Cyclic Oxidation of Titanium Grade 2
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
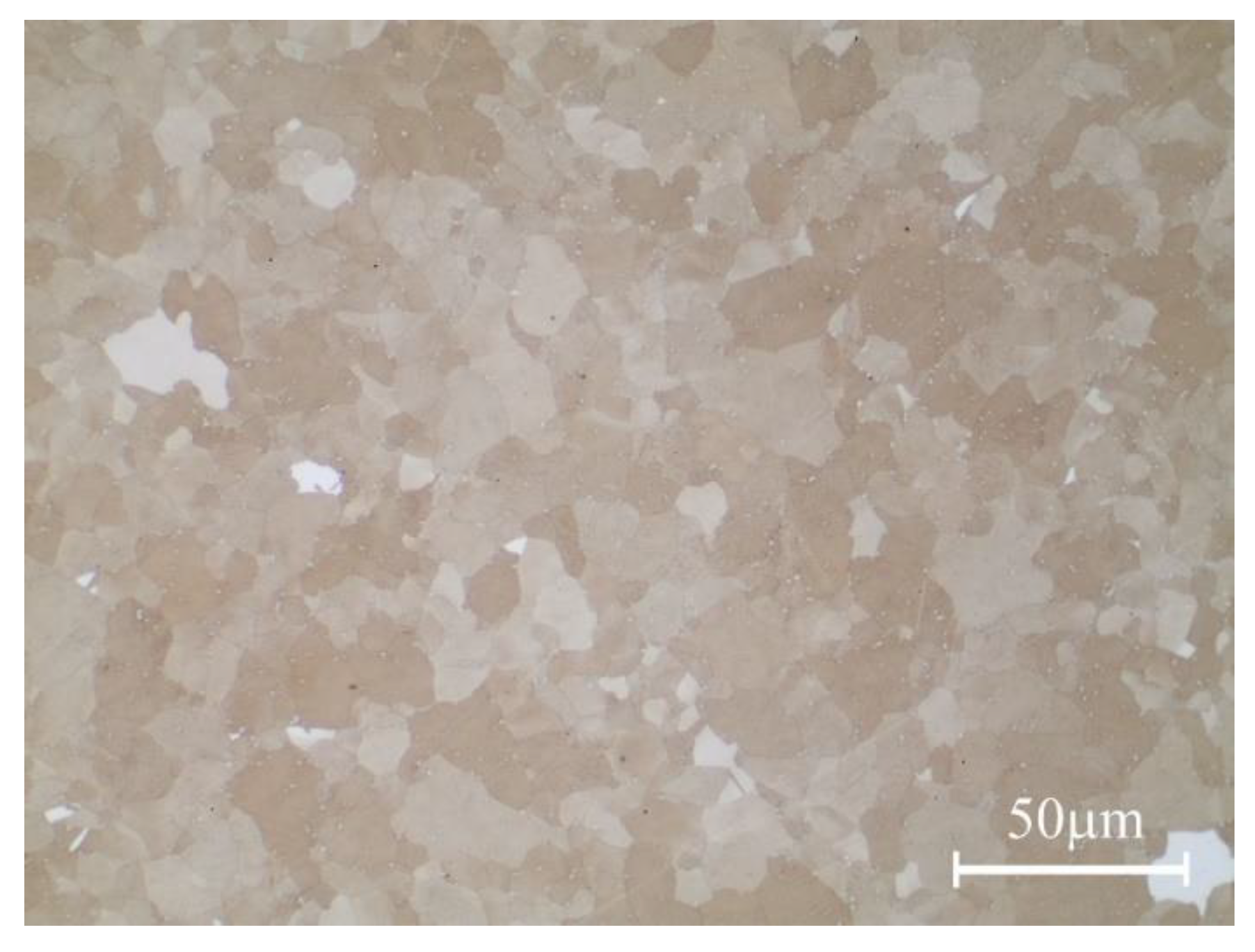
3.1. Characteristics of Research Material
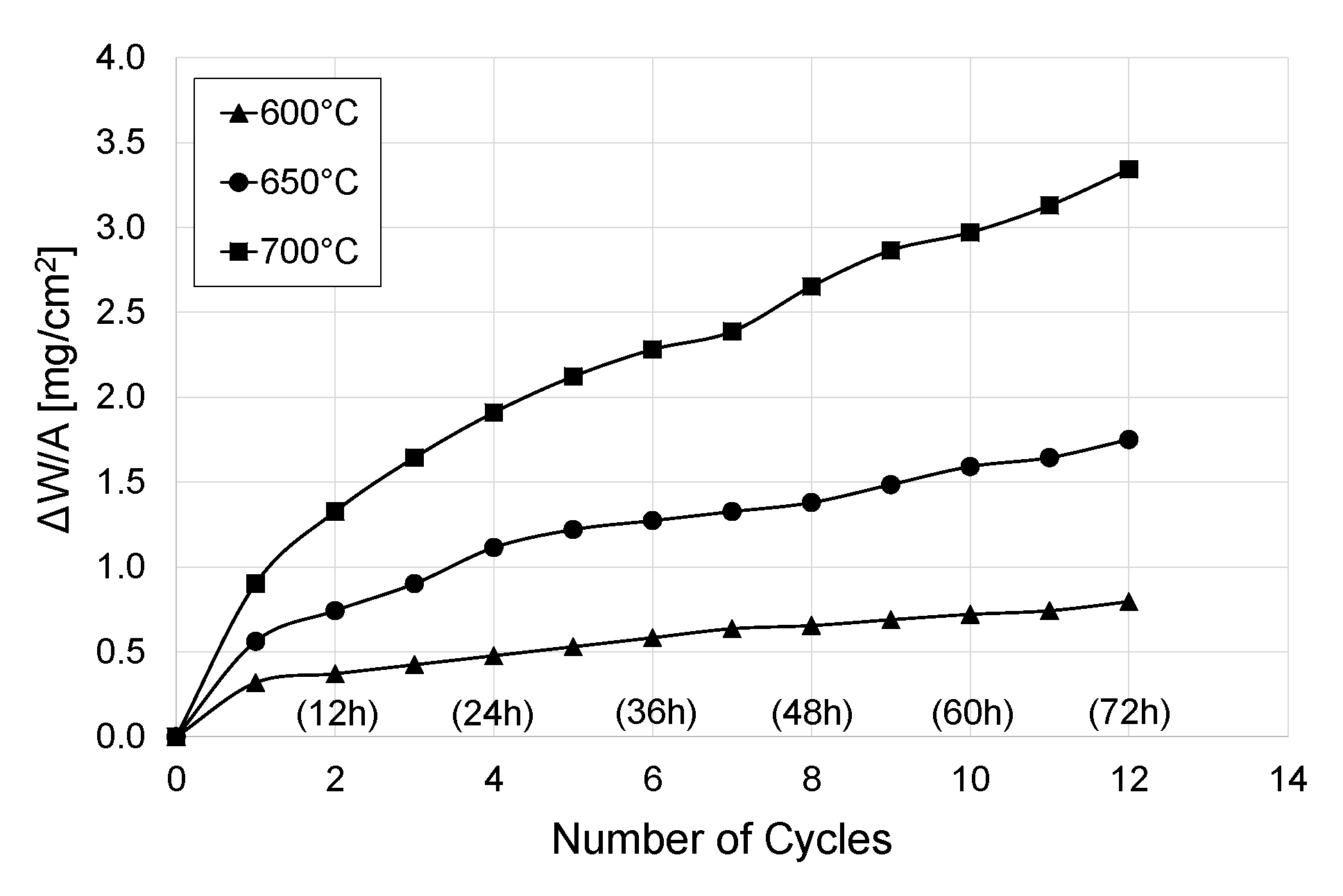
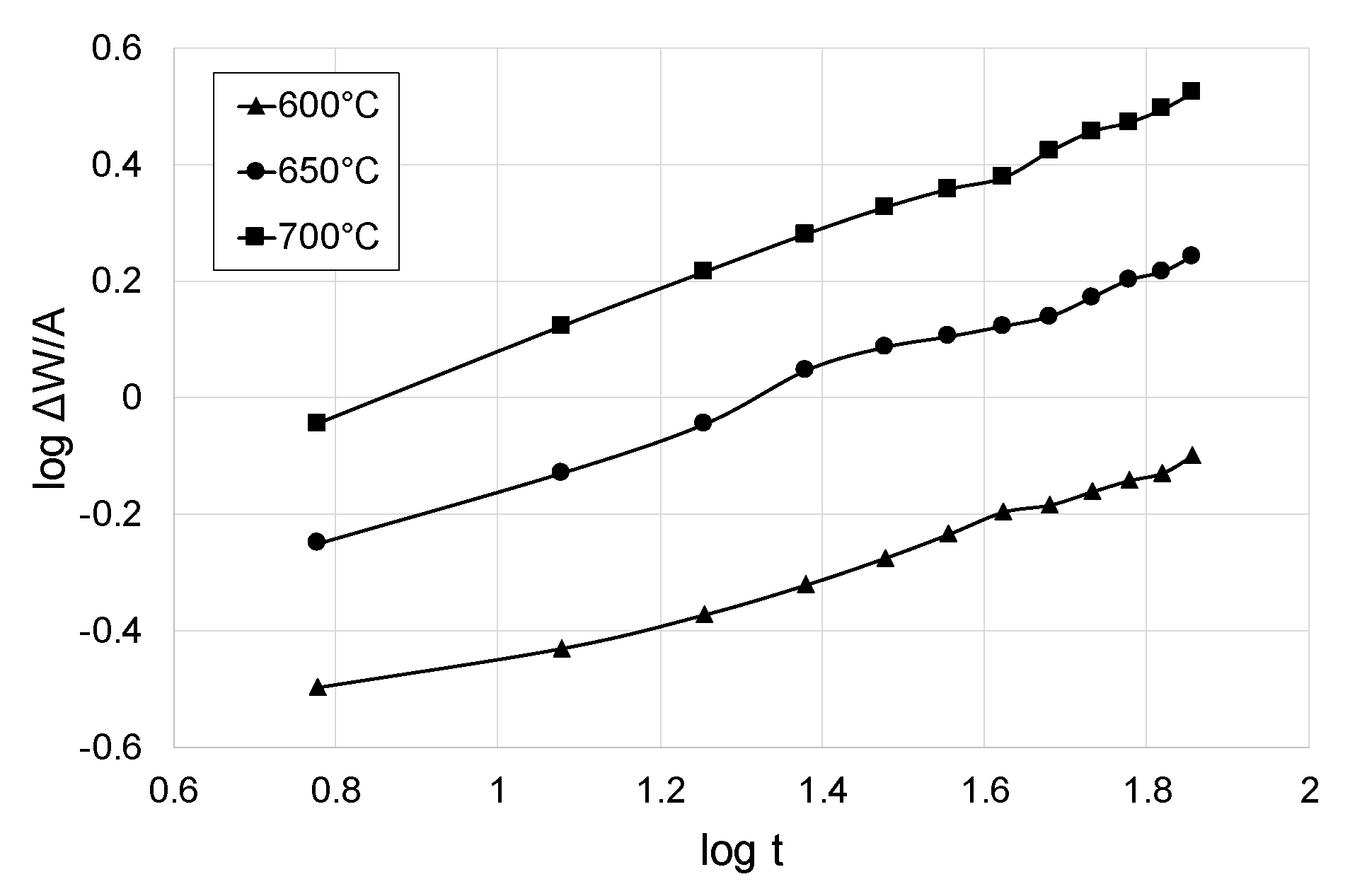
3.2. Kinetics of Cyclic Oxidation of Titanium Grade 2
3.3. Examination of the Morphological Features of the Produced Oxides
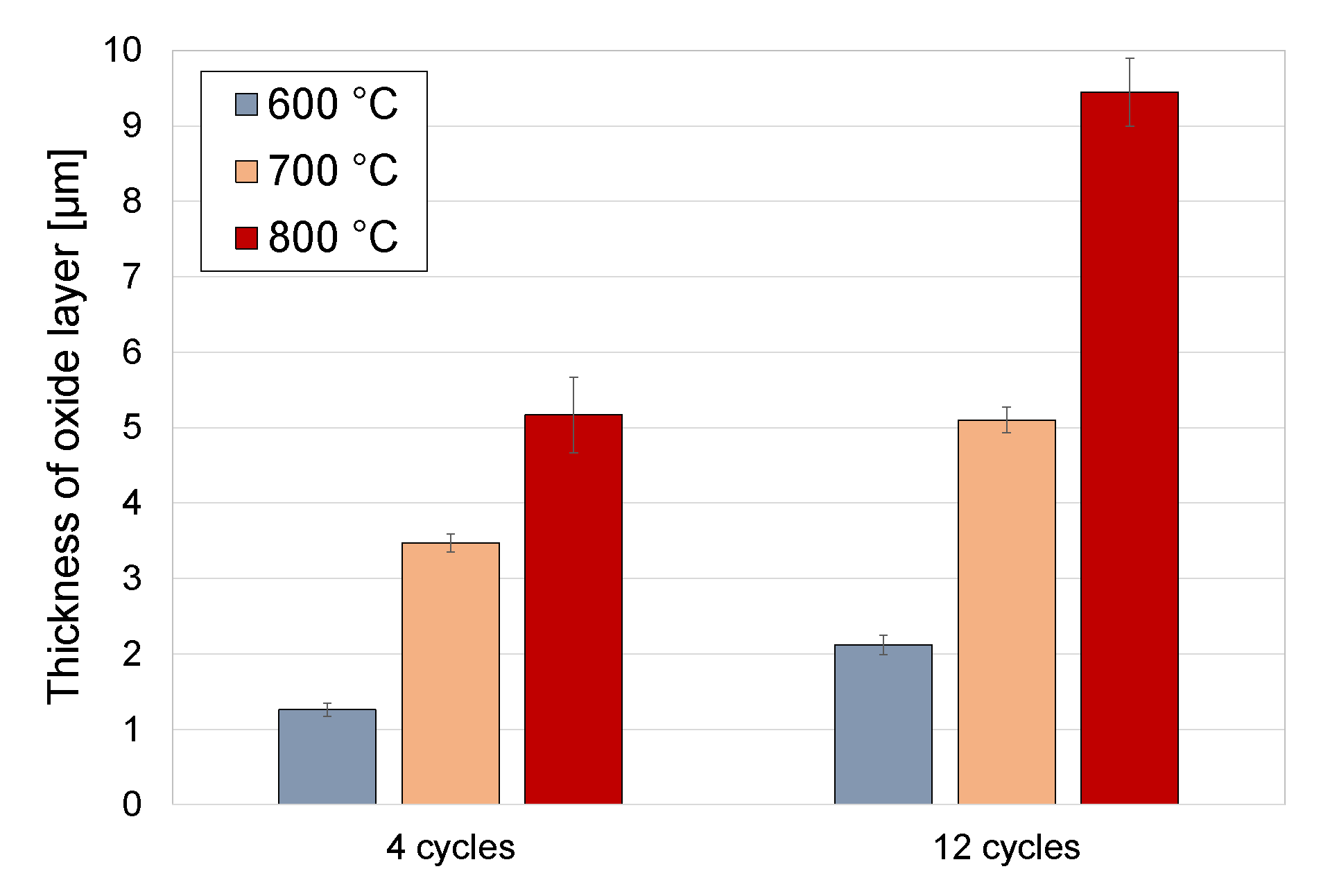
3.4. Oxide Scales Thickness
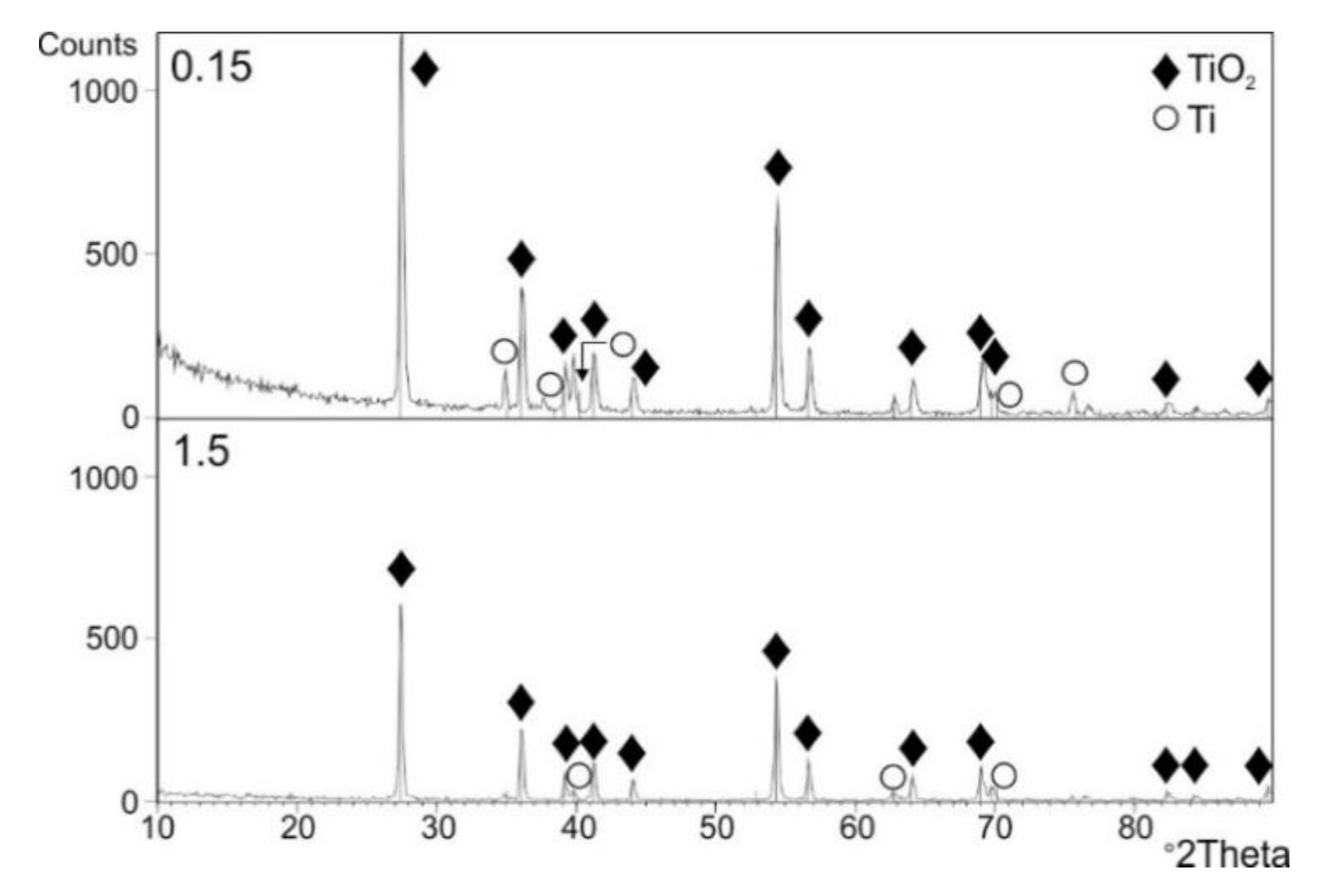
