Digital Image Correlation (DIC) Assessment of the Non-Linear Response of the Anterior Longitudinal Ligament of the Spine during Flexion and Extension
Abstract
1. Introduction
- Characterize the strain distribution of the ALL in situ during flexion-extension;
- Compare the strain in specific regions of interest (ROIs), in front of L4 vertebral body (VB) and L4-L5 IVD (this region of the spine is subject to greater range of motion);
- Analyze the non-linear relationship between the measured strain and the imposed rotation and the resultant moment.
2. Materials and Methods
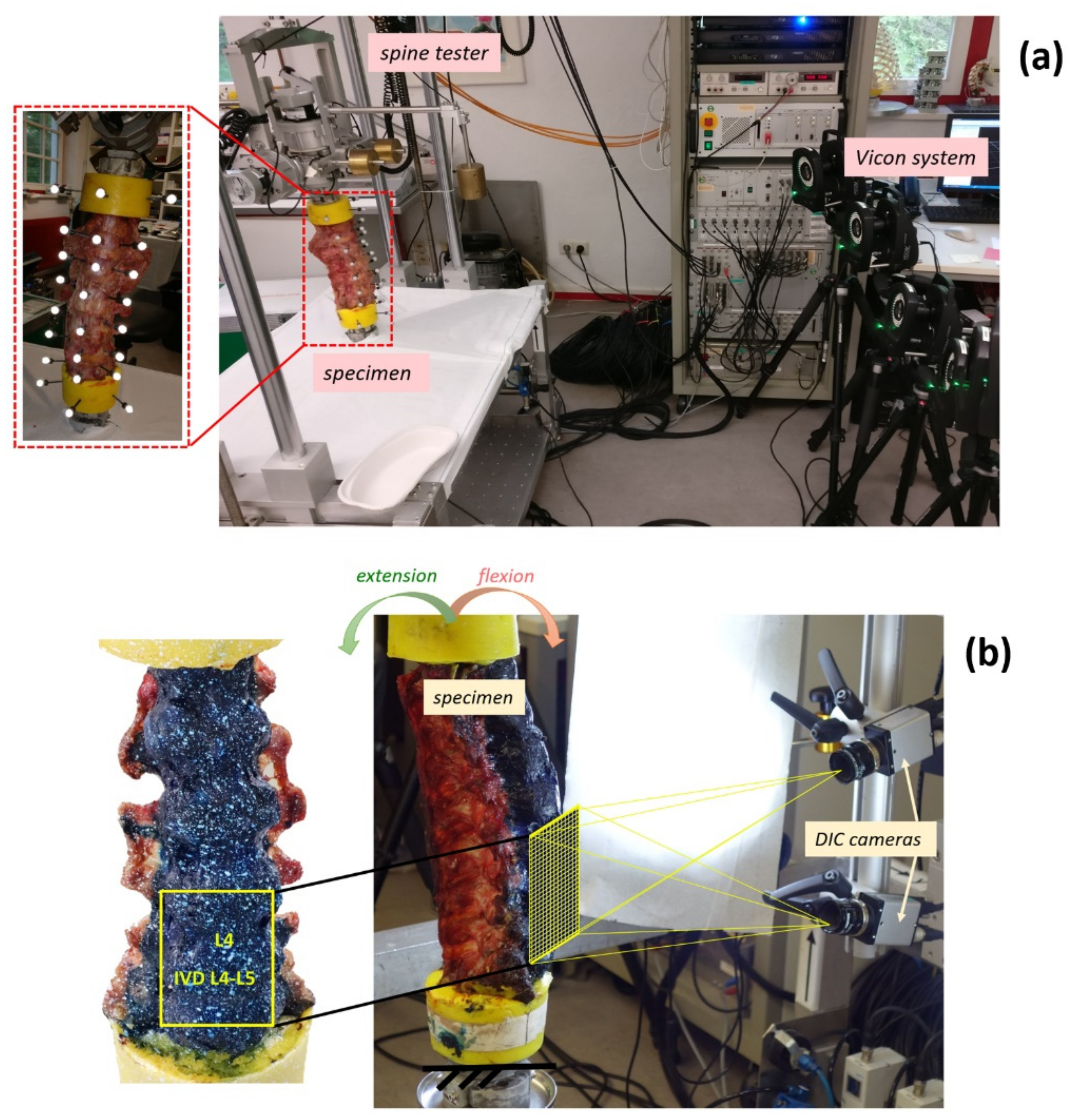
2.1. Specimens
2.2. Mechanical Test
2.3. Measurements of Intervertebral Motions
2.4. Digital Image Correlation
2.5. Analysis of Strain
2.6. Analysis of the Non-Linearity
2.7. Assessment of Measurement Uncertainties
- Range of motion (from the Vicon system): the error on the measurement of the angle between L4–L5 was less than 0.1° [29].
