Fluorescence Microscopy of Superplasticizers in Cementitious Systems: Applications and Challenges
Abstract
1. Introduction
2. Materials and Methods
2.1. Solids
2.2. Superplasticizers
2.3. Fluorescence Microscopy
2.4. Scanning Electron Microscopy
2.5. Calorimetry
2.6. Slump Test
3. Retardation of C3S-Hydration–Results and Discussion
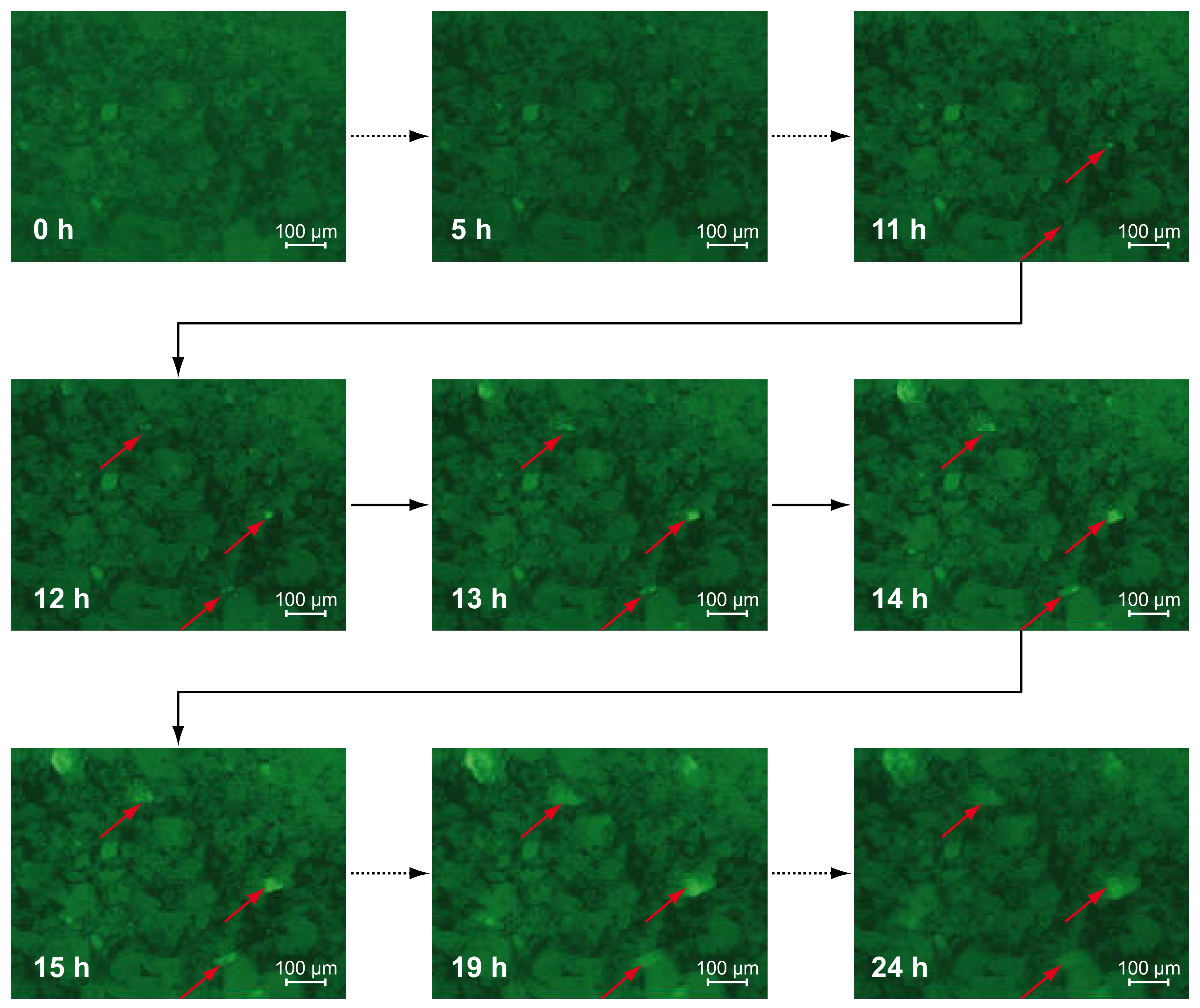
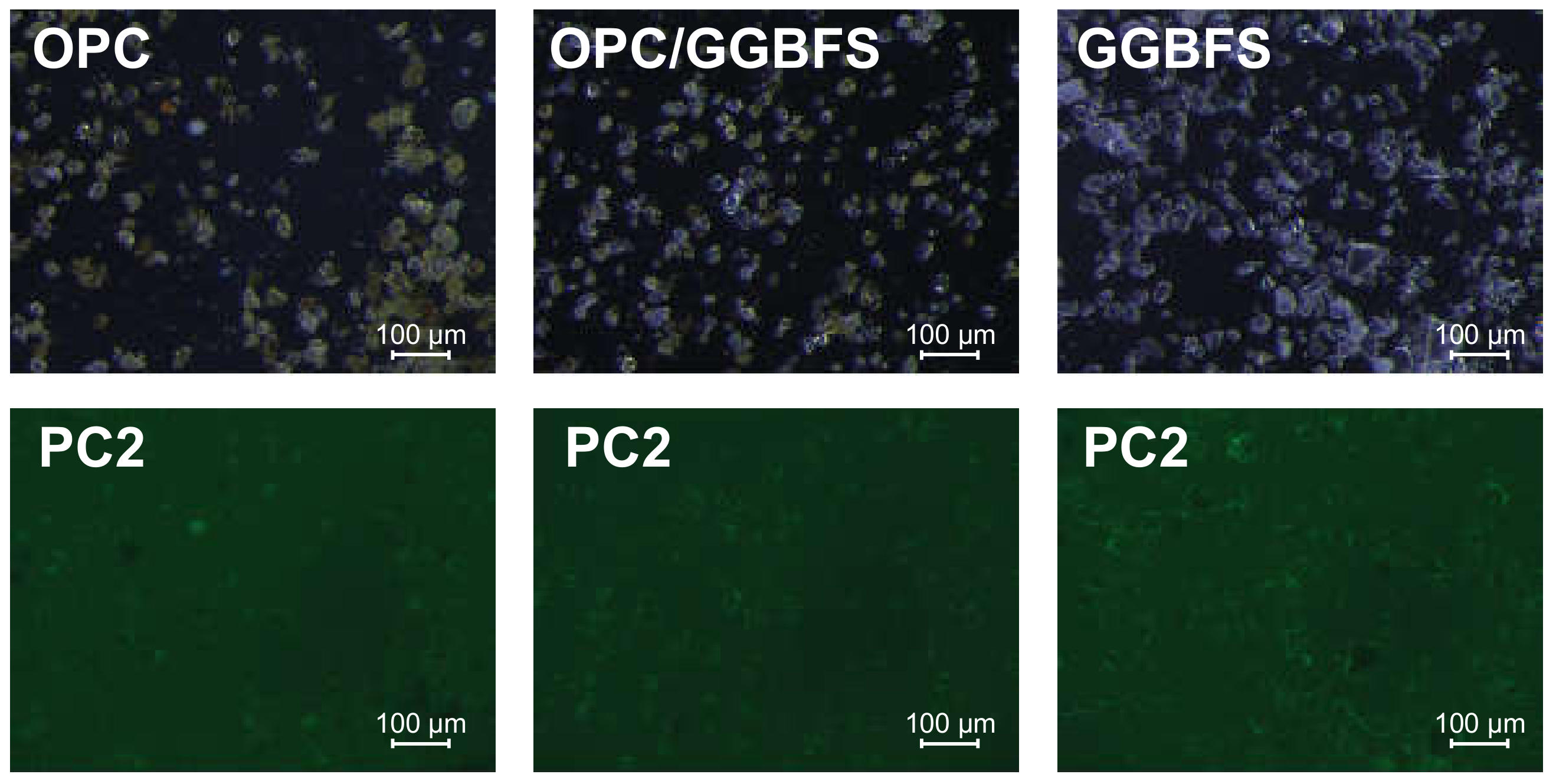
3.1. Fluorescence Microscopic Investigations
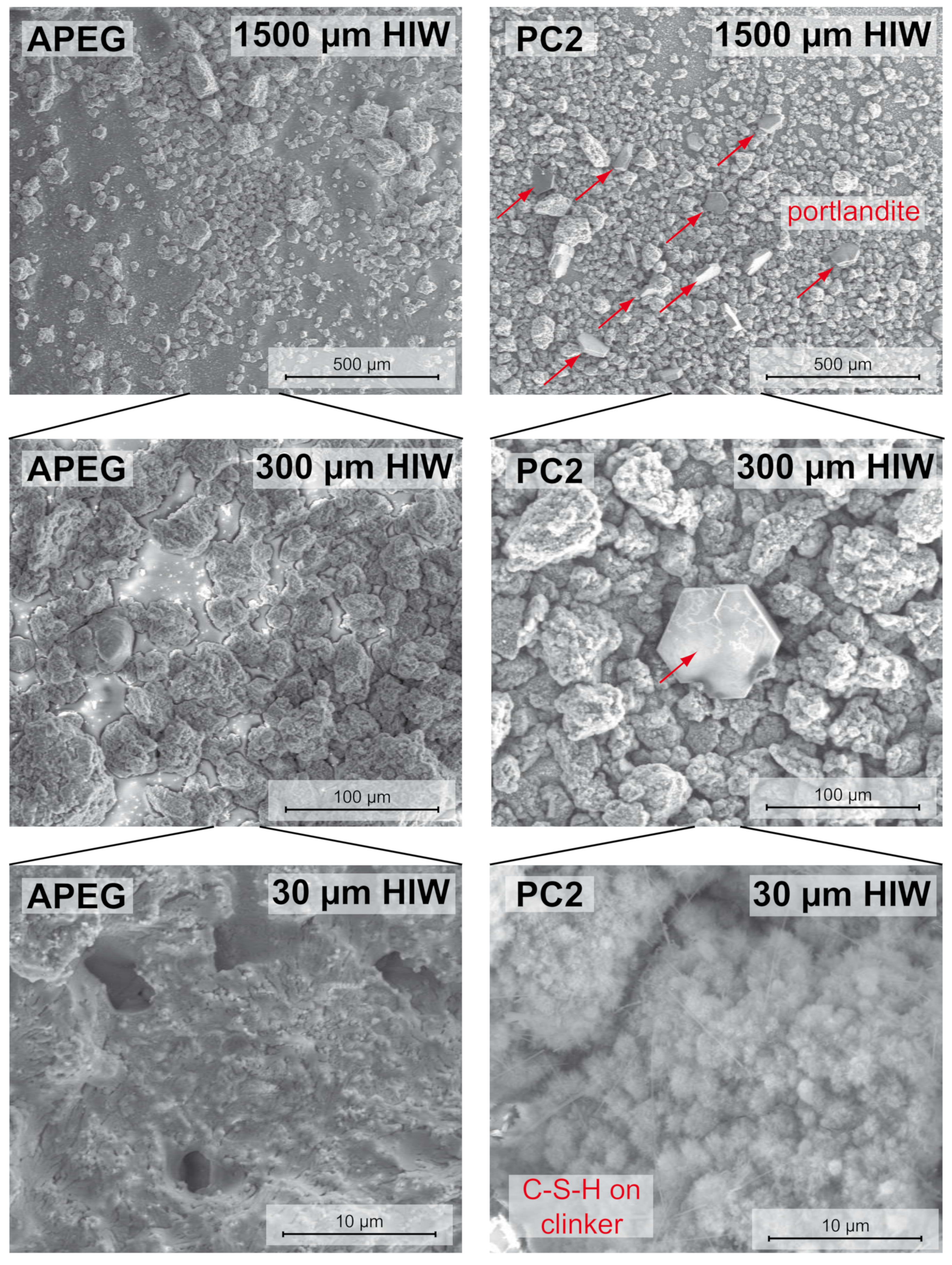