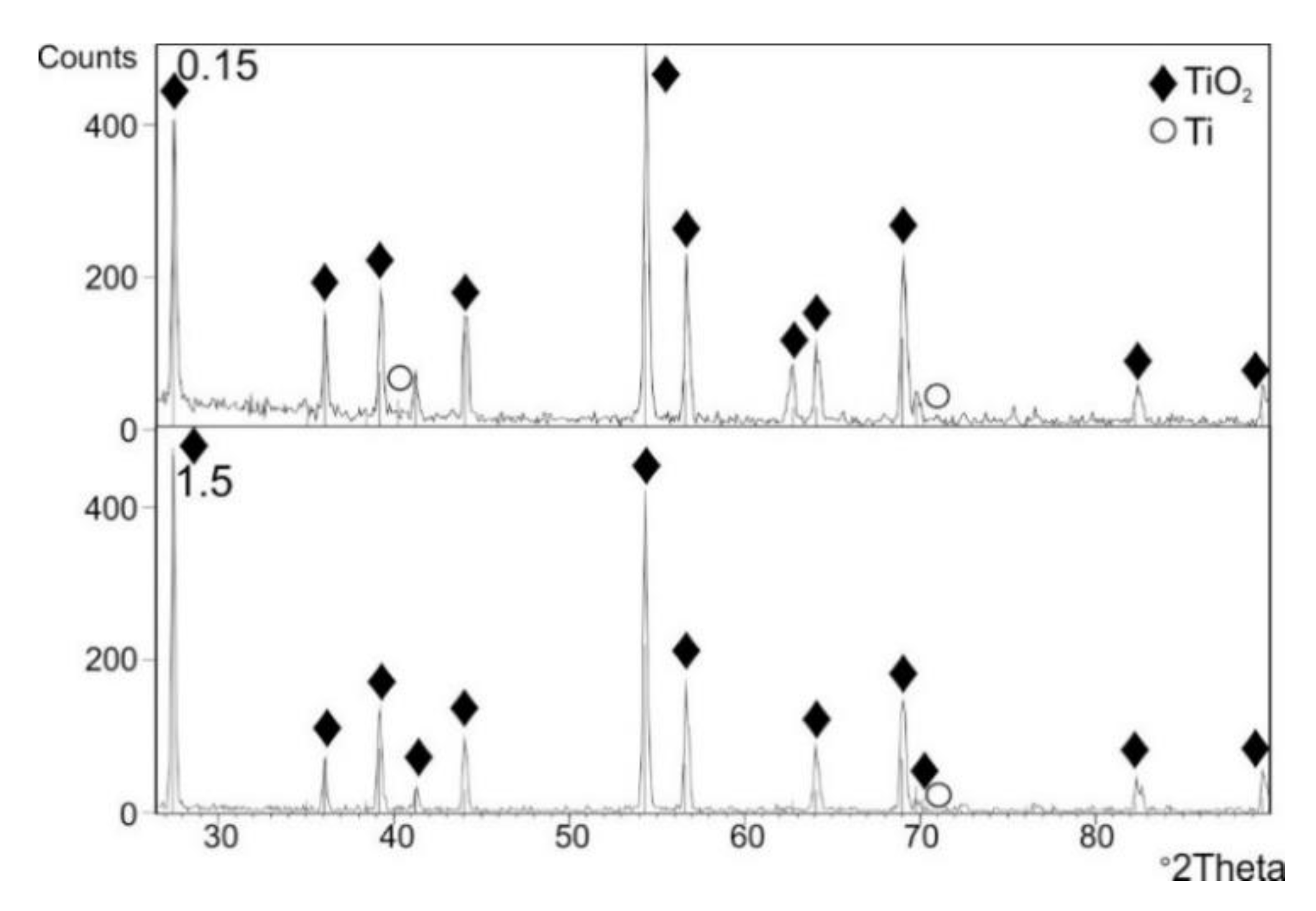
3.5. Phase Composition of the Oxide Scales
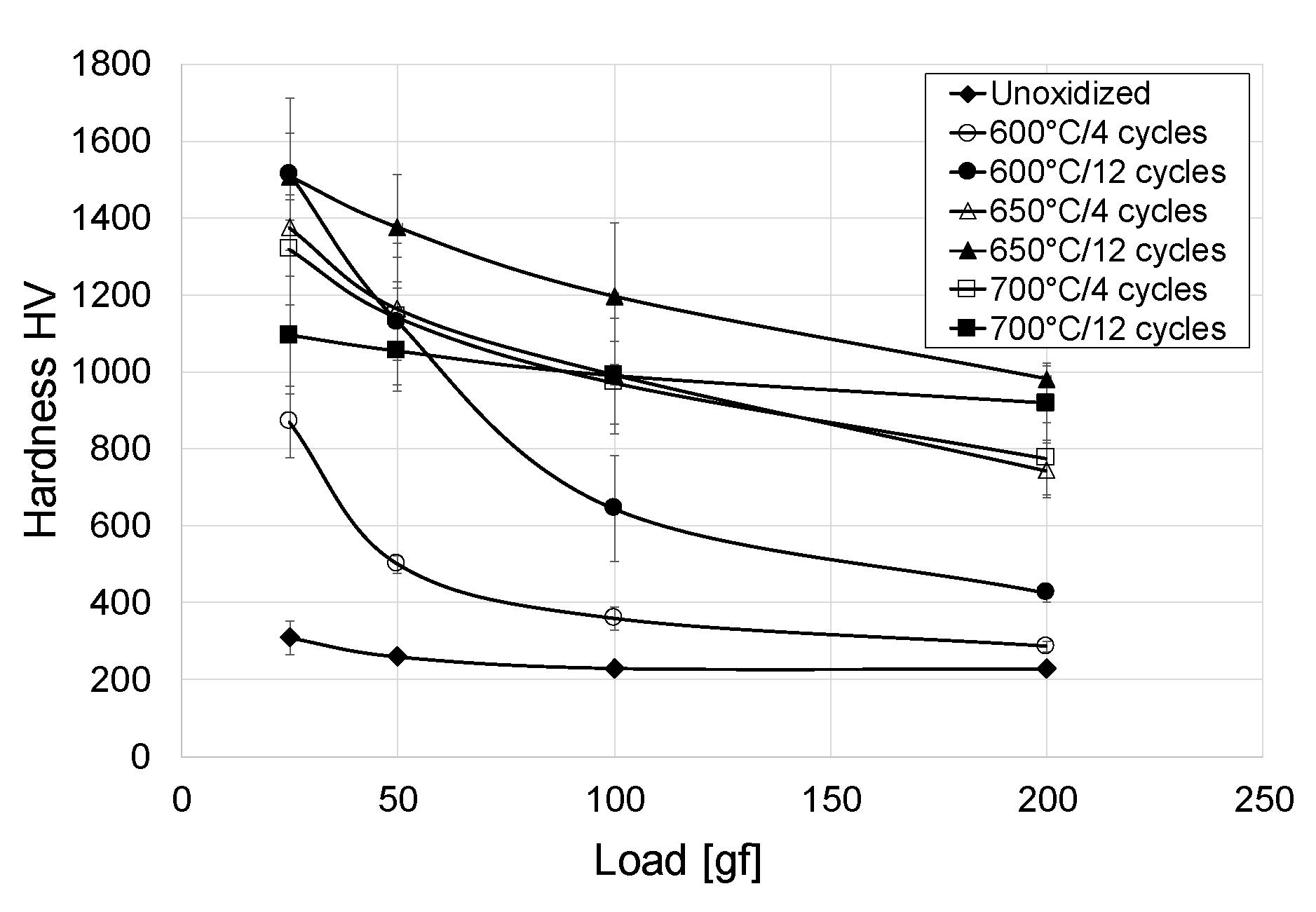
3.6. Oxide Scale Hardness
4. Conclusions
- The cyclic oxidation process allowed the formation on the surface of titanium Grade 2 of oxide scales of good quality, covering the whole surface and characterised by a homogeneous structure.
- The parameters of cyclic oxidation (temperature and number of cycles) had a significant influence on the dynamics of oxide scales growth on titanium Grade 