- Strain uncertainty (DIC system): as the largest component of error in DIC-measured strain is the random error [24,27], a zero-strain analysis was used. Two images of each unloaded specimen were captured with the DIC system and analyzed with the optimal software parameters to evaluate the strain measurement uncertainties in a known configuration (zero-strain) [24]. Being in a zero-strain configuration, any strain different from zero was accounted as measurement error. DIC-measured strains had a systematic error less than 0.002% and a random error less than 0.006%.
- Intra-operator variability: in order to analyze the reliability of ROIs identification, the same operator was asked to identify the ROIs three times on different days in correspondence. The difference among the three repetitions was less than 0.2% of the mean value inside the ROI.
3. Results
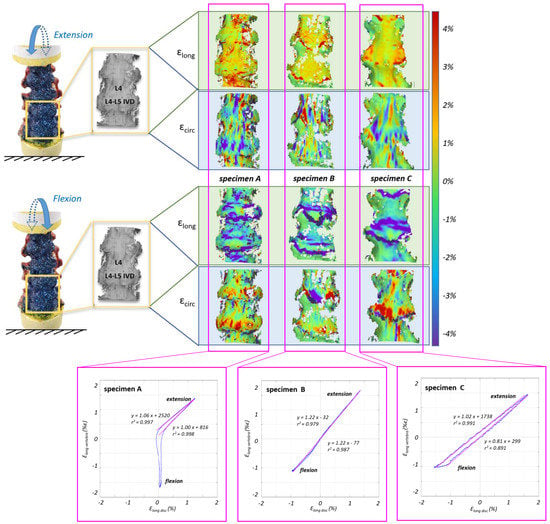
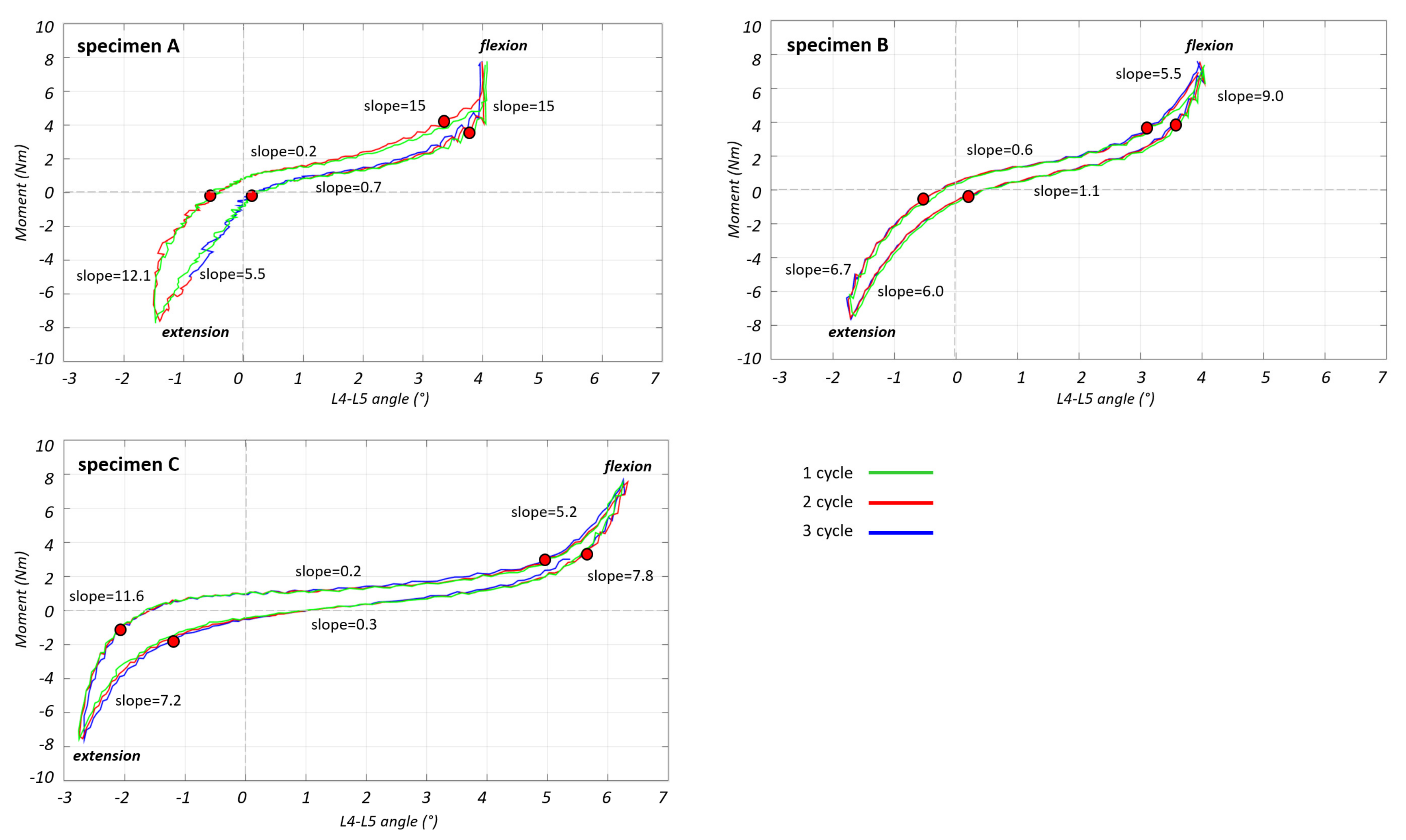
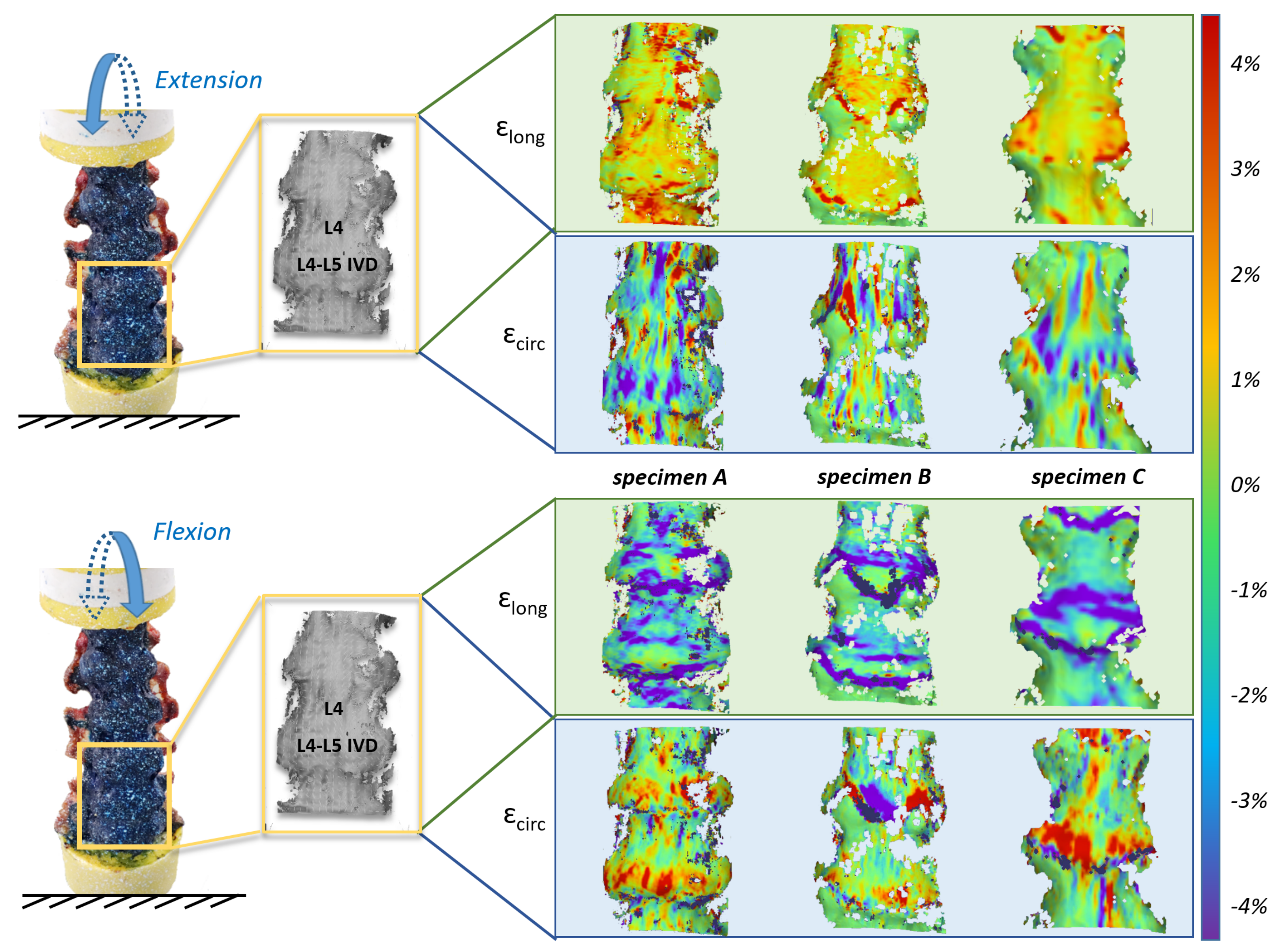
3.1. Range of Motion and Strain Maps
- During flexion, the L4–L5 angle reached 4.0° for specimens A and B, and 6.3° for specimen C;
- During extension the angle reached 1.5° for specimen A, 1.7° for specimen B and 2.7° for specimen C.
