Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
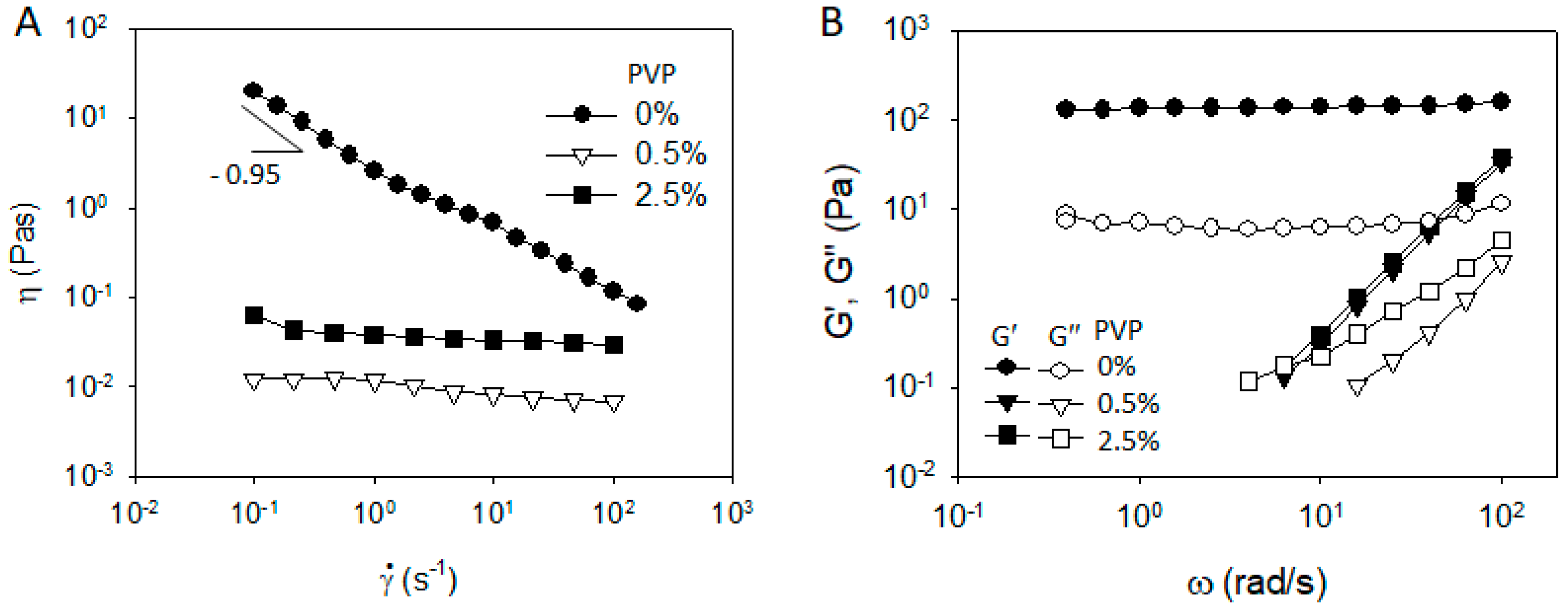
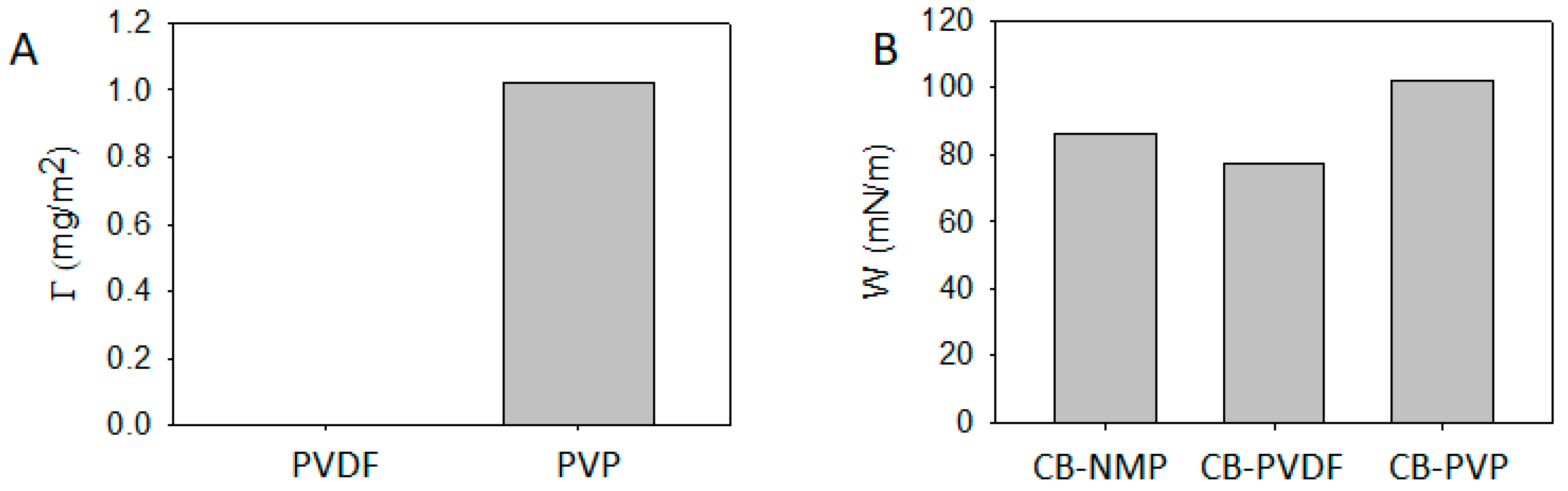
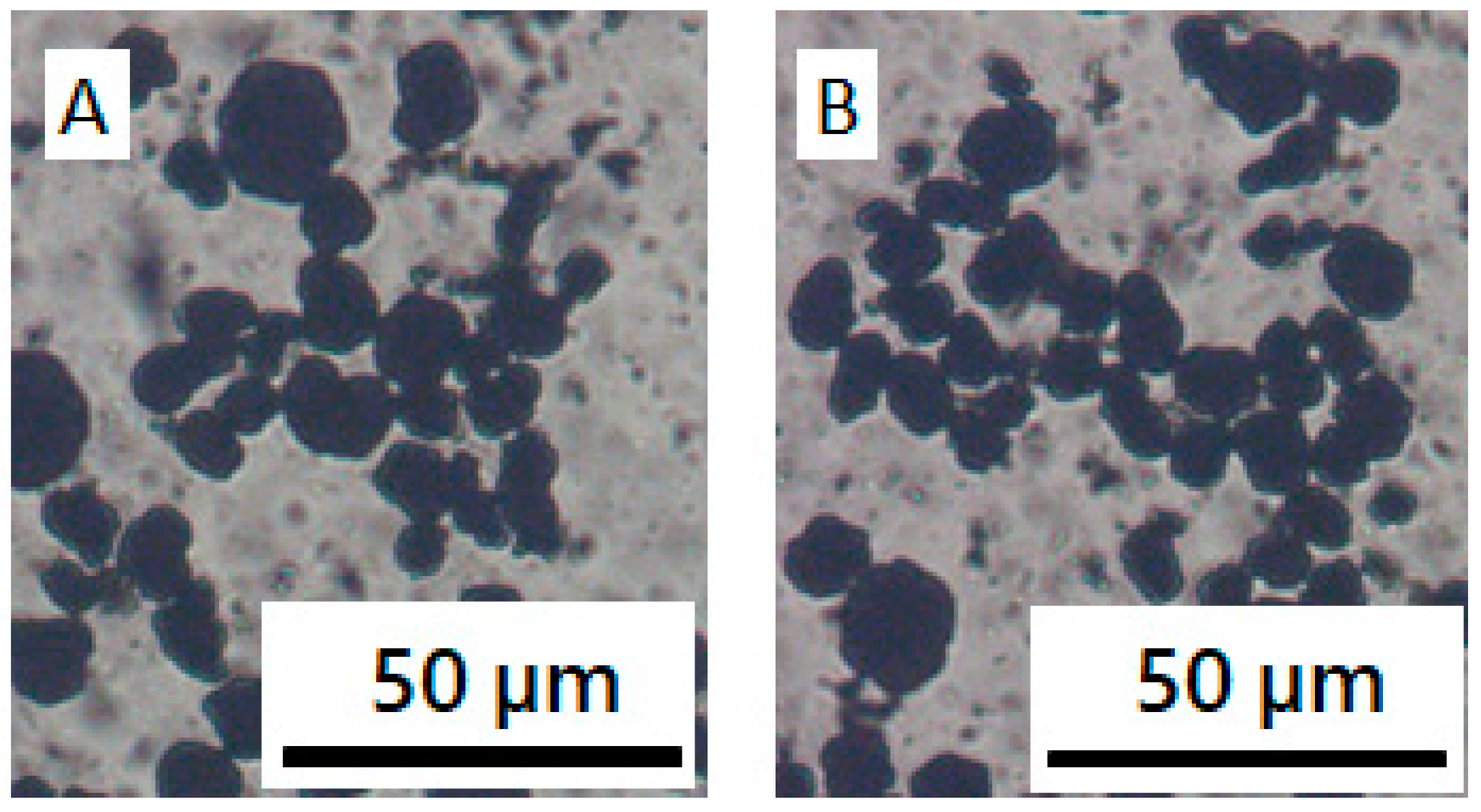
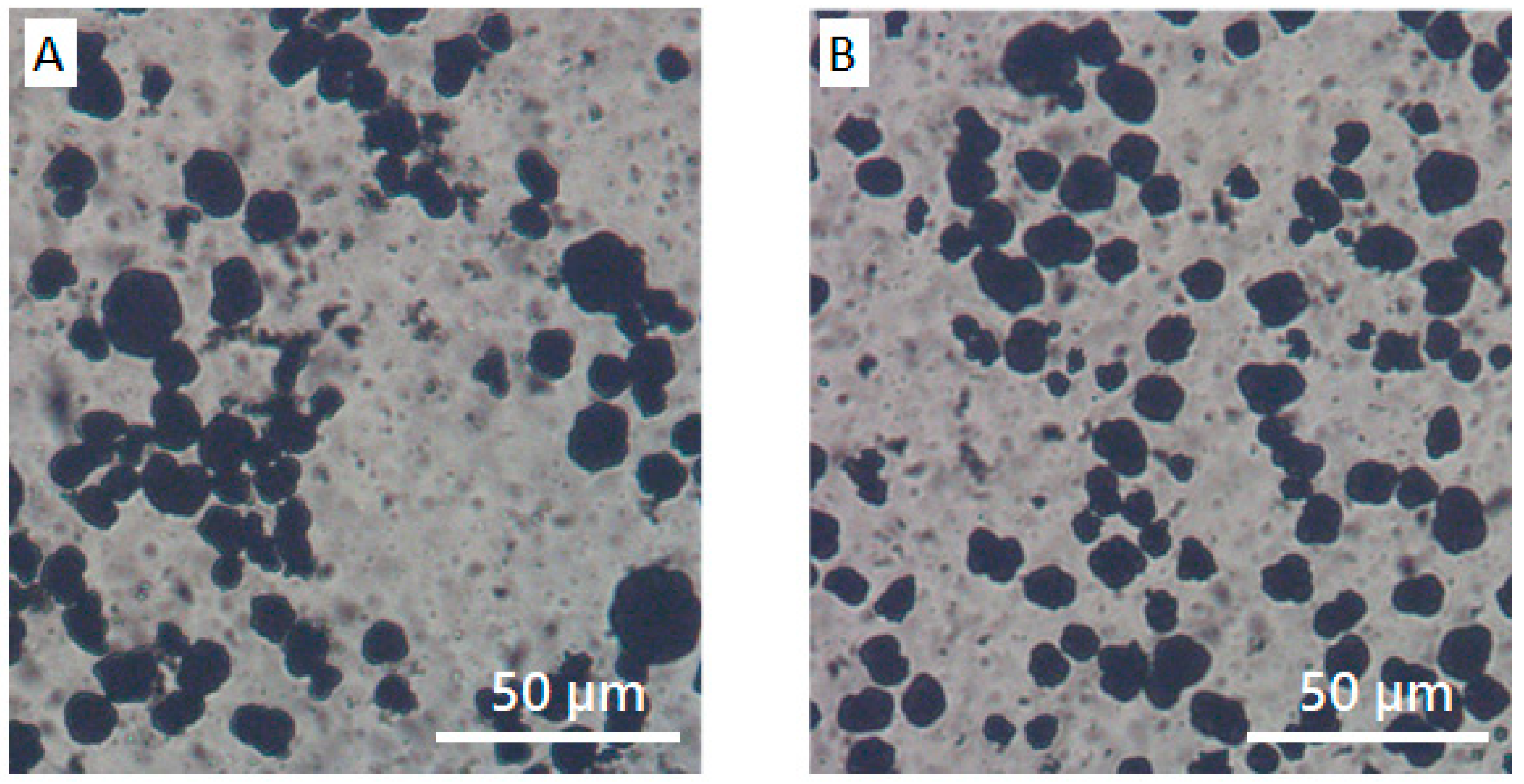
3.1. CB/Polymer/NMP Suspension
3.2. NCM/Polymer/NMP Mixtures
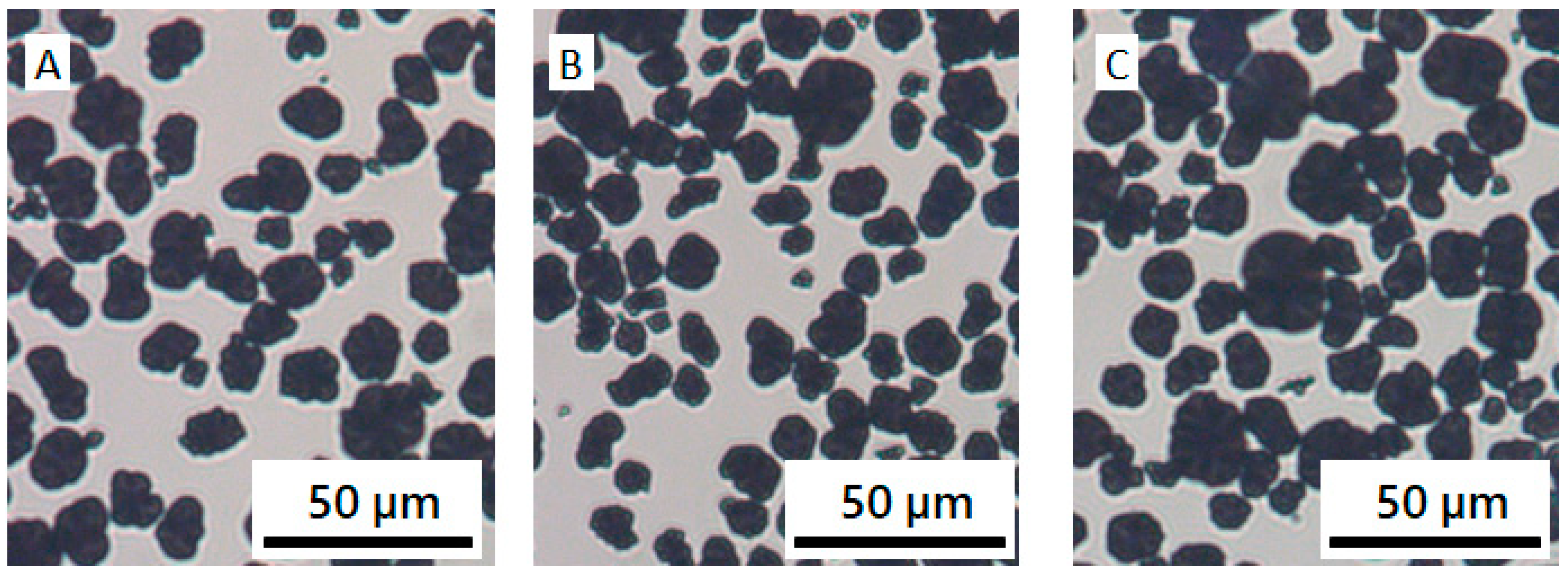
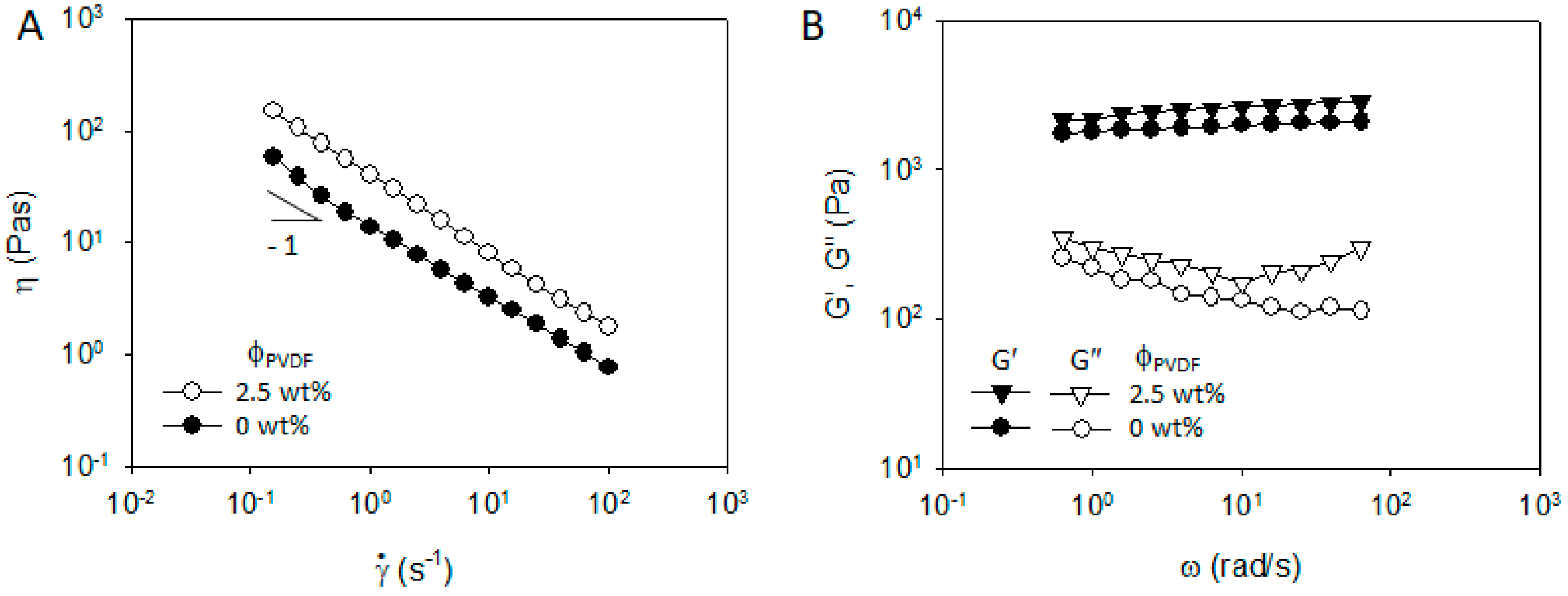
3.3. NCM/CB/Polymer/NMP Suspensions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ha, S.; Lee, K.T. Batteries: Converting to long stability. Nat. Energy 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.J.; Xiong, D.; Dahn, J.R. A Guide to Li-Ion Coin-Cell Electrode Making for Academic Researchers. J. Electrochem. Soc. 2010, 158, A51. [Google Scholar] [CrossRef]
- Wang, N.; NuLi, Y.; Su, S.; Yang, J.; Wang, J. Effects of binders on the electrochemical performance of rechargeable magnesium batteries. J. Power Sources 2017, 341, 219–229. [Google Scholar] [CrossRef]
- Bitsch, B.; Dittmann, J.; Schmitt, M.; Scharfer, P.; Schabel, W.; Willenbacher, N. A novel slurry concept for the fabrication of lithium-ion battery electrodes with beneficial properties. J. Power Sources 2014, 265, 81–90. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, Z.; Wang, J.; Qi, X.; Li, Q.; Wang, J.; Lu, S. The effect of solid content on the rheological properties and microstructures of a Li-ion battery cathode slurry. RSC Adv. 2020, 10, 19360–19370. [Google Scholar] [CrossRef]
- Gordon, R.; Kassar, M.; Willenbacher, N. Effect of Polymeric Binders on Dispersion of Active Particles in Aqueous LiFePO4-Based Cathode Slurries as well as on Mechanical and Electrical Properties of Corresponding Dry Layers. ACS Omega 2020, 5, 11455–11465. [Google Scholar] [CrossRef]
- Park, J.; Willenbacher, N.; Ahn, K.H. How the interaction between styrene-butadiene-rubber (SBR) binder and a secondary fluid affects the rheology, microstructure and adhesive properties of capillary-suspension-type graphite slurries used for Li-ion battery anodes. Colloids Surf. Phys. Eng. Asp. 2019, 579, 123692. [Google Scholar] [CrossRef]
- Lee, G.-W.; Ryu, J.H.; Han, W.; Ahn, K.H.; Oh, S.M. Effect of slurry preparation process on electrochemical performances of LiCoO2 composite electrode. J. Power Sources 2010, 195, 6049–6054. [Google Scholar] [CrossRef]
