Concrete for Precast Blocks: Binary and Ternary Combination of Sewage Sludge Ash with Diverse Mineral Residue
Abstract
1. Introduction
- As an example, in the case of SSA, it has been shown to be adequate to partially replace cement up to proportions of 10%, or with 2% substitution of the mix sand. In such cases, the specimens showed analogous compressive strengths compared with control specimens. Regarding this leaching study, results indicate that the mixture of mortar or concrete with ash, cement and sand induced the stabilization of Mo and Se, so in this way, this constitutes an effective treatment of the ashes [12]. Previously published research revealed the good mechanical properties of mortars with SSA [13,14]. These enhancements have been attributed to the pozzolanic activity of SSA [9,15,16]. Nevertheless, it should also be mentioned that other research focused on reused materials has shown that the pozzolanic activity of ashes should be considered at least as weak when it is compared with other widely known pozzolanic materials [17]. Moreover, the irregular shapes of the particles that limit their performance as a solid lubricant result in a reduction in the workability of the mixes, as has been concluded in references [18,19].
- The use of FA as a mineral admixture has been widely known for more than three decades. The most significant properties rely on its pozzolanic activity for the medium and long term, with spherical particles and high amounts of vitreous SiO2 and Al2O3 [20]. In addition, incorporating FA in Portland cement composites has been shown to improve the workability and consistency of concrete and mortar matrixes [21].
- SSA has been shown to possess significant pozzolanic activity for ternary combinations for the production of cement/SSA/FA binders. This pozzolanic activity increased the mechanical properties of mortars in the age time-period from 7 to 28 days. In these ternary combinations, SSA harmed the fluidity of the mixes while the addition of FA enhanced it [22,23].
- Published research has shown that MD in cementitious composite materials, employed as an addition to substitute up to 10% of sand, enhances the cohesion of the mixture, maintaining the compressive strength, reducing permeability, and even improving the mechanical performance in comparison with the effect of the same amount of limestone filler [24,25]. Regarding the properties of self-compacting concrete by adding MD, the results have been shown to meet the requirements in the standards. Given that with the addition of fine particles with good properties, a greater compactness is achieved, thus improving the mechanical performance [24,26]. The plastic viscosity of the mixtures with the addition of MD tends to increase with increasing content rates and has been rectified by using superplasticizers.
