XANES Measurements for Studies of Adsorbed Protein Layers at Liquid Interfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Protein Film Formation
2.3. X-ray Absorption Near Edge Structure (XANES) Measurements
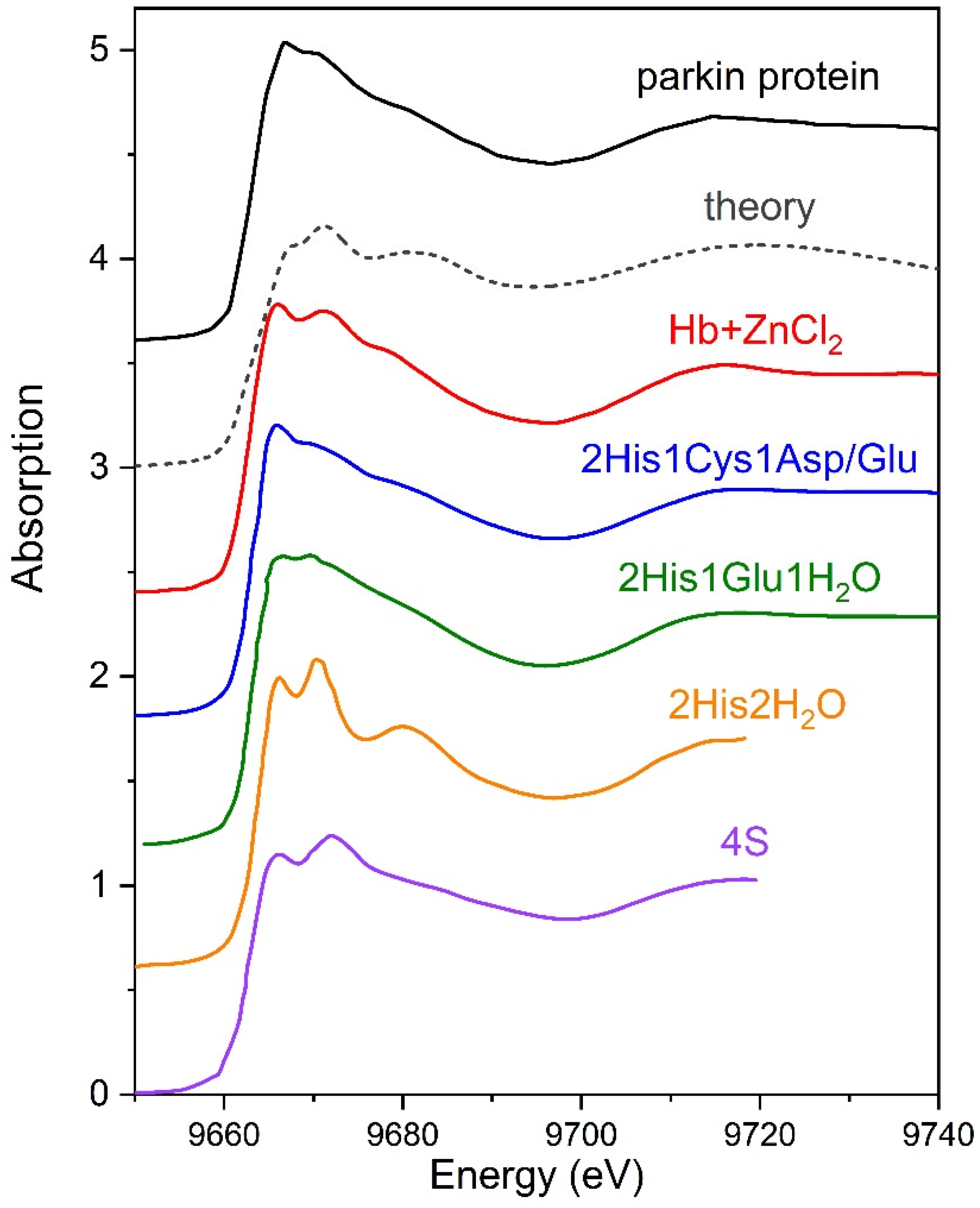
3. Results
3.1. Investigations of Protein Arrangement at the Air/Liquid Interface
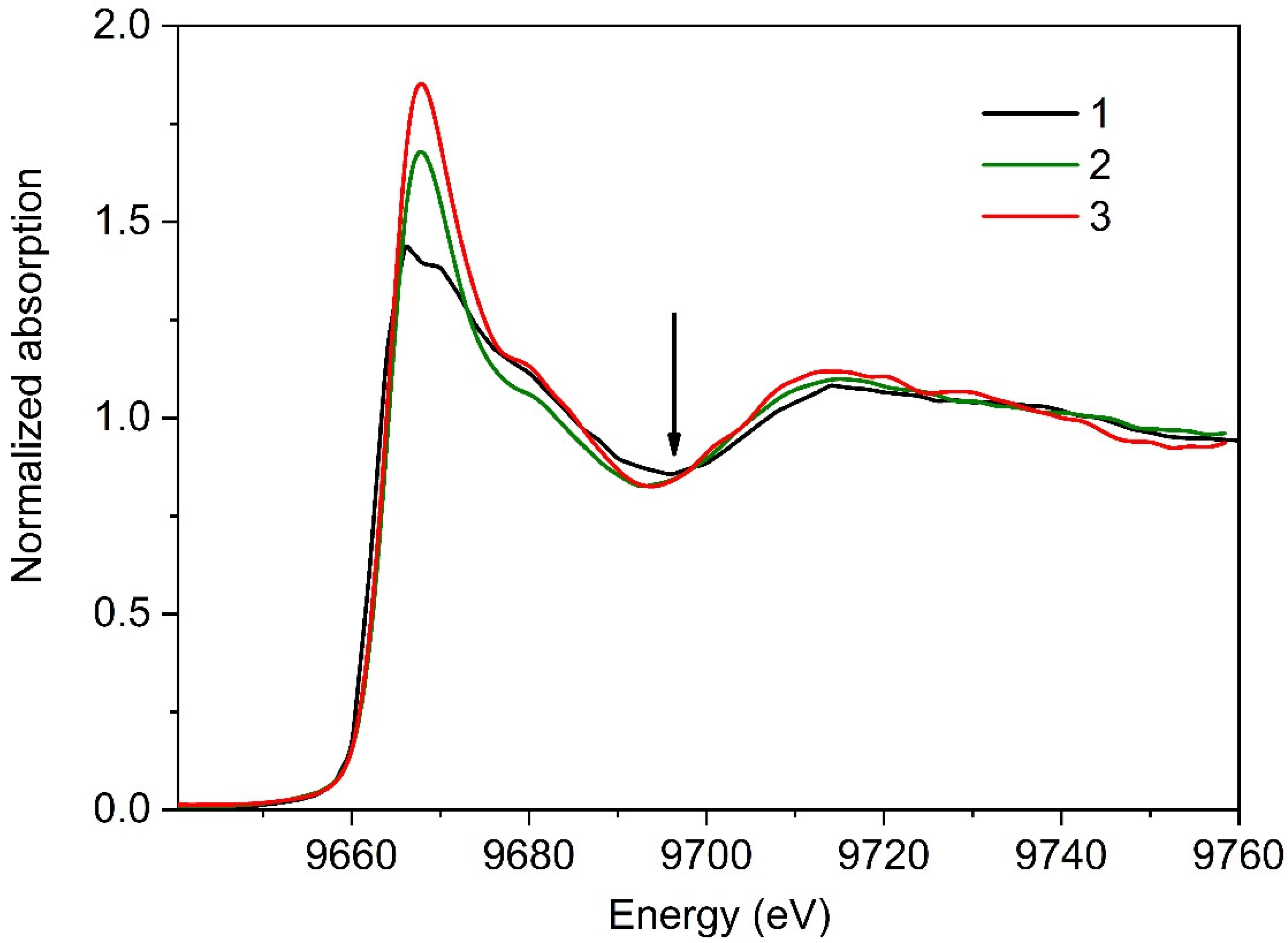
3.2. XANES Measurements for Studying Protein Layer at the Air/Liquid Interface
3.2.1. Series I
3.2.2. Series II
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daillant, J.; Gibaud, A. X-ray and Neutron Reflectivity: Principles and Applications; Springer: Berlin, Germany, 2009; p. 348. [Google Scholar]
- Bu, W.; Schlossman, M.L. Synchrotron X-ray scattering from liquid surfaces and interfaces. In Synchrotron Light Sources and Free-Electron Lasers; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 1579–1616. [Google Scholar]
- Zheludeva, S.I.; Novikova, N.N.; Kovalchuk, M.V.; Stepina, N.D.; Yurieva, É.A.; Tereschenko, E.Y.; Konovalov, O.V. Biomembrane models and organic monolayers on liquid and solid surfaces. In X-ray Standing Wave Technique: Principles and Applications; World Sci. Publ.: New York, NY, USA, 2013; Volume 1, pp. 355–368. [Google Scholar] [CrossRef]
- Porcaro, F.; Roudeau, S.; Carmona, A.; Ortega, R. Advances in element speciation analysis of biomedical samples using synchrotron-based techniques. TrAC Trends Anal. Chem. 2018, 104, 22–41. [Google Scholar] [CrossRef]
- Feiters, M.C.; Meyer-Klaucke, W. X-ray absorption and emission spectroscopy in biology. In Practical Approaches to Biological Inorganic Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 229–273. [Google Scholar] [CrossRef]
- Shi, W.; Chance, M.R. Metalloproteomics: Forward and reverse approaches in metalloprotein structural and functional characterization. Curr. Opin. Chem. Biol. 2011, 15, 144–148. [Google Scholar] [CrossRef]
- Aziz, E.F. X-ray spectroscopies revealing the structure and dynamics of metalloprotein active centers. J. Phys. Chem. Lett. 2011, 2, 320–326. [Google Scholar] [CrossRef]
- Wernet, P. Chemical interactions and dynamics with femtosecond X-ray spectroscopy and the role of X-ray free-electron lasers. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20170464. [Google Scholar] [CrossRef] [PubMed]