- Kim, S.; Sung, J.; Ahn, K.; Lee, S. Drying of the Silica/PVA Suspension: Effect of Suspension Microstructure. Langmuir 2009, 25, 6155–6161. [Google Scholar] [CrossRef]
- Kim, S.; Hyun, K.; Struth, B.; Ahn, K.H.; Clasen, C. Structural Development of Nanoparticle Dispersion during Drying in Polymer Nanocomposite Films. Macromolecules 2016, 49, 9068–9079. [Google Scholar] [CrossRef]
- Lee, J.; Sung, S.; Kim, Y.; Park, J.D.; Ahn, K.H. A new paradigm of materials processing—Heterogeneity control. Curr. Opin. Chem. Eng. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Li, C.-C.; Lee, J.-T.; Peng, X.-W. Improvements of Dispersion Homogeneity and Cell Performance of Aqueous-Processed LiCoO2 Cathodes by Using Dispersant of PAA—NH4. J. Electrochem. Soc. 2006, 153, A809. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, D.H.; Kim, S.; Jeong, M.H.; Nam, J.; Ahn, K.H. Effect of neutralization of poly(acrylic acid) binder on the dispersion heterogeneity of Li-ion battery electrodes. J. Mater. Sci. 2019, 54, 13208–13220. [Google Scholar] [CrossRef]
