Long-Term Behavior and Durability of Alkali-Activated Clay Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mortars Design
2.2. Methods
3. Experimental Results
3.1. Physical Properties of the Mortars
3.2. Mechanical Properties of Mortars
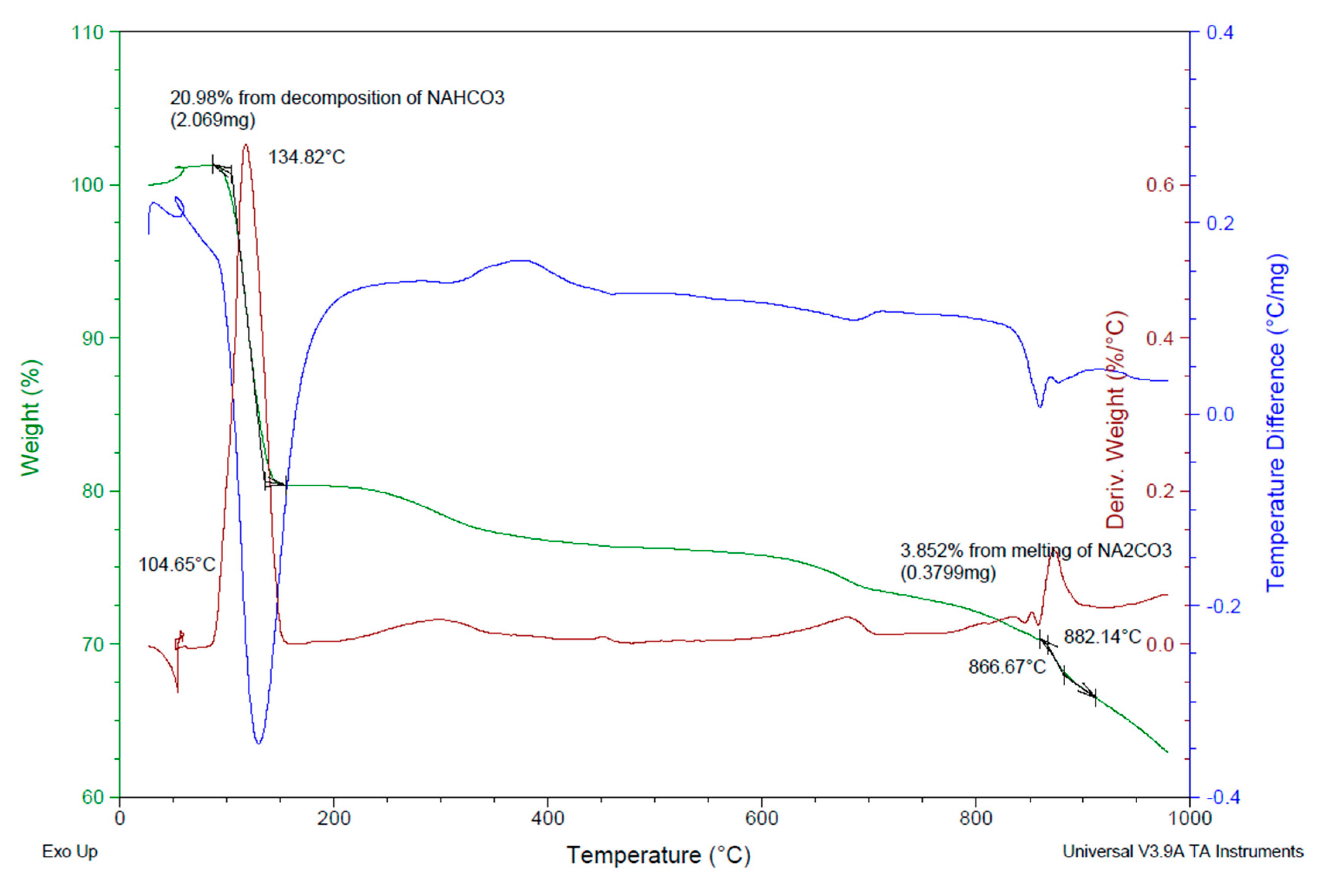
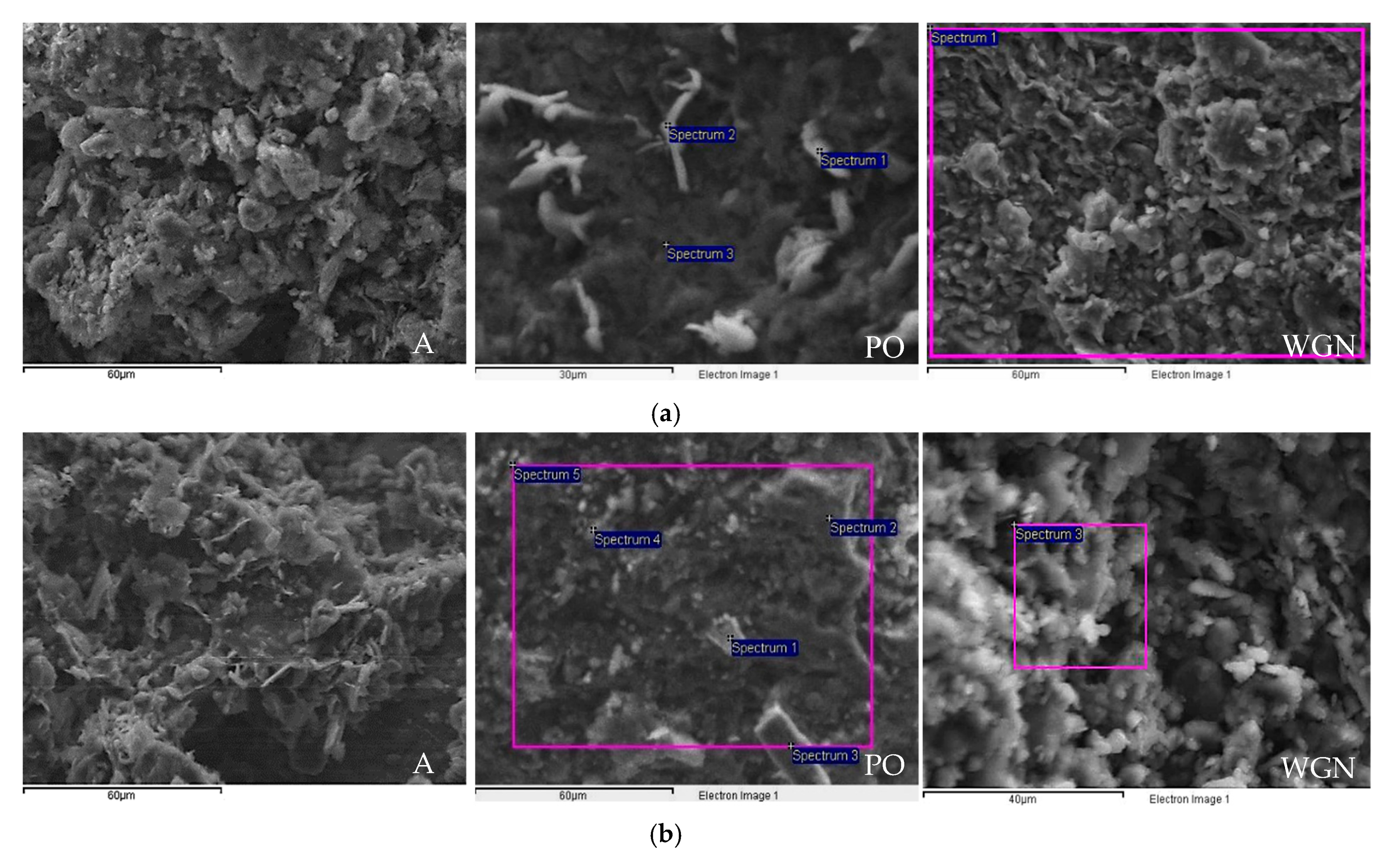
3.3. Durability Properties and Microscopic and Stereoscopic Observation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galán-Marín, C.; Rivera-Gómez, C.; Petric, J. Clay-based composite stabilized with natural polymer and fibre. Constr. Build. Mater. 2010, 24, 1462–1468. [Google Scholar] [CrossRef]
- Bui, Q.B.; Morel, J.C.; Hans, S.; Meunier, N. Compression behaviour of non-industrial materials in civil engineering by three scale experiments: The case of rammed earth. Mater. Struct./Mater. Et Constr. 2009, 42, 1101–1116. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S. (Eds.) Alkali Activated Materials, State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789400776715. [Google Scholar]
- Sameh, S.H. Promoting earth architecture as a sustainable construction technique in Egypt. J. Clean. Prod. 2014, 65, 362–373. [Google Scholar] [CrossRef]
- Faqir, N.M.; Shawabkeh, R.; Al-Harthi, M.; Wahhab, H.A. Fabrication of Geopolymers from Untreated Kaolin Clay for Construction Purposes. Geotech. Geol. Eng. 2019, 37, 129–137. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2017, 114, 40–48. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Heah, C.Y. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Papayianni, I.; Kesikidou, F.; Konopisi, S. Alkali Activation of HCFA Mixtures with Aluminosilicate Additives-Mechanical Characteristics. Key Eng. Mater. 2018, 761, 96–99. [Google Scholar] [CrossRef]
- Papayianni, I.; Konopissi, S. Study on Geopolymerization of Highlime Fly Ashes, Concrete in the Low Carbon Era. In Proceedings of the International Conferenceheld, Scotland, UK, 9–11 July 2012. [Google Scholar]
