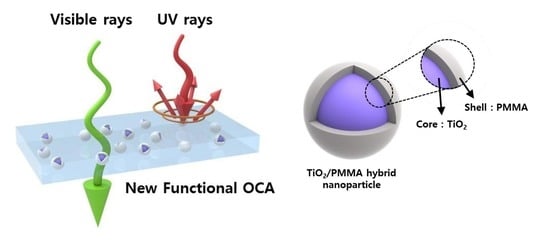
Selectively UV-Blocking and Visibly Transparent Adhesive Films Embedded with TiO2/PMMA Hybrid Nanoparticles for Displays
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
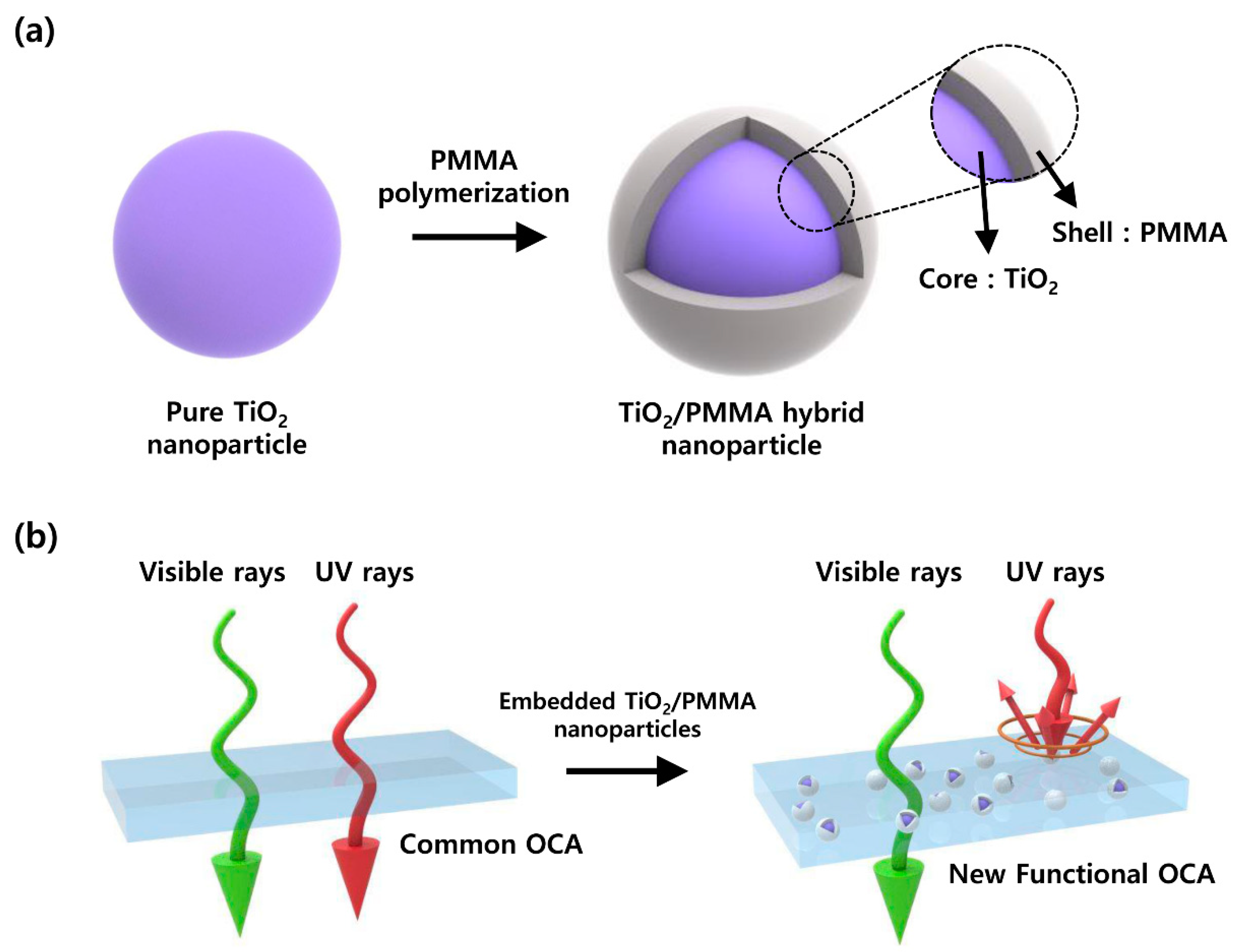
2.2. Synthesis of TiO2/PMMA Hybrid Nanoparticles
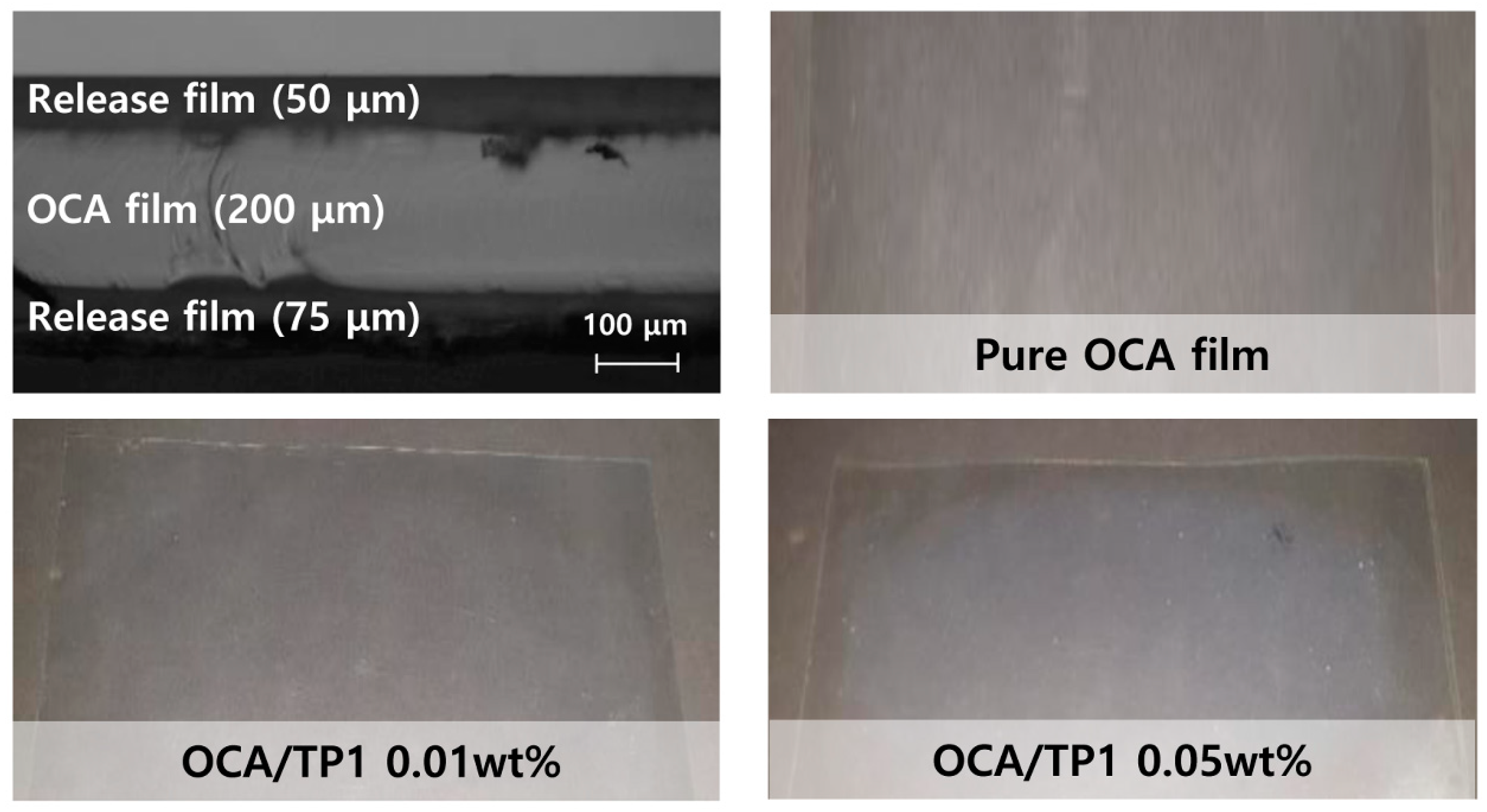
2.3. Fabrication of the OCA Films Embedded with TiO2/PMMA Hybrid Nanoparticles
2.4. Characterization
3. Results and Discussion
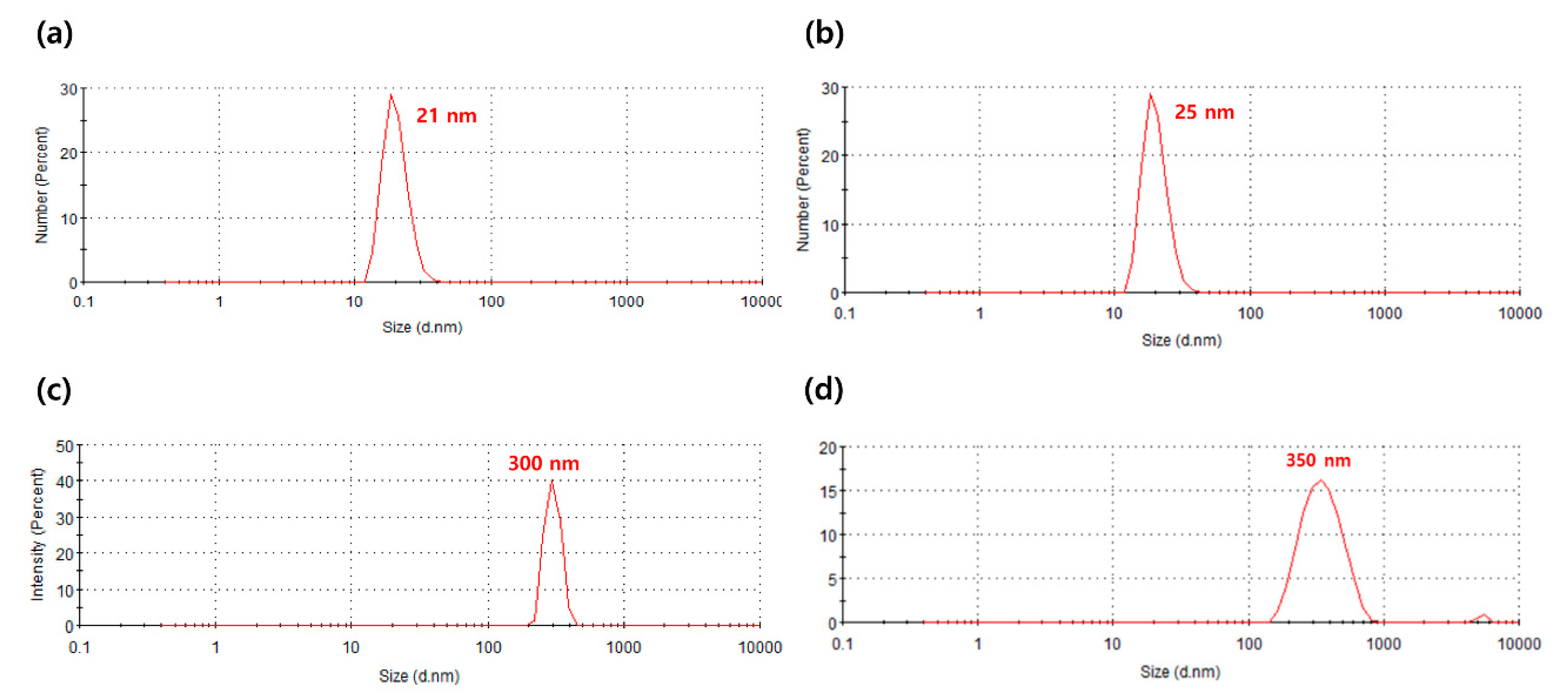
3.1. Particle Size Characteristics of TiO2/PMMA Hybrid Nanoparticles
3.2. Structural Characteristics of TiO2/PMMA Hybrid Nanoparticles
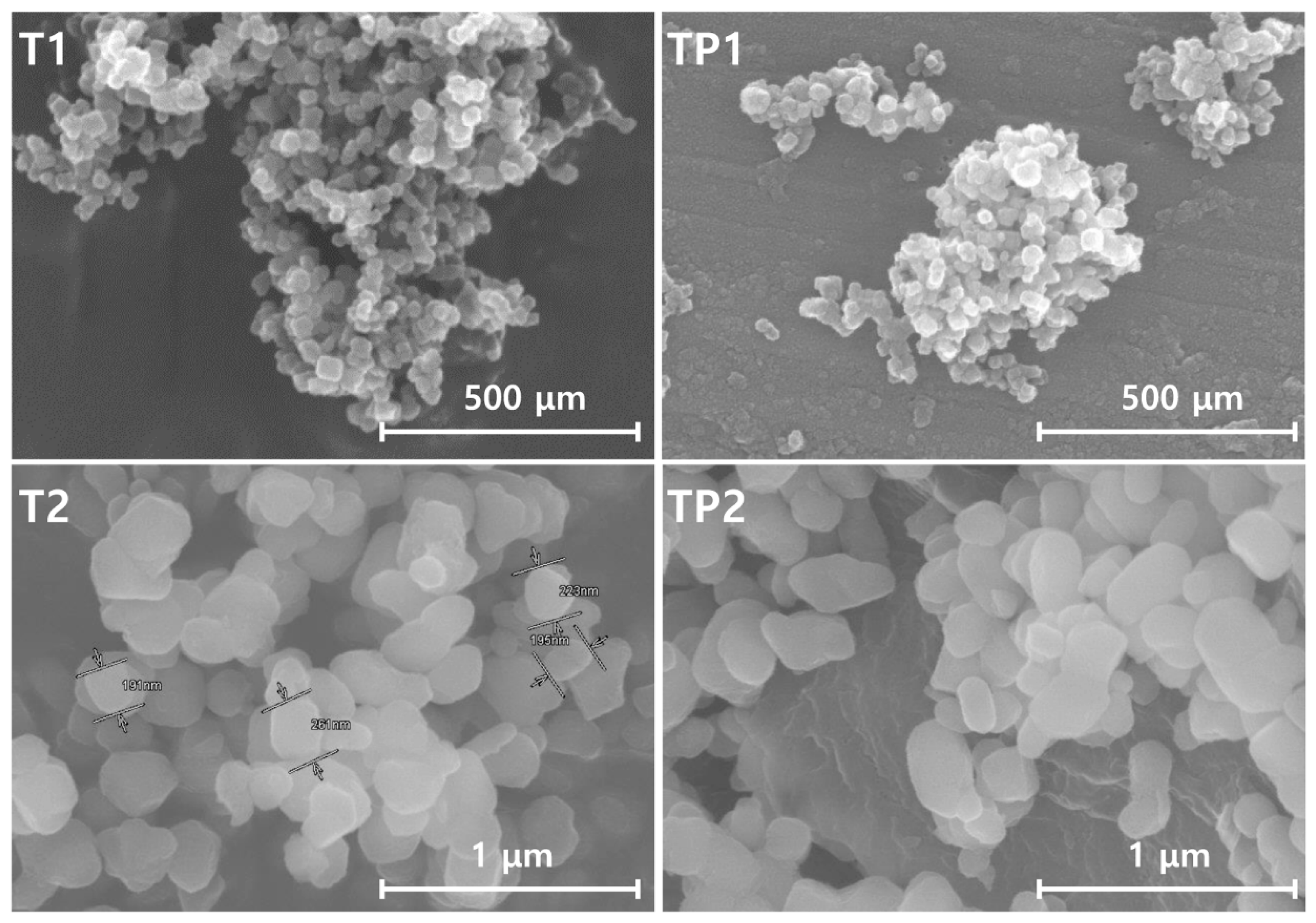
3.3. Morphological and Elemental Characteristics of TiO2/PMMA Hybrid Nanoparticles
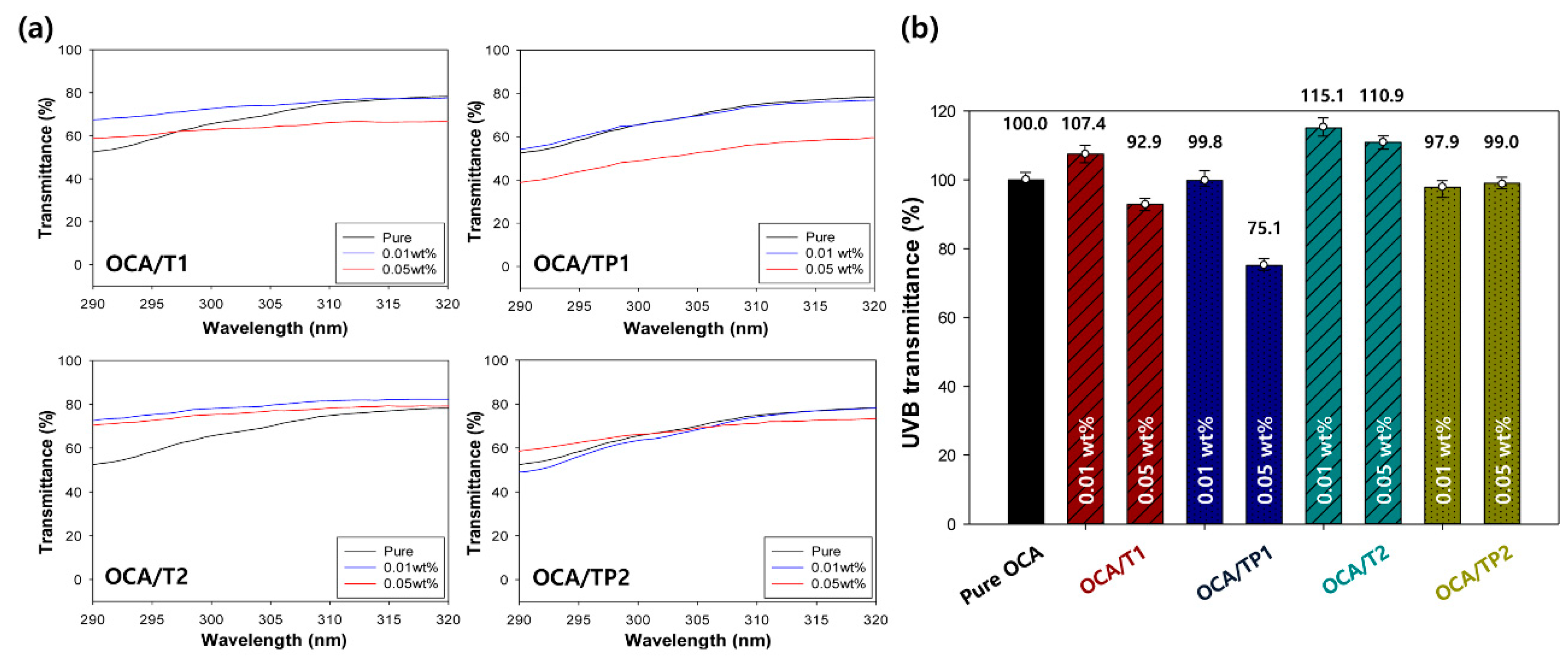
3.4. UV-Blocking Characteristics of the OCA Films Embedded with TiO2/PMMA Hybrid Nanoparticles
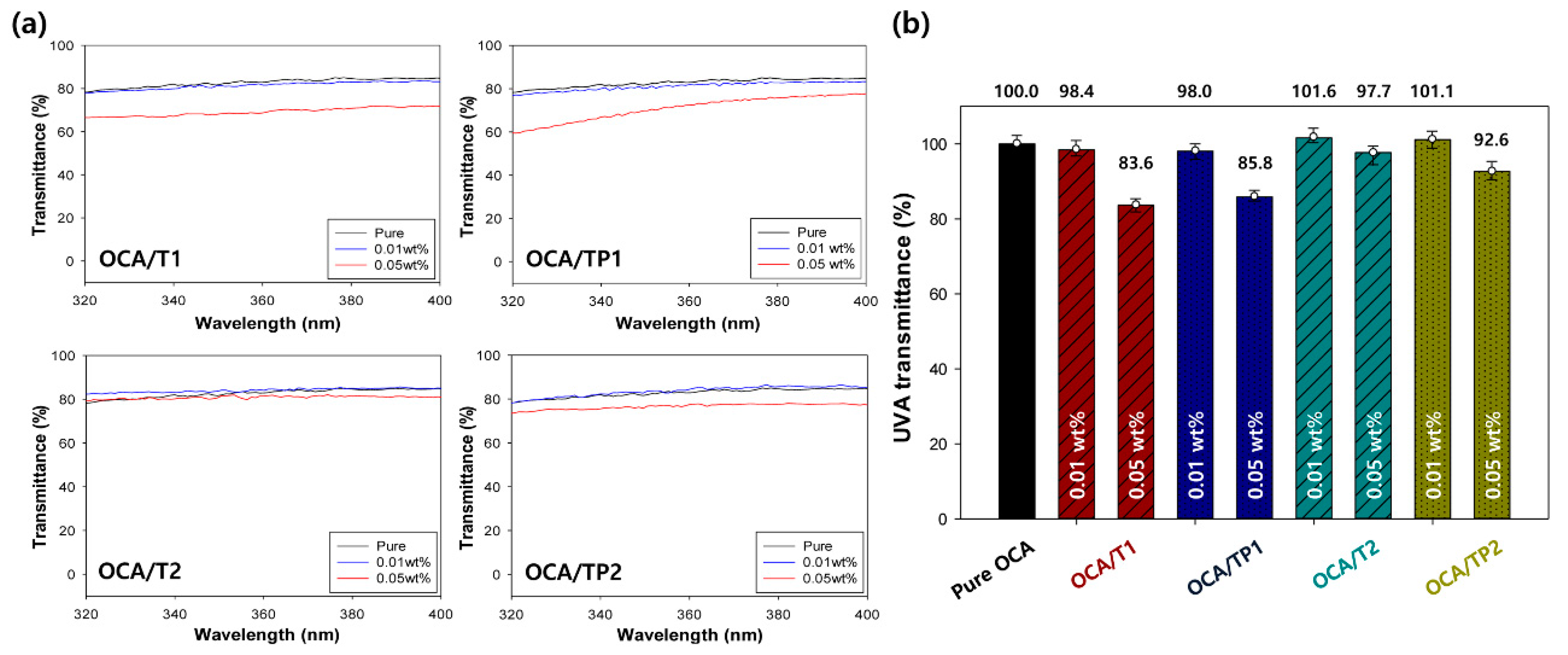
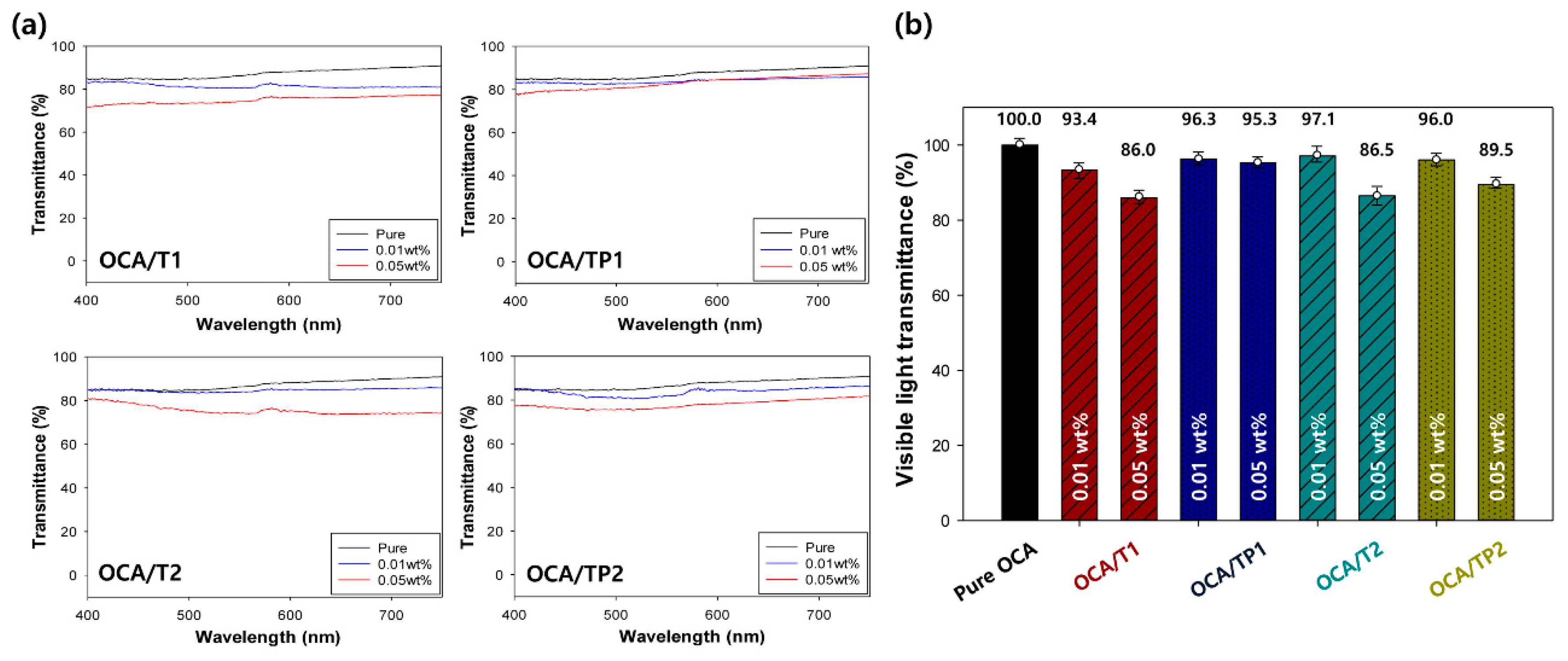