- Lee, J.-H.; Paik, U.; Hackley, V.A.; Choi, Y.-M. Effect of Carboxymethyl Cellulose on Aqueous Processing of Natural Graphite Negative Electrodes and their Electrochemical Performance for Lithium Batteries. J. Electrochem. Soc. 2005, 152, A1763. [Google Scholar] [CrossRef]
- Lim, S.; Kim, S.; Ahn, K.H.; Lee, S.J. The effect of binders on the rheological properties and the microstructure formation of lithium-ion battery anode slurries. J. Power Sources 2015, 299, 221–230. [Google Scholar] [CrossRef]
- Lim, S.; Ahn, K.H.; Yamamura, M. Latex Migration in Battery Slurries during Drying. Langmuir 2013, 29, 8233–8244. [Google Scholar] [CrossRef]
- Li, C.-C.; Lin, Y.-S. Interactions between organic additives and active powders in water-based lithium iron phosphate electrode slurries. J. Power Sources 2012, 220, 413–421. [Google Scholar] [CrossRef]
- Aoki, Y. Rheology of Carbon Black Suspensions: Effect of Carbon Black Structure. AIP Conf. Proc. 2008, 1027, 99–101. [Google Scholar] [CrossRef]
- Aoki, Y. Rheology of carbon black suspensions. IV. Effect of suspending media on the sol–gel transition behavior. Rheol. Acta 2011, 50, 779–785. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Beris, A.N.; Rogers, S.A.; Wagner, N.J. Dynamic shear rheology and structure kinetics modeling of a thixotropic carbon black suspension. Rheol. Acta 2017, 56, 811–824. [Google Scholar] [CrossRef]
- Dullaert, K.; Mewis, J. A model system for thixotropy studies. Rheol. Acta 2005, 45, 23–32. [Google Scholar] [CrossRef]
- Kim, N.; Koo, S. Rheological analysis of particle aggregation in a colloidal suspension of carbon black particles. Korea-Aust. Rheol. J. 2018, 30, 189–197. [Google Scholar] [CrossRef]
- Trappe, V.; Weitz, D.A. Scaling of the Viscoelasticity of Weakly Attractive Particles. Phys. Rev. Lett. 2000, 85, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-Y.; Meeker, S.P.; Trappe, V.; Weitz, D.A.; Diggs, N.Z.; Emert, J.I. Effect of Temperature on Carbon-Black Agglomeration in Hydrocarbon Liquid with Adsorbed Dispersant. Langmuir 2005, 21, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Kwon, Y.I.; Youn, J.R.; Song, Y.S. Interaction analysis between binder and particles in multiphase slurries. Analyst 2013, 138, 2044–2050. [Google Scholar] [CrossRef]