3.2. Scanning Electron Microscopic Investigations
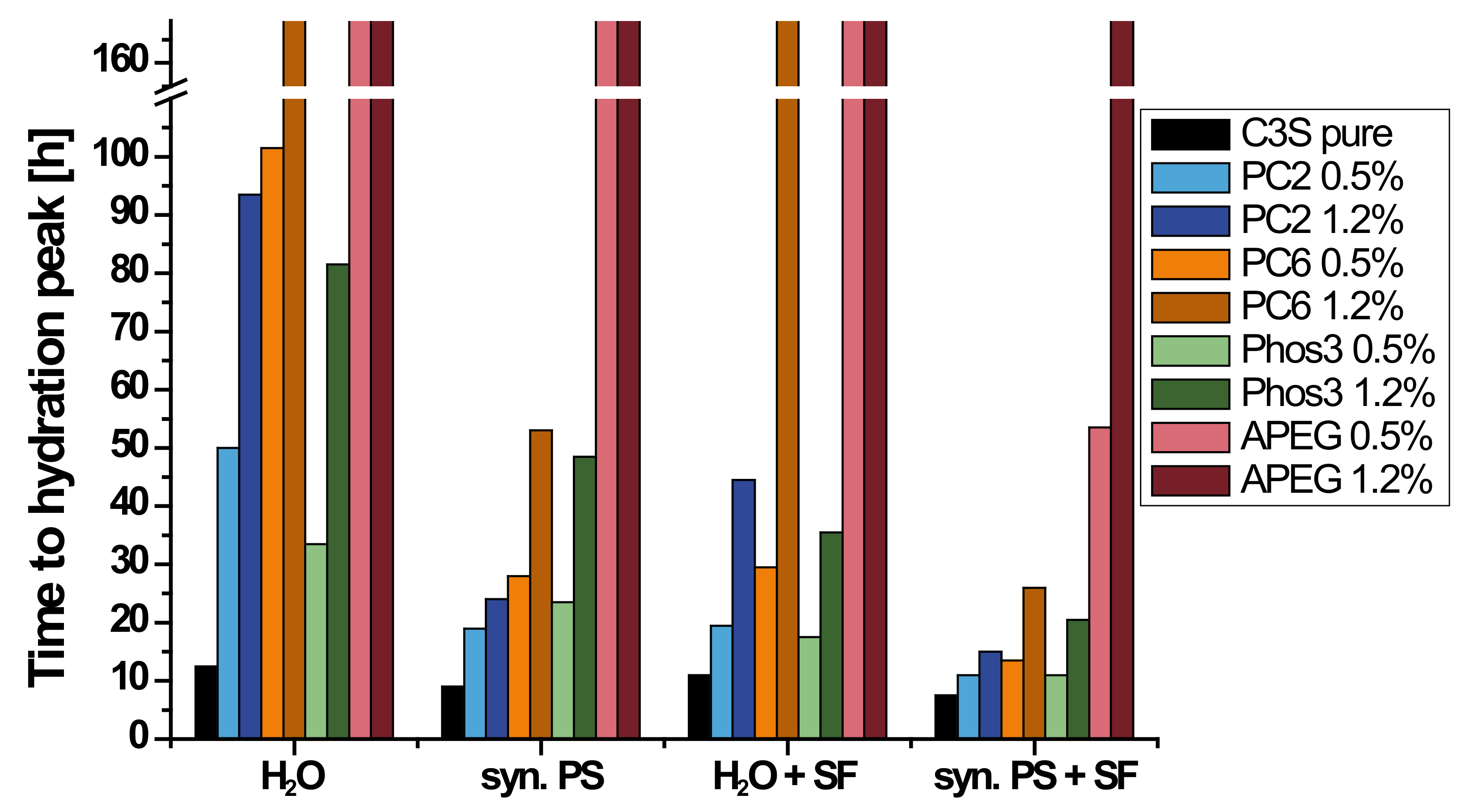
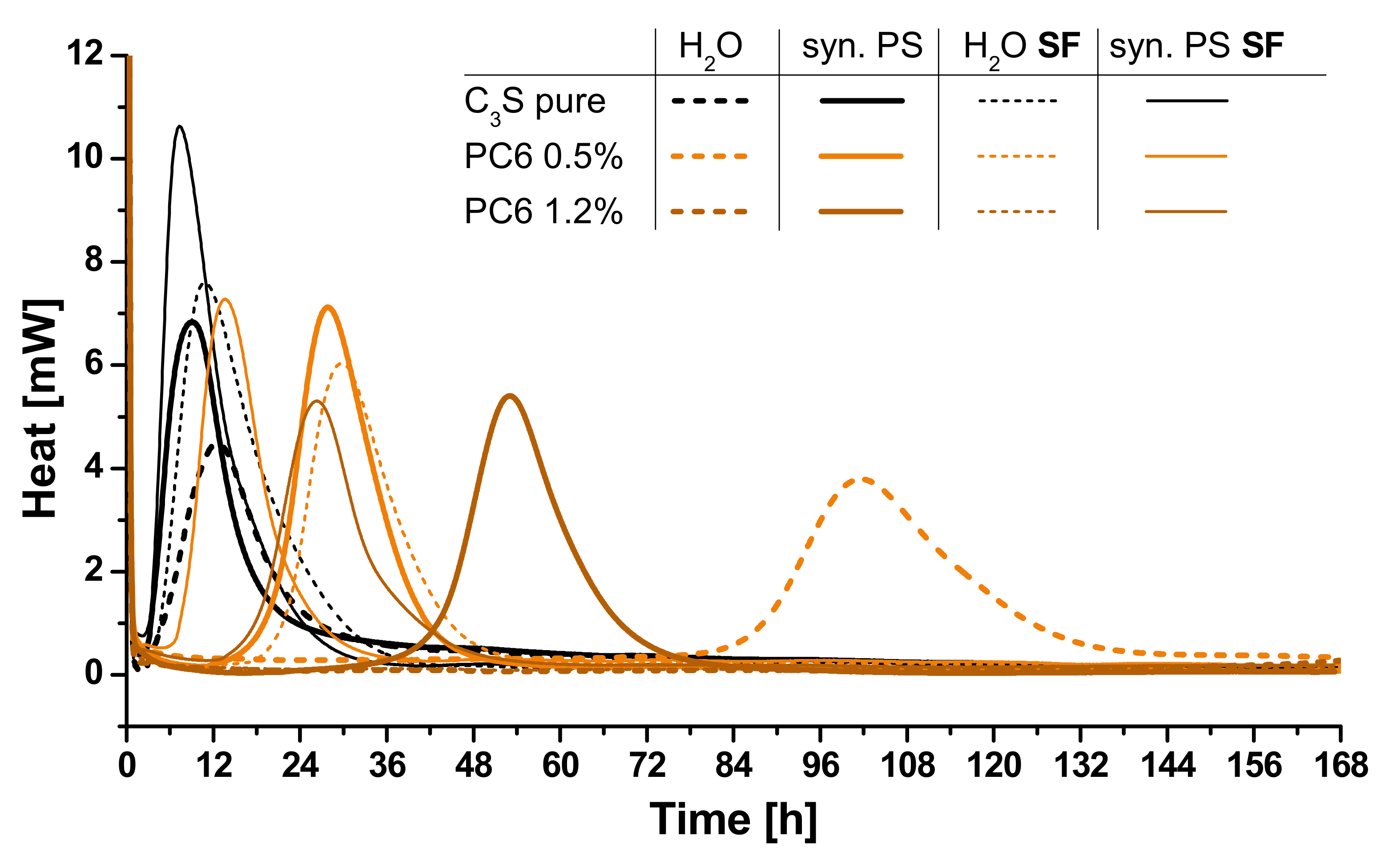
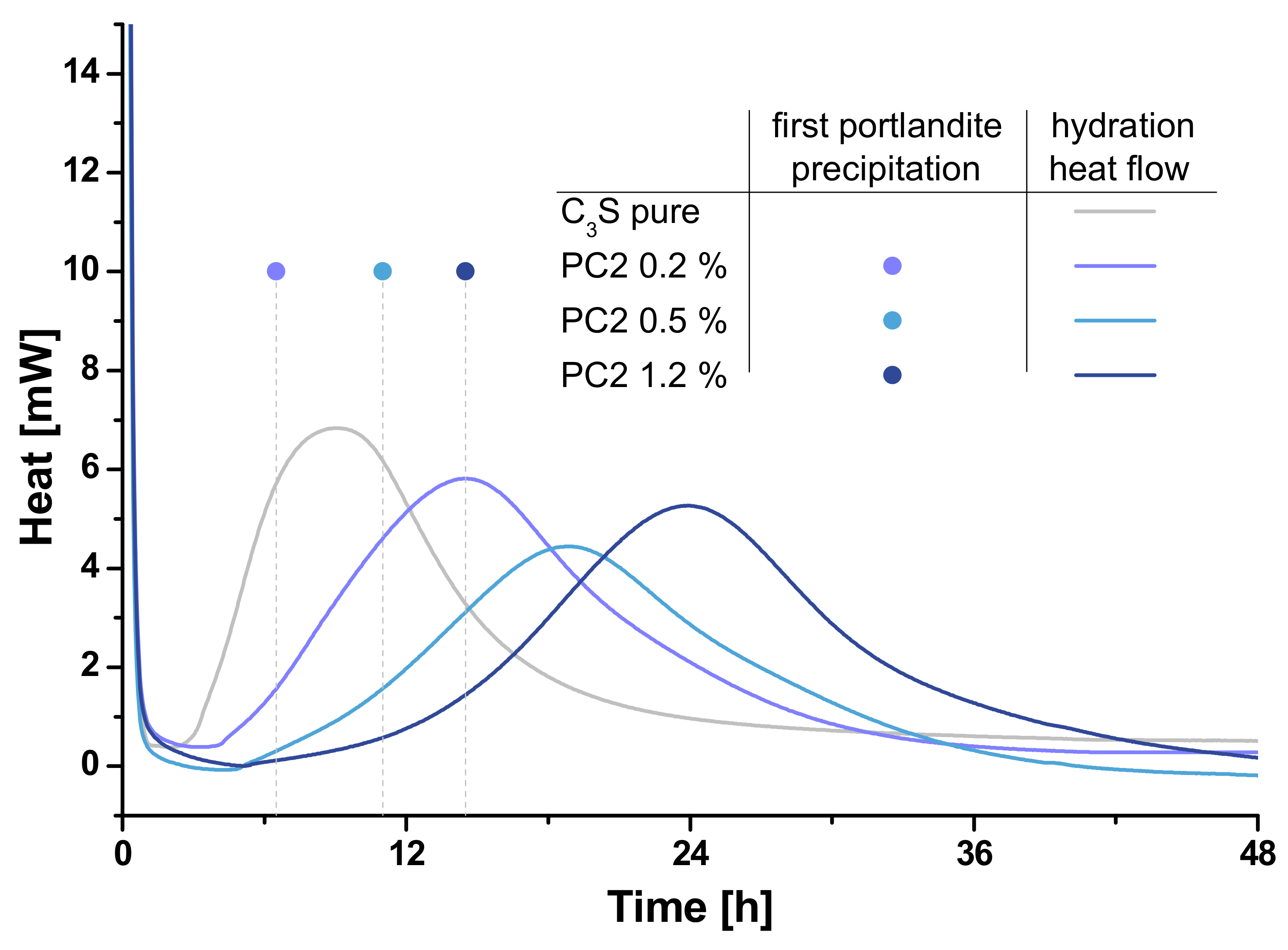
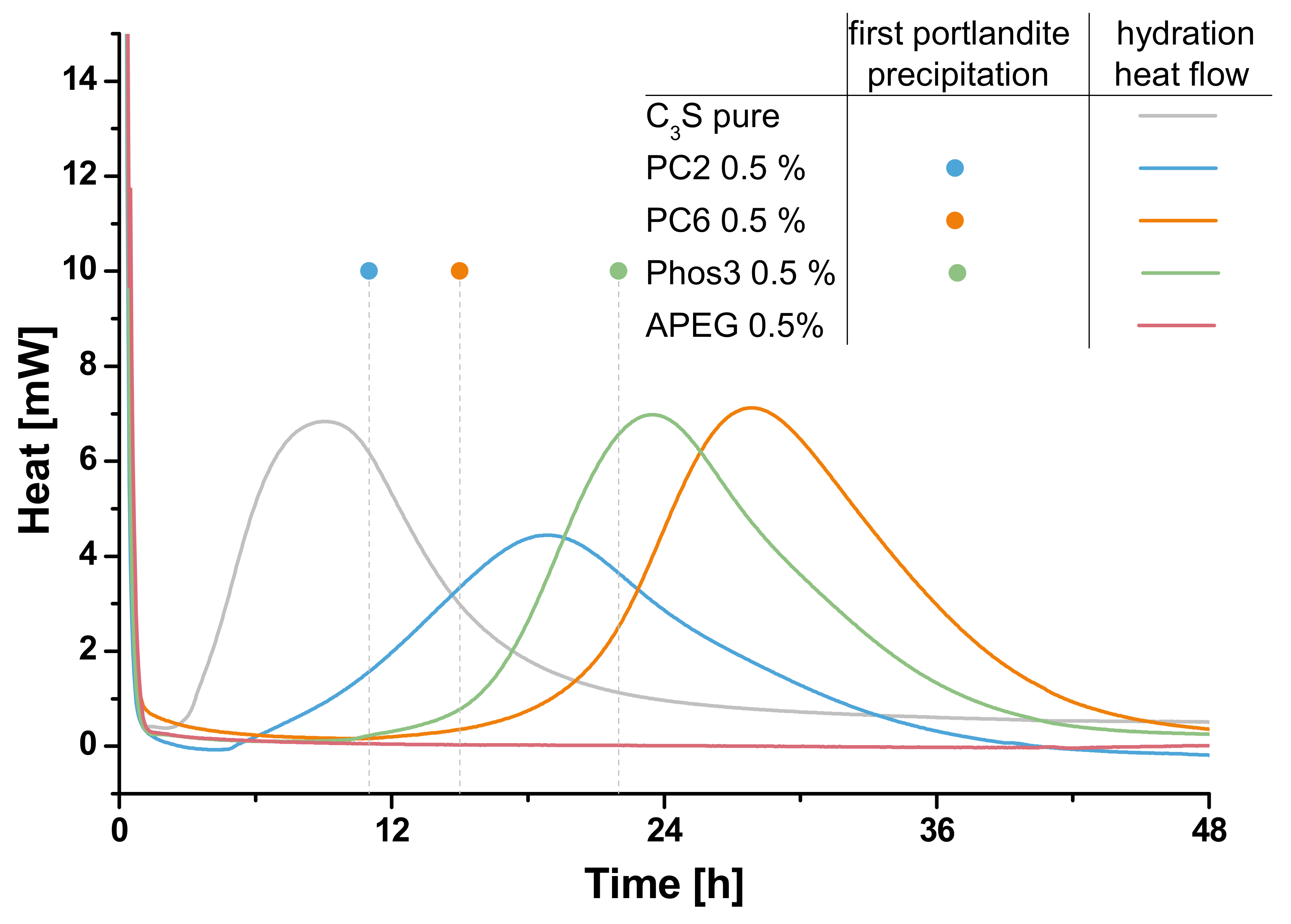
3.3. Calorimetry
3.4. Correlation of Fluorescence Microscopy and Calorimetry
- The passivation of reactive clinker, influenced by charge density and side chain length
- The shift of precipitation equilibria by the different ion coordination capacity
- The influence on the hydrate precipitation, as well determined by the chemical composition