- Giannopoulou, I.; Panias, D. Structure, design and applications of geopolymeric materials. In Proceedings of the 3rd International Conference on Deformation Processing and Structure of Materials, Belgrade, Serbia, 20–22 September 2007; pp. 5–15. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2017, 103, 21–34. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers – Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Rodríguez, E.; Bernal, S.; Mejía De Gutiérrez, R.; Puertas, F. Alternative concrete based on alkali-activated slag. Mater. Constr. 2008, 58, 53–67. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejía De Gutiérrez, R.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Wongpa, J.; Kiattikomol, K.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength, modulus of elasticity, and water permeability of inorganic polymer concrete. Mater. Des. 2010, 31, 4748–4754. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Janssen, D.J.; Snyder, M.B. Resistance of Concrete to Freezing and Thawing; National Sand and Gravel Association: Washington, DC, USA, 1994; ISBN 0309057736. [Google Scholar]
- Valenza, J.J.; Scherer, G.W. Mechanism for salt scaling. J. Am. Ceram. Soc. 2006, 89, 1161–1179. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D.M. Alkali-Activated Cements and Concretes; Taylor & Francis: Abingdon-on-Thames, UK, 2006. [Google Scholar]
- Gifford, P.M.; Gillott, J.E. Freeze–thaw durability of activated blast furnace slag cement concrete. Mater. J. 1996, 93, 242–245. [Google Scholar]
- Guo, J.J.; Wang, K.; Guo, T.; Yang, Z.Y.; Zhang, P. Effect of dry-wet ratio on properties of concrete under sulfate attack. Materials 2019, 12. [Google Scholar] [CrossRef]
- Puertas, F.; Amat, T.; Fernández-Jiménez, A.; Vázquez, T. Mechanical and durable behaviour of alkaline cement mortars reinforced with polypropylene fibres. Cem. Concr. Res. 2003, 33, 2031–2036. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H. Potential application of geopolymers as protection coatings for marine concrete I. Basic properties. Appl. Clay Sci. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Hakkinen, T. The influence of slag content on the microstructure, permeability and mechanical properties of concrete, Part 2 Technical properties and theoretical examinations. Cem. Concr. Res. 1993, 23, 518–530. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Sanchez, F.; Kosson, D.S. Leaching model for a cement mortar exposed to intermittent wetting and drying. Aiche J. 2003, 49, 1317–1333. [Google Scholar] [CrossRef]