- Salgado, E.N.; Ambroggio, X.I.; Brodin, J.D.; Lewis, R.A.; Kuhlman, B.; Tezcan, F.A. Metal templated design of protein interfaces. Proc. Natl. Acad. Sci. USA 2009, 107, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kuzmenko, I.; Vaknin, D. Iron near absorption edge X-ray spectroscopy at aqueous-membrane interfaces. Phys. Chem. Chem. Phys. 2014, 16, 13517–13522. [Google Scholar] [CrossRef]
- Risseeuw, E.P.; Daskalchuk, T.E.; Banks, T.W.; Liu, E.; Cotelesage, J.; Hellmann, H.; Estelle, M.; Somers, D.E.; Crosby, W.L. Protein interaction analystis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 2003, 34, 753–767. [Google Scholar] [CrossRef]
- Hristova, V.A.; Beasley, S.A.; Rylett, R.; Shaw, G.S. Identification of a novel Zn2+-binding domain in the autosomal recessive juvenile parkinson-related E3 ligase parkin. J. Biol. Chem. 2009, 284, 14978–14986. [Google Scholar] [CrossRef]
- Smilgies, D.-M.; Boudet, N.; Struth, B.; Konovalov, O. Troika II: A versatile beamline for the study of liquid and solid interfaces. J. Synchrotron Radiat. 2005, 12, 329–339. [Google Scholar] [CrossRef]
- Parratt, L.G. Surface studies of solids by total reflection of X-rays. Phys. Rev. 1954, 95, 359–369. [Google Scholar] [CrossRef]
- Brooks-Bartlett, J.C.; Batters, R.A.; Bury, C.S.; Lowe, E.D.; Ginn, H.M.; Round, A.; Garman, E.F. Development of tools to automate quantitative analysis of radiation damage in SAXS experiments. J. Synchrotron Radiat. 2017, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bunău, O.; Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 2009, 21, 345501. [Google Scholar] [CrossRef] [PubMed]
- Trempe, J.-F.; Sauvé, V.; Grenier, K.; Seirafi, M.; Tang, M.Y.; Ménade, M.; Al-Abdul-Wahid, S.; Krett, J.; Wong, K.; Kozlov, G.; et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science 2013, 340, 1451–1455. [Google Scholar] [CrossRef]
- Feiters, M.C.; Eijkelenboom, A.P.A.M.; Nolting, H.F.; Krebs, B.; van den Ent, F.M.I.; Plasterk, R.H.A.; Kaptein, R.; Boelens, R. X-ray absorption spectroscopic studies of zinc in the N-terminal domain of HIV-2 integrase and model compounds. J. Synchrotron Radiat. 2003, 10, 86–95. [Google Scholar] [CrossRef]