3.5. Visible Light Transmittance Characteristics of the OCA Films Embedded with TiO2/PMMA Hybrid Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, J.T.; Ko, J.H. Study on the Effects of Ultraviolet Light and Temperature on Optical Films for Display Backlights. New Phys. Sae Mulli 2018, 68, 1029–1034. [Google Scholar] [CrossRef]
- Park, S.K.; Ryu, M.; Hwang, C.; Yang, S.; Byun, C.; Lee, J.; Shin, J.; Yoon, S.M.; Chu, H.Y.; Cho, K.I. 42.3: Transparent ZnO thin film transistor for the application of high aperture ratio bottom emission AM-OLED display. SID Int. Symp. Dig. Tec. 2008, 39, 629–632. [Google Scholar] [CrossRef]
- Godar, D.E.; Thomas, D.P.; Miller, S.A.; Lee, W. Long-wavelength UVA radiation induces oxidative stress, cytoskeletal damage and hemolysis. Photochem. Photobiol. 1993, 57, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Matthews, Y.J.; Halliday, G.M.; Phan, T.A.; Damian, D.L. Wavelength dependency for UVA-induced suppression of recall immunity in humans. J. Dermatol. Sci. 2010, 59, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Djavaheri-Mergny, M.; Pieraggi, M.T.; Maziere, C.; Santus, R.; Lageron, A.; Salvayre, R.; Dubertret, L.; Maziere, J.C. Early alterations