- Karozou, A.; Konopisi, S.; Paulidou, E.; Stefanidou, M. Alkali activated clay mortars with different activators. Constr. Build. Mater. 2019, 212, 85–91. [Google Scholar] [CrossRef]
- ASTM International. ASTM C188-95, Standard Test Method for Density of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2016; pp. 1–2. [Google Scholar] [CrossRef]
- ASTM International. ASTM D248—11 Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM International: West Conshohocken, PA, USA, 2006; pp. 1–5. [Google Scholar] [CrossRef]
- Nicolas, R.S.; Provis, J.L.; Bernal, S.A.; van Deventer, J.S.J. Alkali-activated slag cements produced with a blended sodium carbonate/sodium silicate activator. Adv. Cem. Res. 2015, 1–12. [Google Scholar] [CrossRef]
- Steudel, A.; Mehl, D.; Emmerich, K. Simultaneous thermal analysis of different bentonite–sodium carbonate systems: An attempt to distinguish alkali-activated bentonites from raw materials. Clay Miner. 2013, 48, 117–128. [Google Scholar] [CrossRef]
- Stefanidou, M. Methods for porosity measurement in lime-based mortars. Constr. Build. Mater. 2010, 24, 2572–2578. [Google Scholar] [CrossRef]
- Liliana Sofia, P.N. Drying Index as an In-Service Parameter of Renders Applied on Exterior Facades Supervisor: Prof a Inês dos Santos Flores Barbosa Colen, Co-supervisor: Engº LuísMiguel Cardoso da Silva October 2012 2. Performance of Renders Facing the Water. 2012. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/395144992891/extended%20abstract.pdf (accessed on 22 July 2020).
- EVS EN 16322-13 Conservation of Cultural Heritage—Test Methods—Determination of Drying Properties; European Standard: Brussels, Belgium, 2013.
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Van Deventer, J.S.J. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Grilo, J.; Faria, P.; Veiga, R.; Santos Silva, A.; Silva, V.; Velosa, A. New natural hydraulic lime mortars—Physical and microstructural properties in different curing conditions. Constr. Build. Mater. 2014. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Earth Plasters—Terms and Definitions, Requirements, Test Methods; DIN 18947; Deutsche Norm: Berlin, Germany, 2013.