2. The highest intensification of the process was found after oxidation at 700 °C. For this variant, the mass gain was nearly four times higher than in the case where the oxidation temperature was 600 °C.
- The temperature of cyclic oxidation had a significant influence on the morphology of the oxide scales formed. After oxidation at 600 °C, the produced oxide scale was characterised by the presence of oxides in the acicular system. Increasing the oxidation temperature to 650 and 700 °C led to the formation of oxide scales composed of oxide particles which increased with the increasing oxidation temperature.
- The oxide scales obtained in the cyclic oxidation process had varied thicknesses, depending on the oxidation temperature and the number of cycles. The oxidation process conducted at a temperature of 600 °C enabled the formation of oxide scales with thicknesses of 1.26 µm (4 cycles) and 2.12 µm (12 cycles). Increasing the oxidation temperature to 650 °C induced a further growth of the oxide scale thickness (to 3.47 µm and 5.10 µm, respectively). The oxide scales produced at 700 °C had the greatest thickness (5.17 and 9.45 µm, respectively).
- XRD examination after cyclic oxidation showed presence of TiO2 (rutile) only in the obtained layers, regardless of the oxidation temperature. At the same time, presence of peaks coming from the titanium Grade 2 substrate was found. No presence of other phases was found.
- The cyclic oxidation process contributed to an increase in the surface hardness of titanium Grade 2. The oxide scales which formed at 600 and 650 °C had the greatest hardness (approx. 1500 HV). After oxidation at 700 °C, a considerable decrease in hardness was found (by approx. 400 HV).
- Cyclic oxidation, in comparison with the traditional isothermal oxidation method, is characterised by higher intensity, which allows obtaining oxide scales characterised by higher thickness. This, in turn, may positively contribute to the improvement of functional properties of titanium.
