Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
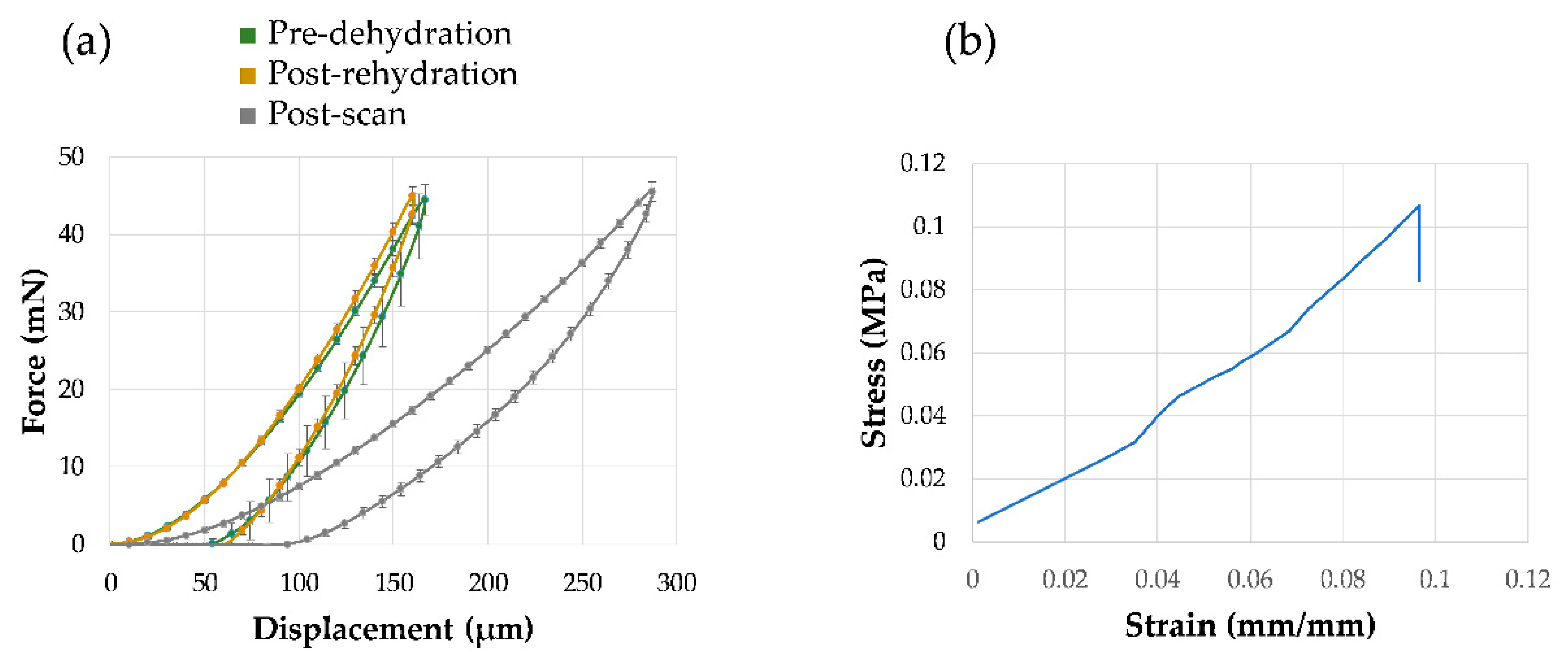
2.2. Micromechanical Probing and Mechanical Testing
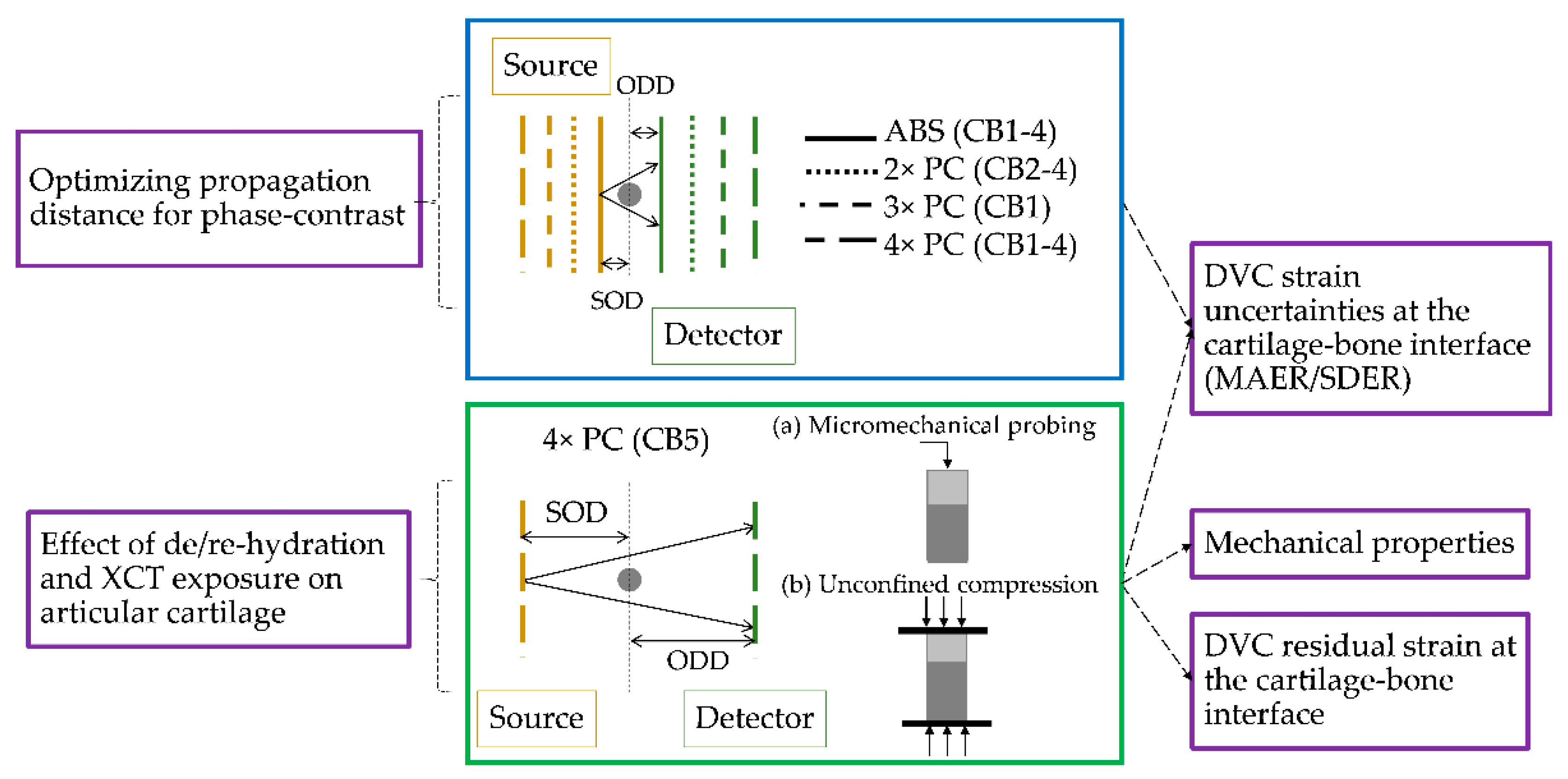
2.3. XCT, Image Post-Processing and Synthetic Deformation
2.4. Digital Volume Correlation
3. Results
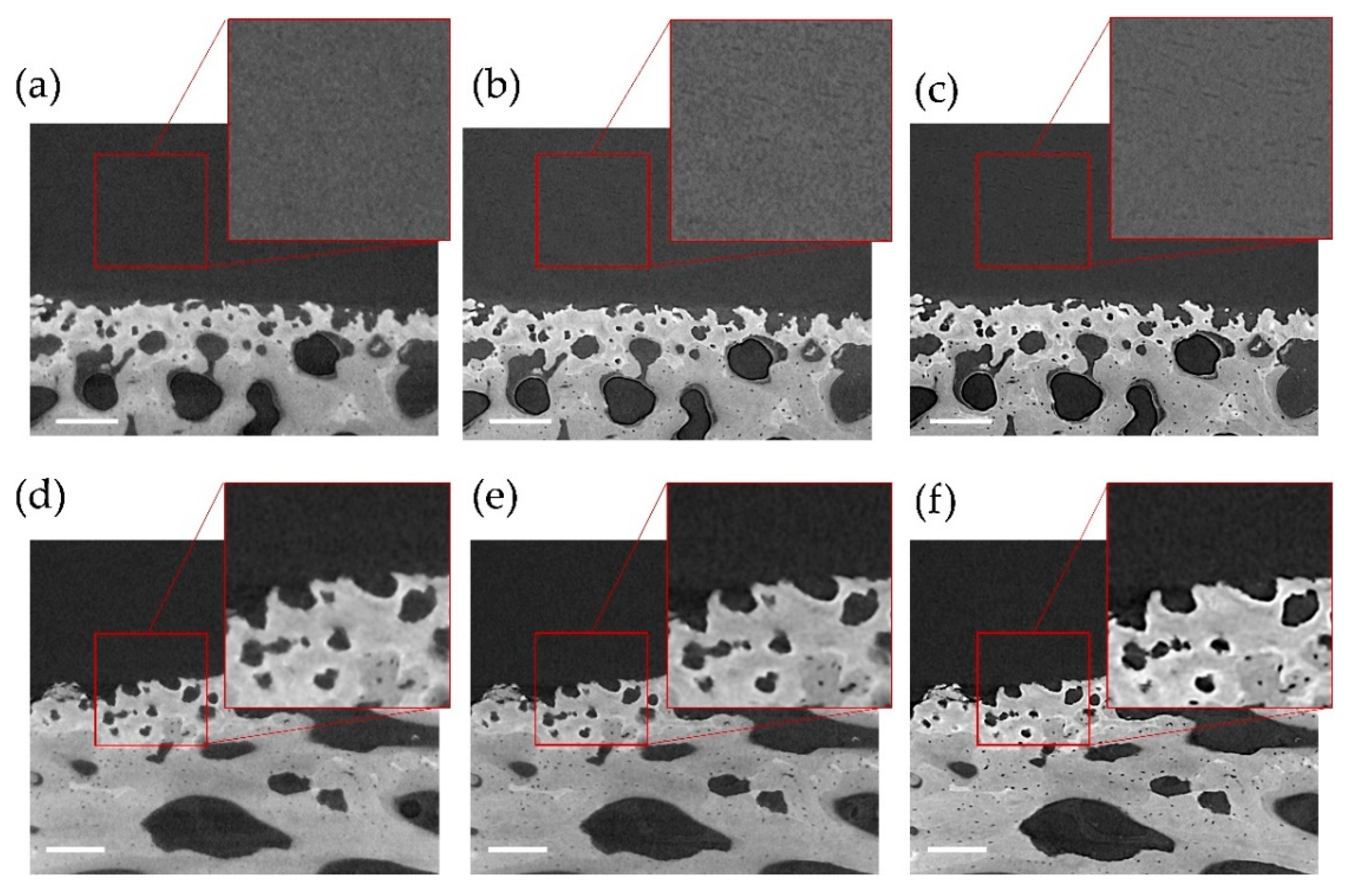
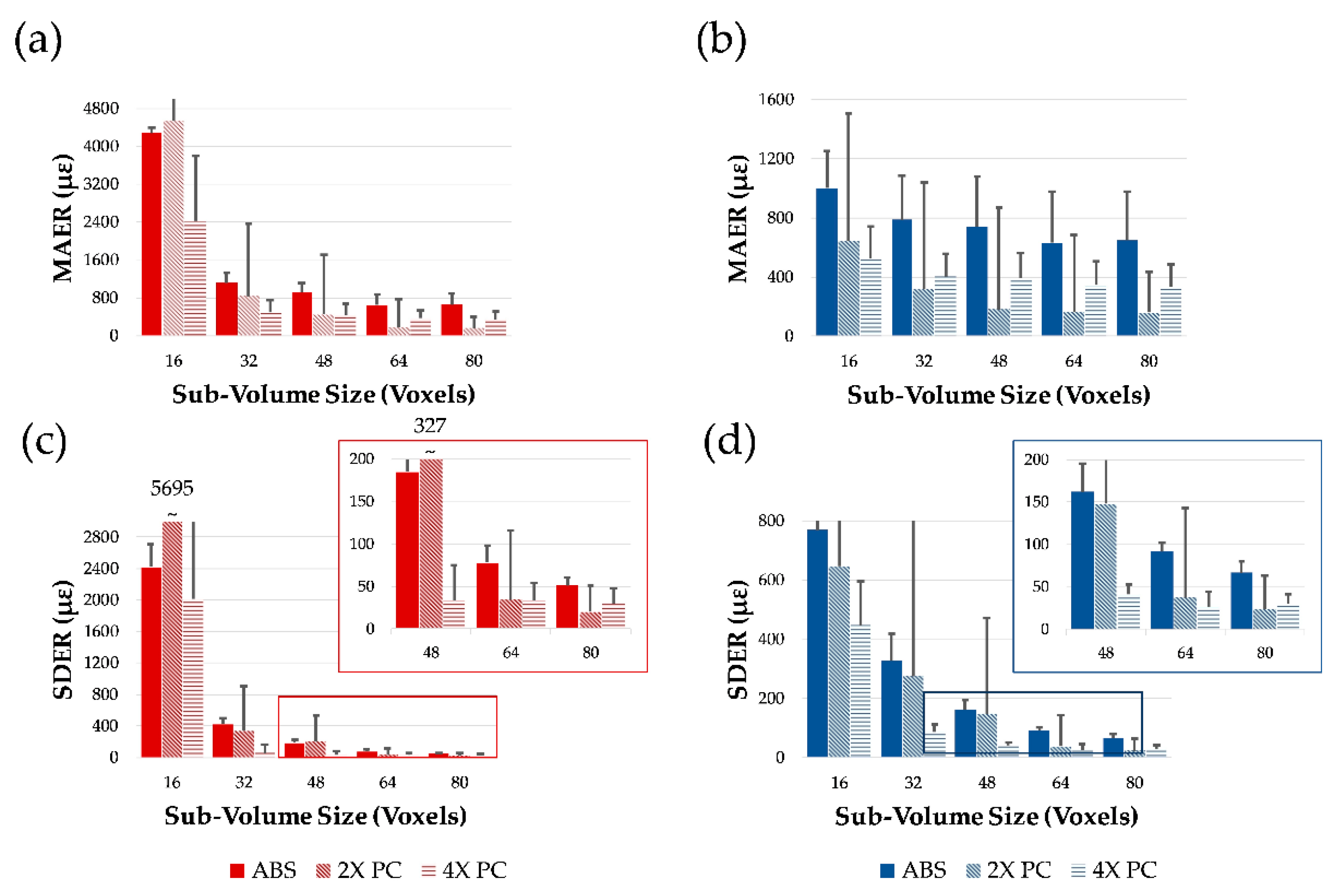
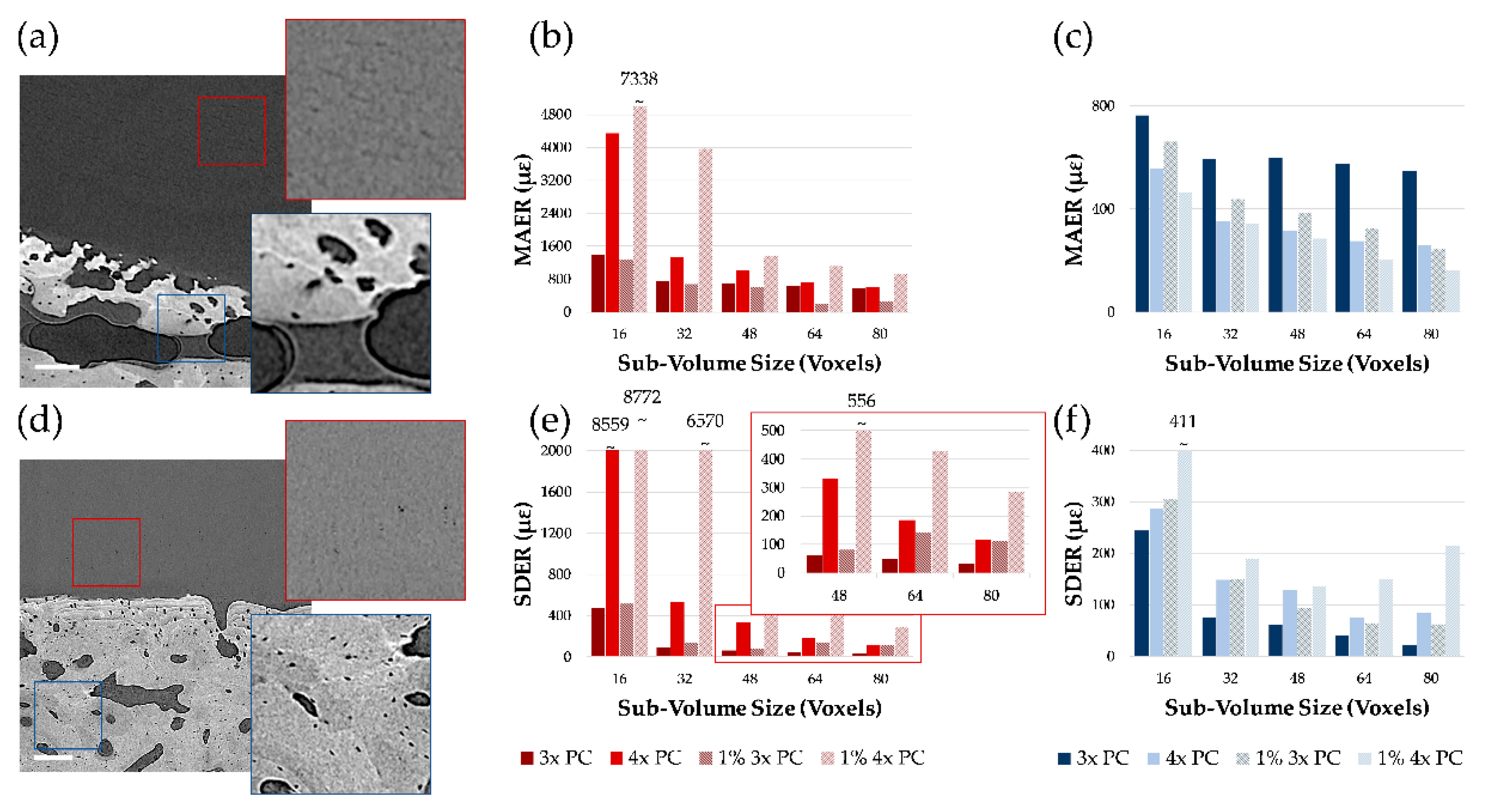
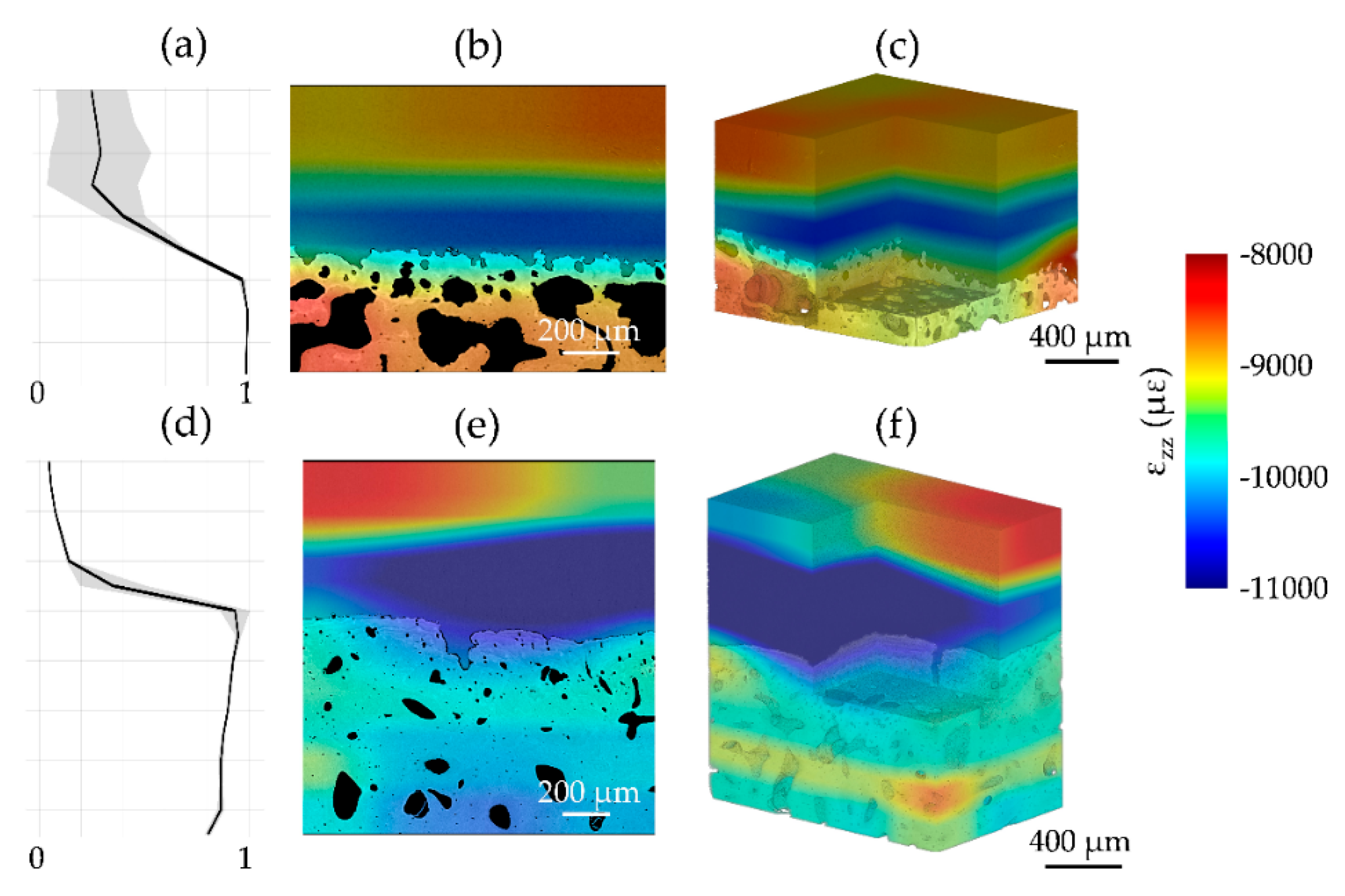
3.1. Phase-Contrast Enhancement and DVC Strain Uncertainties
3.2. Mechanics and Full-Field Residual Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Rose, R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 1987, 213, 34–40. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Gao, L.; Xu, B.; Zhang, C. Test the Mechanical Properties of Articular Cartilage using Digital Image Correlation Technology. Procedia Environ. Sci. 2011, 8, 191–196. [Google Scholar] [CrossRef]
- Wang, S.; Bao, Y.; Guan, Y.; Zhang, C.; Liu, H.; Yang, X.; Gao, L.; Guo, T.; Chen, Q. Strain distribution of repaired articular cartilage defects by tissue engineering under compression loading. J. Orthop. Surg. Res. 2018, 13, 19. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Black, C.; Dawson, J.; Gibbs, D.; Kanczler, J.; Oreffo, R.O.C.; Tozzi, G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials (Basel) 2020, 13, 168. [Google Scholar] [CrossRef]
- Tozzi, G.; Danesi, V.; Palanca, M.; Cristofolini, L. Elastic Full-Field Strain Analysis and Microdamage Progression in the Vertebral Body from Digital Volume Correlation. Strain 2016, 52, 446–455. [Google Scholar] [CrossRef]