- Papayianni, I.; Stefanidou, M. Microstructural Analysis of Old Mortars of Byzantine Period. In Proceedings of the International Conference STREMAH, Bologna, Italy, 28–30 May 2001; pp. 45–52. [Google Scholar]
- Eires, R.; Camões, A.; Jalali, S. Enhancing water resistance of earthen buildings with quicklime and oil. J. Clean. Prod. 2017, 142, 3281–3292. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Darmawan, S.; Wang, J. Impact of field conditions on the strength development of a geopolymer stabilized marine clay. Appl. Clay Sci. 2019, 167, 33–42. [Google Scholar] [CrossRef]
- Slaty, F.; Khoury, H.; Wastiels, J.; Rahier, H. Characterization of alkali activated kaolinitic clay. Appl. Clay Sci. 2013, 75–76, 120–125. [Google Scholar] [CrossRef]
- Edward, G. Nawy Concrete Construction Engineering Handbook; CRC Press: New York, NY, USA, 2008; ISBN 978-0-8493-7492-0. [Google Scholar]
- Liptay, G. Atlas of Thermoanalytical Curves: (TG-, DTG-, DTA-Curves Measured Simultaneously); Erdey, L., Paulik, F., Paulik, J., Eds.; Heyden and Son: London, UK; New York, NY, USA, 1971; ISBN 0855010525. [Google Scholar]
