Enhanced Triboelectric Performance of Modified PDMS Nanocomposite Multilayered Nanogenerators
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
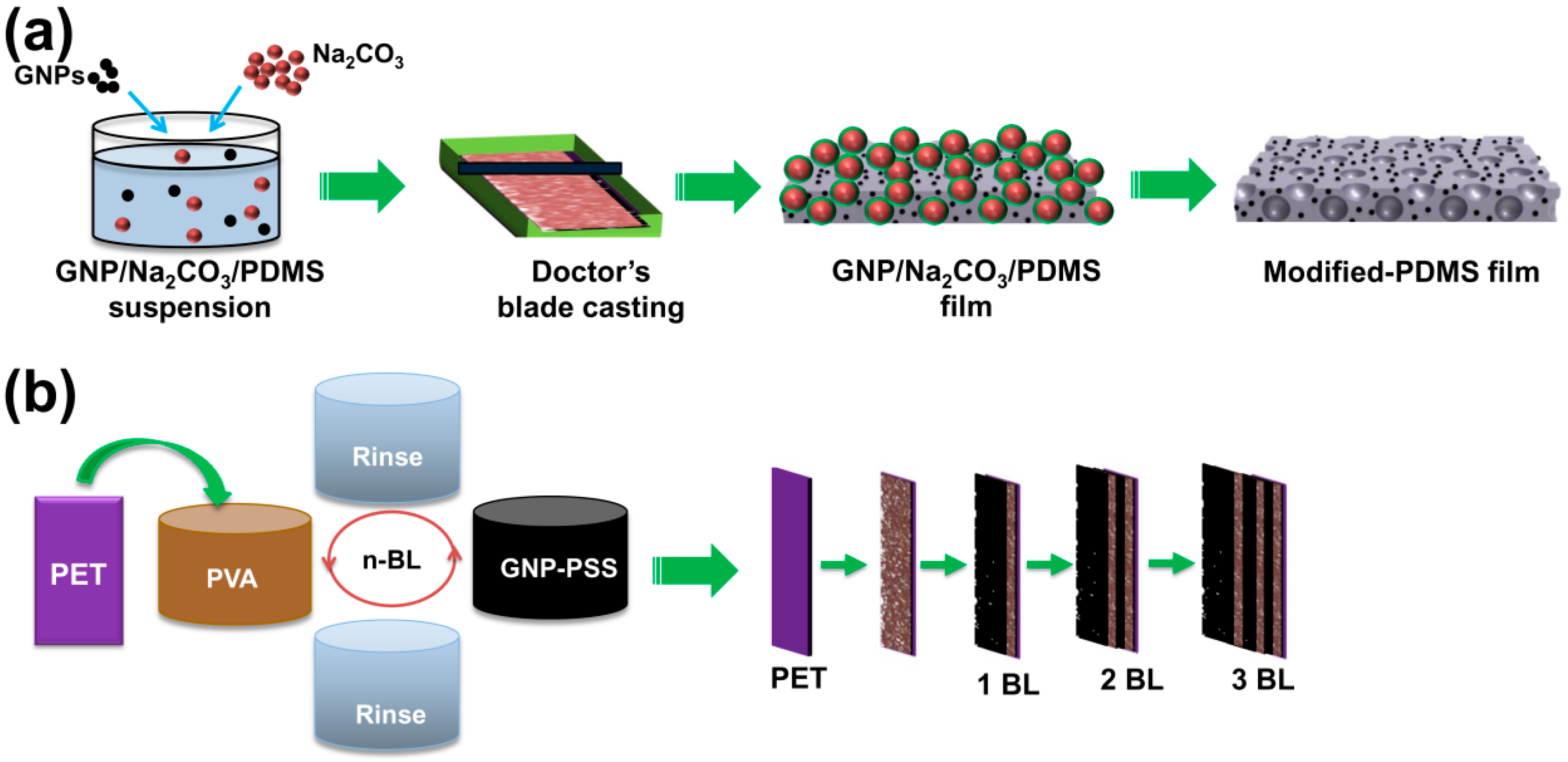
2.2. Fabrication of Modified PDMS Composite Films
2.3. Fabrication of [PVA/GNP-PSS]n Film by LbL Assembly
2.4. Fabrication of GNP-PDMS@[PVA/GNP-PSS]n Composite TENGs
2.5. Characterization
2.6. Electrical Output Performance Measurement
3. Results and Discussion
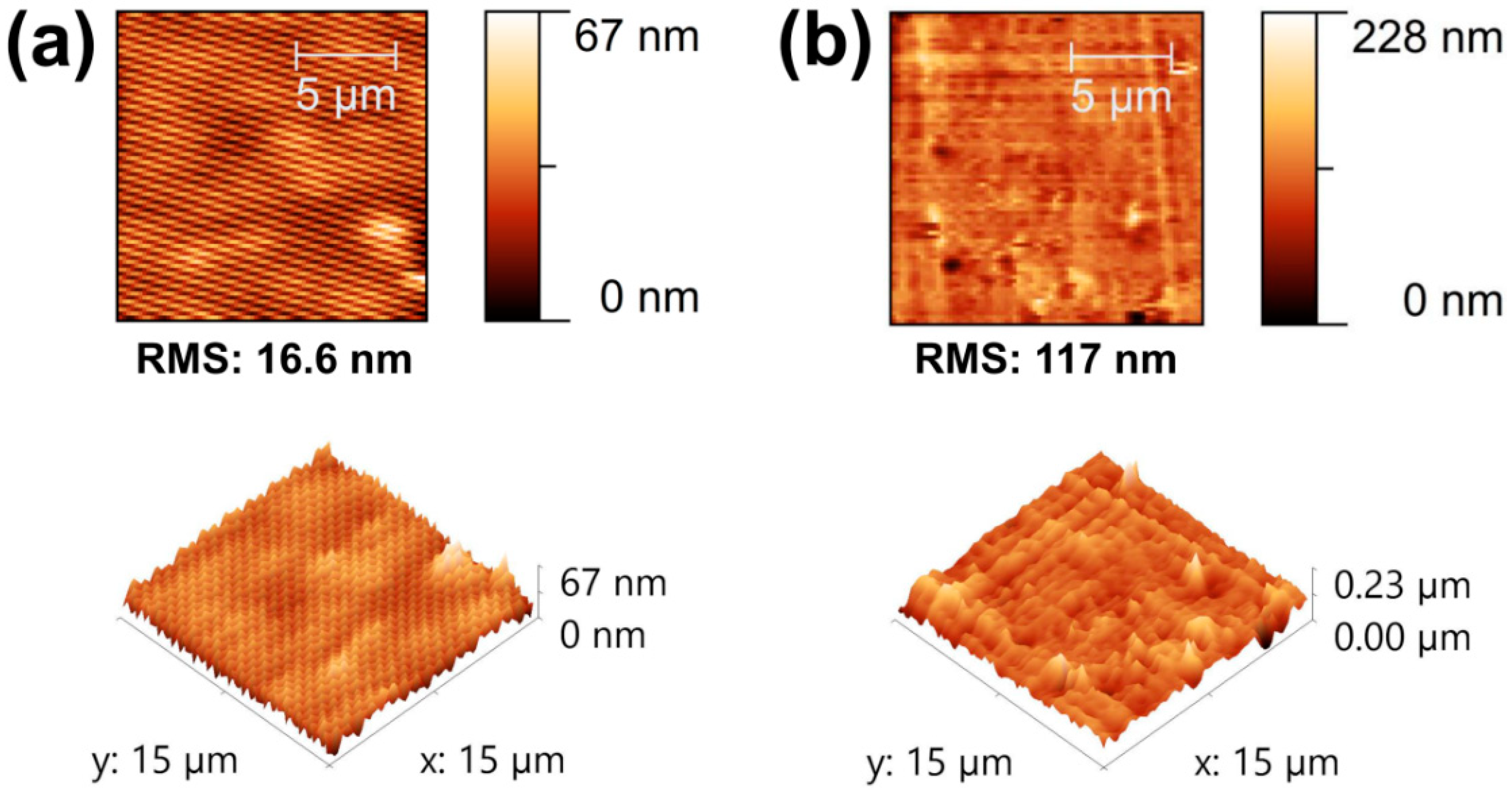
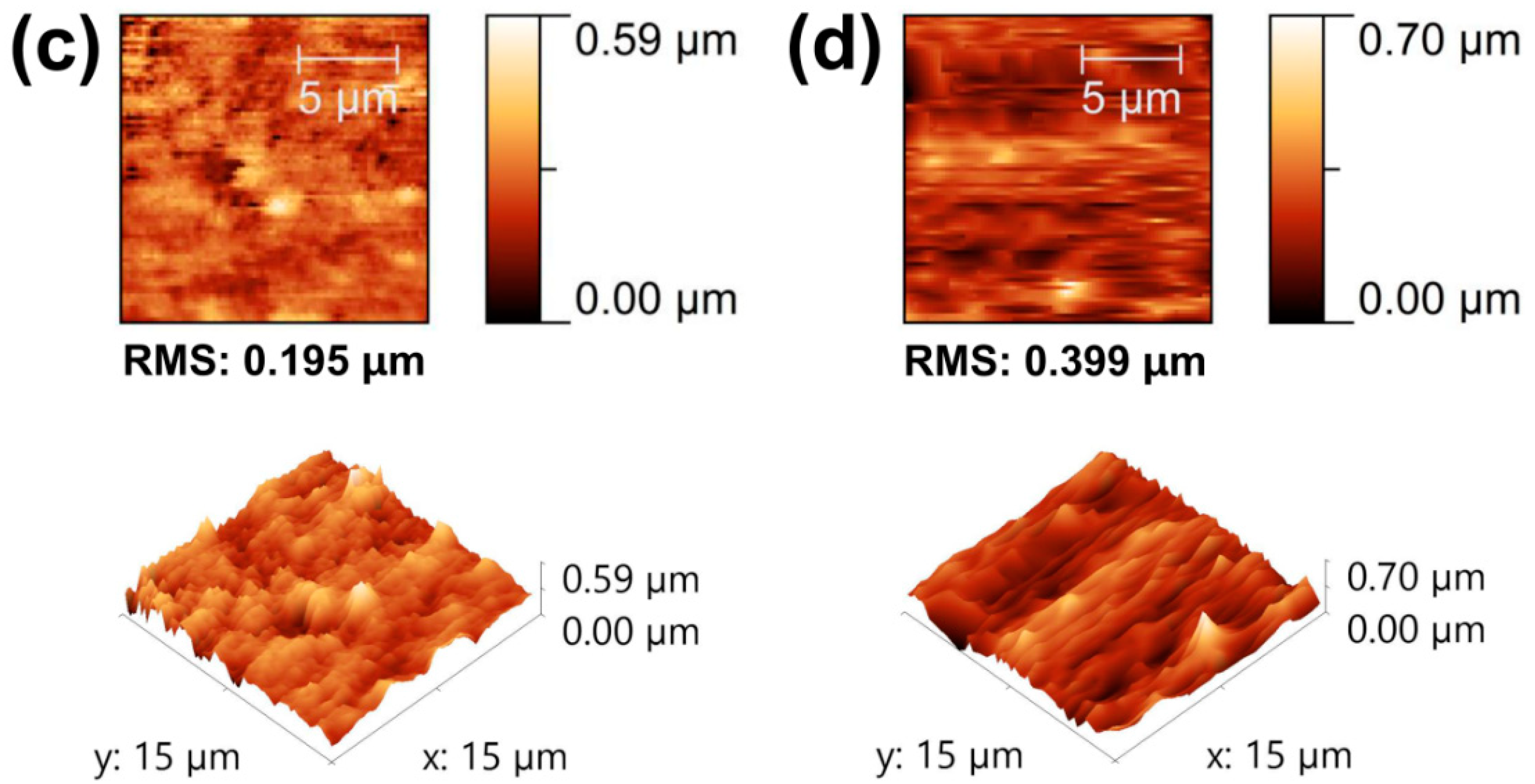
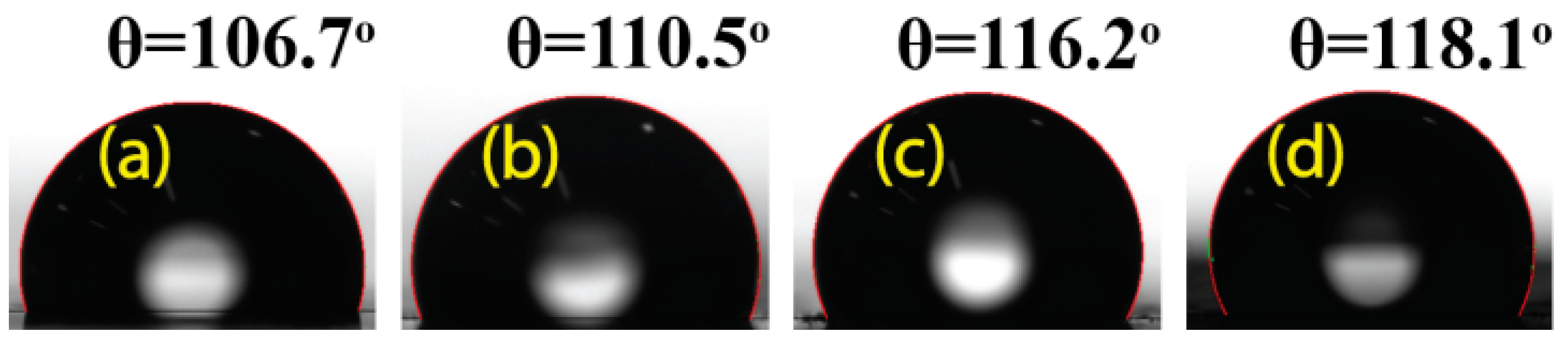
3.1. Characterization of Composite Films
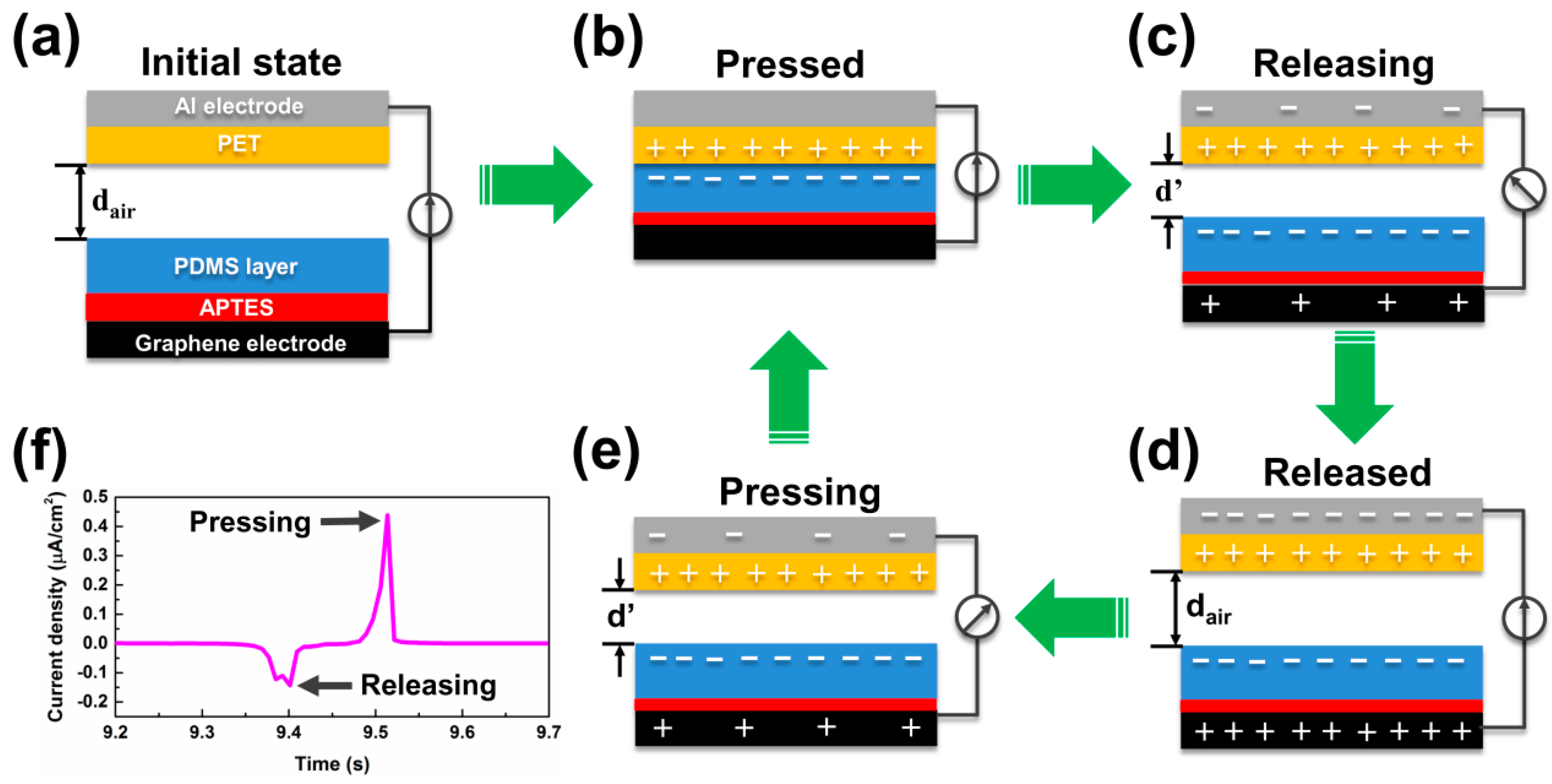
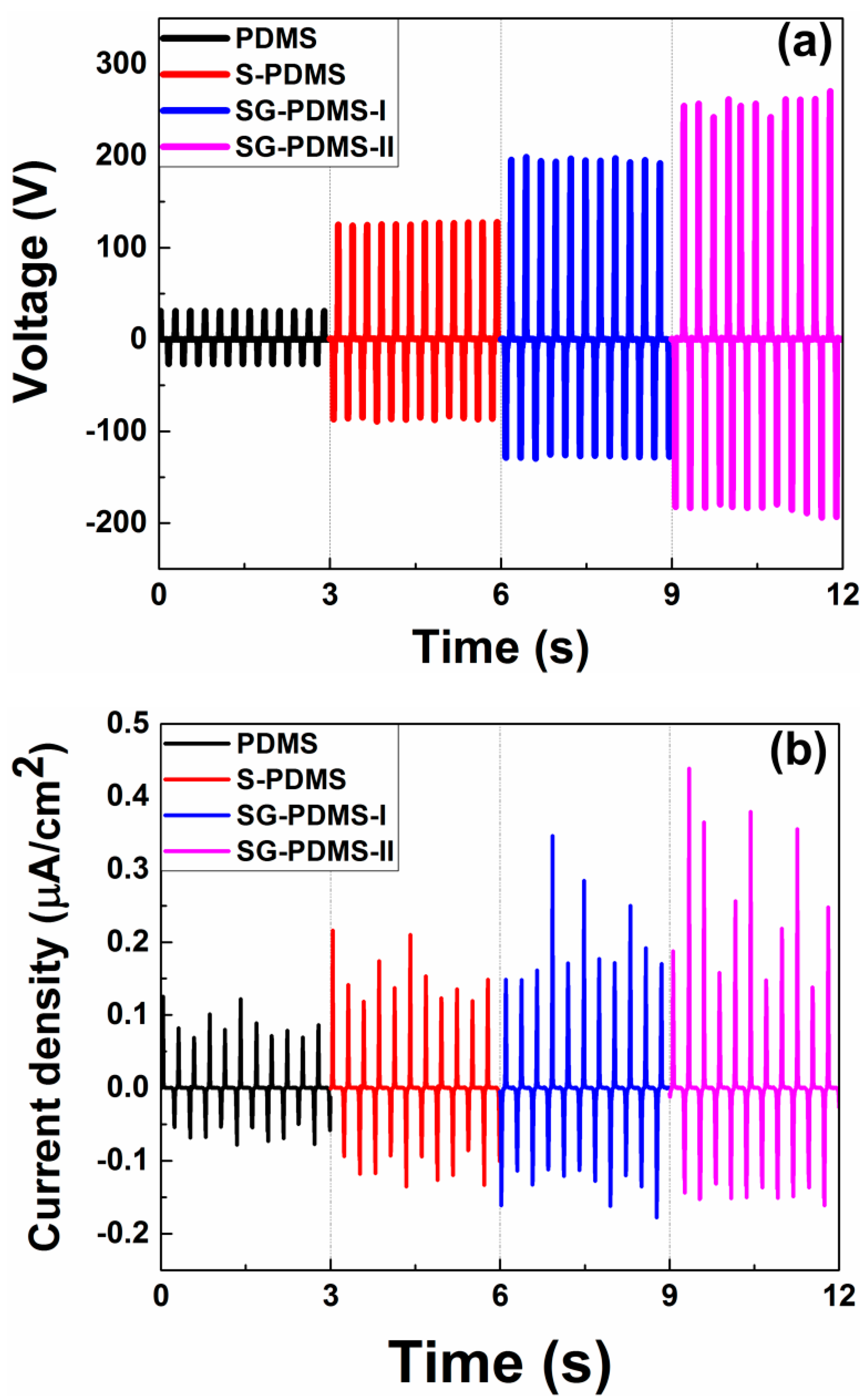
3.2. Electrical Output Performance of TENGs
3.3. Mechanical Durability of SG-PDMS-II@[PVA/GNP-PSS]3 TENG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Sun, H.; Tian, H.; Yang, Y.; Xie, D.; Zhang, Y.-C.; Liu, X.; Ma, S.; Zhao, H.-M.; Ren, T.-L. A novel flexible nanogenerator made of ZnO nanoparticles and multiwall carbon nanotube. Nanoscale 2013, 5, 6117–6123. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenerators as new energy technology and self-powered sensors—Principles, problems and perspectives. Faraday Discuss. 2014, 176, 447–458. [Google Scholar] [CrossRef]
