Synthesis of Steryl Hydroxycinnamates to Enhance Antioxidant Activity of Rapeseed Oil and Emulsions
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Samples
2.3. NMR Analysis
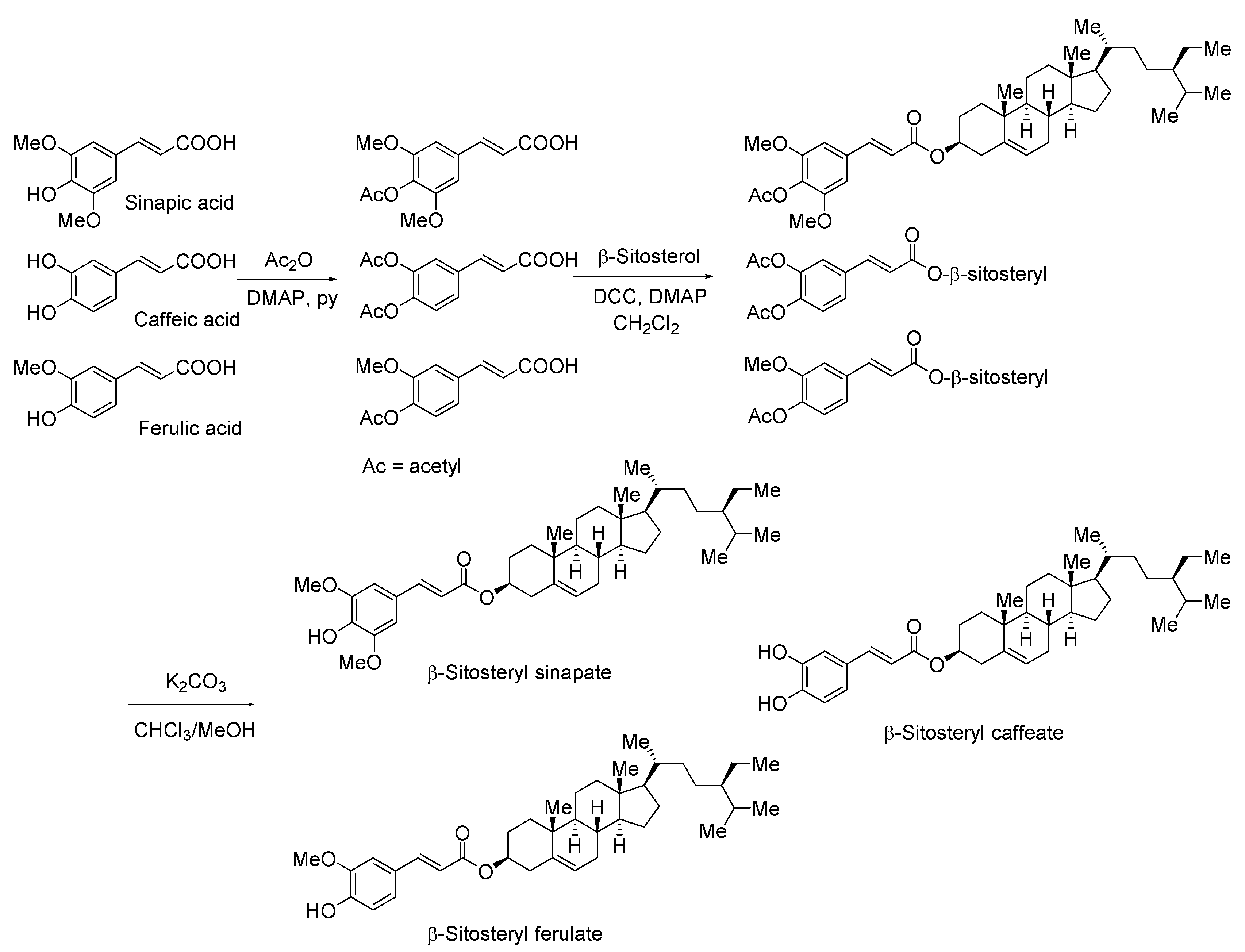
2.4. Chemical Synthesis
2.4.1. Acetylation of SA, CA, and FA to Its 4-OH or 3,4-OH Protected Derivatives
2.4.2. Esterification of 4-O-Acetylsinapic Acid, 3,4-O-Diacetylcaffeic Acid, and 4-O-Acetylferulic Acid with β-Sitosterol
2.4.3. Deprotection of Acetoxy Groups
2.5. Addition of the Synthesized Steryl Hydroxycinnamates to Fat Samples
2.6. Antioxidant Activity Determination
2.6.1. Samples Preparation
2.6.2. DPPH and ABTS Methods
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Steryl Hydroxycinnamates
3.2. Antioxidant Activity of the Synthesized Steryl Hydroxycinnamates
3.3. Effect of Steryl Hydroxycinnamates Concentration on Antioxidant Activity of Real Fat Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calheiros, R.; Machado, N.F.L.; Fiuza, S.M.; Gaspar, A.; Garrido, J.; Milhazes, N.; Borges, F.; Marques, M.P.M. Antioxidant phenolic esters with potential anticancer activity: A Raman spectroscopy study. J. Raman Spectrosc. 2008, 39, 95–107. [Google Scholar] [CrossRef]
- Decker, E.A. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci. Technol. 1998, 9, 241–248. [Google Scholar] [CrossRef]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech. J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef]
- Schär, A.; Liphardt, S.; Nyström, L. Enzymatic synthesis of steryl hydroxycinnamates and their antioxidant activity. Eur. J. Lipid Sci. Technol. 2017, 119, 1600267. [Google Scholar] [CrossRef]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Poural, S.; Saso, L.; Borges, F.; Firuz, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef]
- Begum, A.; Borah, P.; Chowdhury, P. Microwave (MW) promoted high yield expedient synthesis of steryl ferulates—A class of novel biologically active compounds: A comparative study of their antioxidant activity with that of naturally occurring γ-oryzanol. Steroids 2016, 107, 37–44. [Google Scholar] [CrossRef]
- Nyström, L.; Moreau, R.A.; Lampi, A.-M.; Hicks, K.B.; Piironen, V. Enzymatic hydrolysis of steryl ferulates and steryl glycosides. Eur. Food. Res. Technol. 2008, 227, 727–733. [Google Scholar] [CrossRef]
