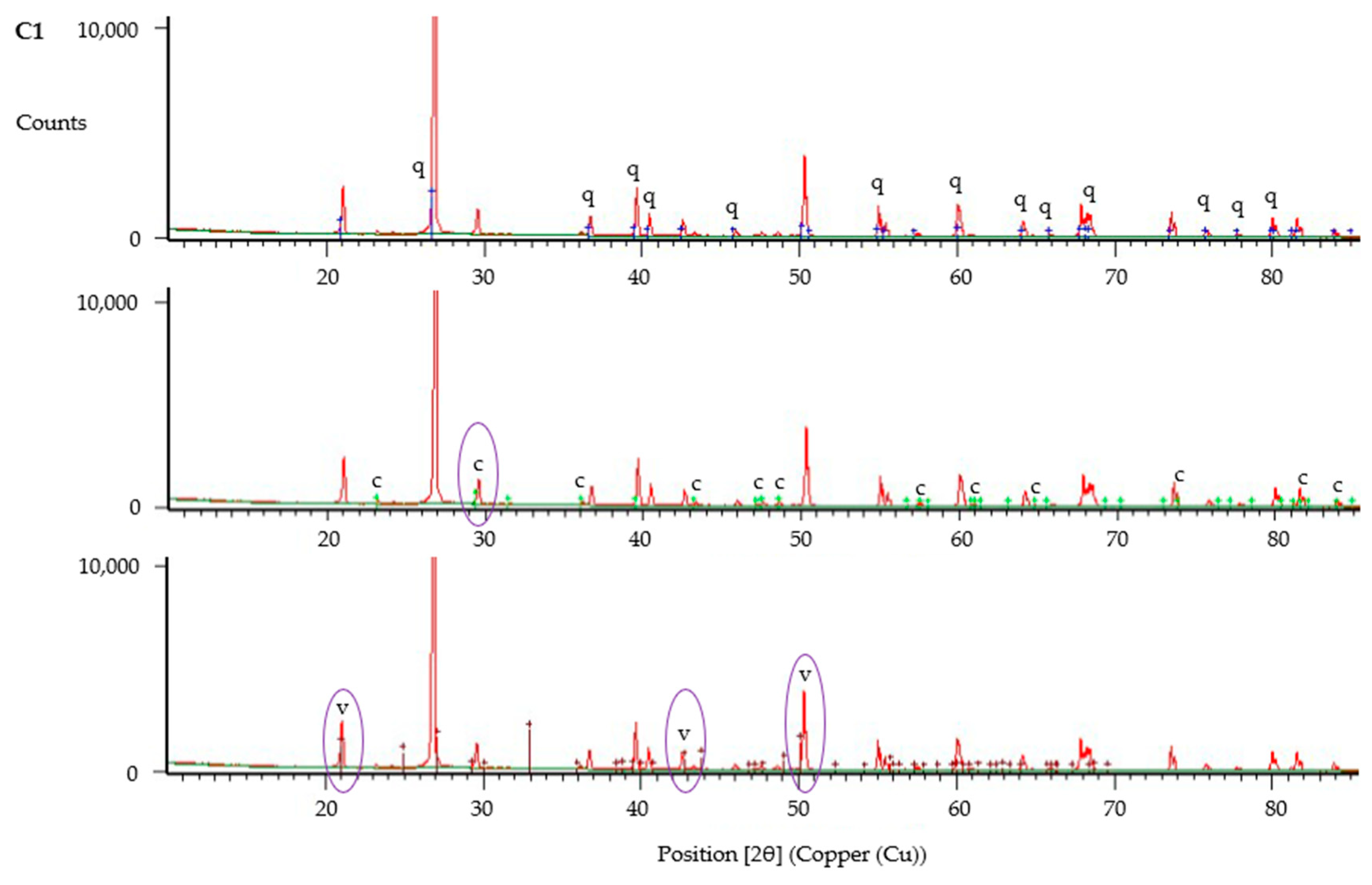
2.11. Mineral Analysis
2.12. Unconfined Compressive Strength Testing
3. Results and Discussion
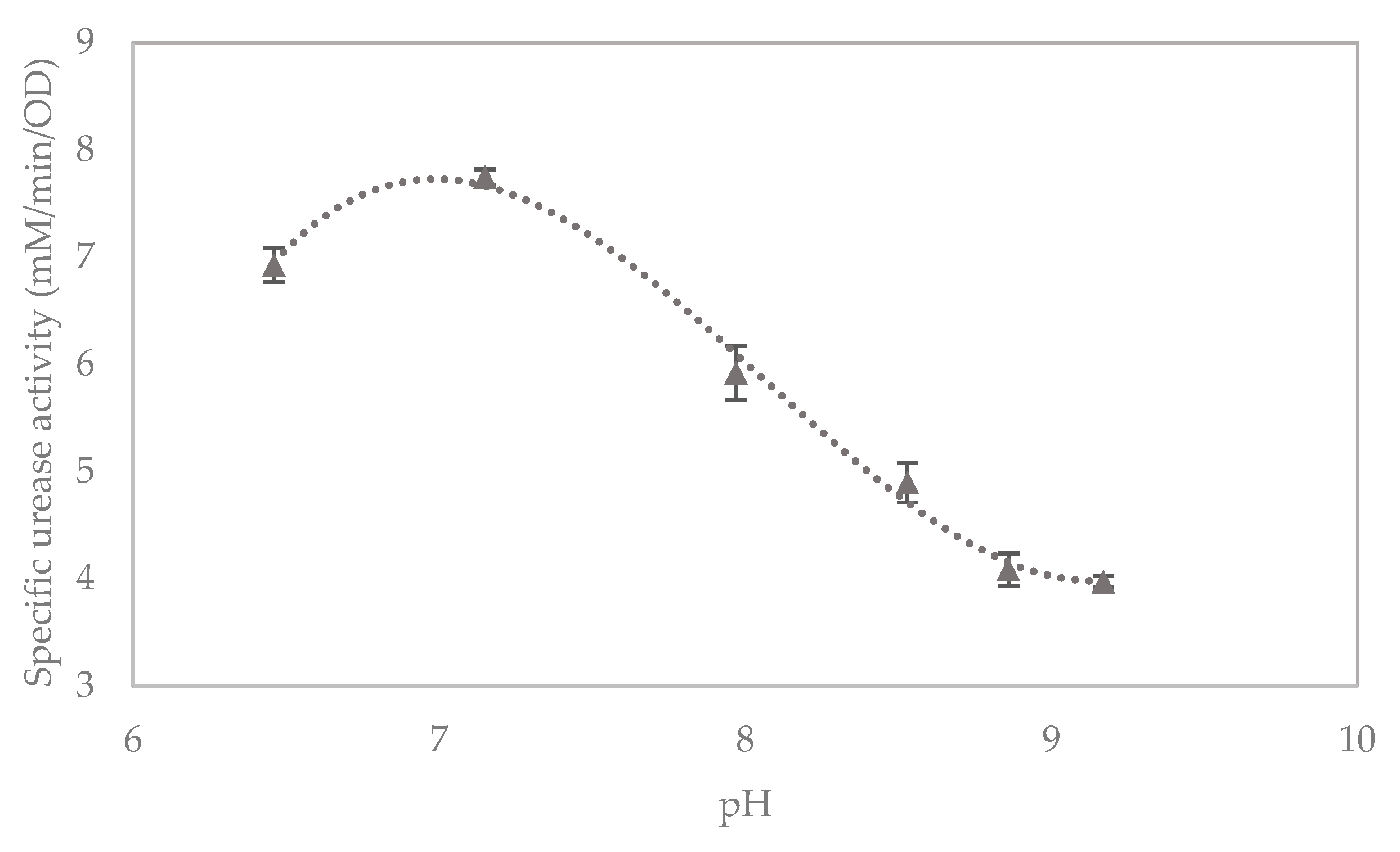
3.1. Urease Activity Batch Test
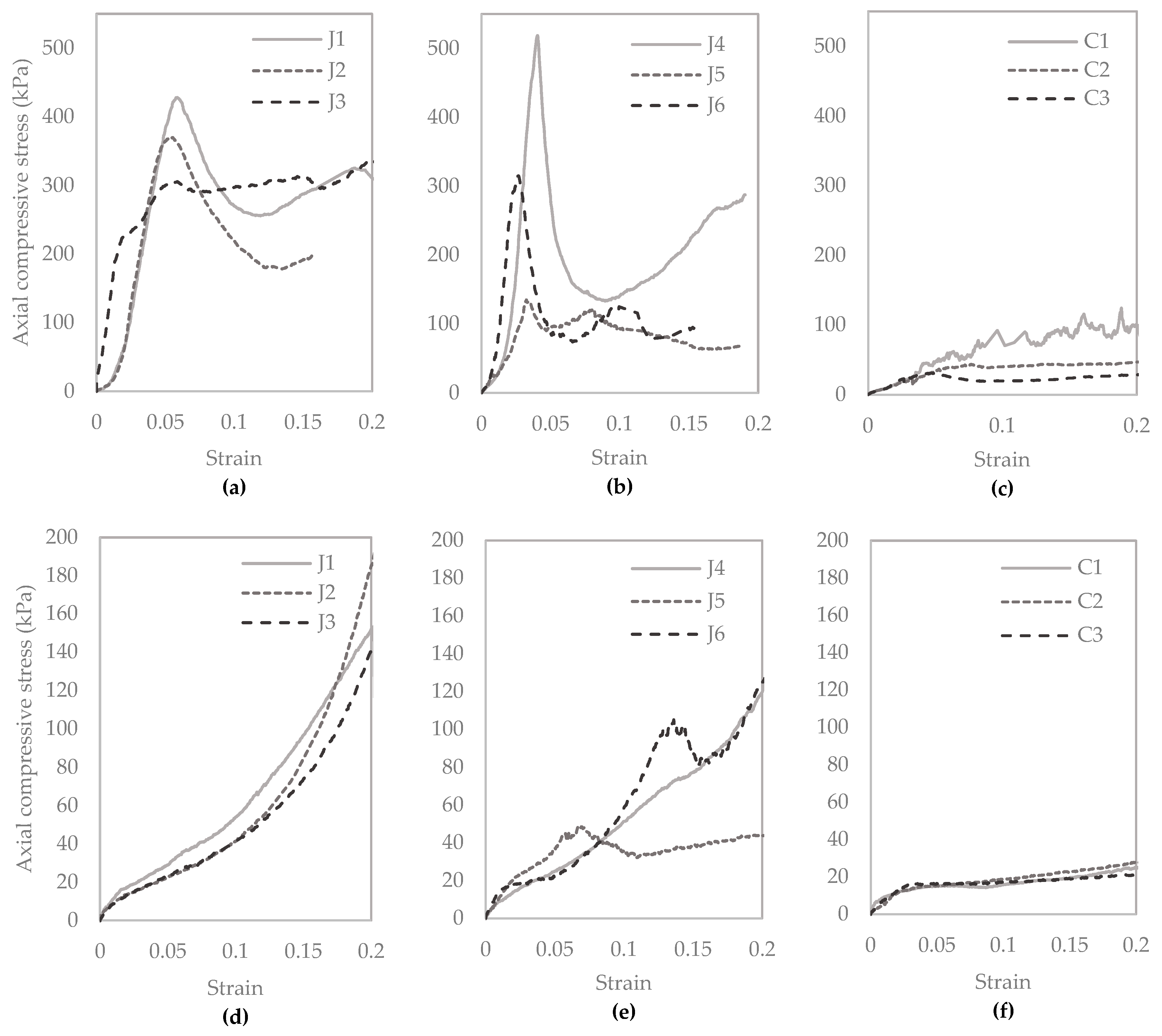
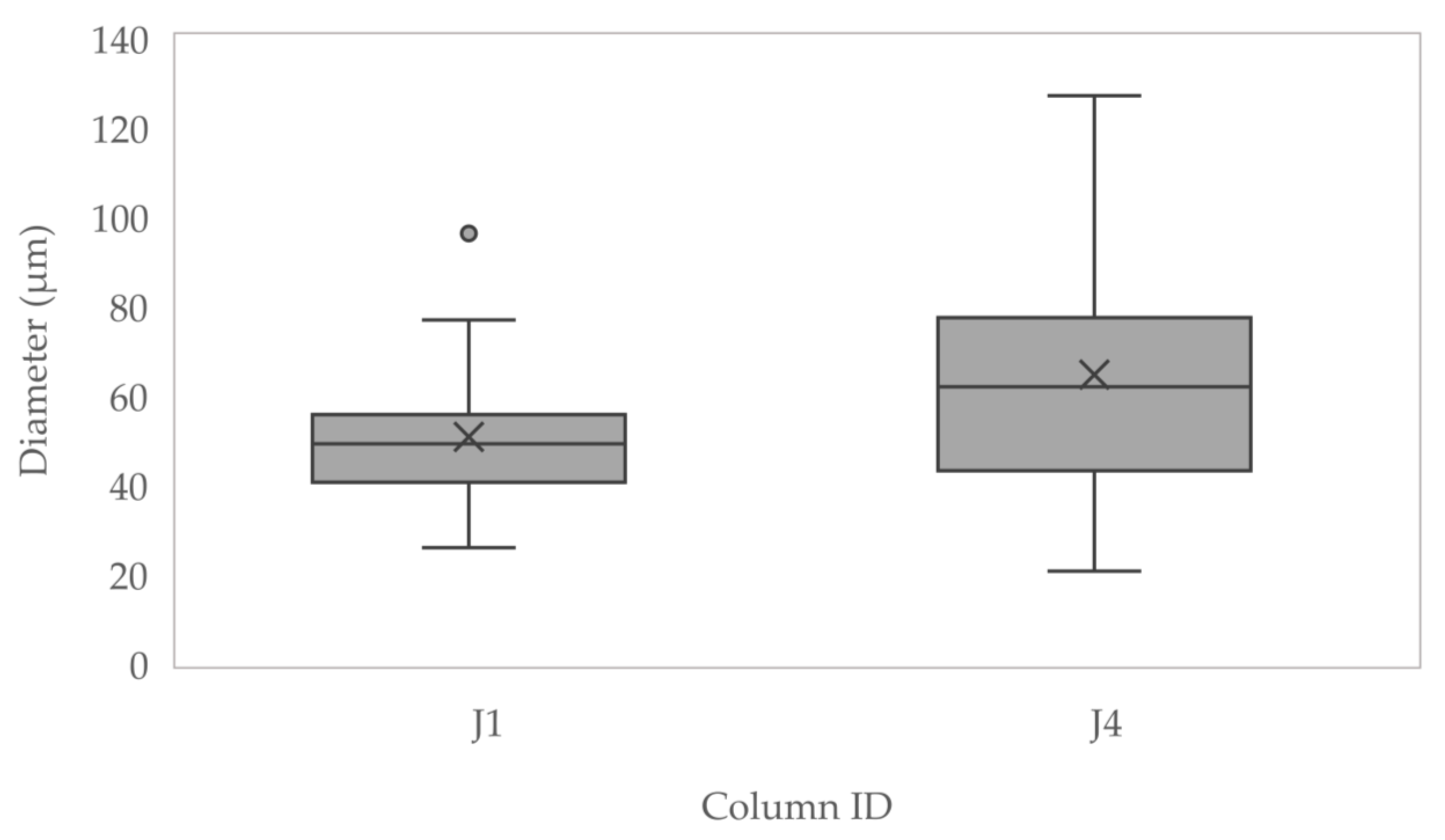
3.2. Column Studies
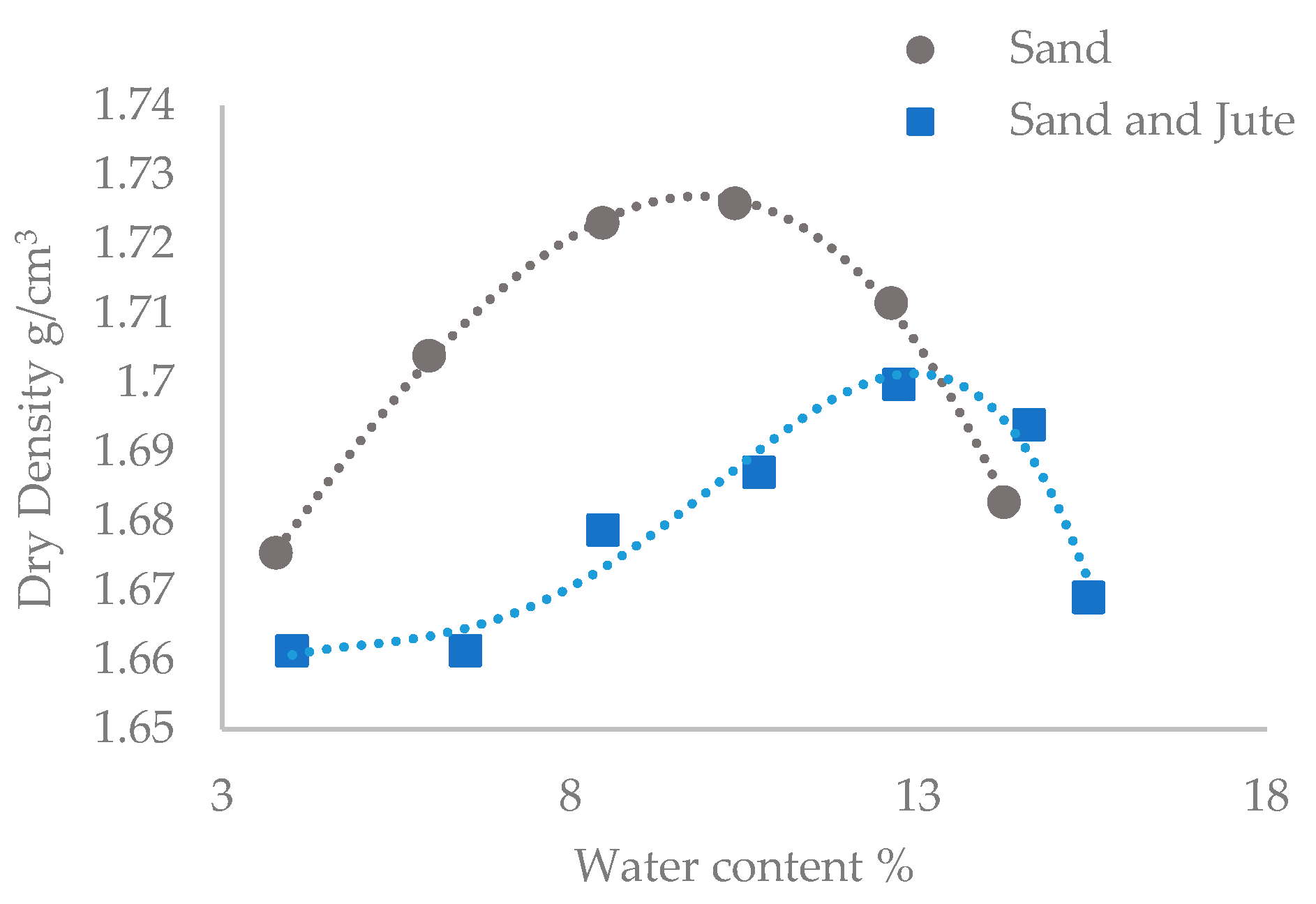
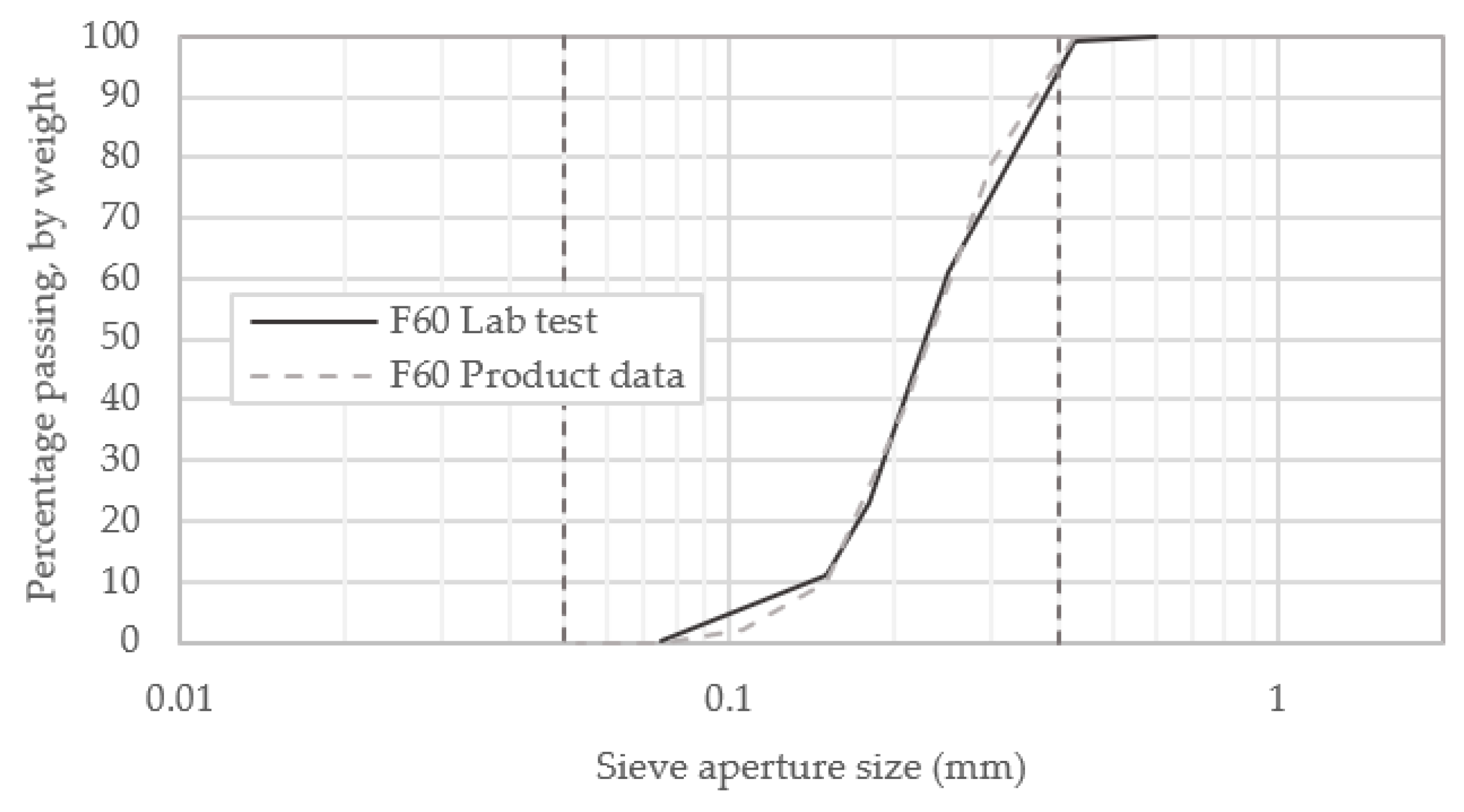
3.2.1. Soil Properties
3.2.2. Bacteria Fixation and Initial Activity
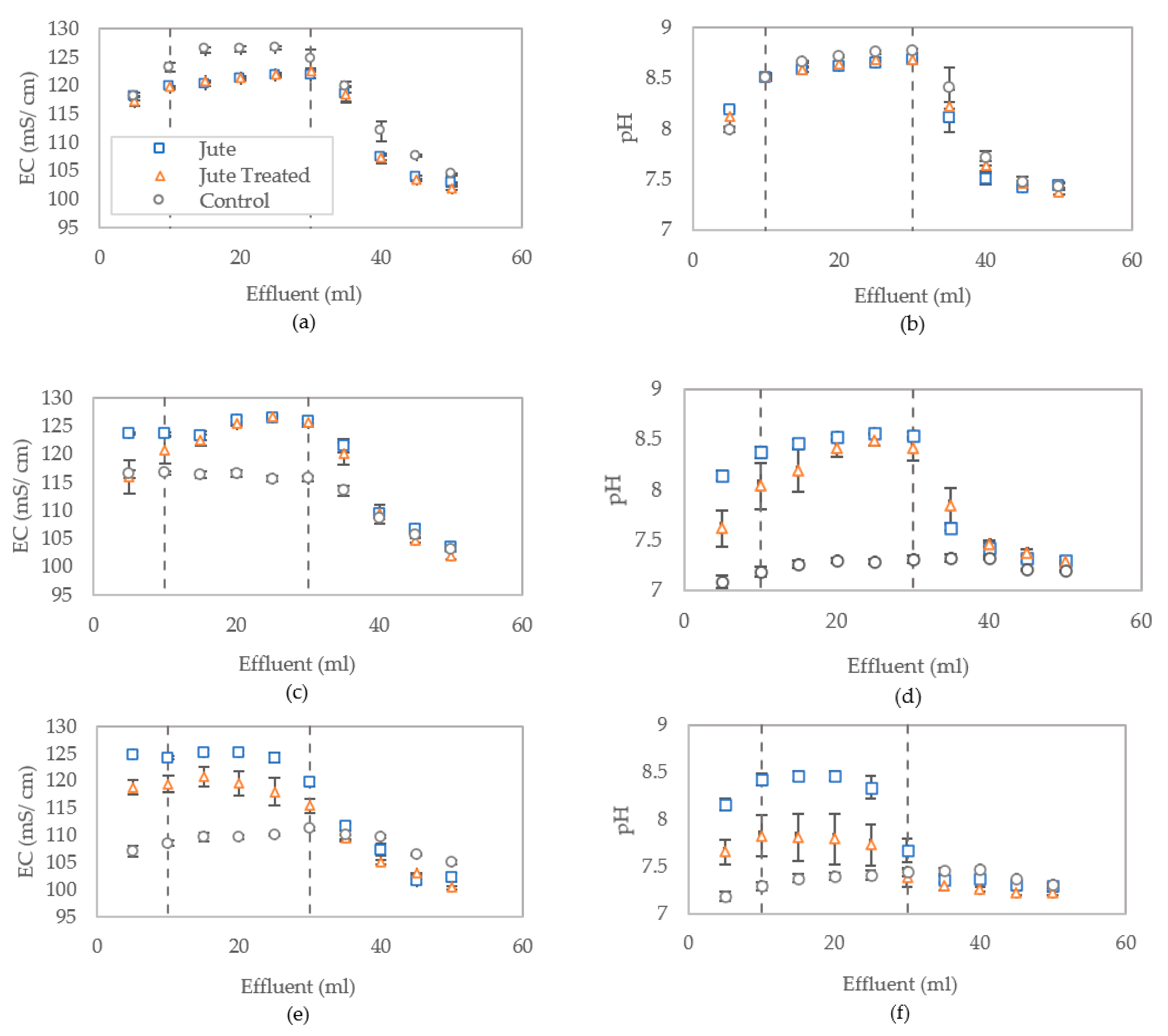
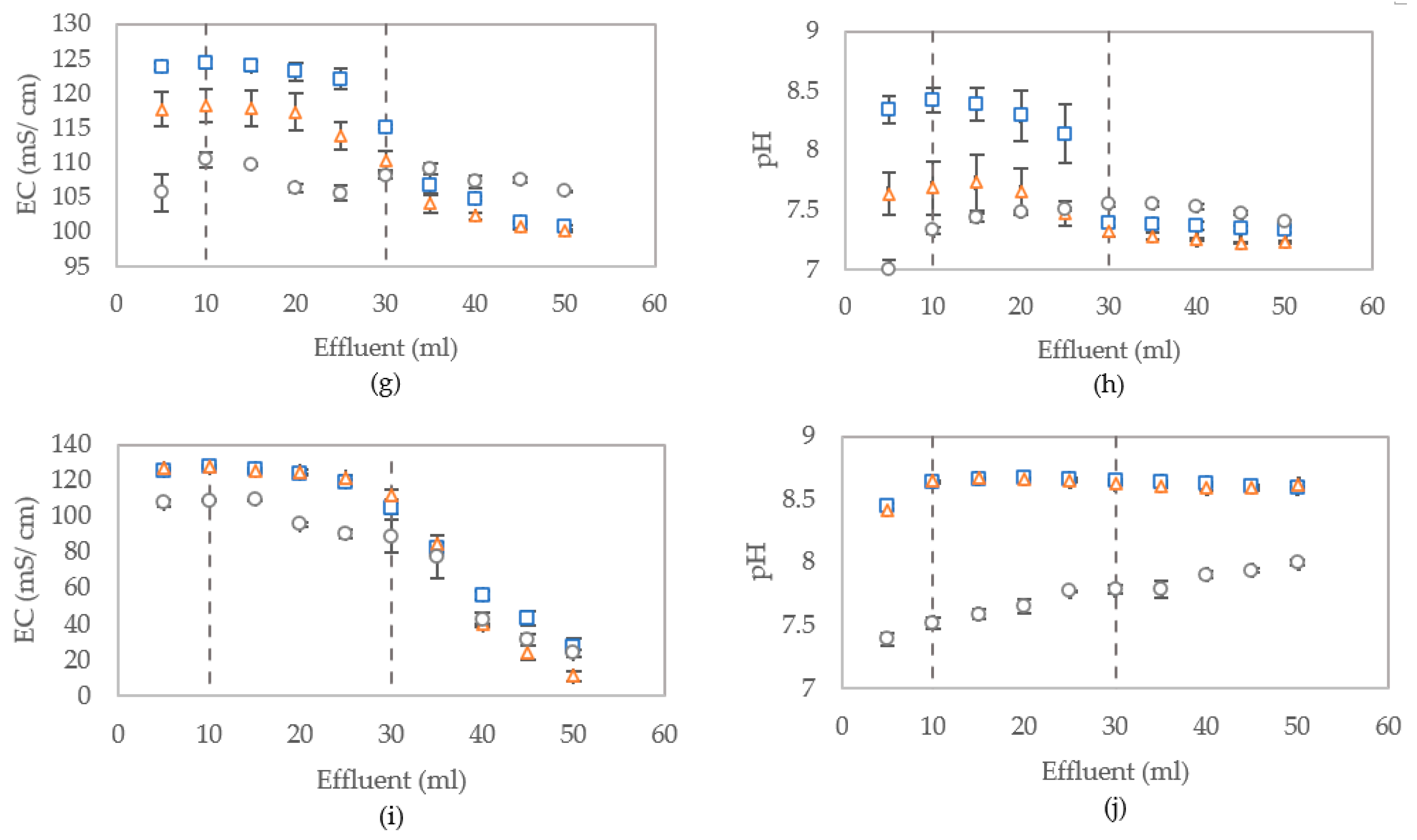
3.2.3. Distribution of Bacterial Activity