Author Contributions
Funding
Conflicts of Interest
References
- Hu, H.Y.; Zhang, L.; He, Z.Y.; Jiang, Y.H.; Tan, J. Microstructure evolution, mechanical properties, and enhanced bioactivity of Ti-13Nb-13Zr based calcium pyrophosphate composites for biomedical applications. Mater. Sci. Eng. 2019, 98, 279–287. [Google Scholar] [CrossRef]
- Lederer, S.; Lutz, P.; Fürbeth, W. Surface modification of Ti 13Nb 13Zr by plasma electrolytic oxidation. Surf. Coat. Technol. 2018, 335, 62–71. [Google Scholar] [CrossRef]
- Koizumi, H.; Takeuchi, Y.; Imai, H.; Kawai, T.; Yoneyama, T. Application of titanium and titanium alloys to fixed dental prostheses. J. Prosthod. Res. 2019, 63, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, H.; Wieciński, P.; Kuczyńska, D.; Kubacka, D.; Kurzydłowski, K.J. The effect of grain size on the surface properties of titanium grade 2 after different treatments. Surf. Coat. Technol. 2018, 335, 13–24. [Google Scholar] [CrossRef]
- Jia, Y.-F.; Pan, R.-J.; Zhang, P.-Y.; Sun, Z.-T.; Chen, X.-R.; Zhang, X.-C.; Wu, X.-J. Enhanced surface strengthening of titanium treated by combined surface deep-rolling and oxygen boost diffusion technique. Corr. Sci. 2019, 157, 256–267. [Google Scholar] [CrossRef]
- Sundqvist, B.; Tolpygo, V.K. Saturation and pressure effects on the resistivity of titanium and two Ti-Al alloys. J. Phys. Chem. Solid. 2018, 122, 41–50. [Google Scholar] [CrossRef]
- Pohrelyuk, I.M.; Sheykin, S.E.; Padgurskas, J.; Lavrys, S.M. Wear resistance of two-phase titanium alloy after deformation-diffusion treatment. Tribol. Int. 2018, 127, 404–411. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Comp. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Bolzoni, F.; Pedeferri, M.P.; Ormellese, M. Chemical oxidation as repairing technique to restore corrosion resistance on damaged anodized titanium. Surf. Coat. Technol. 2019, 364, 225–230. [Google Scholar] [CrossRef]
- Singh, P.; Pungotra, H.; Kalsi, N.S. On the characteristics of titanium alloys for the aircraft applications. Mater. Today Proceed. 2017, 4, 8971–8982. [Google Scholar] [CrossRef]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Character. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Arias-González, F.; Del Val, J.; Comesaña, R.; Penide, J.; Lusquiños, F.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; Javier Gil, F.; Pou, J. Microstructure and crystallographic texture of pure titanium parts generated by laser additive manufacturing. Met. Mater. Int. 2018, 24, 231–239. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Imshinetskiy, I.M.; Piatkova, M.A.; Pleshkova, A.I.; Belov, E.A.; Filonina, V.S.; Suchkov, S.N.; Sinebryukhov, S.L.; et al. Bioactive Coatings Formed on Titanium by Plasma Electrolytic Oxidation: Composition and Properties. Materials 2020, 13, 4121. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.M.R.; Carvalho, O.; Henriques, B.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Electrical potential approaches to inhibit biofilm adhesion on titanium implants. Mater. Lett. 2019, 255, 126577. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.T. Development of a new metastable beta titanium alloy for biomedical applications. Karb. Int. J. Modern Sci. 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Bansal, R.; Singh, J.K.; Singh, V.; Singh, D.D.N.; Das, P. Optimization of oxidation temperature for commercially pure titanium to achieve improved corrosion resistance. J. Mater. Eng. Perform. 2017, 26, 969–977. [Google Scholar] [CrossRef]
- Bombac, D.; Brojan, M.; Fajfar, P.; Kosel, F.; Turk, R. Review of materials in medical applications. RMZ Mater. Geoenvir. 2007, 54, 471–499. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science—An Introduction to Materials in Medicine; Academic Press (Elsevier): Amsterdam, The Netherlands, 2013. [Google Scholar]
- Amanov, A.; Cho, I.-S.; Kim, D.-E.; Pyun, Y.-S. Fretting wear and friction reduction of CP titanium and Ti–6Al–4V alloy by ultrasonic nanocrystalline surface modification. Surf. Coat. Technol. 2012, 207, 135–142. [Google Scholar] [CrossRef]
- Hu, X.; Shen, H.; Cheng, Y.; Xiong, X.; Wang, S.; Fang, J.; Wei, S. One-step modification of nano-hydroxyapatite coating on titanium surface by hydrothermal method. Surf. Coat. Technol. 2010, 205, 2000–2006. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.; Lu, M.; Liu, L.; Yan, Y.; Chu, Z.; Ge, Y.; Wang, T.; Tang, C. Enhanced bioactive and osteogenic activities of titanium by modification with phytic acid and calcium hydroxide. App. Surf. Sci. 2019, 478, 162–175. [Google Scholar] [CrossRef]