- Lee, S.; Yeom, B.; Kim, Y.; Cho, J. Layer-by-layer assembly for ultrathin energy-harvesting films: Piezoelectric and triboelectric nanocomposite films. Nano Energy 2019, 56, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Fan, Y.J.; Meng, X.S.; Li, H.Y.; Kuang, S.Y.; Zhang, L.; Wu, Y.; Wang, Z.L.; Zhu, G. Stretchable porous carbon nanotube-elastomer hybrid nanocomposite for harvesting mechanical energy. Adv. Mater. 2016, 29, 1603115. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.-S.; Kwon, H.-B.; Jo, S.-E.; Kim, Y.-J. Wearable triboelectric nanogenerator using a plasma-etched PDMS-CNT composite for a physical activity sensor. RSC Adv. 2017, 7, 48368–48373. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Q.; Yan, C.; Liu, Y.; Zeng, X.; Wei, X.; Hu, Y.; Zheng, Z. Machine-washable and breathable pressure sensors based on triboelectric nanogenerators enabled by textile technologies. Nano Energy 2020, 70, 104528. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.-J.; Jeon, S.-B.; Seol, M.-L.; Choi, Y.-K. A triboelectric sponge fabricated from a cube sugar template by 3D soft lithography for superhydrophobicity and elasticity. Adv. Electron. Mater. 2016, 2, 1500331. [Google Scholar] [CrossRef]
- Uddin, A.I.; Chung, G.-S. Wide-ranging impact-competent self-powered active sensor using a stacked corrugated-core sandwich-structured robust triboelectric nanogenerator. Sens. Actuators B Chem. 2017, 245, 1–10. [Google Scholar] [CrossRef]
- Xu, Z.; Duan, J.; Li, W.; Wu, N.; Pan, Y.; Lin, S.; Li, J.; Yuan, F.; Chen, S.; Huang, L.; et al. Boosting the efficient energy output of electret nanogenerators by suppressing air breakdown under ambient conditions. ACS Appl. Mater. Interfaces 2019, 11, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Guo, H.; Yue, X.; Gao, J.; Xi, Y.; Hu, C. Improving energy conversion efficiency for triboelectric nanogenerator with capacitor structure by maximizing surface charge density. Nanoscale 2015, 7, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Pei, Y.; Kottapalli, A.G.P. Ultralightweight and 3D squeezable graphene-polydimethylsiloxane composite foams as piezoresistive sensors. ACS Appl. Mater. Interfaces 2019, 11, 35201–35211. [Google Scholar] [CrossRef]