- Danesi, V.; Tozzi, G.; Cristofolini, L. Application of digital volume correlation to study the efficacy of prophylactic vertebral augmentation. Clin. Biomech. 2016, 39, 14–24. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Full-Field Strain Analysis of Bone–Biomaterial Systems Produced by the Implantation of Osteoregenerative Biomaterials in an Ovine Model. ACS Biomater. Sci. Eng. 2019, 5, 2543–2554. [Google Scholar] [CrossRef]
- Tozzi, G.; Zhang, Q.H.; Tong, J. Microdamage assessment of bone-cement interfaces under monotonic and cyclic compression. J. Biomech. 2014, 47, 3466–3474. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Ara, E.D.; Kao, A.P.; Bodey, A.J.; Karali, A.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Preservation of bone tissue integrity with temperature control for in situ SR-microCT experiments. Materials (Basel) 2018, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Palanca, M.; Tozzi, G.; Cristofolini, L.; Viceconti, M.; Dall’Ara, E. Three-Dimensional Local Measurements of Bone Strain and Displacement: Comparison of Three Digital Volume Correlation Approaches. J. Biomech. Eng. 2015, 137, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ara, E.; Peña-Fernández, M.; Palanca, M.; Giorgi, M.; Cristofolini, L.; Tozzi, G. Precision of Digital Volume Correlation Approaches for Strain Analysis in Bone Imaged with Micro-Computed Tomography at Different Dimensional Levels. Front. Mater. 2017, 4. [Google Scholar] [CrossRef]