- RHA has demonstrated a significant positive impact on both the mechanical and durability properties in cementitious matrices [27,28]. Research dealing with RHA, mainly substituting part of the cement in mortars and concrete, has revealed that when replacing up to 25% of the cement, the results obtained were at least the same compared with a reference conventional concrete [29]. When the percentage of substitution was increased up to 30%, durability and consistency remained, showing improvements. In such cases, the compressive strength only showed improvements for older ages [30].
2. Experimental Campaign
2.1. Materials
2.2. Mix Proportioning
2.3. Experimental Program
2.4. Procedure
2.4.1. Previous and Complementary Tests
2.4.2. Manufacture of the Concrete Specimens
- Cubic specimens of dimensions 150 × 150 × 150 mm3 were manufactured by pouring the concrete on PVC molds following the standard UNE-EN 12390-1. Before the molds were filled, a release agent was sprayed on the walls and they were protected with plastic sheets in order to ease the specimen removal. This operation allowed for extracting the concrete element despite the dry consistency of the mixture.
- In order to perform an adequate mix of the components, the humidity was carefully controlled. This is of great importance given the strict consistency requirement of the mix design. Some of the components such as cement and the mineral additions were dry in origin. In the first step of the mix sequence, those dry components were mixed in absence of water. Then, the mixing of aggregates and water was conducted. The concrete was poured into the mold and it was compacted for 30 s at low speed by means of a pneumatic hammer (Milwaukee Kango-900). The specimens were stored in a moisture room for 24 h and were demolded by means of compressed air. After this, the specimens were stored in a curing chamber at 90% RH 20 °C until the testing age. In specimen “30” two forms of curing were used (the one called simply as “30”, cured in a humidity room, and the so-called “30’ ” cured under water.
2.4.3. Tests on Concrete Specimens
2.4.4. Statistical Data Analysis
3. Results and Discussion
3.1. Previous Tests
3.1.1. Characterization of Aggregates