- Davidovits, J. Geopolymer: Chemistry & Applications; Geopolymer Institute: Saint-Quentin, France, 2011; ISBN 9782951482098. [Google Scholar]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
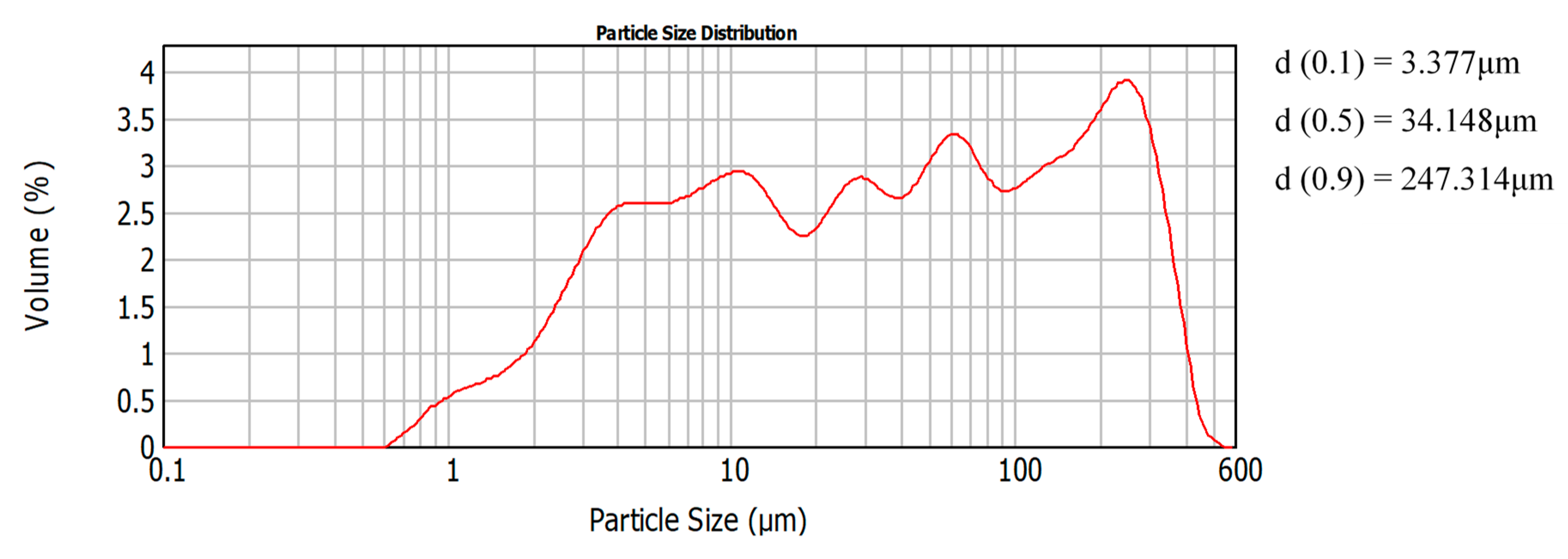
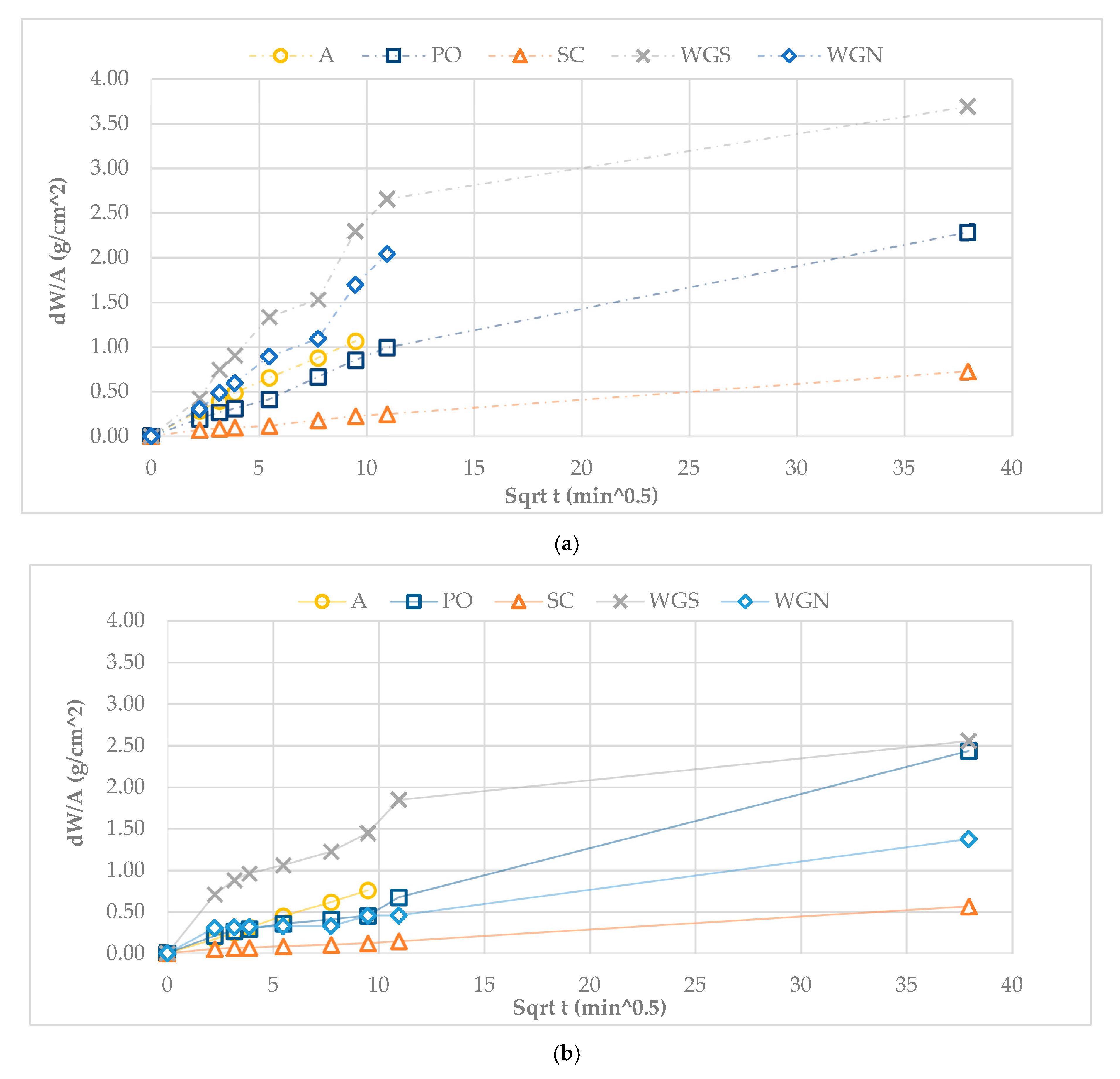
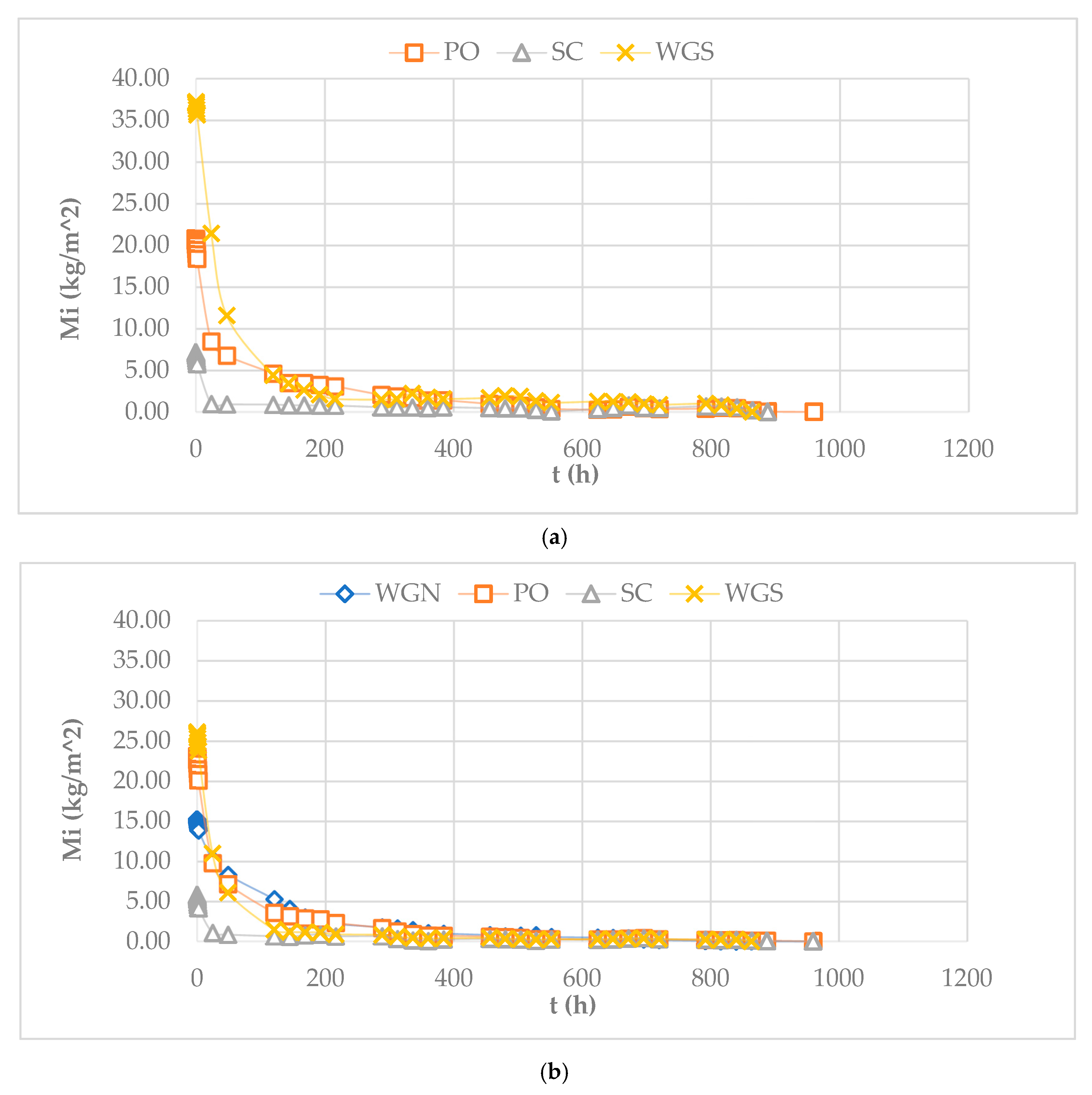
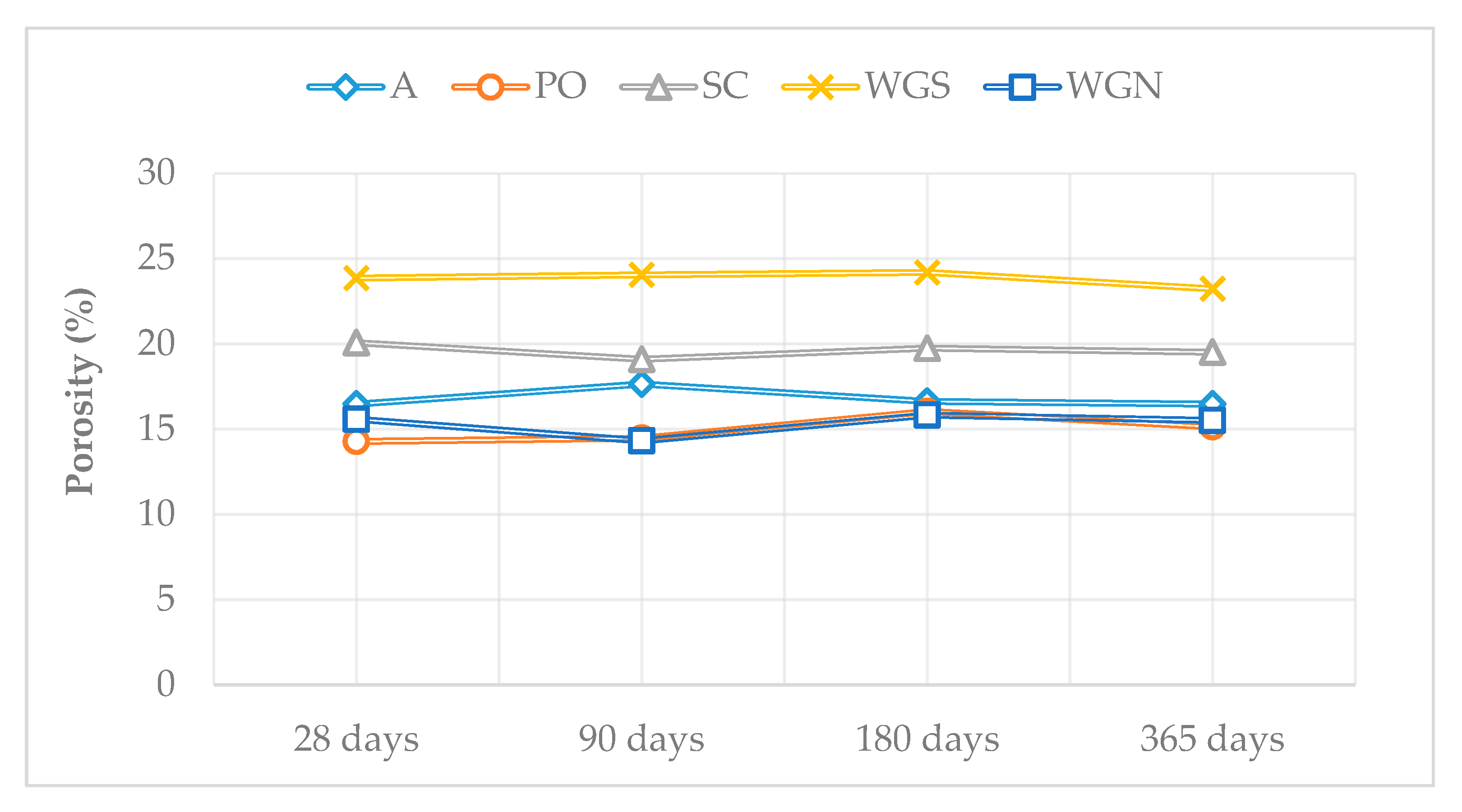


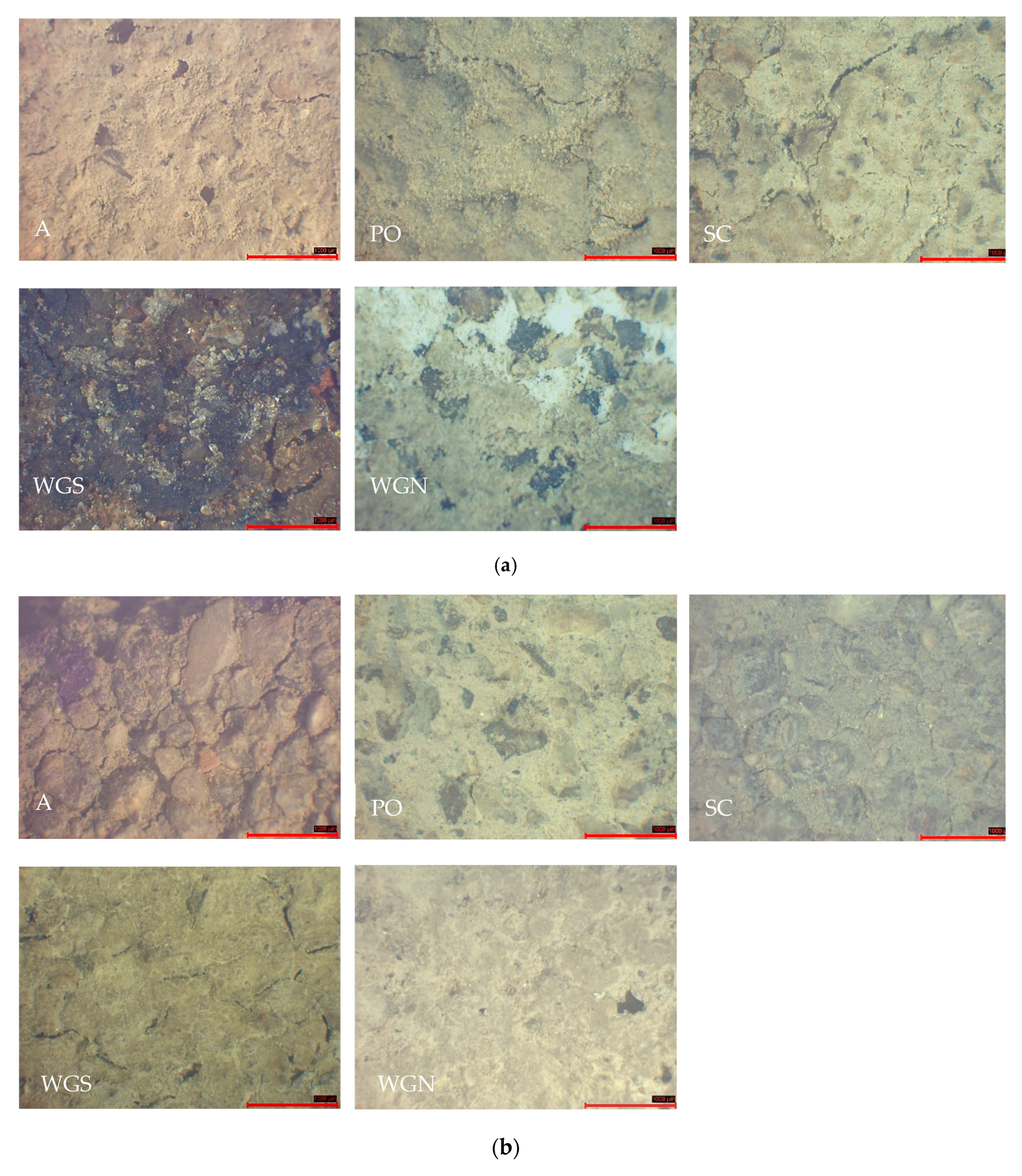
| Mortar | Binder/Aggregate | Activator | L/S 1 | Workability [mm] |
|---|---|---|---|---|
| A | 1:2.5 | no activator | 0.65 | 155 |
| PO | 1:2.5 | potassium metasilicate | 0.62 | 150 |
| SC | 1:2.5 | sodium carbonate solution | 0.71 | 150 |
| WGS | 1:2.5 | water–glass | 0.95 | 155 |
| WGN | 1:2.5 | water–glass with sodium hydroxide solution | 0.69 | 155 |
| Mortar | Capillary Coefficient [g/cm2·min1/2] | Drying Index (ID) | Porosity [%] | |||
|---|---|---|---|---|---|---|
| 180 days | 365 days | 180 days | 365 days | 180 days | 365 days | |
| A | 0.120 | 0.082 | – | – | 16.63 | 16.47 |
| PO | 0.075 | 0.065 | 0.095 | 0.075 | 16.04 | 15.14 |
| SC | 0.021 | 0.015 | 0.090 | 0.072 | 19.74 | 19.51 |