- Comini, F.; Palanca, M.; Cristofolini, L.; Dall’Ara, E. Uncertainties of synchrotron microCT-based digital volume correlation bone strain measurements under simulated deformation. J. Biomech. 2019, 86, 232–237. [Google Scholar] [CrossRef]
- Palanca, M.; Bodey, A.J.; Giorgi, M.; Viceconti, M.; Lacroix, D.; Cristofolini, L.; Dall’Ara, E. Local displacement and strain uncertainties in different bone types by digital volume correlation of synchrotron microtomograms. J. Biomech. 2017, 58, 27–36. [Google Scholar] [CrossRef]
- Ylitalo, T.; Finnilä, M.A.J.; Gahunia, H.K.; Karhula, S.S.; Suhonen, H.; Valkealahti, M.; Lehenkari, P.; Hæggström, E.; Pritzker, K.P.H.; Saarakkala, S.; et al. Quantifying Complex Micro-Topography of Degenerated Articular Cartilage Surface by Contrast-Enhanced Micro-Computed Tomography and Parametric Analyses. J. Orthop. Res. 2019, 37, 855–866. [Google Scholar] [CrossRef]
- Clark, J.N.; Garbout, A.; Ferreira, S.A.; Javaheri, B.; Andrew, A.; Rankin, S.M.; Jeffers, J.R.T.; Hansen, U. Propagation phase-contrast micro-computed tomography allows laboratory-based three-dimensional imaging of articular cartilage down to the cellular level. Osteoarthr. Cartil. 2020, 28, 102–111. [Google Scholar] [CrossRef]
- de Bournonville, S.; Vangrunderbeeck, S.; Ly, H.G.T.; Geeroms, C.; De Borggraeve, W.M.; Parac-Vogt, T.N.; Kerckhofs, G. Exploring polyoxometalates as non-destructive staining agents for contrast-enhanced microfocus computed tomography of biological tissues. Acta Biomater. 2020, 105, 253–262. [Google Scholar] [CrossRef]
- West, M.J. Tissue Shrinkage and Stereological Studies. Cold Spring Harb. Protoc. 2013, 2013. [Google Scholar] [CrossRef]