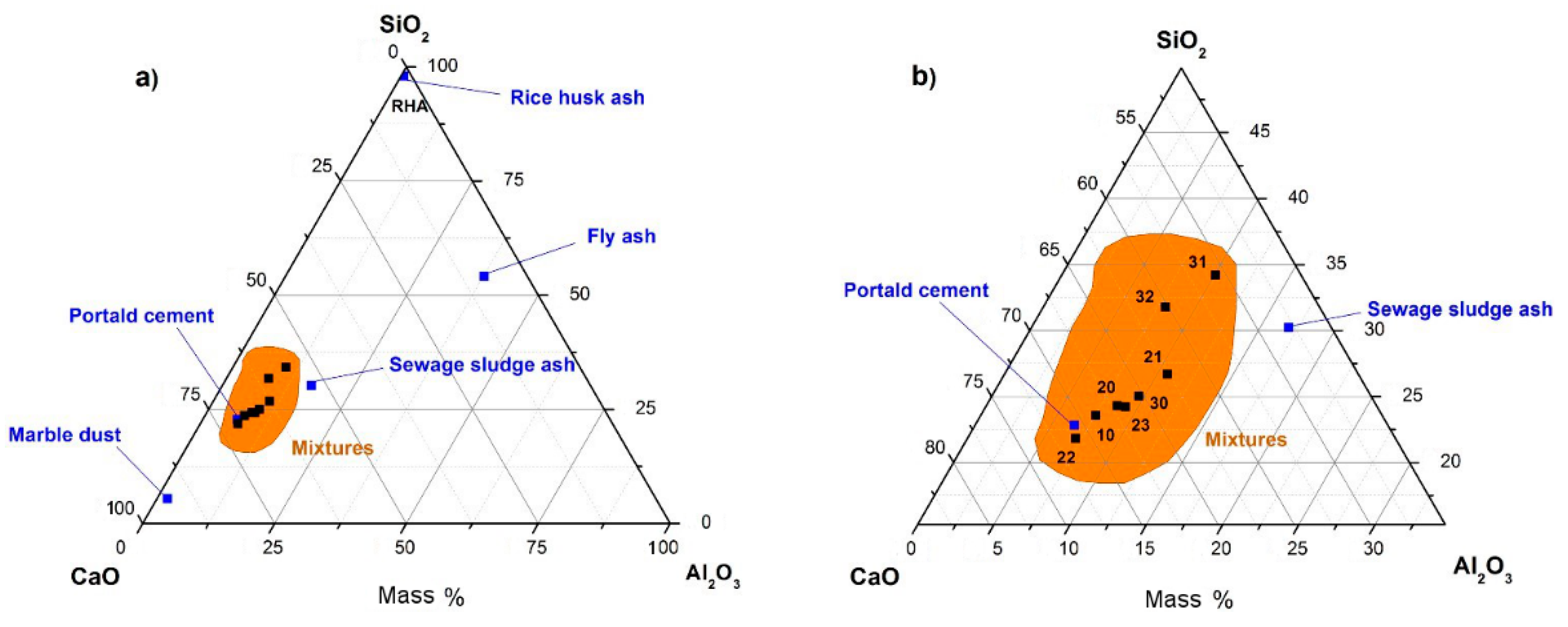
3.1.2. X-ray Fluorescence Analysis on Mineral Additions
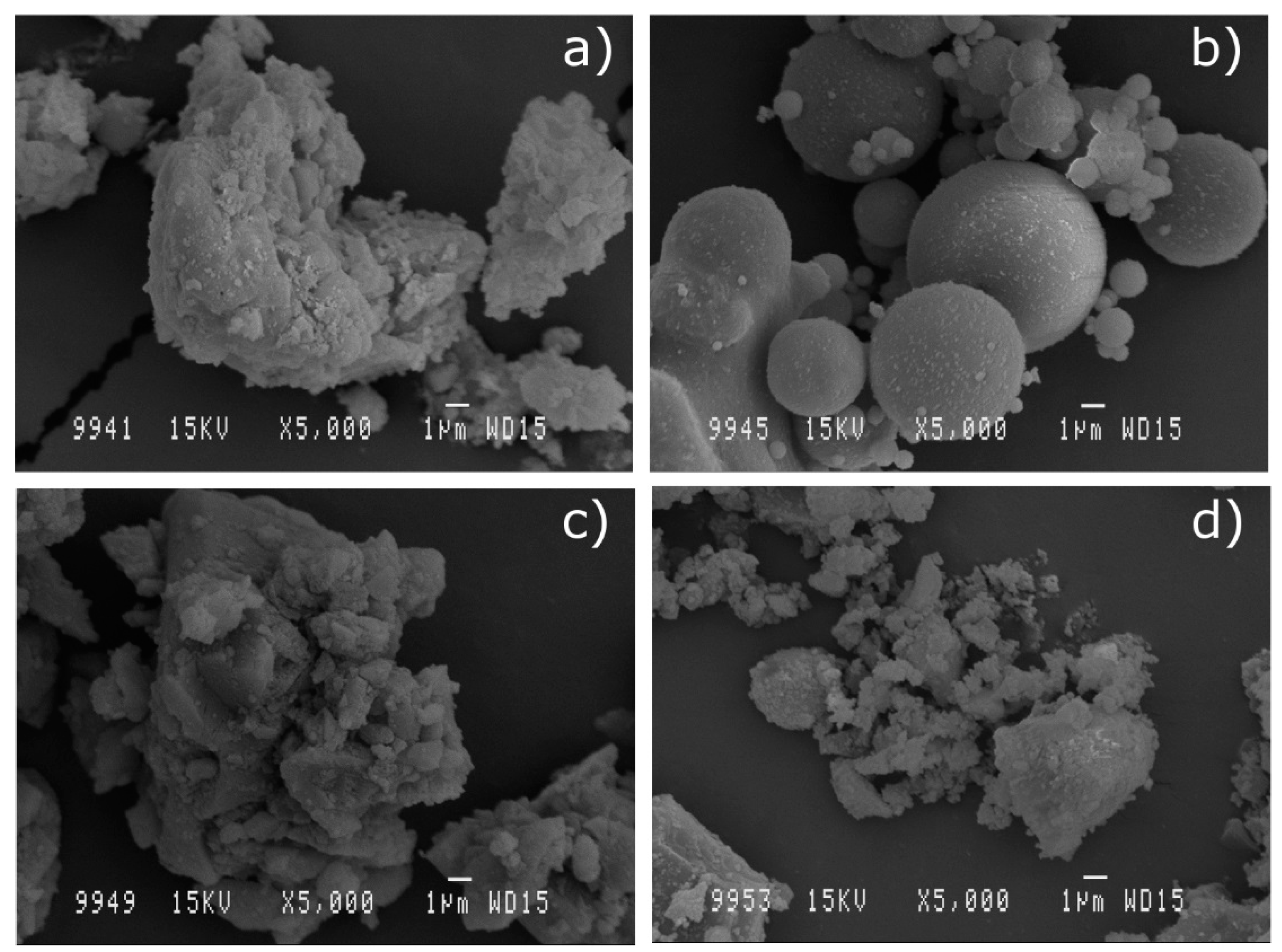
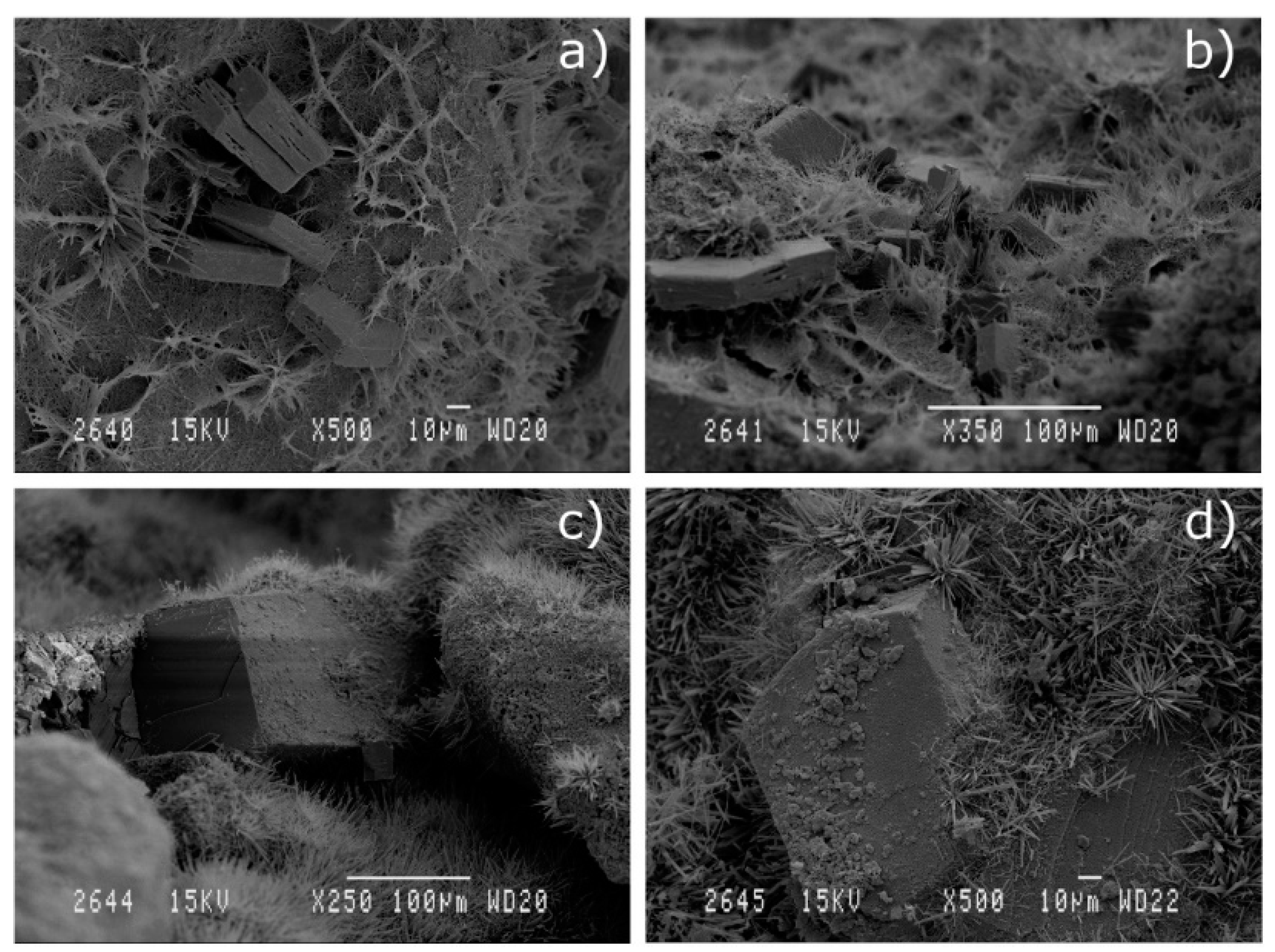
3.1.3. Scanning Electron Microscopy (SEM) of the Mineral Additions
3.2. Results in Concrete
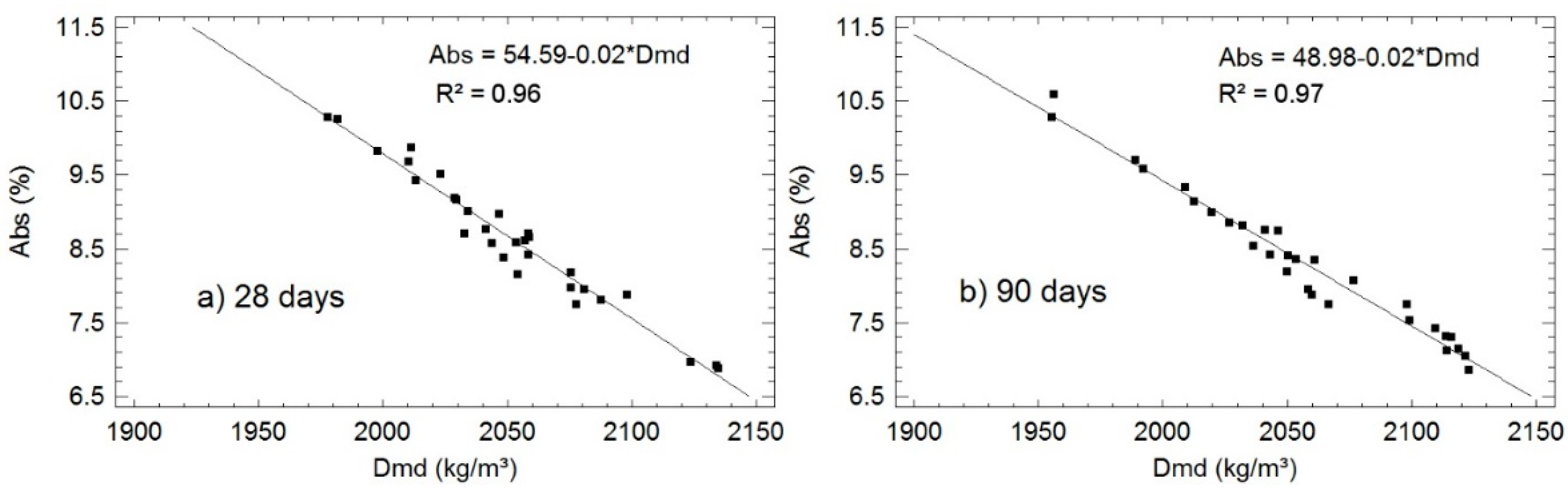
3.2.1. Density and Water Absorption
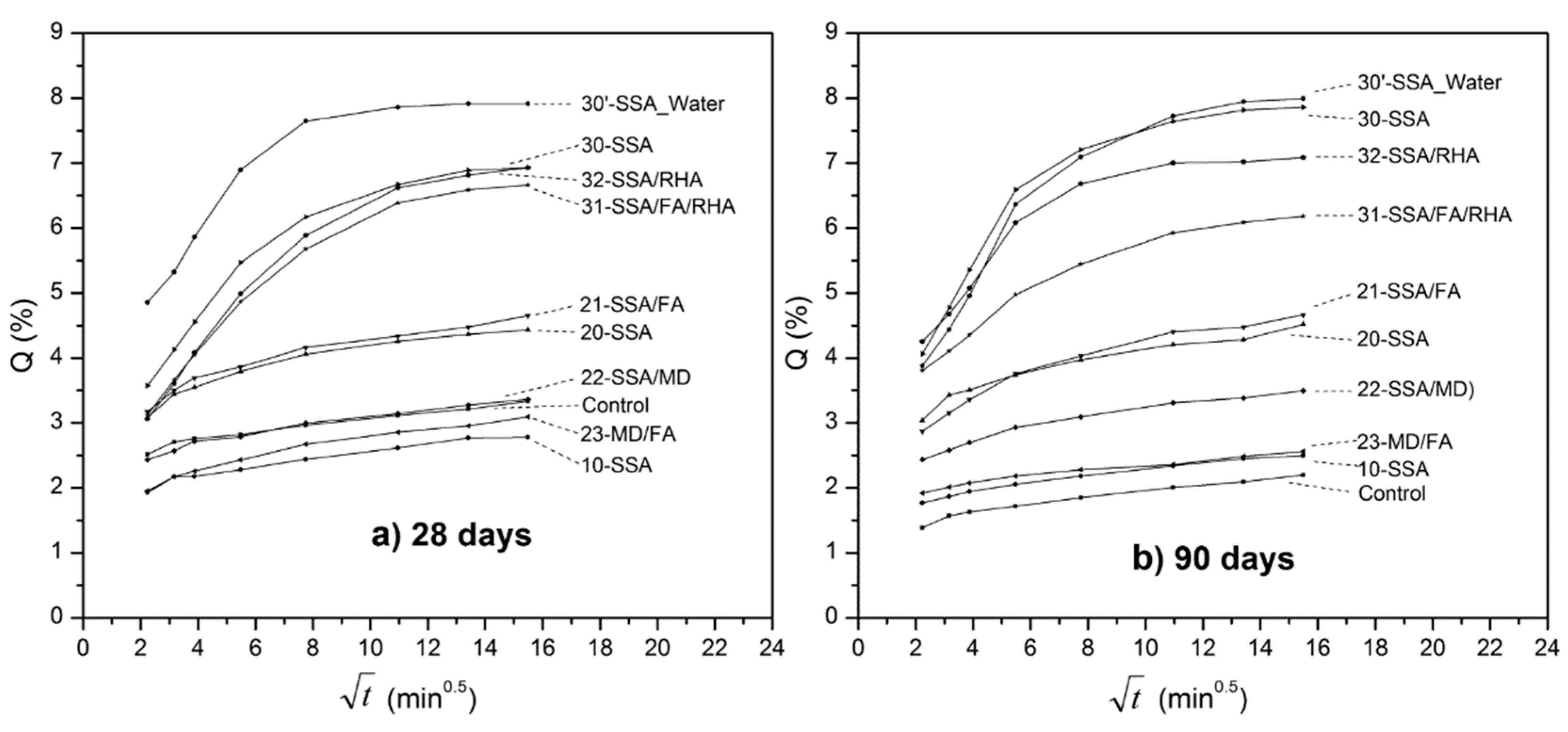
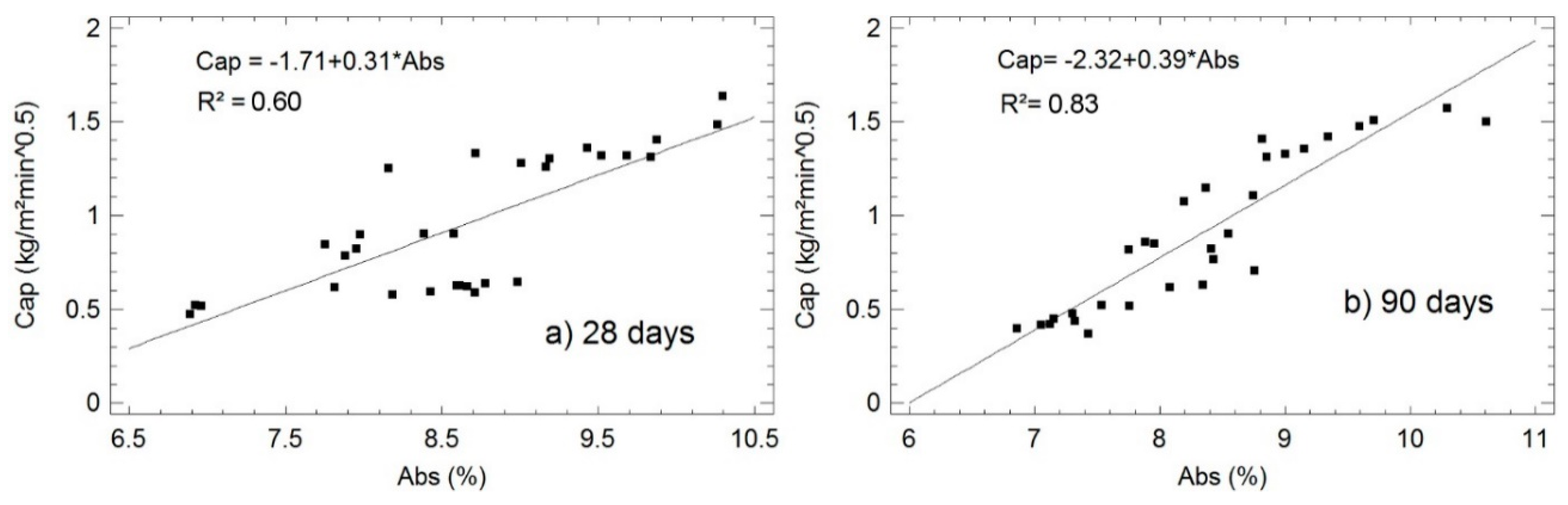
3.2.2. Capillary Water Absorption
- In the bottom of the graphs, therefore with lower values of capillary absorption, including the reference sample, samples “10” and “20”, and the binary combinations of mineral admixtures (“21”, “22” and “23”). These mixtures show lines parallel to each other, which indicates similar behavior during the test. After four hours of testing, the upslope remains, which means that water do not reach the upper surface in any of these samples, so that the filling is produced by water absorption through the capillary pores (state 1 of the Fagerlund model);
- Upper half of the graphics, therefore with higher capillary absorption values, including samples “30” and “30’ ” (cured under water), and the ternary combinations of mineral additions (“31” and “32”). A similar behavior between the specimens of this series is observed (parallel lines), but the main novelty of the parameters analyzed so far is that the slope is very low when the test is finished, which means that the water front reaches the top of the specimens, saturating the specimens faster. The time tn, which would correspond to the continuity of the capillary refill through pores of air by the process of diffusion and dissolution of air, will be approximately 120 min in sample “32” and between 180–240 min in samples “30” and “30’ ” (specimens cured for 90 days).
3.2.3. Compressive Strength
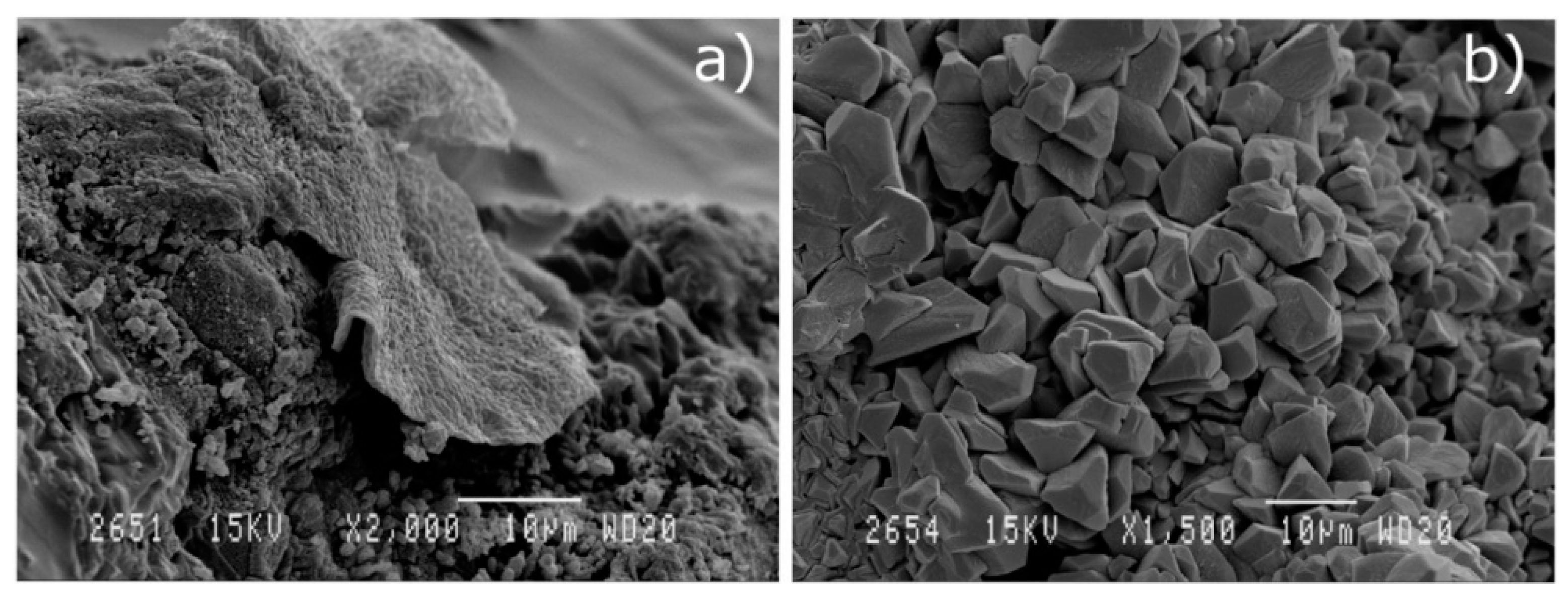
3.2.4. Scanning Electron Microscopy (SEM) of Concrete
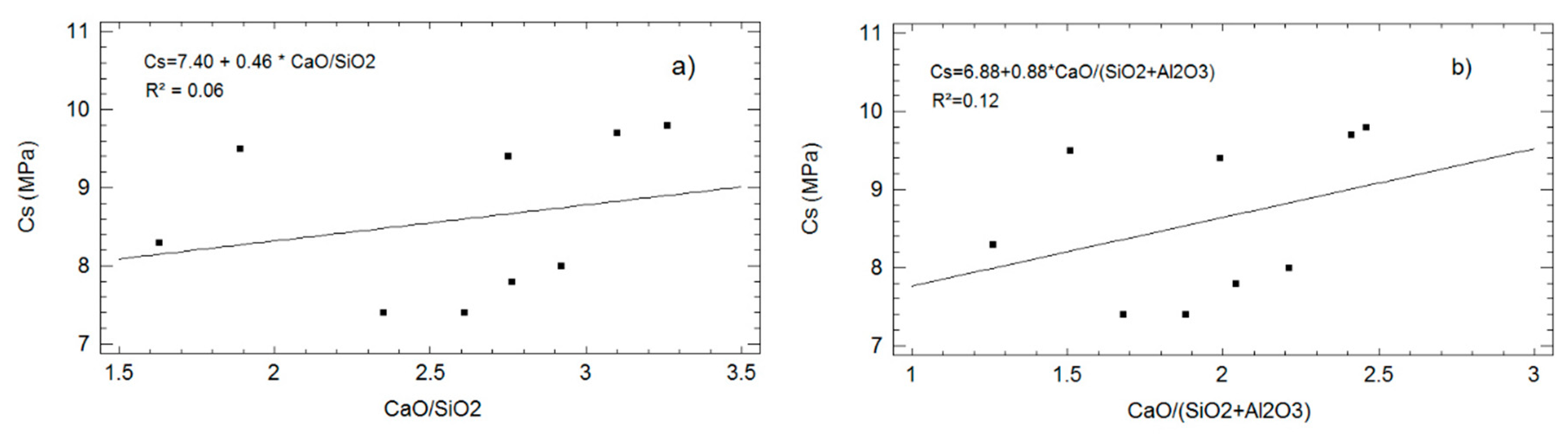
3.2.5. Correlation Matrix
- (a)
- The Spearman correlations between each variable pair are shown at the top, on the diagonal matrix. These values, ranking between +1 and −1, measure the strength of the linear relationship between the variables. At the bottom of the cell, the p-value shown is derived from the analysis of variance (ANOVA). ANOVA tests the statistical significance of the estimated correlations (for p-value < 0.05, the correlations are said to be significantly different from zero, with a reliability of 95%);
- (b)
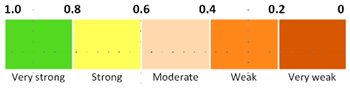