of actin in cultured human keratinocytes and fibroblasts exposed to long-wavelength radiations. Possible involvement in the UVA-induced perturbations of endocytotic processes. Photochem. Photobiol. 1994, 59, 48–52. [Google Scholar] [CrossRef]
- Coldiron, B.M. Thinning of the ozone layer: Facts and consequences. JAAD 1992, 27, 653–662. [Google Scholar] [CrossRef]
- Montazer, M.; Seifollahzadeh, S. Enhanced self-cleaning, antibacterial and UV protection properties of nano TiO2 treated textile through enzymatic pretreatment. Photochem. Photobiol. 2011, 87, 877–883. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, J.H. Oxidation mechanism of as (III) in the UV/TiO2 system: Evidence for a direct hole oxidation mechanism. Environ. Sci. Technol. 2005, 39, 9695–9701. [Google Scholar] [CrossRef]
- Wen, C.; Ishikawa, K.; Kishima, M.; Yamada, K. Effects of silver particles on the photovoltaic properties of dye-sensitized TiO2 thin films. Sol. Energy Mater. Sol. 2000, 61, 339–351. [Google Scholar] [CrossRef]
- Frank, A.J.; Kopidakis, N.; Van De Lagemaat, J. Electrons in nanostructured TiO2 solar cells: Transport, recombination and photovoltaic properties. Coord. Chem. Rev. 2004, 248, 1165–1179. [Google Scholar] [CrossRef]
- Lee, I.S.; Lee, J.Y.; Sung, J.H.; Choi, H.J. Synthesis and electrorheological characteristics of polyaniline-titanium dioxide hybrid suspension. Synth. Met. 2005, 152, 173–176. [Google Scholar] [CrossRef]
- Bilenberg, B.; Nielsen, T.; Clausen, B.; Kristensen, A. PMMA to SU-8 bonding for polymer based lab-on-a-chip systems with integrated optics. J. Micromech. Microeng. 2004, 14, 814. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abu-Elnader, M. Structural and optical properties of pure PMMA and metal chloride-doped PMMA films. Phys. Rev. B Condens. Matter 2005, 355, 308–317. [Google Scholar] [CrossRef]
- Demir, M.M.; Koynov, K.; Akbey, Ü.; Bubeck, C.; Park, I.; Lieberwirth, I.; Wegner, G. Optical properties of composites of PMMA and surface-modified zincite nanoparticles. Macromolecules 2007, 40, 1089–1100. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, J.W.; Kwon, Y.E.; Kim, S.; Kim, H.J.; Kim, E.A.; Woo, H.S.; Swiderska, J. Optical properties and UV-curing behaviors of optically clear semi-interpenetrated structured acrylic pressure sensitive adhesives. Int. J. Adhes. Adhes. 2012, 38, 5–10. [Google Scholar] [CrossRef]
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of organic acids on TiO2 nanoparticles: Effects of pH, nanoparticle size, and nanoparticle aggregation. Langmuir 2008, 24, 6659–6667. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater. Sci. J. 2009, 4, 9–14. [Google Scholar] [CrossRef]
- Wu, J.; Yu, C. Aligned TiO2 nanorods and nanowalls. J. Phys. Chem. B. 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Gao, Z.; Qu, Y.; Zhou, X.; Wang, L.; Song, Y.; Schmuki, P. Pt-Decorated g-C3N4/TiO2 Nanotube Arrays with Enhanced Visible-Light Photocatalytic Activity for H2 Evolution. ChemistryOpen 2016, 5, 197–200. [Google Scholar] [CrossRef]
- Fukushima, K.; Yamada, I.; Takagi, T. Characteristics of TiO2 films deposited by a reactive ionized cluster beam. J. Appl. Phys. 1985, 58, 4146–4149. [Google Scholar] [CrossRef]