3.2. Non-Linear Trend of the Strain in the Different Parts of the ALL
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tkaczuk, H. Tensile properties of human lumbar longitudinal ligaments. Acta Orthop. Scand. 2014, 39, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Hukins, D.W.L.; Kirby, M.C.; Sikoryn, T.A.; Aspden, R.M.; Cox, A.J. Comparison of structure, mechanical properties, and functions of lumbar spinal ligaments. Spine 1990, 15, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.; Phty, B.; Bogduk, N. The ligaments and anulus fibrosus of human adult cervical intervertebral discs. Spine 1999, 24, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Behrsin, J.; Briggs, C. Ligaments of the lumbar spine: A review. Surg. Radiol. Anat. 1988, 10, 211–219. [Google Scholar] [CrossRef]
- Neumann, P.; Keller, T.S.; Ekstrom, L.; Hansson, T.H.; Spengler, D.M. Mechanical properties of the human lumbar anterior longitudinal ligament. J. Biomech. 1992, 25, 1185–1194. [Google Scholar] [CrossRef]
- Neumann, P.; Keller, T.S.; Ekstrom, L.; Hansson, T.H. Effect of strain rate and bone mineral on the structural properties of the human anterior longitudinal ligament. Spine 1994, 19, 205–211. [Google Scholar] [CrossRef]
- Benzel, E.C. Biomechanically relevant anatomy and material properties of the spine and associated elements. In Biomechanics of Spine Stabilization; American Association of Neurological Surgeons: Rolling Meadows, IL, USA, 2001; pp. 8–10. [Google Scholar]
- Yoganandan, N.; Kumaresan, S.; Pintar, F.A. Geometric and mechanical properties of human cervical spine ligaments. J. Biomech. Eng. 2000, 122, 623–629. [Google Scholar] [CrossRef]
- Robertson, D.J.; Von Forell, G.A.; Alsup, J.; Bowden, A.E. Thoracolumbar spinal ligaments exhibit negative and transverse pre-strain. J. Mech. Behav. Biomed. Mater. 2013, 23, 44–52. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Goel, V.K.; Takata, K. Physiologic strains in the lumbar spine ligaments: An in vitro biomechanical study. Spine 1982, 7, 192–203. [Google Scholar] [CrossRef]
- Schendel, M.J.; Wood, K.; Butterman, G.R.; Lewis, J.L.; Ogilvie, J.W. Experimental measurement of ligament force, facet force, and segment motion in the human lumbar spine. J. Biomech. 1993, 26, 427–438. [Google Scholar] [CrossRef]