- The determination coefficients R2 between variables are located below the diagonal matrix. These variables are colored according to the scale of Evans [41], (1–0.8 very strong, 0.8–0.6 strong, 0.6–0.4 moderate, 0.4–0.2 weak and 0.2–0 very weak). This matrix makes clear the strong relationship among capillarity, absorption and. Accordingly, the analysis of the simple regression models (see Figure 4, Figure 5 and Figure 6) included in the corresponding section is completed.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Government of Spain. PNIR-Integrated National Waste Plan Spain; Government of Spain: Madrid, Spain, 2008.
- Bribián, I.Z.; Capilla, A.V.; Usón, A.A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Ambiente, M.D.; Marino, M.R. Study on the Generation and Management of Construction and Demolition Waste in Spain; Prointec: Madrid, Spain, 2006. [Google Scholar]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Science: Oxford, UK, 2003. [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Damtoft, J.S.; Herfort, D.; Yde, E. Concrete Binders, Mineral Additions and Chemical Admixtures: State of the Art and Challenges for the 21st Century; Damtoft, J.S., Herfort, D., Yde, E., Eds.; Thomas Telford Publishing: Dundee, Scotland, 1999; pp. 1–15. [Google Scholar]
- European Commission. Environmental, economic and social impacts of the use of sewage sludge on land. In Final Report. Part I: Overview Report; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Ministerio de Agricultura Alimentación y Medio Ambiente. Available online: http://www.magrama.gob.es/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos/flujos/lodos-dep (accessed on 10 May 2013).
- Cyr, M.; Coutand, M.; Clastres, P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cem. Concr. Res. 2007, 37, 1278–1289. [Google Scholar] [CrossRef]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Marble Association of Alicante. Available online: http://www.marmoldealicante.es (accessed on 20 July 2013).
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and technical assessments of the potential utilization of sewage sludge ashes (SSAs) as secondary raw materials in construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Monzó, J.; Payá, J.; Borrachero, M.V.; Córcoles, A. Use of sewage sludge ash(SSA)-cement admixtures in mortars. Cem. Concr. Res. 1996, 26, 1389–1398. [Google Scholar] [CrossRef]