- Degenhardt, K.; Wright, A.C.; Horng, D.; Padmanabhan, A.; Epstein, J.A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ. Cardiovasc. Imaging 2010, 3, 314–322. [Google Scholar] [CrossRef]
- Vickerton, P.; Jarvis, J.; Jeffery, N. Concentration-dependent specimen shrinkage in iodine-enhanced microCT. J. Anat. 2013, 223, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, J.; Goyens, J.; De Greef, D.; Aerts, P.; Dirckx, J. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-CT and light sheet fluorescence microscopy (LSFM). Microsc. Microanal. 2014, 20, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.M.R.; Carr, A.J.; Czernuszka, J.T. Atomic Force Microscopy of bulk tendon samples: Affect of location and fixation on tissue ultrastructure. Micron 2011, 42, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, M.B.; Coan, P.; Huber, M.B.; Diemoz, P.C.; Sciences, I.; States, U.; Facility, S.R. Phase contrast imaging X-ray computed tomography: Quantitative characterization of human patellar cartilage matrix with topological and geometrical features. SPIE Int. Soc. Opt. Eng. 2014, 9038, 903811. [Google Scholar]
- Coan, P.; Bamberg, F.; Diemoz, P.; Bravin, A.; Timpert, K.; Mützel, E.; Raya, J.; Adam-Neumair, A.; Reiser, M.; Glaser, C. Characterization of Osteoarthritic and Normal Human Patella Cartilage by Computed Tomography X-ray Phase-Contrast Imaging: A Feasibility Study. Investig. Radiol. 2010, 45, 437–444. [Google Scholar] [CrossRef]