| WGS | 0.244 | 0.194 | 0.087 | 0.056 | 24.19 | 23.23 |
| WGN | 0.163 | 0.059 | – | 0.123 | 15.83 | 15.51 |
| Mortar | Water Penetration [mL/min·cm2] | Linear Shrinkage [%] | Volume Loss [%] | |||
|---|---|---|---|---|---|---|
| 90 days | 365 days | 180 days | 365 days | 180 days | 365 days | |
| A | 0.053 | 0.035 | 1.48 | 1.92 | 10.82 | 12.93 |
| PO | 0.139 | 0.076 | 0.60 | 0.64 | 2.31 | 2.61 |
| SC | 0.014 | 0.023 | 1.70 | 1.97 | 20.14 | 21.40 |
| WGS | 0.377 | 0.728 | 1.14 | 1.21 | 10.22 | 11.11 |
| WGN | 0.897 | 0.413 | 1.05 | 1.10 | 4.05 | 4.64 |
| Mortar | Compressive Strength [MPa] | Flexural Strength [MPa] | ||||||
|---|---|---|---|---|---|---|---|---|
| 180 days | 365 days | 180 days | 365 days | |||||
| – | – | st.dev | – | st.dev | – | st.dev | st.dev | |
| A | 1.28 | 0.074 | 1.52 | 0.118 | 0.7 | 0.081 | 0.9 | 0.026 |
| PO | 5.12 | 0.690 | 6.13 | 0.548 | 1.84 | 0.611 | 2.55 | 0.390 |
| SC | 1.06 | 0.050 | 1.08 | 0.055 | 0.49 | 0.375 | 0.5 | 0.041 |
| WGS | 0.85 | 0.036 | 1.43 | 0.154 | 0.38 | 0.007 | 0.59 | 0.042 |
| WGN | 1.66 | 0.723 | 4.5 | 0.444 | 1.06 | 0.462 | 1.26 | 0.219 |