- Alcocel, E.G.; Garcés, P.; Martínez, J.J.; Payá, J.; Andión, L.G. Effect of sewage sludge ash (SSA) on the mechanical performance and corrosion levels of reinforced Portland cement mortars. Materiales de Construcción 2006, 56, 282. [Google Scholar]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Amahjour, F.; Girbés, I.; Velázquez, S.; Ordóñez, L.M. Advantages in the use of fly ashes in cements containing pozzolanic combustion residues: Silica fume, sewage sludge ash, spent fluidized bed catalyst and rice husk ash. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2002, 77, 331–335. [Google Scholar] [CrossRef]
- Tay, J.-H.; Show, K.-Y. Municipal wastewater sludge as cementitious and blended cement materials. Cem. Concr. Compos. 1994, 16, 39–48. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Gonfalves, A.; Esteves, A.M.; Carvalho, M.; Machado, A.; Correia, E. Incorporation of sludge from a water treatment plant in cement mortars. Int. RILEM Conf. Use Recycl. Mater. Build. Struct. 2004, 2, 843. [Google Scholar]
- GIQUIMA. Research Group in Chemistry Building Materials—Universitat Politècnica de València. Proyecto PEL-CEN. Available online: http://epsar.cop.gva.es/depuradorasv (accessed on 15 July 2018).
- Malhotra, V.M.; Ramezanianpour, A.A. C.C. for M. and E. Technology, Fly Ash in Concrete; CANMET: Hamilton, Canada, 1994; ISBN 978-0660157641. [Google Scholar]
- Peris, E.; Paya, J.; Monzó, J. Influence of different sized fractions of a fly ash on workability of mortars. Cem. Concr. Res. 1993, 23, 917–924. [Google Scholar] [CrossRef]
- Monzó, J.; Borrachero, M.V.; Payá, J.; Girbés, I. Morteros de cementos compuestos a base de cenizas volantes de central termoeléctrica de carbón (CV) y cenizas procedentes de la incineración de lodos de depuradora (CLD); Actas del III Congreso Nacional de Materiales Compuestos: Benalmádena Málaga, Spain, 1999; pp. 477–483. [Google Scholar]
- Borrachero, M.V.; Payá, J.; Monzó, J.; Bonilla, M.; Girbés, I. Evolución de las resistencias mecánicas de sistemas ternarios cemento/ceniza volante/ceniza de lodo de depuradora: Efectos puzolánicos complementarios. Available online: https://www.upv.es/pms2002/Comunicaciones/038 PAYA.PDF (accessed on 5 January 2019).
- Corinaldesi, V.; Moriconi, G.; Naik, T.R. Characterization of marble powder for its use in mortar and concrete. Constr. Build. Mater. 2010, 24, 113–117. [Google Scholar] [CrossRef]
- Binici, H.; Kaplan, H.; Yilmaz, S. Influence of Marble and Limestone Dusts as Additives on Some Mechanical Properties of Concrete, SCI RES ESSAYS 2 (2007) 372-379. Available online: http://www.academicjournals.org/SRE (accessed on 5 January 2019).