- Giachini, L.; Veronesi, G.; Francia, F.; Venturoli, G.; Boscherini, F. Synergic approach to XAFS analysis for the identification of most probable binding motifs for mononuclear zinc sites in metalloproteins. J. Synchrotron Radiat. 2010, 17, 41–52. [Google Scholar] [CrossRef]
- Veronesi, G.; Whitehead, S.J.; Francia, F.; Giachini, L.; Boscherini, F.; Venturoli, G.; Cotton, N.P.; Jackson, J.B. X-ray absorption studies of Zn2+-binding sites in Escherichia coli transhydrogenase and its βH91K mutant. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 494–500. [Google Scholar] [CrossRef]
- Novikova, N.N.; Kovalchuk, M.V.; Yurieva, E.A.; Konovalov, O.V.; Stepina, N.D.; Rogachev, A.; Yalovega, G.E.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakunin, S.N. The enhancement of metal-binding properties in hemoglobin: The role of mild damaging factors. J. Phys. Chem. B 2019, 123, 8370–8377. [Google Scholar] [CrossRef]
- Gilman, J.G.; Brewer, G.J. The oxygen-linked zinc-binding site of human haemoglobin. Biochem. J. 1978, 169, 625–632. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Heim, J.M. Interaction of zinc with hemoglobin: Binding of zinc and the oxygen affinity. Biochemistry 1977, 16, 4438–4443. [Google Scholar] [CrossRef]
- Boffi, F.; Ascone, I.; Della-Longa, S.; Girasole, M.; Yalovega, G.É.; Soldatov, A.V.; Varoli-Piazza, A.; Castellano, A.C. X-ray absorption near-edge spectroscopy of transferrins: A theoretical and experimental probe of the metal site local structure. Eur. Biophys. J. 2003, 32, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Penner-Hahn, J.E. Characterization of “spectroscopically quiet” metals in biology. Co-ord. Chem. Rev. 2005, 249, 161–177. [Google Scholar] [CrossRef]
- Novikova, N.N.; Yakunin, S.N.; Koval’chuk, M.V.; Yur’eva, E.A.; Stepina, N.D.; Rogachev, A.V.; Kremennaya, M.A.; Yalovega, G.E.; Kosmachevskaya, O.V.; Topunov, A.F. Possibilities of X-ray absorption spectroscopy in the total external reflection geometry for studying protein films on liquids. Crystallogr. Rep. 2019, 64, 952–957. [Google Scholar] [CrossRef]
- Weik, M.; Ravelli, R.B.G.; Kryger, G.; McSweeney, S.; Raves, M.L.; Harel, M.; Gros, P.; Silman, I.; Kroon, J.; Sussman, J.L. Specific chemical and structural damage to proteins produced by synchrotron radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 623–628. [Google Scholar] [CrossRef]