| Mortar | Mass Change [%] | Compressive Strength [MPa] | Porosity [%] | |||
|---|---|---|---|---|---|---|
| Freeze–Thaw | Wet–Dry | Freeze–Thaw | Wet–Dry | Freeze–Thaw | Wet–Dry | |
| A | –1.16 | –2.56 | 0.97 | 1.38 | 15.42 | 14.82 |
| PO | 1.34 | –10.41 | 5.46 | 4.88 | 15.38 | 15.19 |
| SC | 0.11 | –0.01 | 0.62 | 1.10 | 19.17 | 19.33 |
| WGS | 0.66 | –0.31 | 0.43 | 0.71 | 23.37 | 24.58 |
| WGN | –0.27 | –3.27 | 2.25 | 2.41 | 15.24 | 15.13 |
| (1) PO (a) | |||||||
| Spectrum (Early Age) | Na | Al | Si | K | Ca | Fe | O |
| Spectrum 1 | 1.93 | 6.77 | 18.90 | 35.14 | –1.19 | 1.08 | 36.52 |
| Spectrum 2 | 4.14 | 7.63 | 22.01 | 23.46 | 2.49 | 0.58 | 39.52 |
| Spectrum 3 | 6.08 | 10.45 | 32.51 | 1.48 | 0.26 | 0.68 | 48.74 |
| PO (b) | |||||||
| Spectrum (365 days) | Na | Al | Si | K | Ca | Fe | O |
| Spectrum 1 | – | 10.27 | 19.84 | 5.2 | – | 3.14 | 56.26 |
| Spectrum 2 | 7.15 | 9.81 | 30.01 | – | 1.58 | – | 51.46 |
| Spectrum 3 | – | 5.23 | 25.21 | – | 4.88 | 3.72 | 51.24 |
| Spectrum 4 | 7.34 | 7.43 | 27.28 | 1.26 | – | – | 56.68 |
| Spectrum 5 | 5.05 | 8.33 | 23.43 | 3.96 | 1.91 | – | 57.31 |