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Auda, E.M. Re-use of waste marble dust in the production of cement and concrete. Constr. Build. Mater. 2014, 50, 28–41. [Google Scholar] [CrossRef]
- Givi, N.A.; Rashid, S.A.; Nora, F.; Aziz, F.A.; Amran, M.; Salleh, A. Contribution of rice husk ash to the properties of mortar and concrete: A review. J. Am. Sci. 2010, 6, 157–165. [Google Scholar]
- de Sensale, G.R. Strength development of concrete with rice-husk ash. Cem. Concr. Compos. 2006, 28, 158–160. [Google Scholar] [CrossRef]
- Khan, R.; Jabbar, A.; Ahmad, I.; Khan, W.; Khan, A.N.; Mirza, J. Reduction in environmental problems using rice-husk ash in concrete. Constr. Build. Mater. 2012, 30, 360–365. [Google Scholar] [CrossRef]
- Madandoust, R.; Ranjbar, M.M.; Moghadam, H.A.; Mousavi, S.Y. Mechanical properties and durability assessment of rice husk ash concrete. Biosyst. Eng. 2011, 110, 144–152. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Stoica, A.E.; Vrabec, M.; Rogan, N.S.; Sturm, S.; Ow-Yang, C.; Gulgun, M.A.; Bundur, Z.B.; Ciuca, I.; Vasile, B.S. End-of-life materials used as supplementary cementitious materials in the concrete industry. Materials 2020, 13, 1954. [Google Scholar] [CrossRef]
- Arenas, C.G.; Marrero, M.; Leiva, C.; Solís-Guzmán, J.; Arenas, L.F.V. High fire resistance in blocks containing coal combustion fly ashes and bottom ash. Waste Manag. 2011, 31, 1783–1789. [Google Scholar] [CrossRef]
- Poon, C.-S.; Kou, S.; Wan, H.; Etxeberria, M. Properties of concrete blocks prepared with low grade recycled aggregates. Waste Manag. 2009, 29, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Sabai, M.M.; Cox, M.G.D.M.; Mato, R.R.; Egmond, E.L.C.; Lichtenberg, J.J.N. Concrete block production from construction and demolition waste in Tanzania. Resour. Conserv. Recycl. 2013, 72, 9–19. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2010, 252, 119610. [Google Scholar] [CrossRef]
- Xiao, R.; Polaczyk, P.; Zhang, M.; Jiang, X.; Zhang, Y.; Huang, B.; Hu, W. Evaluation of glass powder-based geopolymer stabilized road bases containing recycled waste glass aggregate. Transp. Res. Rec. 2020, 2674, 22–32. [Google Scholar] [CrossRef]
- Brotons, F.B.; Garcés, P.; Bernabeu, J.P.; Malo, O.G. Valuation of sewage sludge ash as a component of precast concrete. ALCONPAT 2015, 5, 44–57. [Google Scholar]
- AENOR. UNE-EN 83982:2008 Concrete Durability. Test Methods. Determination of the Capillar Suction in Hardened Concrete. Fagerlund Method; AENOR: Madrid, Spain, 2008. [Google Scholar]
- Antoni, M.; Rossen, J.; Martinera, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Scrivener, K.; Martinera, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Pacific Grove, CA, USA, 1996; ISBN 978-0534231002. [Google Scholar]

| Reference | Aggregate Fraction | Cement | Mineral Addition | Water | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-0/4 | F-2/8 | SSA | FA | MD | RHA | ||||||||
| 0 | C | 1227 | 571 | 125.6 | - | - | - | - | - | - | - | - | 85.6 |
| 10 | SSA | 1227 | 571 | 113.0 | 12.6 | 10% | - | - | - | - | - | - | 85.4 |
| 20 | SSA | 1227 | 571 | 100.4 | 25.1 | 20% | - | - | - | - | - | - | 85.4 |
| 21 | SSA/FA | 1227 | 571 | 100.4 | 12.6 | 10% | 12.6 | 10% | - | - | - | - | 85.4 |
| 22 | SSA/MD | 1227 | 571 | 100.4 | 12.6 | 10% | - | - | 12.6 | 10% | - | - | 85.4 |
| 23 | MD/FA | 1227 | 571 | 100.4 | - | - | 12.6 | 10% | 12.6 | 10% | - | - | 85.4 |
| 30 | SSA | 1227 | 571 | 87.9 | 37.7 | 30% | - | - | - | - | - | - | 85.4 |
| 31 | SSA/FA/RHA | 1227 | 571 | 87.9 | 12.6 | 10% | 12.6 | 10% | - | - | 12.6 | 10% | 85.4 |
| 32 | SSA/RHA | 1227 | 571 | 87.9 | 25.1 | 20% | - | - | - | - | 12.6 | 10% | 85.4 |
| Sieve (mm) | PR (g) | CR (g) | % CR | % PASSING | ||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | F1 | F2 | |
| 63 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| 31.5 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| 8 | 0 | 18.9 | 0 | 18.9 | 0 | 1.4 | 100 | 98.6 |
| 4 | 0 | 1020.0 | 0 | 1038.9 | 0 | 78.3 | 100 | 21.7 |
| 2 | 84.1 | 187.8 | 84.1 | 1226.7 | 26.9 | 92.5 | 73.1 | 7.5 |
| 1 | 80.9 | 31.2 | 165.0 | 1257.9 | 52.7 | 94.9 | 47.3 | 5.1 |
| 0.5 | 47.5 | 13.2 | 212.5 | 1271.1 | 67.9 | 95.9 | 32.1 | 4.1 |
| 0.25 | 29.2 | 7.9 | 241.7 | 1279.0 | 77.2 | 96.5 | 22.8 | 3.5 |
| 0.125 | 18.0 | 5.7 | 259.7 | 1284.7 | 83.0 | 96.9 | 17.0 | 3.1 |