- Heuer, F.; Schmidt, H.; Wilke, H.J. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J. Biomech. 2008, 41, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Hausfeld, J.N.; White, A.A. A biomechanical study of the ligamentous stability of the thoracic spine in man. Acta Orthop. Scand. 1981, 52, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kettler, A.; Wilke, H.J.; Haid, C.; Claes, L. Effects of specimen length on the monosegmental motion behavior of the lumbar spine. Spine 2000, 25, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Schmidt, H.; Klezl, Z.; Claes, L.; Wilke, H.J. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J. Biomech. 2007, 40, 271–280. [Google Scholar] [CrossRef]
- Palanca, M.; Tozzi, G.; Cristofolini, L. The use of digital image correlation in the biomechanical area: A review. Int. Biomech. 2015, 3, 1–21. [Google Scholar] [CrossRef]
- Palanca, M.; Marco, M.; Ruspi, M.L.; Cristofolini, L. Full-field strain distribution in multi-vertebra spine segments: An in vitro application of digital image correlation. Med. Eng. Phys. 2017, 52, 76–83. [Google Scholar] [CrossRef]
- Palanca, M.; Ruspi, M.L.; Cristofolini, L.; Liebsch, C.; Villa, T.; Brayda-Bruno, M.; Galbusera, F.; Wilke, H.J.; La Barbera, L. The strain distribution in the lumbar anterior longitudinal ligament is affected by the loading condition and bony features: An in vitro full-field analysis. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Jordan, J.; Konstantinou, K.; O’Dowd, J. Herniated lumbar disc. Musculoskelet. Disord. 2008, 3, 1–34. [Google Scholar]
- Wilke, H.J.; Rohlmann, F.; Neidlinger-Wilke, C.; Werner, K.; Claes, L.; Kettler, A. Validity and interobserver agreement of a new radiographic grading system for intervertebral disc degeneration: Part I. Lumbar spine. Eur. Spine J. 2006, 15, 720–730. [Google Scholar] [CrossRef]
- Al-Rawahi, M.; Luo, J.; Pollintine, P.; Dolan, P.; Adams, M.A. Mechanical function of vertebral body osteophytes, as revealed by experiments on cadaveric spines. Spine 2011, 36, 770–777. [Google Scholar] [CrossRef]