| (2) WGN (a) and (b) | |||||||
| Spectrum | Na | Al | Si | K | Ca | Fe | O |
| Spectrum 1 (early age) | 9.01 | 4.28 | 19.57 | 1.50 | 4.00 | 1.66 | 59.10 |
| Spectrum 3 (365 days) | 1.54 | 5.57 | 19.49 | 0.83 | 3.70 | 2.25 | 60.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karozou, A.; Konopisi, S.; Pavlidou, E.; Stefanidou, M. Long-Term Behavior and Durability of Alkali-Activated Clay Mortars. Materials 2020, 13, 3790. https://doi.org/10.3390/ma13173790
Karozou A, Konopisi S, Pavlidou E, Stefanidou M. Long-Term Behavior and Durability of Alkali-Activated Clay Mortars. Materials. 2020; 13(17):3790. https://doi.org/10.3390/ma13173790
Chicago/Turabian StyleKarozou, Aspasia, Stavroula Konopisi, Eleni Pavlidou, and Maria Stefanidou. 2020. "Long-Term Behavior and Durability of Alkali-Activated Clay Mortars" Materials 13, no. 17: 3790. https://doi.org/10.3390/ma13173790
APA StyleKarozou, A., Konopisi, S., Pavlidou, E., & Stefanidou, M. (2020). Long-Term Behavior and Durability of Alkali-Activated Clay Mortars. Materials, 13(17), 3790. https://doi.org/10.3390/ma13173790