- Cui, C.; Wang, X.; Yi, Z.; Yang, B.; Wang, X.; Chen, X.; Liu, J.-Q.; Yang, C. Flexible single-electrode triboelectric nanogenerator and body moving sensor based on porous Na2CO3/polydimethylsiloxane film. ACS Appl. Mater. Interfaces 2018, 10, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, H.; He, X.; Liu, G.; Xi, Y.; Shi, H.; Hu, C. Enhancing performance of triboelectric nanogenerator by filling high dielectric nanoparticles into sponge PDMS film. ACS Appl. Mater. Interfaces 2015, 8, 736–744. [Google Scholar] [CrossRef]
- Jang, S.; Oh, J.H. Rapid fabrication of microporous batio 3/pdms nanocomposites for triboelectric nanogenerators through one-step microwave irradiation. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Seol, M.-L.; Woo, J.-H.; Lee, D.-I.; Im, H.; Hur, J.; Choi, Y.-K. Nature-replicated nano-in-micro structures for triboelectric energy harvesting. Small 2014, 10, 3887–3894. [Google Scholar] [CrossRef]
- Park, K.-I.; Bin Bae, S.; Yang, S.H.; Lee, H.I.; Lee, K.; Lee, S.J. Lead-free BaTiO3 nanowires-based flexible nanocomposite generator. Nanoscale 2014, 6, 8962–8968. [Google Scholar] [CrossRef]