- Wilke, H.J.; Schmitt, H.; Wolf, S. A universal spine tester for in vitro experiments with muscle force simulation. Eur. Spine J. 1994, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Jungkunz, B.; Wenger, K.; Claes, L. Spinal segment range of motion as a function of in vitro test conditions: Effects of exposure period, accumulated cycles, angular-deformation rate, and moisture condition. Anat. Rec. 1998, 251, 15–19. [Google Scholar] [CrossRef]
- Palanca, M.; Brugo, T.M.; Cristofolini, L. Use of digital image correlation to investigate the biomechanics of the vertebra. J. Mech. Med. Biol. 2015, 15. [Google Scholar] [CrossRef]
- Ruspi, M.L.; Palanca, M.; Faldini, C.; Cristofolini, L. Full-field in vitro investigation of hard and soft tissue strain in the spine by means of Digital Image Correlation. Muscles Ligaments Tendons J. 2017, 7, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Lionello, G.; Sirieix, C.; Baleani, M. An effective procedure to create a speckle pattern on biological soft tissue for digital image correlation measurements. J. Mech. Behav. Biomed. Mater. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Freddi, A.; Olmi, G.; Cristofolini, L. Experimental Stress Analysis for Materials and Structures: Stress Analysis Models for Developing Design Methodologies; Springer: Cham, Switzerland, 2015; Volume 5, pp. 187–212. [Google Scholar]
- Jones, E.M.C. A Good Practices Guide for Digital Image Correlation; Internation Digital Image Correlation Society, 2018; Available online: https://doi.org/10.32720/idics/gpg.ed1 (accessed on 9 January 2020).
- Graf, N. Entwicklung einer Messmethode für Biomechanische In-Vitro-Untersuchungen am Humanen Brustkorb. Master’s Thesis, Universitätsklinikum Ulm, Ulm, Germany, 2009. [Google Scholar]
- Heuer, F.; Schmidt, H.; Wilke, H.J. The relation between intervertebral disc bulging and annular fiber associated strains for simple and complex loading. J. Biomech. 2008, 41, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Pintar, F.A.; Yoganandan, N.; Myers, T.; Elhagediab, A.; Sances, A. Biomechanical properties of human lumbar spine ligaments. J. Biomech. 1992, 25, 1351–1356. [Google Scholar] [CrossRef]