4. Rheology of Pastes—Results and Discussion
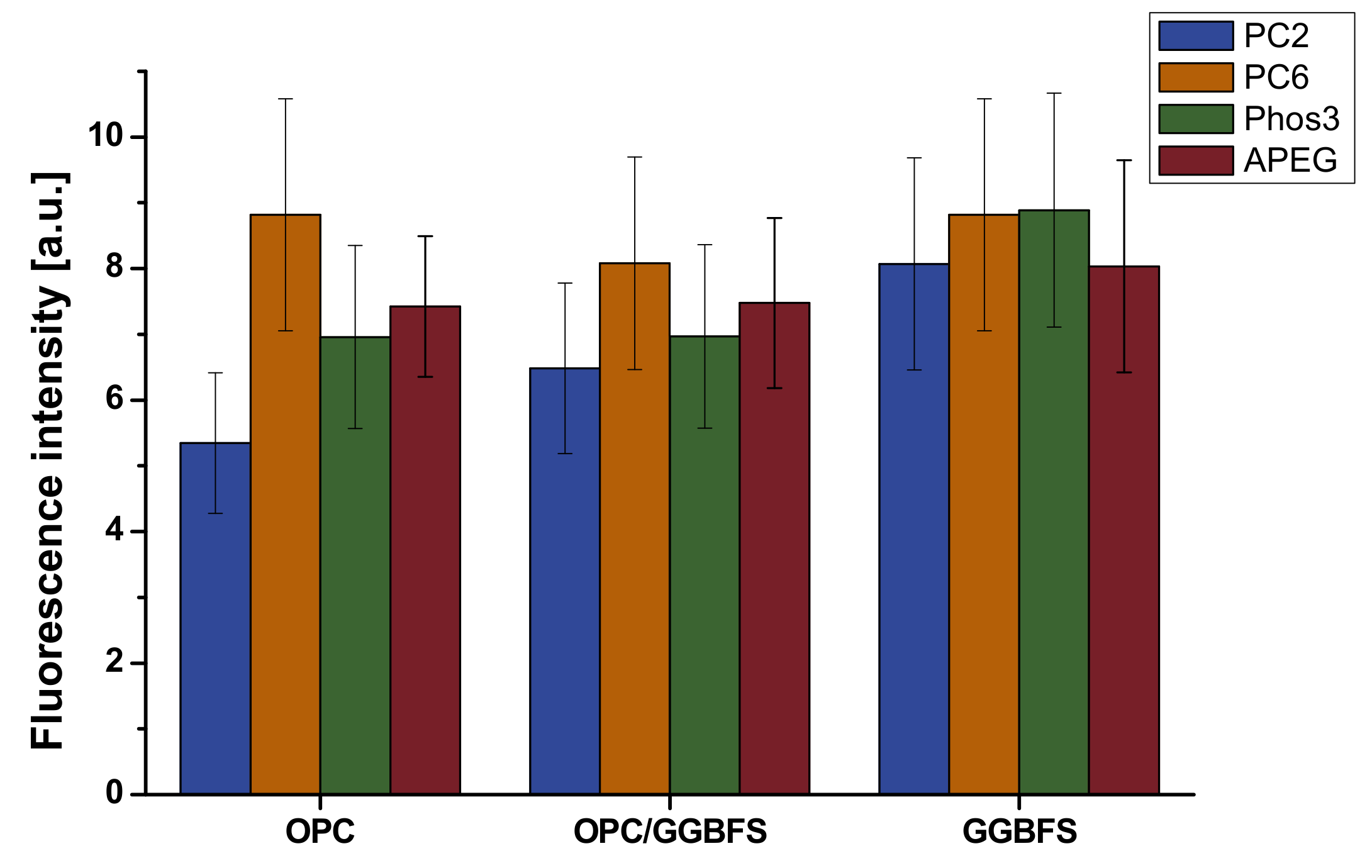
4.1. Fluorescence Microscopic Adsorption Measurements
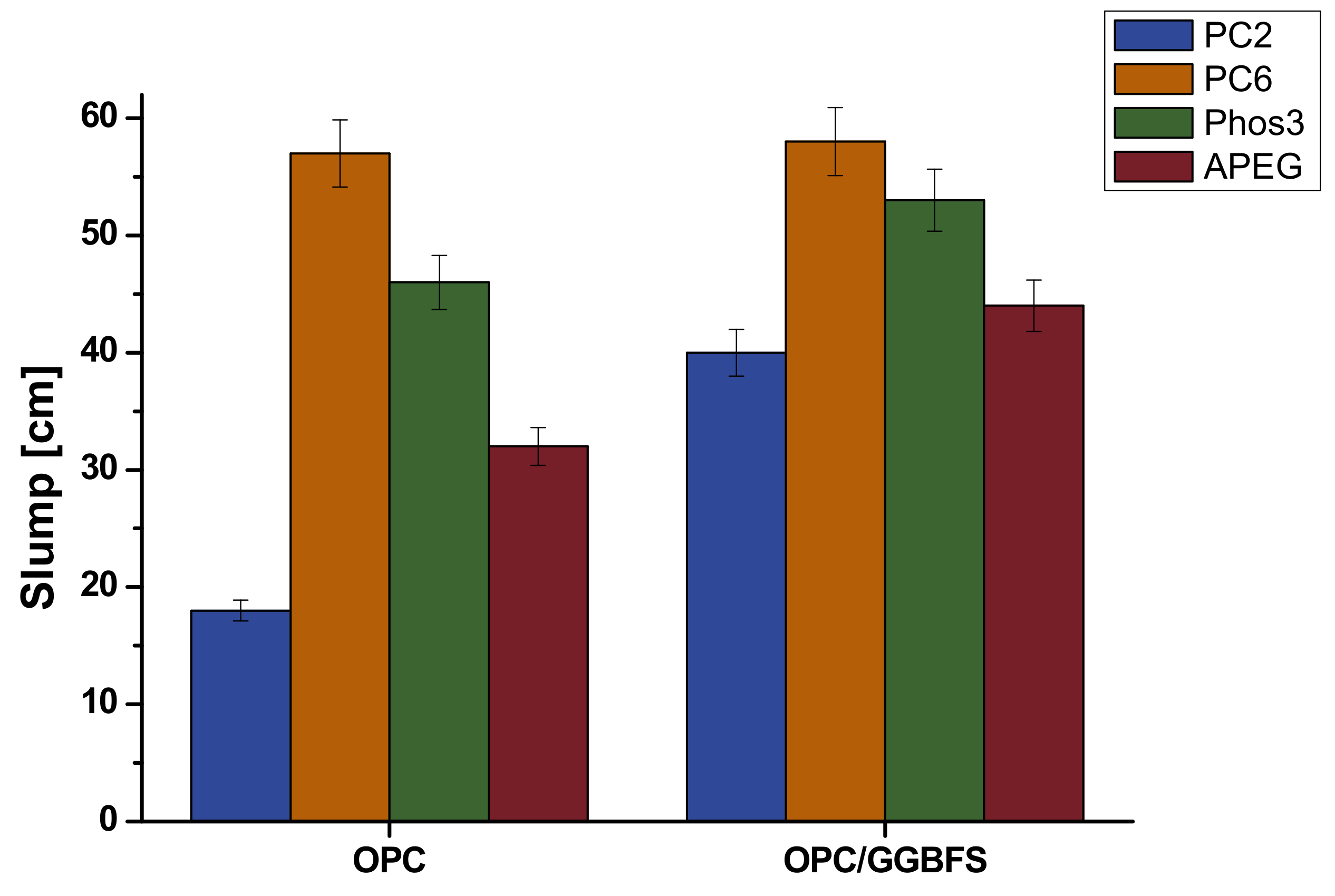
4.2. Slump Flow
4.3. Correlation of Fluorescence Microscopy and Rheology
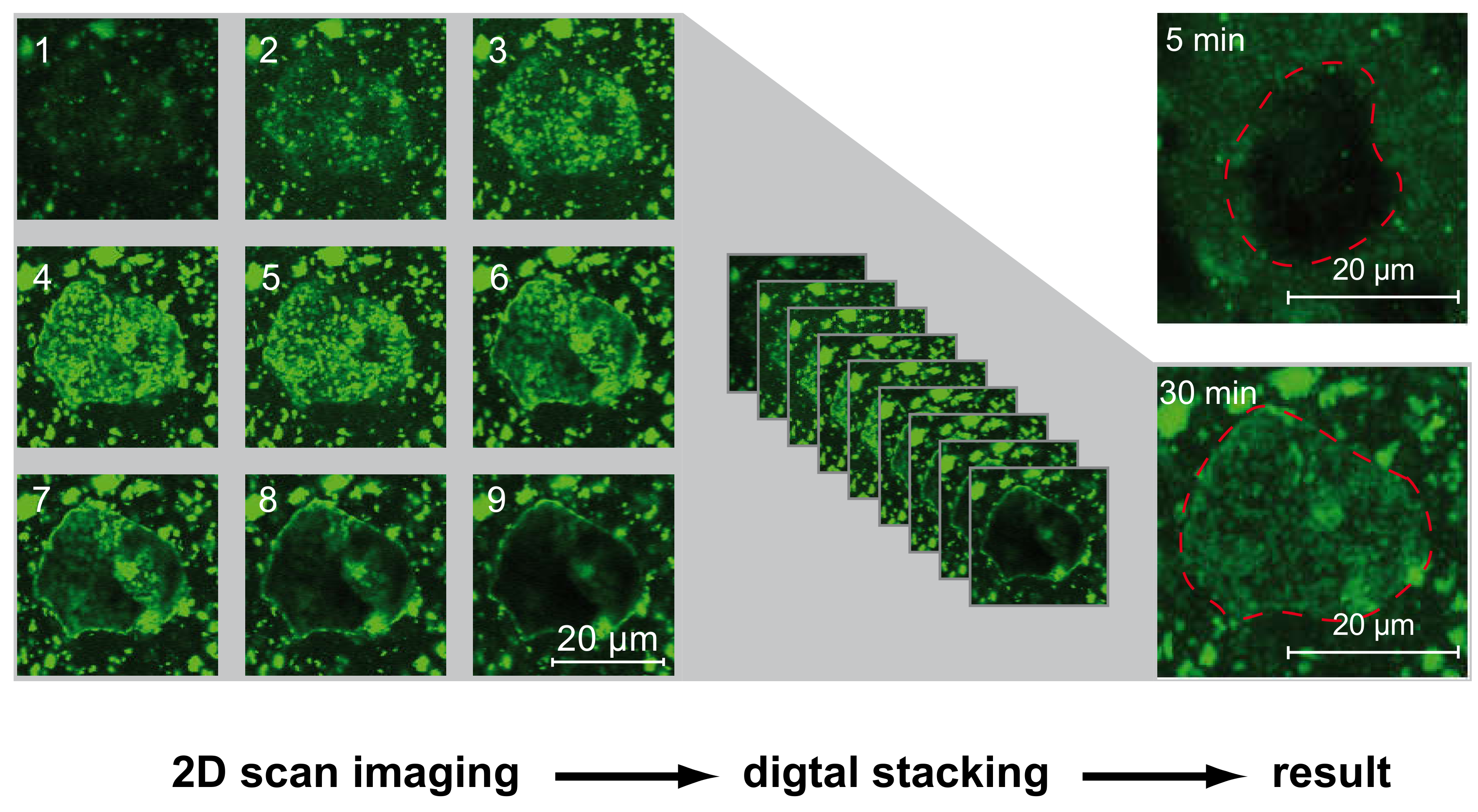
5. Confocal Laser Scanning Microscopy (CLSM)
6. Conclusions and Outlook
- The shown correlation present SP and retardation is in line with previous results obtained by other methods such as in-situ XRD. It was shown by