| 0.063 | 10.4 | 4.8 | 270.1 | 1289.5 | 86.3 | 97.2 | 13.7 | 2.8 |
| Receiver | 42.9 | 36.5 | 313.0 | 1326.0 | 100 | 100 | 0 | 0 |
| Total (without fines) | 270.1 | 1289.5 | Designation | |||||
| Total (with fines) | 313.0 | 1326.0 | d | 0 | 2 | |||
| % fines | 13.71 | 2.75 | D | 4 | 8 | |||
| Oxide | SSA | FA | MD | RHA | Oxide | SSA | FA | MD | RHA | Oxide | SSA | FA | MD | RHA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | 0.94 | 0.17 | 0.39 | 0.09 | TiO2 | 0.92 | 0.90 | - | 0.02 | Rb2O | 0.01 | 0.01 | - | - |
| MgO | 3.22 | 1.06 | 6.90 | 0.67 | Cr2O3 | 0.17 | 0.03 | - | - | SrO | 0.25 | 0.11 | 0.04 | 0.01 |
| Al2O3 | 9.64 | 25.57 | 1.39 | 0.44 | MnO | 0.07 | 0.04 | - | 0.16 | SnO2 | 0.03 | - | - | - |
| SiO2 | 17.27 | 36.70 | 3.77 | 81.57 | Fe2O3 | 8.52 | 15.72 | 0.35 | 0.16 | BaO | 0.14 | 0.10 | - | - |
| P2O5 | 14.25 | 0.65 | 0.09 | 0.95 | NiO | 0.03 | 0.02 | - | - | PbO | 0.04 | 0.01 | - | - |
| SO3 | 8.95 | 1.53 | 1.27 | 0.33 | CuO | 0.18 | - | - | Cl | 0.15 | - | 0.13 | 0.28 | |
| K2O | 1.28 | 1.28 | 0.30 | 3.51 | ZnO | 0.32 | 0.03 | - | 0.01 | Y2O3 | - | 0.01 | - | - |
| CaO | 30.24 | 5.56 | 64.25 | 1.23 | As2O3 | 0.00 | 0.01 | - | - | ZrO2 | - | 0.02 | - | - |
| LOI | 3.38 | 10.47 | 21.12 | 10.57 |
| Reference Specimen | Dmd | σ | cv | rel. | ∆ | Abs | σ | cv | rel. | ∆ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (kg/m3) | (kg/m3) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | ||
| 0 | C-28 | 2058 | 1 | 0.0 | 100 | - | 8.7 | 0.1 | 0.6 | 100 | - |
| C-90 | 2115 | 7 | 0.3 | 100 | 2.8 | 7.1 | 0.3 | 4.0 | 100 | −18.4 | |
| 10 | SSA-28 | 2131 | 6 | 0.3 | 103.5 | - | 6.9 | 0.0 | 0.5 | 80.0 | - |
| SSA-90 | 2118 | 3 | 0.1 | 100.1 | −0.6 | 7.2 | 0.1 | 1.8 | 100.9 | 4.4 | |
| 20 | SSA-28 | 2085 | 11 | 0.5 | 101.0 | - | 7.7 | 0.2 | 2.5 | 88.0 | - |
| SSA-90 | 2062 | 4 | 0.2 | 97.5 | −1.2 | 7.9 | 0.1 | 1.3 | 110.7 | 2.6 | |
| 21 | SSA/FA-28 | 2056 | 17 | 0.8 | 99.9 | - | 8.3 | 0.3 | 3.7 | 96.0 | - |
| SSA/FA-90 | 2043 | 7 | 0.3 | 96.6 | −0.6 | 8.5 | 0.1 | 0.9 | 118.6 | 2.4 | |
| 22 | SSA/MD-28 | 2047 | 6 | 0.3 | 99.5 | - | 8.8 | 0.2 | 2.2 | 101.4 | - |
| SSA/MD-90 | 2060 | 2 | 0.9 | 97.4 | 0.6 | 8.4 | 0.3 | 4.1 | 117.6 | −4.6 | |
| 23 | MD/FA-28 | 2074 | 15 | 0.7 | 100.8 | - | 8.1 | 0.3 | 3.8 | 94.0 | - |
| MD/FA-90 | 2103 | 9 | 0.4 | 99.4 | 1.4 | 7.5 | 0.2 | 2.9 | 105.6 | −7.4 | |
| 30 | SSA-28 | 2010 | 18 | 0.9 | 97.7 | - | 9.7 | 0.2 | 2.3 | 111.7 | - |
| SSA-90 | 1972 | 23 | 1.2 | 93.2 | −1.9 | 10.2 | 0.6 | 6.2 | 142.3 | 5.2 | |
| 30’ | SSA_water-28 | 1990 | 18 | 0.9 | 96.7 | - | 10.1 | 0.2 | 2.3 | 117.1 | - |
| SSA_water-90 | 2000 | 12 | 0.6 | 94.6 | 0.5 | 9.5 | 0.2 | 1.9 | 132.7 | −5.9 | |
| 31 | SSA/FA/RHA-28 | 2039 | 13 | 0.7 | 99.1 | - | 8.7 | 0.5 | 5.8 | 100.2 | - |
| SSA/FA/RHA-90 | 2050 | 3 | 0.2 | 96.9 | 0.5 | 8.4 | 0.3 | 3.3 | 118.2 | −3.5 | |
| 32 | SSA/RHA-28 | 2025 | 11 | 0.5 | 98.4 | - | 9.2 | 0.2 | 2.3 | 106.3 | - |
| SSA/RHA-90 | 2019 | 10 | 0.5 | 95.5 | −0.3 | 9.0 | 0.2 | 2.3 | 126.1 | 2.2 | |
| Reference Specimen | Cap | σ | cv | rel. | ∆ | |
|---|---|---|---|---|---|---|
| (kg/m2min0.5) | (kg/m2min0.5) | (%) | (%) | (%) | ||
| 0 | C-28 | 0.61 | 0.02 | 3.1 | 100 | - |
| C-90 | 0.40 | 0.04 | 9.9 | 100 | −34.4 | |
| 10 | SSA-28 | 0.51 | 0.03 | 5.4 | 82.5 | - |
| SSA-90 | 0.45 | 0.03 | 6.7 | 112.5 | −11.8 | |
| 20 | SSA-28 | 0.82 | 0.03 | 3.8 | 134.0 | - |
| SSA-90 | 0.84 | 0.04 | 4.8 | 210.8 | 2.4 | |
| 21 | SSA/FA-28 | 0.90 | 0.00 | 0.1 | 147.5 | - |
| SSA/FA-90 | 0.83 | 0.07 | 8.3 | 209.7 | −7.8 | |
| 22 | SSA/MD-28 | 0.64 | 0.01 | 1.6 | 103.9 | - |
| SSA/MD-90 | 0.65 | 0.05 | 7.4 | 164.8 | 1.56 | |
| 23 | MD/FA-28 | 0.60 | 0.02 | 3.3 | 97.5 | - |
| MD/FA-90 | 0.49 | 0.05 | 9.7 | 124.4 | −18.3 | |
| 30 | SSA-28 | 1.32 | 0.01 | 0.4 | 214.8 | - |
| SSA-90 | 1.47 | 0.05 | 3.7 | 371.4 | 11.4 | |
| 30’ | SSA_water-28 | 1.51 | 0.12 | 7.8 | 246.1 | - |
| SSA_water-90 | 1.49 | 0.08 | 5.1 | 375.9 | −1.3 | |
| 31 | SSA/FA/RHA-28 | 1.28 | 0.04 | 3.4 | 209.0 | - |
| SSA/FA/RHA-90 | 1.11 | 0.05 | 4.5 | 280.5 | −13.3 | |
| 32 | SSA/RHA-28 | 1.31 | 0.04 | 3.3 | 214.4 | - |
| SSA/RHA-90 | 1.33 | 0.02 | 1.7 | 336.1 | 1.5 | |
| Reference Specimen | Cs | σ | cv | rel. | ∆ | Upv | σ | cv | rel. | |
|---|---|---|---|---|---|---|---|---|---|---|
| (MPa) | (MPa) | (%) | (%) | (%) | (km/s) | (km/s) | (%) | (%) | ||
| 0 | C-28 | 7.0 | 0.2 | 2.7 | 100 | - | - | - | - | - |