- Kowalski, R.J.; Ferrara, L.A.; Benzel, E.C. Biomechanics of the spine. Neurosurg. Q. 2005, 15, 42–59. [Google Scholar] [CrossRef]
- White, A.A.; Panjabi, M.M. The basic kinematics of the human spine. Spine 1978, 3, 12–20. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Oxland, T.R.; Yamamoto, I.; Crisco, J.J. Mechanical behaviour of the human lumbar and lumbosacral spine as shown by three-dimensional load displacement curves. J. Bone Jt. Surg. 1994, 76, 413–424. [Google Scholar] [CrossRef]
- Bermel, E.A.; Barocas, V.H.; Ellingson, A.M. The role of the facet capsular ligament in providing spinal stability. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 712–721. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Brayda-Bruno, M.; Liebsch, C.; Villa, T.; Luca, A.; Galbusera, F.; Wilke, H.J. Biomechanical advantages of supplemental accessory and satellite rods with and without interbody cages implantation for the stabilization of pedicle subtraction osteotomy. Eur. Spine J. 2018, 27, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
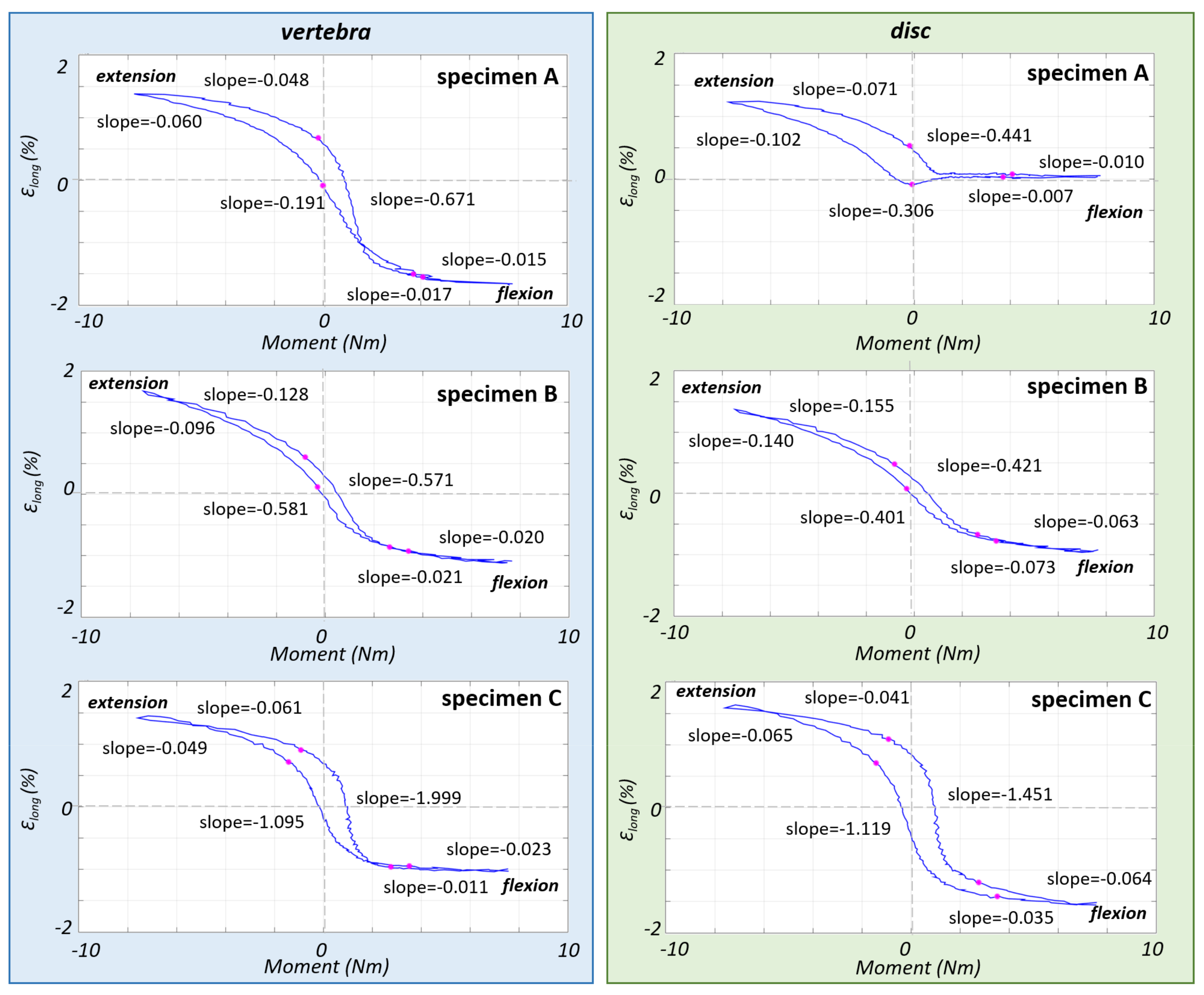
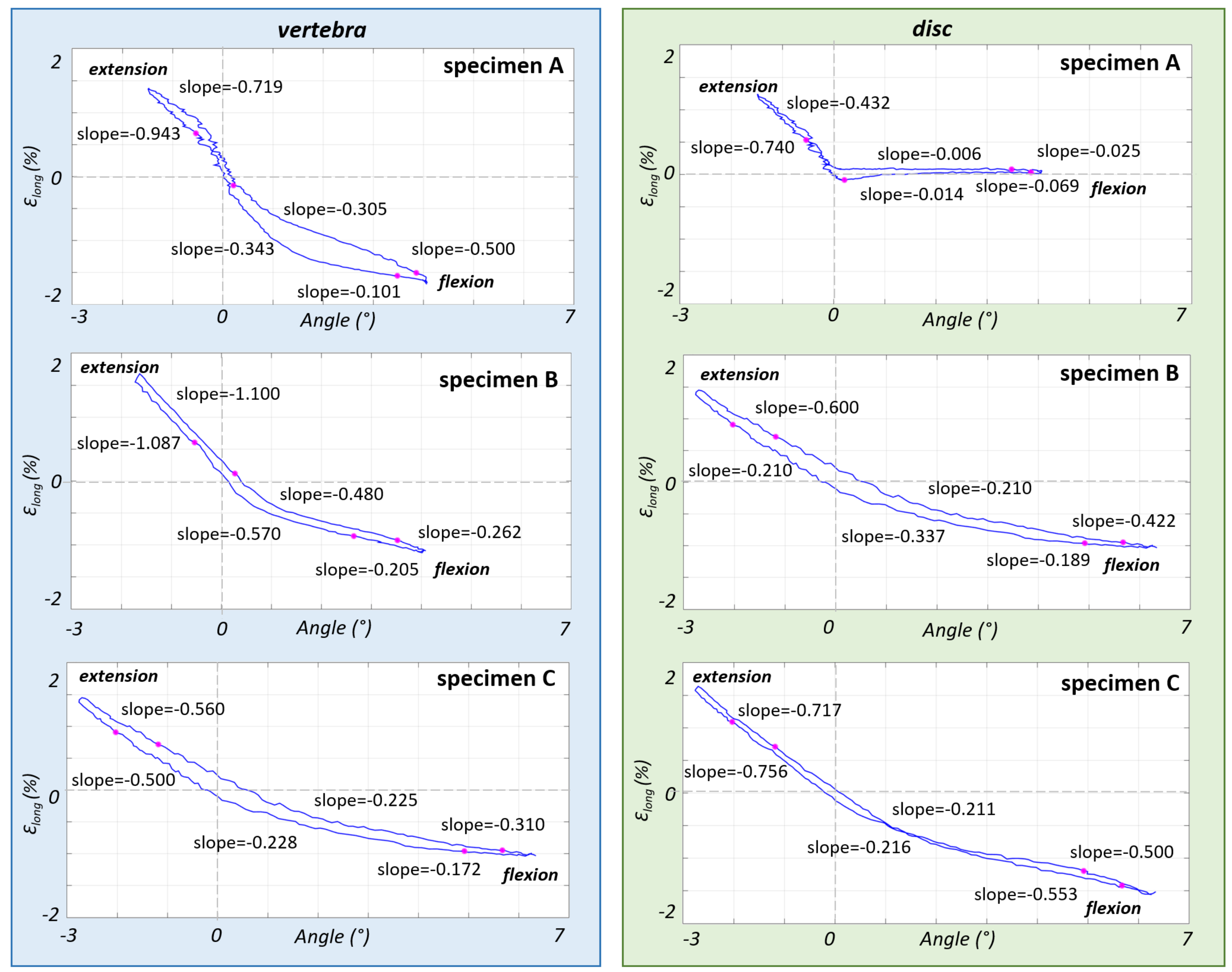
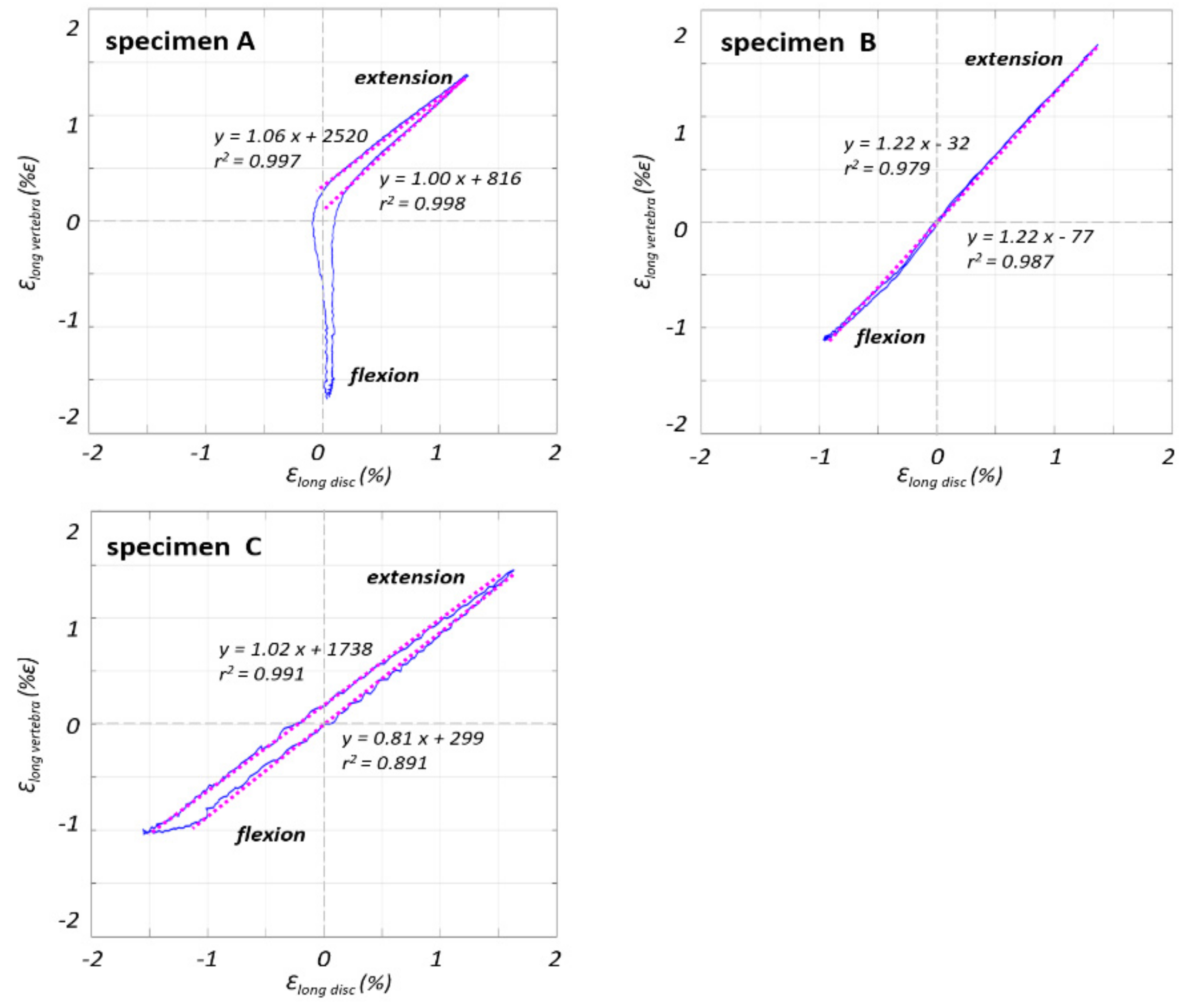
| Specimen | Segment | Sex | Age at Death (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) | BMD (mg/cm3) | Assessment of the Osteophytes in the L4–L5 Area |
|---|---|---|---|---|---|---|---|---|
| A | T11-S1 | M | 66 | 183 | 141 | 42.1 | 82 | 2 osteophytes (both grade 2) centrally located on the endplate of both L4 and L5 |
| B | T11-S1 | M | 62 | 178 | 164 | 51.7 | 94 | 1 osteophyte (grade 2) centrally located on the endplate of L5 |
| C | T12-S1 | F | 63 | 157 | 125 | 50.7 | 157 | 2 osteophytes (grade 1 and grade 2) centrally located on the endplate of respectively L4 and L5 |
| Parameters for the Correlation Analysis | |
|---|---|
| DIC Software Package Name and Manufacturer | Instra 4D, v. 4.3.1, Dantec Dynamics |
| Distance of the cameras | 540 mm |
| Field of view | about 120 mm by 160 mm |
| Depth of field | 70 mm |
| Lens aperture | f/22 |
| Frame rate | 5 frames per second |
| Grid spacing | 4 pixels |
| Facet size | between 39 and 59 pixels |
| Pixel size | about 0.08 mm |
| Contour smoothing | kernel size 5 × 5 |