- Disney, C.M.; Eckersley, A.; McConnell, J.C.; Geng, H.; Bodey, A.J.; Hoyland, J.A.; Lee, P.D.; Sherratt, M.J.; Bay, B.K. Synchrotron tomography of intervertebral disc deformation quantified by digital volume correlation reveals microstructural influence on strain patterns. Acta Biomater. 2019, 92, 290–304. [Google Scholar] [CrossRef]
- Madi, K.; Staines, K.A.; Bay, B.K.; Javaheri, B.; Geng, H.; Bodey, A.J.; Cartmell, S.; Pitsillides, A.A.; Lee, P.D. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat. Biomed. Eng. 2020, 4, 343–354. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Cipiccia, S.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Pani, M.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Effect of SR-microCT radiation on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef]
- Barth, H.D.; Launey, M.E.; MacDowell, A.A.; Ager, J.W.; Ritchie, R.O. On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 2010, 46, 1475–1485. [Google Scholar] [CrossRef]
- Cicek, E. Effect of X-ray irradiation on articular cartilage mechanical properties. Acta Phys. Pol. A 2016, 129, 200–202. [Google Scholar] [CrossRef]
- Lindburg, C.A.; Willey, J.S.; Dean, D. Effects of low dose X-ray irradiation on porcine articular cartilage explants. J. Orthop. Res. 2013, 31, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jasiuk, I. Effects of freeze-thaw and micro-computed tomography irradiation on structure-property relations of porcine trabecular bone. J. Biomech. 2014, 47, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Katsamenis, O.L.; Olding, M.; Warner, J.A.; Chatelet, D.S.; Jones, M.G.; Sgalla, G.; Smit, B.; Larkin, O.J.; Haig, I.; Richeldi, L.; et al. X-ray Micro-Computed Tomography for Nondestructive Three-Dimensional (3D) X-ray Histology. Am. J. Pathol. 