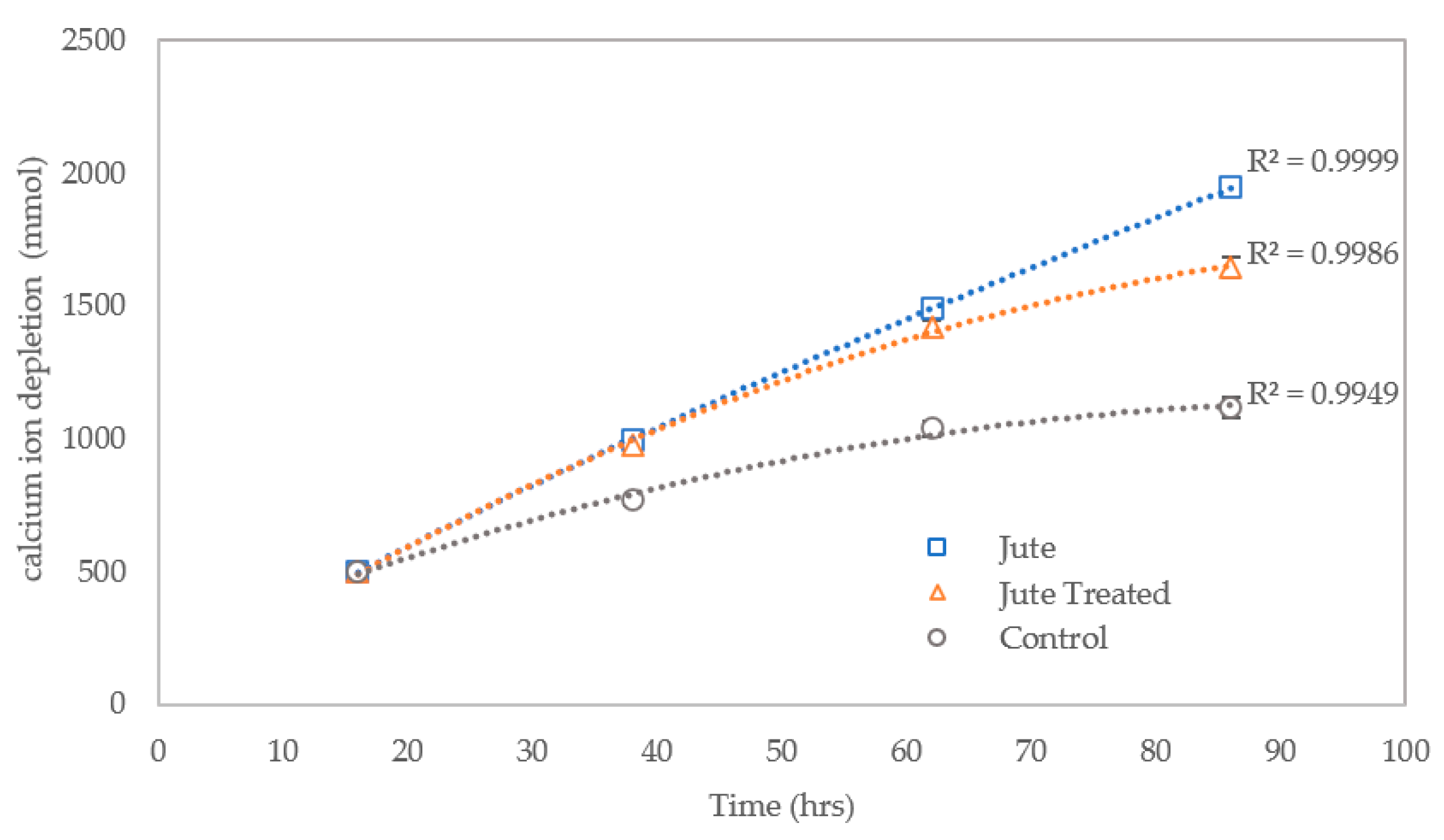
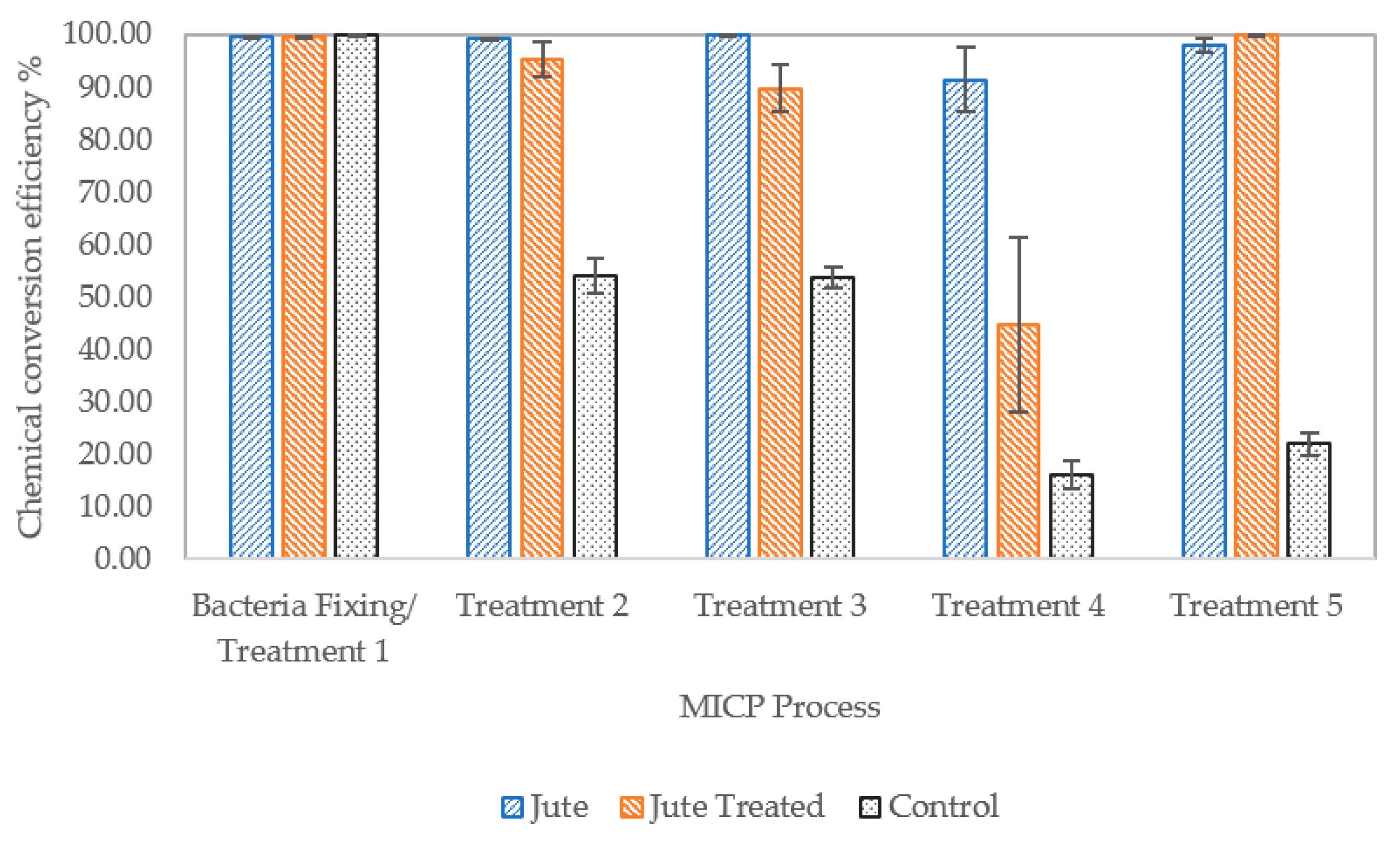
3.2.4. Efficiency of Calcium Ion Conversion
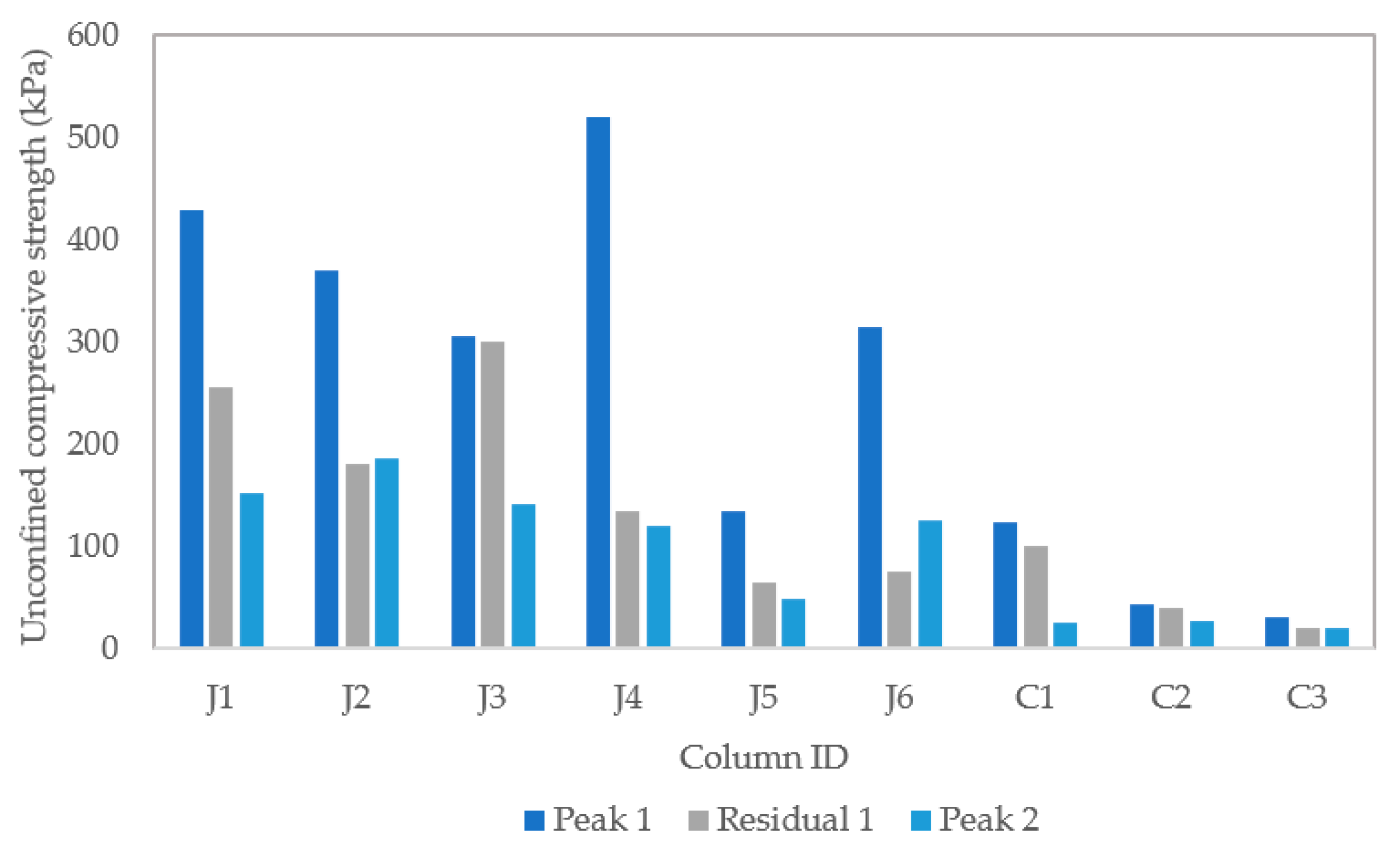
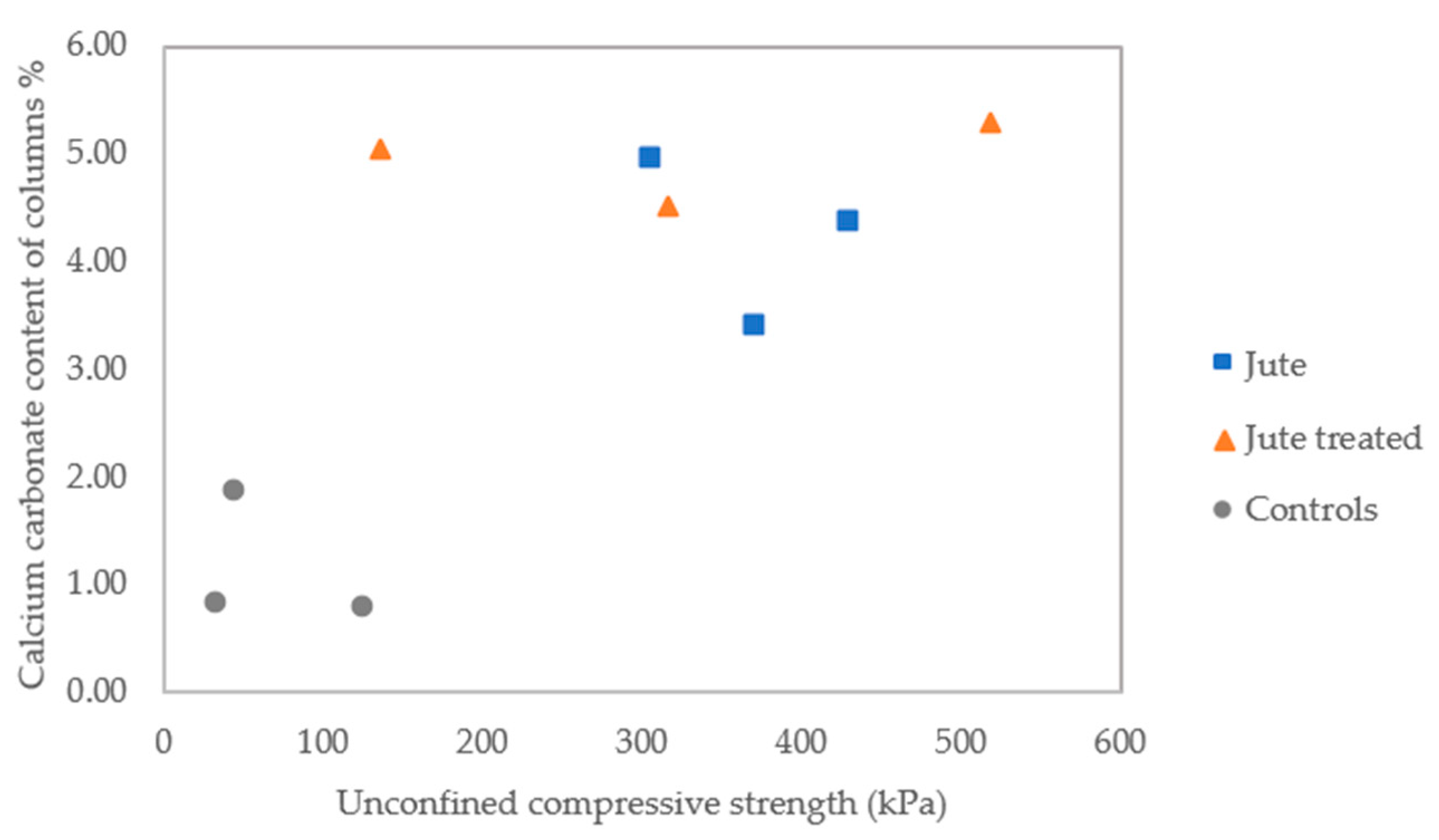
3.2.5. Unconfined Compressive Strength, and CaCO3 Precipitated
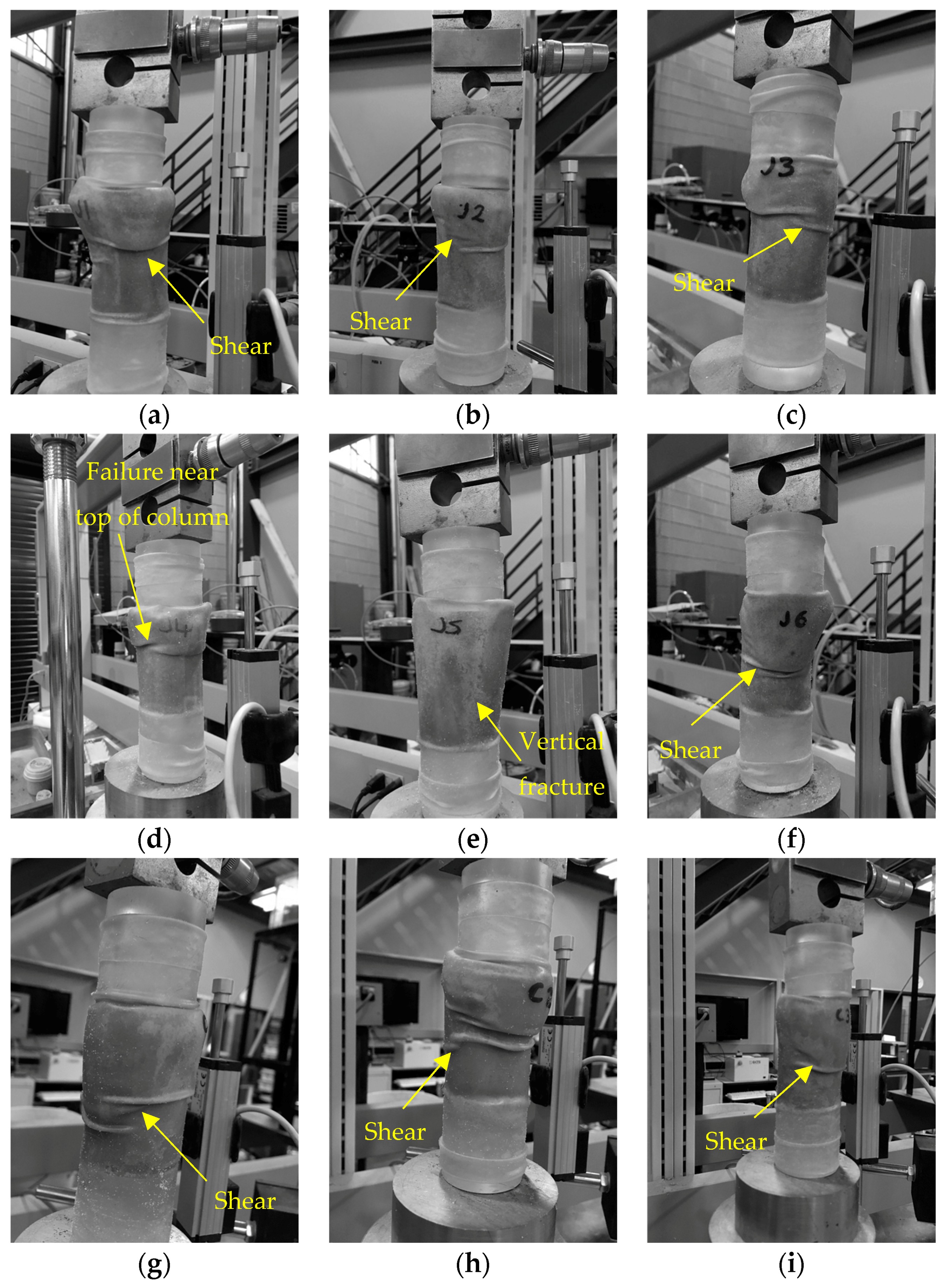
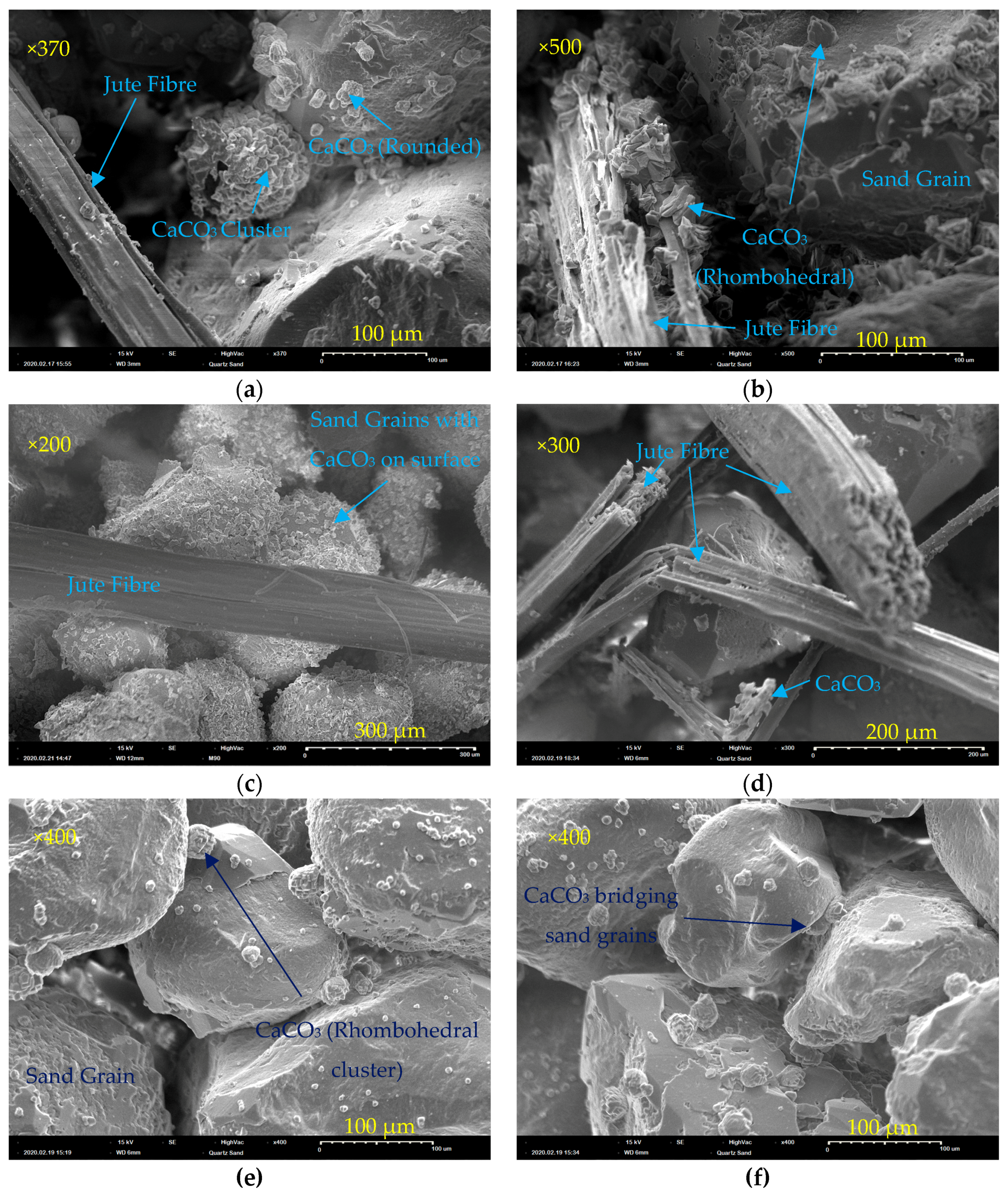
3.2.6. Morphology of CaCO3 Precipitate and Jute Fibres
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ρ | Bulk density |
| Gs | Specific Gravity |
| D10 | Diameter corresponding to 10% finer in the particle-size distribution |