- Marin, E.; Offoiach, R.; Regis, M.; Fusi, S.; Lanzutti, A.; Fedrizzi, L. Diffusive thermal treatments combined with PVD coatings for tribological protection of titanium alloys. Mater. Des. 2016, 89, 314–322. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Zhu, Q.; Fan, C.; Lv, P.; Xiang, M. Low-temperature chemical vapor deposition (CVD) of metallic titanium film from a novel precursor. Surf. Coat. Technol. 2018, 353, 18–24. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation temperature on the microstructural and tribological behavior of Ti6Al4V alloy. Surf. Coat. Technol. 2014, 240, 470–477. [Google Scholar] [CrossRef]
- Kumar, S.; Sankara Narayanan, T.S.N.; Raman, S.G.S.; Seshadri, S.K. Thermal oxidation of Ti6Al4V alloy: Microstructural and electrochemical characterization. Mater. Chem. Phys. 2010, 119, 337–346. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, W.; Tian, M.; Teng, S. Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Int. 2015, 89, 67–71. [Google Scholar] [CrossRef]
- Li, L.; Yu, K.; Zhang, K.; Liu, Y. Study of Ti–6Al–4V alloy spectral emissivity characteristics during thermal oxidation process. Int. J. Heat Mass Transf. 2016, 101, 699–706. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Effect of thermal oxidation on corrosion and corrosion–wear behaviour of a Ti–6Al–4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef]
- Teng, Y.; Chen, Q.; Xin, L. The effect of coatings on the cyclic oxidation behaviour of Ti6Al4V at 750°C. Adv. Mater. Res. 2012, 393-395, 428–435. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, A.; Jiang, H.; Fan, X.; Duan, X.; Yan, X. Cyclic Oxidation Behavior of the Ti–6Al–4V Alloy. Oxid. Met. 2014, 81, 467–476. [Google Scholar] [CrossRef]
- Kim, M.J.; Yadav, P.; Lee, D.B. Isothermal and Cyclic Oxidation of Ti-6Al-4V Alloy. Defect Diff. Forum 2016, 369, 99–103. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Barylski, A.; Dercz, G. Mechanical and tribological properties of oxide layers obtained on titanium in the thermal oxidation process. App. Surf. Sci. 2015, 357, 1419–1426. [Google Scholar] [CrossRef]
- Król, S. Mechanizm i kinetyka utleniania tytanu oraz wybranych stopów tytanu. In Studia i Monografie; Komitet Redakcyjny Wydawnictw WSI: Opole, Poland, 1994. (In Polish) [Google Scholar]
- Kumar, S.; Sankara Narayanan, T.S.N.; Sundara Raman, S.G.; Seshadri, S.K. Thermal oxidation of CP Ti—An electrochemical and structural characterization. Mater. Character. 2010, 61, 589–597. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation duration on the microstructure and fretting wear behavior of Ti6Al4V alloy. Mater. Chem. Phys. 2015, 159, 139–151. [Google Scholar] [CrossRef]
- Vache, N.; Cadoret, Y.; Dod, B.; Monceau, D. Modeling the oxidation kinetics of titanium alloys: Review, method and application to Ti-64 and Ti-6242s alloys. Corr. Sci. 2021, 178, 109041. [Google Scholar] [CrossRef]
- Aniołek, K.; Barylski, A.; Kupka, M.; Leszek, I. The tensile Properties, scratch behaviors and sliding wear of oxide scale formed on titanium Grade 2. Materials 2020, 13, 3048. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti–6Al–4V alloy. J. All. Comp. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Dercz, G. Cyclic oxidation of Ti–6Al–7Nb alloy. Vacuum 2019, 168, 108859. [Google Scholar] [CrossRef]
- Aniołek, K. The influence of thermal oxidation parameters on the growth of oxide layers on titanium. Vacuum 2017, 144, 94–100. [Google Scholar] [CrossRef]
- Biswas, A.; Majumdar, J.D. Surface characterization and mechanical property evaluation of thermally oxidized Ti-6Al-4V. Mater. Character. 2009, 60, 513–518. [Google Scholar] [CrossRef]






| Material | Component Content, wt.-% | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Fe | H | N | O | Al | Nb | Ti | |
| TiGr2 | 0.008 | 0.13 | 0.0019 | 0.010 | 0.18 | - | - | the rest |
| ASTM Grain Size Number | 11 |
| Mean Grain Area [µm2] | 63 |
| Total Number of Grains | 822 |
| Analyzed Area [µm2] | 52,988 |
| Temperature [°C] | |||
|---|---|---|---|
| 600 | 650 | 700 | |
| Kp [(mg2/cm4s)] × 10−8 | 229 | 1120 | 4189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniołek, K.; Barylski, A.; Kupka, M.; Dercz, G. Cyclic Oxidation of Titanium Grade 2. Materials 2020, 13, 5431. https://doi.org/10.3390/ma13235431
Aniołek K, Barylski A, Kupka M, Dercz G. Cyclic Oxidation of Titanium Grade 2. Materials. 2020; 13(23):5431. https://doi.org/10.3390/ma13235431
Chicago/Turabian StyleAniołek, Krzysztof, Adrian Barylski, Marian Kupka, and Grzegorz Dercz. 2020. "Cyclic Oxidation of Titanium Grade 2" Materials 13, no. 23: 5431. https://doi.org/10.3390/ma13235431
APA StyleAniołek, K., Barylski, A., Kupka, M., & Dercz, G. (2020). Cyclic Oxidation of Titanium Grade 2. Materials, 13(23), 5431. https://doi.org/10.3390/ma13235431