- Bernardi, M.; Lee, E.; Lisboa-Filho, P.N.; Leite, E.R.; Longo, E.; Varela, J.A. TiO2 thin film growth using the MOCVD method. Mater. Res. 2001, 4, 223–226. [Google Scholar] [CrossRef]
- Repp, J.; Meyer, G.; Rieder, K. Snell’s law for surface electrons: Refraction of an electron gas imaged in real space. Phys. Rev. Lett. 2004, 92, 036803. [Google Scholar] [CrossRef] [PubMed]
- Parazzoli, C.G.; Greegor, R.B.; Li, K.; Koltenbah, B.; Tanielian, M. Experimental verification and simulation of negative index of refraction using Snell’s law. Phys. Rev. Lett. 2003, 90, 107401. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Sung, J.H.; Kim, K.S.; Chin, I.; Choi, H.J. Preparation and characterization of poly (methyl methacrylate) coated TiO2 nanoparticles. J. Macromol. Sci. 2006, 45, 53–60. [Google Scholar] [CrossRef]
- Choi, H.J.; Cho, Y.H.; Cho, M.S.; Jhon, M.S. Electrorheology of polyaniline-coated poly (methyl methacrylate) microsphere suspensions in silicone oil. Int. J. Mod. Phys. B 2002, 16, 2507–2513. [Google Scholar] [CrossRef]