- Song, G.; Kim, Y.; Yu, S.; Kim, M.-O.; Park, S.-H.; Cho, S.M.; Velusamy, D.B.; Cho, S.H.; Kim, K.L.; Kim, J.; et al. Molecularly engineered surface triboelectric nanogenerator by self-assembled monolayers (METS). Chem. Mater. 2015, 27, 4749–4755. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Liu, G.; Javed, M.S.; Wang, X.; Hu, C. Aligning graphene sheets in PDMS for improving output performance of triboelectric nanogenerator. Carbon 2017, 111, 569–576. [Google Scholar] [CrossRef]
- Kim, W.-G.; Tcho, I.-W.; Kim, D.; Jeon, S.-B.; Park, S.-J.; Seol, M.-L.; Choi, Y.-K. Performance-enhanced triboelectric nanogenerator using the glass transition of polystyrene. Nano Energy 2016, 27, 306–312. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Han, M.-D.; Wang, R.; Meng, B.; Zhu, F.; Sun, X.-M.; Hu, W.; Wang, W.; Li, Z.-H.; Zhang, H. High-performance triboelectric nanogenerator with enhanced energy density based on single-step fluorocarbon plasma treatment. Nano Energy 2014, 4, 123–131. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Niu, S.; Lin, L.; Liu, C.; Zhou, Y.S.; Wang, Z.L. Maximum surface charge density for triboelectric nanogenerators achieved by ionized-air injection: Methodology and theoretical understanding. Adv. Mater. 2014, 26, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kim, J.W.; Jung, W.-S.; Kang, C.-Y.; Kim, S.; Wang, Z.L.; Baik, J.M. Mesoporous pores impregnated with Au nanoparticles as effective dielectrics for enhancing triboelectric nanogenerator performance in harsh environments. Energy Environ. Sci. 2015, 8, 3006–3012. [Google Scholar] [CrossRef]
- Cui, N.; Gu, L.; Lei, Y.; Liu, J.; Qin, Y.; Ma, X.-H.; Hao, Y.; Wang, Z.L. Dynamic behavior of the triboelectric charges and structural optimization of the friction layer for a triboelectric nanogenerator. ACS Nano 2016, 10, 6131–6138. [Google Scholar] [CrossRef]
- Wu, C.; Kim, T.W.; Choi, H.Y. Reduced graphene-oxide acting as electron-trapping sites in the friction layer for giant triboelectric enhancement. Nano Energy 2017, 32, 542–550. [Google Scholar] [CrossRef]
- Park, H.-W.; Huynh, N.D.; Kim, W.; Lee, C.; Nam, Y.; Lee, S.; Chung, K.-B.; Choi, D. Electron blocking layer-based interfacial design for highly-enhanced triboelectric nanogenerators. Nano Energy 2018, 50, 9–15. [Google Scholar] [CrossRef]