2019, 189, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Bidola, P.; Morgan, K.; Williner, M.; Fehringer, A.; Allner, S.; Prade, F.; Pfeiffer, F.; Achterhold, K. Application of sensitive, high-resolution imaging at a commercial lab-based X-ray micro-CT system using propagation-based phase retrieval. J. Microsc. 2017, 266, 211–220. [Google Scholar] [CrossRef]
- Ruan, M.Z.C.; Dawson, B.; Jiang, M.M.; Gannon, F.; Heggeness, M.; Lee, B.H.L. Quantitative imaging of murine osteoarthritic cartilage by phase-contrast micro-computed tomography. Arthritis Rheum. 2013, 65, 388–396. [Google Scholar] [CrossRef]
- Paterson, S.I.; Amin, A.K.; Hall, A.C. Airflow accelerates bovine and human articular cartilage drying and chondrocyte death. Osteoarthr. Cartil. 2015, 23, 257–265. [Google Scholar] [CrossRef][Green Version]
- Boettcher, K.; Kienle, S.; Nachtsheim, J.; Burgkart, R.; Hugel, T.; Lieleg, O. The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater. 2016, 29, 180–187. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Santos, S.; Emery, N.; Neu, C.P.; Pierce, D.M. Propagation of microcracks in collagen networks of cartilage under mechanical loads. Osteoarthr. Cartil. 2019, 27, 1392–1402. [Google Scholar] [CrossRef]
- Nesterets, Y.I.; Wilkins, S.W.; Gureyev, T.E.; Pogany, A.; Stevenson, A.W. On the optimization of experimental parameters for x-ray in-line phase-contrast imaging. Rev. Sci. Instrum. 2005, 76, 093706. [Google Scholar] [CrossRef]