- Yap, M.; Que, Y.T.; Chia, L. FTIR characterization of tropical wood–polymer composites. J. Appl. Polym. Sci. 1991, 43, 2083–2090. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, K.I.; Basu, S.C. IR studies of wood plastic composites. J. Appl. Polym. Sci. 1993, 49, 1547–1551. [Google Scholar] [CrossRef]
- Sugumaran, D.; Karim, K. Removal of copper (II) ion using chitosan-graft-poly (methyl methacrylate) as adsorbent. Eproc. Chem. 2017, 2, 1–11. [Google Scholar]
- Judith, L.; Rottman, G.J.; Kyle, H.L.; Woods, T.N. Detection and parameterization of variations in solar mid-and near-ultraviolet radiation (200–400 nm). J. Geophys. Res. 1997, 102, 29939–29956. [Google Scholar]
- Savenije, B.; Geesink, G.H.; Van der Palen, J.; Hemke, G. Prediction of pork quality using visible/near-infrared reflectance spectroscopy. Meat Sci. 2006, 73, 181–184. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Gulliver, E.A.; Khinast, J.G.; Riman, R.E. Highly dispersible polymer-coated silver nanoparticles. Surf. Coat. Technol. 2009, 19, 2841–2844. [Google Scholar] [CrossRef]

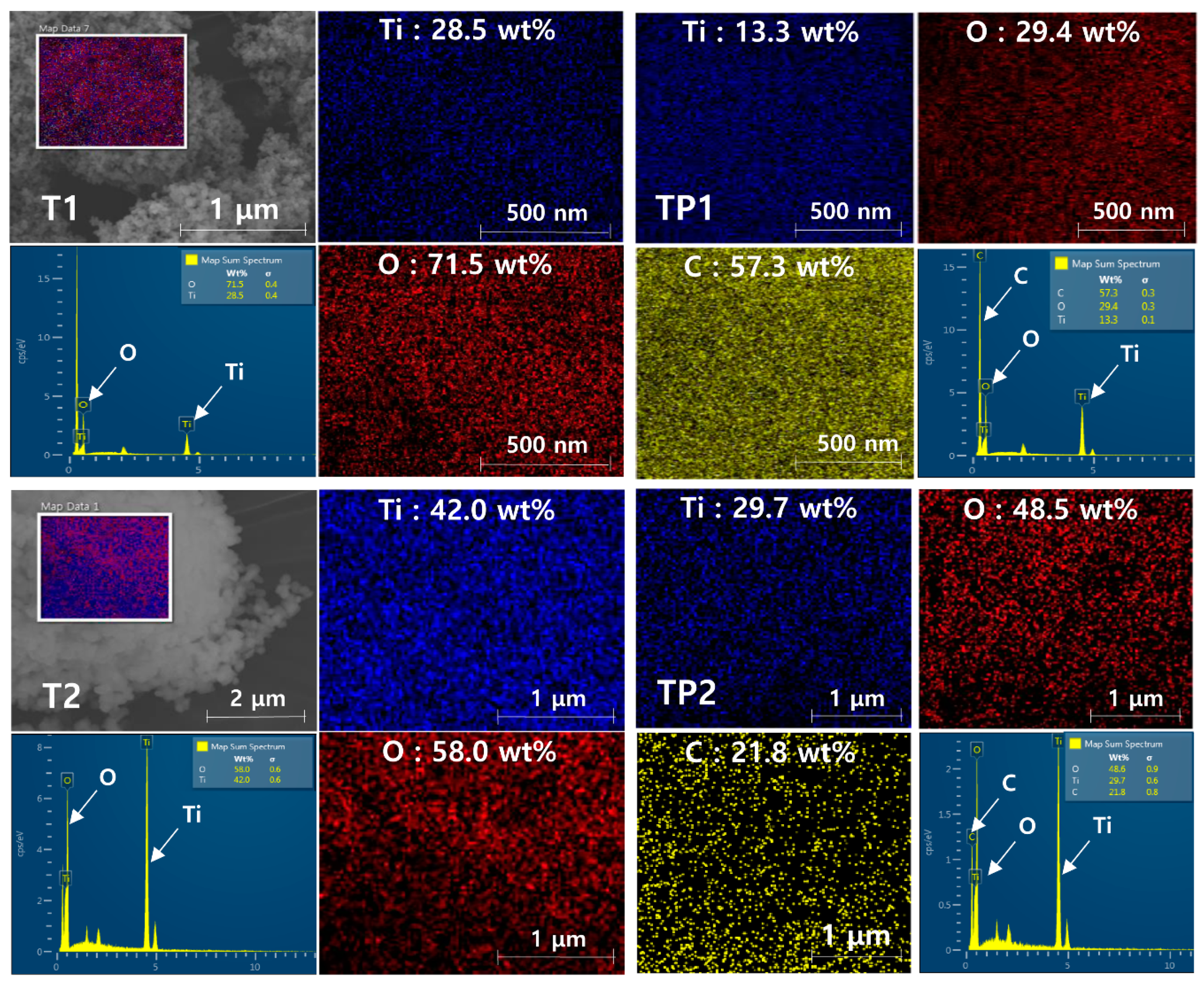
| No. | Code | Material | Average Particle Size | Surface Characteristic |
|---|---|---|---|---|