- Chung, I.J.; Kim, W.; Jang, W.; Park, H.-W.; Sohn, A.; Chung, K.B.; Kim, H.-T.; Choi, D.; Park, Y.T. Layer-by-layer assembled graphene multilayers on multidimensional surfaces for highly durable, scalable, and wearable triboelectric nanogenerators. J. Mater. Chem. A 2018, 6, 3108–3115. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Ko, Y.; Kwon, C.H.; Cho, J. Layer-by-layer assembly-induced triboelectric nanogenerators with high and stable electric outputs in humid environments. Nano Energy 2018, 44, 228–239. [Google Scholar] [CrossRef]
- Huang, L.-B.; Xu, W.; Tian, W.; Han, J.-C.; Zhao, C.-H.; Wu, H.; Hao, J. Ultrasonic-assisted ultrafast fabrication of polymer nanowires for high performance triboelectric nanogenerators. Nano Energy 2020, 71, 104593. [Google Scholar] [CrossRef]
- Beal, J.H.; Bubendorfer, A.; Kemmitt, T.; Hoek, I.; Arnold, W.M. A rapid, inexpensive surface treatment for enhanced functionality of polydimethylsiloxane microfluidic channels. Biomicrofluidics 2012, 6, 36503. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Antoniadou, E.; Lee, M.; Jeong, J.H.; Ahmed, W.W.; Saif, M.T.A.; Boppart, S.A.; Kong, H. Tailoring hydrogel adhesion to polydimethylsiloxane substrates using polysaccharide glue. Angew. Chem. Int. Ed. Engl. 2013, 52, 6949–6952. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menge, H.G.; Kim, J.O.; Park, Y.T. Enhanced Triboelectric Performance of Modified PDMS Nanocomposite Multilayered Nanogenerators. Materials 2020, 13, 4156. https://doi.org/10.3390/ma13184156
Menge HG, Kim JO, Park YT. Enhanced Triboelectric Performance of Modified PDMS Nanocomposite Multilayered Nanogenerators. Materials. 2020; 13(18):4156. https://doi.org/10.3390/ma13184156
Chicago/Turabian StyleMenge, Habtamu Gebeyehu, Jin Ok Kim, and Yong Tae Park. 2020. "Enhanced Triboelectric Performance of Modified PDMS Nanocomposite Multilayered Nanogenerators" Materials 13, no. 18: 4156. https://doi.org/10.3390/ma13184156
APA StyleMenge, H. G., Kim, J. O., & Park, Y. T. (2020). Enhanced Triboelectric Performance of Modified PDMS Nanocomposite Multilayered Nanogenerators. Materials, 13(18), 4156. https://doi.org/10.3390/ma13184156