- Schofield, R.; King, L.; Tayal, U.; Castellano, I.; Stirrup, J.; Pontana, F.; Earls, J.; Nicol, E. Image reconstruction: Part 1–understanding filtered back projection, noise and image acquisition. J. Cardiovasc. Comput. Tomogr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Studholme, C.; Hill, D.L.G.; Hawkes, D.J. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit. 1999, 32, 71–86. [Google Scholar] [CrossRef]
- Peña-Fernández, M.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Optimization of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Javed, M.; Otto, I.A.; Combellack, E.J.; Morgan, S.; Breugem, C.C.; Archer, C.W.; Khan, I.M.; Lineaweaver, W.C.; Kon, M.; et al. Combining regenerative medicine strategies to provide durable reconstructive options: Auricular cartilage tissue engineering. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Abidin, A.Z.; D’Souza, A.M.; Nagarajan, M.B.; Coan, P.; Wismüller, A. Characterizing cartilage microarchitecture on phase-contrast x-ray computed tomography using deep learning with convolutional neural networks. Med. Imaging 2017 Comput. Diagn. 2017, 10134, 1013412. [Google Scholar]
- Barker, M.K.; Seedhom, B.B. The relationship of the compressive modulus of articular cartilage with its deformation response to cyclic loading: Does cartilage optimize its modulus so as to minimize the strains arising in it due to the prevalent loading regime? Rheumatology 2001, 40, 274–284. [Google Scholar] [CrossRef]
- Palanca, M.; Cristofolini, L.; Dall’Ara, E.; Curto, M.; Innocente, F.; Danesi, V.; Tozzi, G. Digital volume correlation can be used to estimate local strains in natural and augmented vertebrae: An organ-level study. J. Biomech. 2016, 49, 3882–3890. [Google Scholar] [CrossRef]