| D50 | Diameter corresponding to 50% finer in the particle-size distribution |
| D60 | Diameter corresponding to 60% finer in the particle-size distribution |
| D30 | Diameter corresponding to 30% finer in the particle-size distribution |
| Cu | Uniformity Coefficient of uniformity |
| Cz | Coefficient of curvature |
References
- Montoya, B.M.; Dejong, J.T. Healing of biologically induced cemented sands. Geotech. Lett. 2013, 3, 147–151. [Google Scholar] [CrossRef]
- Park, S.-S.; Choi, S.-G.; Nam, I.-H. Effect of Plant-Induced Calcite Precipitation on the Strength of Sand. J. Mater. Civ. Eng. 2014, 26, 06014017. [Google Scholar] [CrossRef]
- Dhami, N.K.; Alsubhi, W.R.; Watkin, E.; Mukherjee, A. Bacterial Community Dynamics and Biocement Formation during Stimulation and Augmentation: Implications for Soil Consolidaton. Front. Microbiol. 2017, 8, 1267. [Google Scholar] [CrossRef] [PubMed]
- Ersan, Y.C.; de Belie, N.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenvironmental Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Khodadadi, T.H.; Kavazanjian, E.; Paassen, L.V.; Dejong, J. Bio-Grout Materials: A Review. Geotech. Spec. Publ. 2017, 1–12. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; deVlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. Eng.-Ground Improv. 2015, 168, 206–216. [Google Scholar] [CrossRef]
- Spencer, C.A.; Sass, H. Use of carrier materials to immobilise and supply cementation medium for microbially mediated self-healing of biocement. IOP Conf. Ser. Mater. Sci. Eng. 2019, 660, 012067. [Google Scholar] [CrossRef]
- Botusharova, S. Self-Healing Geotechnical Structures Via Microbial Action. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2017. [Google Scholar]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of Calcite by Indigenous Microorganisms to Strengthen Liquefiable Soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Choi, S.-G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of Fiber Addition on Mechanical Properties of MICP-Treated Sand. J. Mater. Civ. Eng. 2016, 28, 04015166. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Evans, T.M.; Stuedlein, A.W.; Liu, H. Unconfined Compressive and Splitting Tensile Strength of Basalt Fiber-Reinforced Biocemented Sand. J. Geotech. Geoenvironmental Eng. 2019, 145, 04019048. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, C.; Ge, F.; Cheng, X.; Liu, P. Enhancing Strength of MICP-Treated Sand with Scrap of Activated Carbon-Fiber Felt. J. Mater. Civ. Eng. 2020, 32, 04020061. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Chu, J.; He, J. Microbially Induced Calcite Precipitation for Seepage Control in Sandy Soil. Geomicrobiol. J. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Vidal, H. The Principle of Reinforced Earth. Highw. Res. Rec. 1969. Available online: http://onlinepubs.trb.org/Onlinepubs/hrr/1969/282/282-001.pdf (accessed on 2 September 2020).