- Schär, A.; Nyström, L. Enzymatic synthesis of steryl ferulates. Eur. J. Lipid Sci. Technol. 2016, 118, 1557–1565. [Google Scholar] [CrossRef]
- Zhu, D.; Brambilla, D.; Leroux, J.-C.; Nystrom, L. Permeation of steryl ferulates through an in vitro intestinal barrier model. Mol. Nutr. Food Res. 2015, 59, 1182–1189. [Google Scholar] [CrossRef]
- Mandak, E.; Nystrom, L. Steryl ferulates, bioactive compounds in cereal grains. Lipid Technol. 2012, 24, 80–83. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R.; Rudzinska, M.; Klensporf-Pawlik, D. γ-Oryzanols of North American wild rice (Zizania palustris). J. Am. Oil Chem. Soc. 2013, 90, 1101–1109. [Google Scholar] [CrossRef]
- Fang, N.B.; Yu, S.G.; Badger, T.M. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J. Agric. Food Chem. 2003, 51, 3260–3267. [Google Scholar] [CrossRef]
- Takagi, T.; Iida, T. Antioxidant for fats and oils from canary seed—sterol and triterpene alcohol esters of caffeic acid. J. Am. Oil Chem. Soc. 1980, 57, 326–330. [Google Scholar] [CrossRef]
- Wen-Sen, H.; Hanyue, Z.; Zhen-Yu, C. Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar]
- Nystrom, L.; Achrenius, T.; Lampi, A.-M.; Moreau, R.A.; Piironen, V. A comparison of the antioxidant properties of steryl ferulates with tocopherol at high temperatures. Food Chem. 2007, 101, 947–954. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Hwang, H.-S.; Bakota, E.L.; Palmquist, D.A. Synthesis of steryl ferulates with various sterol structures and comparison of their antioxidant activity. Food Chem. 2015, 169, 92–101. [Google Scholar] [CrossRef]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2012, 79, 1201–1207. [Google Scholar]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the ‘‘cut-off’’ effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. Phytosteryl sinapates and vanillates: Chemoenzymatic synthesis and antioxidant capacity assessment. Food Chem. 2013, 138, 1438–1447. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. A novel chemoenzymatic synthesis of phytosteryl caffeates and assessment of their antioxidant activity. Food Chem. 2012, 133, 1427–1434. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. Chemoenzymatic synthesis of phytosteryl ferulates and evaluation of their antioxidant activity. J. Agric. Food Chem. 2011, 59, 12375–12383. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Shahidi, F. Antioxidant activity of phytosteryl phenolates in different model systems. Food Chem. 2013, 138, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Ming-Ming, Z.; Lian, W.; Feng-Hong, H.; Ling, D.; Ping-Mei, G.; Qian-Chun, D.; Wen-Lin, L.; Chang, Z. Ultrasonic pretreatment for lipase-catalyed synthesis of phytosterol esters with different acyl donors. Ultrason. Sonochem. 2012, 19, 1015–1020. [Google Scholar]
- Szydłowska-Czerniak, A.; Rabiej, D.; Krzemiński, M. Synthesis of novel octyl sinapate to enhance antioxidant capacity of rapeseed–linseed oil mixture. J. Sci. Food Agric. 2018, 98, 1625–1631. [Google Scholar] [CrossRef]
- Ebenezer, W.J. Synthesis of 2880-II, a metabolite related to ferulic acid. Synth. Commun. 1991, 21, 351–358. [Google Scholar] [CrossRef]
- Condo, A.M.; Baker, D.C.; Moreau, R.A.; Hicks, K.B. Improved method for the synthesis of trans-feruloyl-β-sitostanol. J. Agric. Food Chem. 2001, 49, 4961–4964. [Google Scholar] [CrossRef]
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Meyer, A.S.; Adler-Nissen, J.; Holstborg, J.; Hølmer, G. Oxidation in fish-oil-enriched mayonnaise 1. Assessment of propyl gallate as an antioxidant by discriminant partial least squares regression analysis. Eur. Food Res. Technol. 1999, 210, 13–30. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Adler-Nissen, J.; Hølmer, G.; Meyer, A.S. Oxidation in fish-oil-enriched mayonnaise 2. Assessment of the efficacy of different tocopherol antioxidant systems by discriminant partial least squares regression analysis. Eur. Food Res. Technol. 2000, 210, 242–257. [Google Scholar] [CrossRef]
| Antioxidant | IC50(DPPH) ± SD (µmol/L) | IC50(ABTS) ± SD (µmol/L) |
|---|---|---|
| SA | 106.3 ± 0.3 c | 82.8 ± 0.3 b |
| CA | 55.8 ± 2.5 a | 71.3 ± 0.8 a |
| FA | 161.6 ± 0.5 d | 101.2 ± 1.0 c |
| β-SSA | 238.9 ± 6.9 e | 174.6 ± 6.7 d |
| β-SCA | 78.3 ± 1.5 b | 106.7 ± 4.1 c |
| β-SFA | 290.0 ± 5.4 f | 206.0 ± 7.9 e |
| Sample | Concentration (%) | DPPH ± SD (µmol TE/100 g) | ABTS ± SD (µmol TE/100 g) |
|---|---|---|---|
| Oil | - | 390 ± 13 e,f | 1258 ± 28 d,e,f |
| RO + β-SSA | 0.01 | 440 ± 13 g,h | 1277 ± 21 e,f |
| 0.02 | 417 ± 10 f,g | 1241 ± 13 d,e | |
| 0.1 | 491 ± 5 h | 1412 ± 38 g | |
| 0.5 | 740 ± 3 k | 2321 ± 25 l | |
| RO + β-SCA | 0.01 | 475 ± 14 h | 1238 ± 30 d,e |
| 0.02 | 534 ± 10 i | 1269 ± 43 d,e,f | |