- Cho, K.Y.; Kwon, Y.I.; Youn, J.R.; Song, Y.S. Evaluation of slurry characteristics for rechargeable lithium-ion batteries. Mater. Res. Bull. 2013, 48, 2922–2926. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Kim, J.D.; Song, Y.S. Agitation Effect on the Rheological Behavior of Lithium-Ion Battery Slurries. J. Electron. Mater. 2015, 44, 475–481. [Google Scholar] [CrossRef]
- Doo, S.W.; Lee, S.; Kim, H.; Choi, J.H.; Lee, K.T. Hydrophobic Ni-Rich Layered Oxides as Cathode Materials for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6246–6253. [Google Scholar] [CrossRef]
- Kang, H.S.; Santhoshkumar, P.; Park, J.W.; Sim, G.S.; Nanthagopal, M.; Lee, C.W. Glass ceramic coating on LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries. Korean J. Chem. Eng. 2020, 37, 1331–1339. [Google Scholar] [CrossRef]
- Aoki, Y.; Hatano, A.; Watanabe, H. Rheology of carbon black suspensions. I. Three types of viscoelastic behavior. Rheol. Acta 2003, 42, 209–216. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Y.; Wu, J.; Gao, C.; Keyshar, K.; Zhang, X.; Yang, Y.; Ye, M.; Vajtai, R.; Lou, J.; et al. Liquid Phase Exfoliation of Two-Dimensional Materials by Directly Probing and Matching Surface Tension Components. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Demajo, L.P.; Rimai, D.S.; Sharpe, L.H. Fundamentals of Adhesion and Interfaces; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-90-5699-682-6. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.H.; Kim, S.; Park, J.H.; Park, J.D.; Ahn, K.H. Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery. Materials 2020, 13, 4544. https://doi.org/10.3390/ma13204544
Sung SH, Kim S, Park JH, Park JD, Ahn KH. Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery. Materials. 2020; 13(20):4544. https://doi.org/10.3390/ma13204544
Chicago/Turabian StyleSung, Sang Hoon, Sunhyung Kim, Jeong Hoon Park, Jun Dong Park, and Kyung Hyun Ahn. 2020. "Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery" Materials 13, no. 20: 4544. https://doi.org/10.3390/ma13204544
APA StyleSung, S. H., Kim, S., Park, J. H., Park, J. D., & Ahn, K. H. (2020). Role of PVDF in Rheology and Microstructure of NCM Cathode Slurries for Lithium-Ion Battery. Materials, 13(20), 4544. https://doi.org/10.3390/ma13204544