- Narmoneva, D.A.; Wang, J.Y.; Setton, L.A. Nonuniform swelling-induced residual strains in articular cartilage. J. Biomech. 1999, 32, 401–408. [Google Scholar] [CrossRef]
- Cristofolini, L. In vitro evidence of the structural optimization of the human skeletal bones. J. Biomech. 2015, 48, 787–796. [Google Scholar] [CrossRef]


| Specimen | Position | SOD (mm) | ODD (mm) | Voxel Size (µm) | Exposure Time (s) |
|---|---|---|---|---|---|
| CB1 | ABS | 8.5 | 20 | 2.02 | 2 |
| 3× | 25.6 | 60 | 2.02 | 20 | |
| 4× | 34.2 | 80 | 2.02 | 30 | |
| CB2-4 | ABS | 8.5 | 20 | 2.56 | 2 |
| 2× | 17 | 40 | 2.56 | 7.5 | |
| 4× | 34.1 | 80 | 2.56 | 30 | |
| CB5 a | 4× | 44.4 | 104 | 2.03 | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozzi, G.; Peña Fernández, M.; Davis, S.; Karali, A.; Kao, A.P.; Blunn, G. Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials 2020, 13, 2579. https://doi.org/10.3390/ma13112579
Tozzi G, Peña Fernández M, Davis S, Karali A, Kao AP, Blunn G. Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials. 2020; 13(11):2579. https://doi.org/10.3390/ma13112579
Chicago/Turabian StyleTozzi, Gianluca, Marta Peña Fernández, Sarah Davis, Aikaterina Karali, Alexander Peter Kao, and Gordon Blunn. 2020. "Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation" Materials 13, no. 11: 2579. https://doi.org/10.3390/ma13112579
APA StyleTozzi, G., Peña Fernández, M., Davis, S., Karali, A., Kao, A. P., & Blunn, G. (2020). Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials, 13(11), 2579. https://doi.org/10.3390/ma13112579