- Ireland, S.M.; Martin, A.C. ZincBind—the database of zinc binding sites. Database 2019, 2019, 6. [Google Scholar] [CrossRef]
- Padjasek, M.; Kocyła, A.; Kluska, K.; Kerber, O.; Tran, J.B.; Krężel, A. Structural zinc binding sites shaped for greater works: Structure-function relations in classical zinc finger, hook and clasp domains. J. Inorg. Biochem. 2020, 204, 110955. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Auld, D.S. The ins and outs of biological zinc sites. BioMetals 2009, 22, 141–148. [Google Scholar] [CrossRef]
- Torrance, J.W.; MacArthur, M.W.; Thornton, J.M. Evolution of binding sites for zinc and calcium ions playing structural roles. Proteins Struct. Funct. Bioinform. 2008, 71, 813–830. [Google Scholar] [CrossRef]
- Rosato, A.; Valasatava, Y.; Andreini, C. Minimal functional sites in metalloproteins and their usage in structural bioinformatics. Int. J. Mol. Sci. 2016, 17, 671. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konovalov, O.V.; Novikova, N.N.; Kovalchuk, M.V.; Yalovega, G.E.; Topunov, A.F.; Kosmachevskaya, O.V.; Yurieva, E.A.; Rogachev, A.V.; Trigub, A.L.; Kremennaya, M.A.; et al. XANES Measurements for Studies of Adsorbed Protein Layers at Liquid Interfaces. Materials 2020, 13, 4635. https://doi.org/10.3390/ma13204635
Konovalov OV, Novikova NN, Kovalchuk MV, Yalovega GE, Topunov AF, Kosmachevskaya OV, Yurieva EA, Rogachev AV, Trigub AL, Kremennaya MA, et al. XANES Measurements for Studies of Adsorbed Protein Layers at Liquid Interfaces. Materials. 2020; 13(20):4635. https://doi.org/10.3390/ma13204635
Chicago/Turabian StyleKonovalov, Oleg V., Natalia N. Novikova, Mikhail V. Kovalchuk, Galina E. Yalovega, Alexey F. Topunov, Olga V. Kosmachevskaya, Eleonora A. Yurieva, Alexander V. Rogachev, Alexander L. Trigub, Maria A. Kremennaya, and et al. 2020. "XANES Measurements for Studies of Adsorbed Protein Layers at Liquid Interfaces" Materials 13, no. 20: 4635. https://doi.org/10.3390/ma13204635
APA StyleKonovalov, O. V., Novikova, N. N., Kovalchuk, M. V., Yalovega, G. E., Topunov, A. F., Kosmachevskaya, O. V., Yurieva, E. A., Rogachev, A. V., Trigub, A. L., Kremennaya, M. A., Borshchevskiy, V. I., Vakhrameev, D. D., & Yakunin, S. N. (2020). XANES Measurements for Studies of Adsorbed Protein Layers at Liquid Interfaces. Materials, 13(20), 4635. https://doi.org/10.3390/ma13204635