| C-90 | 9.7 | 1.6 | 16.1 | 100 | 38.6 | 3.66 | 0.06 | 1.73 | 100 | |
| 10 | SSA-28 | 6.4 | 0.4 | 5.9 | 90.8 | - | - | - | - | - |
| SSA-90 | 8.0 | 0.3 | 4.0 | 82.3 | 25.0 | 3.62 | 0.08 | 2.22 | 97.8 | |
| 20 | SSA-28 | 5.2 | 0.1 | 1.9 | 75.0 | - | - | - | - | - |
| SSA-90 | 7.8 | 0.2 | 3.0 | 80.5 | 50.0 | 3.56 | 0.05 | 1.26 | 96.2 | |
| 21 | SSA/FA-28 | 6.6 | 0.5 | 7.2 | 95.5 | - | - | - | - | - |
| SSA/FA-90 | 7.4 | 1.6 | 16.1 | 76.3 | 12.1 | 3.53 | 0.08 | 2.14 | 94.9 | |
| 22 | SSA/MD-28 | 7.1 | 0.7 | 10.0 | 102.8 | - | - | - | - | - |
| SSA/MD-90 | 9.8 | 0.6 | 6.4 | 101.0 | 38.0 | 3.52 | 0.04 | 1.21 | 96.2 | |
| 23 | MD/FA-28 | 6.2 | 0.7 | 10.7 | 89.0 | - | - | - | - | - |
| MD/FA-90 | 9.4 | 1.6 | 17.5 | 96.8 | 51.6 | 3.60 | 0.05 | 1.34 | 98.4 | |
| 30 | SSA-28 | 4.6 | 0.5 | 11.8 | 66.8 | - | - | - | - | - |
| SSA-90 | 7.4 | 0.5 | 6.7 | 76.7 | 60.9 | 3.59 | 0.03 | 0.97 | 98.1 | |
| 30’ | SSA_water-28 | 3.4 | 0.5 | 14.7 | 49.3 | - | - | - | - | - |
| SSA_water-90 | 5.6 | 0.4 | 7.5 | 58.1 | 64.7 | 3.55 | 0.02 | 0.63 | 97.3 | |
| 31 | SSA/FA/RHA-28 | 6.2 | 0.3 | 4.3 | 89.6 | - | - | - | - | - |
| SSA/FA/RHA-90 | 8.3 | 0.5 | 5.5 | 85.6 | 33.9 | 3.63 | 0.06 | 1.54 | 100.4 | |
| 32 | SSA/RHA-28 | 7.0 | 0.3 | 4.8 | 101.0 | - | - | - | - | - |
| SSA/RHA-90 | 9.5 | 0.8 | 8.2 | 98.4 | 35.7 | 3.54 | 0.10 | 2.73 | 96.8 | |
| Statistical | Dmd 28 | Dmd 90 | Abs 28 | Abs 90 | Cap 28 | Cap 90 | Cs 28 | Cs 90 | Upv 90 |
|---|---|---|---|---|---|---|---|---|---|
| Count | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Percentage | 2052 | 2055 | 8.6 | 8.3 | 1.0 | 0.9 | 6.0 | 8.3 | 3.6 |
| Standard deviation | 39.7 | 47.9 | 0.9 | 1.0 | 0.4 | 0.4 | 1.2 | 1.4 | 0.1 |
| Coefficient of Variation (%) | 1.9 | 2.3 | 10.5 | 11.5 | 37.9 | 45.0 | 20.4 | 17.5 | 1.9 |
| Minimum | 1978 | 1955 | 6.9 | 6.9 | 0.5 | 0.4 | 3.1 | 5.3 | 3.4 |
| Maximum | 2135 | 2123 | 10.3 | 10.6 | 1.6 | 1.6 | 7.9 | 11.2 | 3.7 |
| Range | 156.6 | 167.5 | 3.4 | 3.7 | 1.2 | 1.2 | 4.8 | 5.9 | 0.3 |
| Standardized bias | 0.8 | −0.7 | −0.3 | 1.2 | 0.7 | 0.6 | −2.1 | 0.2 | 0.1 |
| Standardized Kurtosis | 0.0 | −0.6 | −0.3 | −0.2 | −1.7 | −1.6 | 0.3 | 0.2 | −0.1 |
| Skewness | 0.3 | −0.3 | −0.1 | 0.5 | 0.3 | 0.2 | −0.9 | 0.1 | 0.1 |
| Dmd 28 | Dmd 90 | Abs 28 | Abs 90 | Cap 28 | Cap 90 | Cs 28 | Cs 90 | Upv 90 | |
|---|---|---|---|---|---|---|---|---|---|
| Dmd 28 | 0.83 | −0.96 | −0.85 | −0.86 | −0.80 | 0.22 | 0.26 | 0.15 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 | 0.16 | 0.41 | ||
| Dmd 90 | 0.63 | −0.75 | −0.99 | −0.91 | −0.94 | 0.42 | 0.48 | 0.40 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.03 | |||
| Abs 28 | 0.96 | 0.60 | 0.77 | 0.78 | 0.70 | −0.19 | −0.19 | −0.12 | |
| 0.00 | 0.00 | 0.00 | 0.31 | 0.31 | 0.50 | ||||
| Abs 90 | 0.64 | 0.97 | 0.63 | 0.88 | 0.93 | −0.37 | −0.43 | −0.41 | |
| 0.00 | 0.00 | 0.05 | 0.02 | 0.03 | |||||
| Cap 28 | 0.67 | 0.77 | 0.60 | 0.72 | 0.92 | −0.39 | −0.47 | −0.18 | |
| 0.00 | 0.04 | 0.01 | 0.34 | ||||||
| Cap 90 | 0.62 | 0.87 | 0.58 | 0.83 | 0.93 | −0.53 | −0.51 | −0.26 | |
| 0.00 | 0.01 | 0.15 | |||||||
| Cs 28 | 0.15 | 0.34 | 0.18 | 0.31 | 0.29 | 0.34 | 0.67 | 0.00 | |
| 0.00 | 1.00 | ||||||||
| Cs 90 | 0.08 | 0.28 | 0.08 | 0.25 | 0.23 | 0.25 | 0.57 | 0.05 | |
| 0.80 | |||||||||
| Upv 90 | 0.04 | 0.17 | 0.04 | 0.17 | 0.03 | 0.07 | 0.00 | 0.01 | |
 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Brotons, F.; Payá, J.; Galao, O.; Alberti, M.G.; Garcés, P. Concrete for Precast Blocks: Binary and Ternary Combination of Sewage Sludge Ash with Diverse Mineral Residue. Materials 2020, 13, 4634. https://doi.org/10.3390/ma13204634
Baeza-Brotons F, Payá J, Galao O, Alberti MG, Garcés P. Concrete for Precast Blocks: Binary and Ternary Combination of Sewage Sludge Ash with Diverse Mineral Residue. Materials. 2020; 13(20):4634. https://doi.org/10.3390/ma13204634
Chicago/Turabian StyleBaeza-Brotons, Francisco, Jordi Payá, Oscar Galao, Marcos G. Alberti, and Pedro Garcés. 2020. "Concrete for Precast Blocks: Binary and Ternary Combination of Sewage Sludge Ash with Diverse Mineral Residue" Materials 13, no. 20: 4634. https://doi.org/10.3390/ma13204634
APA StyleBaeza-Brotons, F., Payá, J., Galao, O., Alberti, M. G., & Garcés, P. (2020). Concrete for Precast Blocks: Binary and Ternary Combination of Sewage Sludge Ash with Diverse Mineral Residue. Materials, 13(20), 4634. https://doi.org/10.3390/ma13204634