| Specimen | Moment (Nm) | Angle (°) | Strain in Front of Vertebra (%) | Strain in Front of Disc (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flexion | Extension | Flexion | Extension | Flexion | Extension | Flexion | Extension | |||||||||
| f–e | e–f | f–e | e–f | f–e | e–f | f–e | e–f | f–e | e–f | f–e | e–f | f–e | e–f | f–e | e–f | |
| A | 3.70 | 4.09 | −0.10 | −0.20 | 3.86 | 3.48 | 0.21 | −0.53 | −1.55% | −1.50% | −0.14% | 0.73% | 0.04% | 0.08% | −0.08% | 0.54% |
| B | 3.42 | 2.65 | −0.31 | −0.82 | 3.52 | 2.65 | 0.27 | −0.53 | −0.92% | −0.86% | −0.12% | 0.66% | −0.77% | −0.67% | 0.08% | 0.48% |
| C | 3.52 | 2.77 | −1.42 | −0.92 | 5.68 | 4.92 | −1.18 | −2.03 | −0.95% | −0.96% | 0.97% | 0.79% | −1.42% | −1.19% | 1.09% | 0.71% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruspi, M.L.; Palanca, M.; Cristofolini, L.; Liebsch, C.; Villa, T.; Brayda-Bruno, M.; Galbusera, F.; Wilke, H.-J.; La Barbera, L. Digital Image Correlation (DIC) Assessment of the Non-Linear Response of the Anterior Longitudinal Ligament of the Spine during Flexion and Extension. Materials 2020, 13, 384. https://doi.org/10.3390/ma13020384
Ruspi ML, Palanca M, Cristofolini L, Liebsch C, Villa T, Brayda-Bruno M, Galbusera F, Wilke H-J, La Barbera L. Digital Image Correlation (DIC) Assessment of the Non-Linear Response of the Anterior Longitudinal Ligament of the Spine during Flexion and Extension. Materials. 2020; 13(2):384. https://doi.org/10.3390/ma13020384
Chicago/Turabian StyleRuspi, Maria Luisa, Marco Palanca, Luca Cristofolini, Christian Liebsch, Tomaso Villa, Marco Brayda-Bruno, Fabio Galbusera, Hans-Joachim Wilke, and Luigi La Barbera. 2020. "Digital Image Correlation (DIC) Assessment of the Non-Linear Response of the Anterior Longitudinal Ligament of the Spine during Flexion and Extension" Materials 13, no. 2: 384. https://doi.org/10.3390/ma13020384
APA StyleRuspi, M. L., Palanca, M., Cristofolini, L., Liebsch, C., Villa, T., Brayda-Bruno, M., Galbusera, F., Wilke, H.-J., & La Barbera, L. (2020). Digital Image Correlation (DIC) Assessment of the Non-Linear Response of the Anterior Longitudinal Ligament of the Spine during Flexion and Extension. Materials, 13(2), 384. https://doi.org/10.3390/ma13020384