| 0.1 | 812 ± 20 l | 1952 ± 21 k | |
| 0.5 | 2526 ±18 o | 3451 ± 130 m | |
| RO + β-SFA | 0.01 | 365 ± 2 d,e | 1221 ± 54 d,e |
| 0.02 | 361 ±11 d,e | 1299 ± 7 e,f | |
| 0.1 | 464 ± 18 h | 1313 ± 11 f | |
| 0.5 | 630 ± 4 j | 1869 ± 14 j | |
| Margarine | - | 212 ± 8 a,b | 811 ± 28 a |
| MR + β-SSA | 0.01 | 248 ± 12 a,b,c | 870 ± 15 a,b |
| 0.02 | 244 ± 8 a,b,c | 859 ± 13 a,b | |
| 0.1 | 336 ± 13 d | 873 ± 25 a,b | |
| 0.5 | 456 ± 10 g,h | 1578 ± 19 h | |
| MR + β-SCA | 0.01 | 204 ± 4 a | 880 ± 25 a,b |
| 0.02 | 251 ± 7 a,b,c | 924 ± 23 b | |
| 0.1 | 459 ± 19 g,h | 1232 ± 40 d,e | |
| 0.5 | 1364 ± 22 m | 3492 ± 54 m | |
| MR + β-SFA | 0.01 | 246 ± 8 a,b,c | 842 ± 17 a,b |
| 0.02 | 215 ± 3 a,b | 897 ± 21 b | |
| 0.1 | 216 ± 9 a,b | 822 ± 30 a,b | |
| 0.5 | 459 ± 8 g,h | 1203 ± 24 d | |
| Mayonnaise | - | 267 ± 10 b,c | 856 ± 42 a,b |
| M + β-SSA | 0.01 | 258 ± 4 b,c | 891 ± 11 b |
| 0.02 | 233 ± 7 a,b | 847 ± 35 a,b | |
| 0.1 | 224 ± 3 a,b | 864 ± 12 a,b | |
| 0.5 | 329 ± 10 d | 1790 ± 16 i | |
| M + β-SCA | 0.01 | 221 ± 4 a,b | 876 ± 25 a,b |
| 0.02 | 233 ± 7 a,b | 877 ± 23 a,b | |
| 0.1 | 331 ± 2 d | 1044 ± 40 c | |
| 0.5 | 1644 ± 22 n | 4339 ± 54 n | |
| M + β-SFA | 0.01 | 226 ± 8 a,b | 848 ± 17 a,b |
| 0.02 | 281 ± 3 c | 855 ± 21 a,b | |
| 0.1 | 286 ± 9 c | 950 ± 30 b | |
| 0.5 | 478 ± 8 h | 1562 ± 19 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiej-Kozioł, D.; Krzemiński, M.P.; Szydłowska-Czerniak, A. Synthesis of Steryl Hydroxycinnamates to Enhance Antioxidant Activity of Rapeseed Oil and Emulsions. Materials 2020, 13, 4536. https://doi.org/10.3390/ma13204536
Rabiej-Kozioł D, Krzemiński MP, Szydłowska-Czerniak A. Synthesis of Steryl Hydroxycinnamates to Enhance Antioxidant Activity of Rapeseed Oil and Emulsions. Materials. 2020; 13(20):4536. https://doi.org/10.3390/ma13204536
Chicago/Turabian StyleRabiej-Kozioł, Dobrochna, Marek P. Krzemiński, and Aleksandra Szydłowska-Czerniak. 2020. "Synthesis of Steryl Hydroxycinnamates to Enhance Antioxidant Activity of Rapeseed Oil and Emulsions" Materials 13, no. 20: 4536. https://doi.org/10.3390/ma13204536
APA StyleRabiej-Kozioł, D., Krzemiński, M. P., & Szydłowska-Czerniak, A. (2020). Synthesis of Steryl Hydroxycinnamates to Enhance Antioxidant Activity of Rapeseed Oil and Emulsions. Materials, 13(20), 4536. https://doi.org/10.3390/ma13204536