- Vigneswaran, C.; Jayapriya, J. Effect on physical characteristics of jute fibres with cellulase and specific mixed enzyme systems. J. Text. Inst. 2010, 101, 506–513. [Google Scholar] [CrossRef]
- Shukla, S.K. Fundamentals of Fibre-Reinforced Soil Engineering; Springer: Singapore, 2017; ISBN 978-981-10-3063-5. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behaviour. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2007. [Google Scholar]
- ASTM D6913/D6913M-17. Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM D1557-12e1. Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Modified Effort (56,000 ft-lbf/ft3 (2,700 kN-m/m3)); ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Van de Velde, K.; Baetens, E. Thermal and Mechanical Properties of Flax Fibres as Potential Composite Reinforcement. Macromol. Mater. Eng. 2001, 286, 342–349. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of Concrete Using Microorganisms. Mater. J. 2001, 98, 3–9. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Geotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Ibraim, E.; Diambra, A.; Russell, A.R.; Muir Wood, D. Assessment of laboratory sample preparation for fibre reinforced sands. Geotext. Geomembr. 2012, 34, 69–79. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Eijkelkamp Soil & Water. Calcimeter: Manual; Eijkelkamp Soil & Water, Netherlands; 2018; Available online: https://www.eijkelkamp.com/download.php?file=M0853e_Calcimeter_b21b.pdf (accessed on 20 November 2019).
- British Standards Institution. BS 1377-7:1990 - Methods of test for soils for civil engineering purposes. Part 7: Shear strength tests (total stress); British Standards Institution: London, UK, 1999. [Google Scholar]
- Craig, R.F. Craig’s Soil Mechanics: Seventh Edition; E & FN Spon: Abingdon, UK, 2004. [Google Scholar]
- Doran, P.M. Chapter 10 - Mass Transfer. In Bioprocess Engineering Principles (Second Edition); Doran, P.M., Ed.; Academic Press: London, UK, 2013; ISBN 978-0-12-220851-5. [Google Scholar]
- van Paassen, L. Biogrout, ground improvement by microbial induced carbonate precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Kawano, J.; Shimobayashi, N.; Kitamura, M.; Shinoda, K.; Aikawa, N. Formation process of calcium carbonate from highly supersaturated solution. J. Cryst. Growth 2002, 237–239, 419–423. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiation of Calcium Carbonate Polymorphs by Surface Analysis Techniques – An XPS and TOF-SIMS study. Surf. Interface Anal. SIA 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Grasby, S.E. Naturally precipitating vaterite (μ-CaCO3) spheres: Unusual carbonates formed in an extreme environment. Geochim. Cosmochim. Acta 2003, 67, 1659–1666. [Google Scholar] [CrossRef]
- Nawarathna, T.H.K.; Nakashima, K.; Kawasaki, S. Chitosan enhances calcium carbonate precipitation and solidification mediated by bacteria. Int. J. Biol. Macromol. 2019, 133, 867–874. [Google Scholar] [CrossRef]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed]


| Soil Origin | Gs | ρ (g/cm3) | Mineralogy | Shape |
|---|---|---|---|---|
| Ottawa | 2.65 | 1.522 | Quartz | Round |
| Precursor Chemicals and Nutrients | CM1 (g/L) | CM2 (g/L) | CM3 (g/L) | Sterilisation Method |
|---|---|---|---|---|
| Calcium chloride dihydrate (CaCl2∙2H2O) | 73.51 | 73.51 | 147.02 | Autoclaved |
| Urea (NH2(CO)NH2) | 40 | 40 | 80 | Syringe filtered |
| Ammonium chloride (NH4Cl) | 0 | 20 | - | Autoclaved |
| Sodium bicarbonate (NaHCO3) | 0 | 2.12 | - | Syringe filtered |
| Oxoid CM0001 nutrient broth | 3 | 6 | 12 | Autoclaved |
| Column ID | Sand, g | Jute, g | Immobilised CM, g |
|---|---|---|---|
| J1 | 133 | 1 | 0 |
| J2 | 133 | 1 | 0 |
| J3 | 133 | 1 | 0 |
| J4 | 133 | 1 | 1.687 |
| J5 | 133 | 1 | 1.681 |
| J6 | 133 | 1 | 1.686 |
| C1 | 143 | 0 | 0 |
| C2 | 143 | 0 | 0 |
| C3 | 143 | 0 | 0 |
| Day | Time Since Prior Injection (h) | Column Injection (1.5 × Pore Volume) | Treatment |
|---|---|---|---|
| 0 | 0 | CM1 | 1 |
| 1 | 16 | CM2 | 2 |
| 2 | 22 | CM2 | 3 |
| 3 | 24 | CM2 | 4 |
| 4 | 24 | CM2 | 5 |
| 12 | 192 | Tap Water | None |
| ID | Water | Inoculant | pH | Culture Time | OD | Electrical Conductivity (mS/cm/min) | Urea Hydrolysed (mM/min) | Specific Urease Activity (mM/min/OD) |
|---|---|---|---|---|---|---|---|---|
| 1 | Tap | PC | 8.37 | 19 | 0.940 | 0.56 | 6.22 | 6.62 |
| 2 | DI | PC | 7.97 | 19 | 0.937 | 0.41 | 4.59 | 4.90 |
| 3 | Tap | LBC | 8.37 | 19 | 1.308 | 0.75 | 8.30 | 6.34 |
| 4 | Tap | LBC | 8.37 | 16 | 1.222 | 0.69 | 7.63 | 6.24 |
| 5 | Tap | LBC | 8.37 | 14 | 1.068 | 0.57 | 6.30 | 5.89 |
| 6 | DI | LBC | 6.46 | 19 | 1.005 | 0.63 | 6.96 | 6.93 |
| 7 | DI | LBC | 7.15 | 19 | 0.822 | 0.57 | 6.37 | 7.75 |
| 8 | DI | LBC | 7.97 | 19 | 1.038 | 0.55 | 6.15 | 5.92 |
| 9 | DI | LBC | 8.53 | 19 | 1.134 | 0.50 | 5.56 | 4.90 |
| 10 | DI | LBC | 8.86 | 19 | 1.196 | 0.44 | 4.89 | 4.09 |
| 11 | DI | LBC | 9.17 | 19 | 1.156 | 0.41 | 4.59 | 3.97 |
| D10 | D50 | D60 | D30 | Cu | Cz |
|---|---|---|---|---|---|
| 0.140 | 0.226 | 0.250 | 0.193 | 1.786 | 1.064 |
| Column | J1 | J2 | J3 | J4 | J5 | J6 | C1 | C2 | C3 |
|---|---|---|---|---|---|---|---|---|---|
| Effluent OD600 (5–10 mL) | 0.018 | 0.01 | 0.015 | 0.011 | 0.008 | 0.011 | 0.055 | 0.02 | 0.044 |
| Columns | Moisture Content % | CaCO3 Content% |
|---|---|---|
| J1–J3 | 18.35 ± 0.34 | 3.98 ± 0.59 |
| J4–J6 | 16.82 ± 1.33 | 4.63 ± 0.31 |
| C1–C3 | 17.03 ± 0.39 | 1.08 ± 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spencer, C.A.; van Paassen, L.; Sass, H. Effect of Jute Fibres on the Process of MICP and Properties of Biocemented Sand. Materials 2020, 13, 5429. https://doi.org/10.3390/ma13235429
Spencer CA, van Paassen L, Sass H. Effect of Jute Fibres on the Process of MICP and Properties of Biocemented Sand. Materials. 2020; 13(23):5429. https://doi.org/10.3390/ma13235429
Chicago/Turabian StyleSpencer, Christine Ann, Leon van Paassen, and Henrik Sass. 2020. "Effect of Jute Fibres on the Process of MICP and Properties of Biocemented Sand" Materials 13, no. 23: 5429. https://doi.org/10.3390/ma13235429
APA StyleSpencer, C. A., van Paassen, L., & Sass, H. (2020). Effect of Jute Fibres on the Process of MICP and Properties of Biocemented Sand. Materials, 13(23), 5429. https://doi.org/10.3390/ma13235429