| 1 | T1 | TiO2 | 21 ± 2.7 nm | Inorganic |
| 2 | TP1 | TiO2/PMMA | 25 ± 3.3 nm | Organic |
| 3 | T2 | TiO2 | 300 ± 23.9 nm | Inorganic |
| 4 | TP2 | TiO2/PMMA | 350 ± 93.2 nm | Organic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Lee, J.H. Selectively UV-Blocking and Visibly Transparent Adhesive Films Embedded with TiO2/PMMA Hybrid Nanoparticles for Displays. Materials 2020, 13, 5273. https://doi.org/10.3390/ma13225273
Choi J-W, Lee JH. Selectively UV-Blocking and Visibly Transparent Adhesive Films Embedded with TiO2/PMMA Hybrid Nanoparticles for Displays. Materials. 2020; 13(22):5273. https://doi.org/10.3390/ma13225273
Chicago/Turabian StyleChoi, Jin-Wook, and Jun Hyup Lee. 2020. "Selectively UV-Blocking and Visibly Transparent Adhesive Films Embedded with TiO2/PMMA Hybrid Nanoparticles for Displays" Materials 13, no. 22: 5273. https://doi.org/10.3390/ma13225273
APA StyleChoi, J.-W., & Lee, J. H. (2020). Selectively UV-Blocking and Visibly Transparent Adhesive Films Embedded with TiO2/PMMA Hybrid Nanoparticles for Displays. Materials, 13(22), 5273. https://doi.org/10.3390/ma13225273