fluorescence microscopic, calorimetric and electron microscopic investigations that the different chemical composition of the four SPs is responsible for the retardation of clinker hydration. The formation of passivating polymer layers is found by the results, but different extents of ion coordination in solution have to be considered as well to interpret all results.
- The amount of adsorbed polymer correlates in the conducted experiments roughly with rheological investigation, remembering this parameter is not standing alone in most cases. The best correlation was found for MPEG-type SPs (PC2 and PC6), while the APEG and phosphate-SP did not show sufficient differences for clear interpretation. It is assumed that the UHPC-specific high SP dosage is responsible and will be reduced in further work.
- The general approach to use CLSM in terms of high resolved in-situ experiments to investigate early hydration is presented as well, illustrating the potential of the approach.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, C.; Wu, Z.; Xiao, J.; Wang, D.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part I. Raw materials and mixture design. Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Schmidt, M.; Fehling, E.; Fröhlich, S. (Eds.) Sustainable Building with Ultra-High Performance Concrete. Results of the German Priority Programme 1182 Funded by Deutsche Forschungsgemeinschaft (DFG); Kassel University Press: Kassel, Germany, 2014; ISBN 9783862194810. [Google Scholar]
- Uchikawa, H.; Hanehara, S.; Sawaki, D. The role of steric repulsive force in the dispersion of cement particles in fresh paste prepared with organic admixture. Cem. Concr. Res. 1997, 27, 37–50. [Google Scholar] [CrossRef]
- Hirata, T.; Tsubakimoto, T.; Hosoido, M.; Tawara, H. Cement Dispersant 84 2022. JP Patent S59-018338, 26 April 1984. [Google Scholar]
- Janowska-Renkas, E. The effect of superplasticizers’ chemical structure on their efficiency in cement pastes. Constr. Build. Mater. 2013, 38, 1204–1210. [Google Scholar] [CrossRef]
- Jansen, D.; Neubauer, J.; Goetz-Neunhoeffer, F.; Haerzschel, R.; Hergeth, W.-D. Change in reaction kinetics of a Portland cement caused by a superplasticizer—Calculation of heat flow curves from XRD data. Cem. Concr. Res. 2012, 42, 327–332. [Google Scholar] [CrossRef]
- Liu, M.; Lei, J.; Guo, L.; Du, X.; Li, J. The application of thermal analysis, XRD and SEM to study the hydration behavior of tricalcium silicate in the presence of a polycarboxylate superplasticizer. Thermochim. Acta 2015, 613, 54–60. [Google Scholar] [CrossRef]
- Marchon, D.; Boscaro, F.; Flatt, R.J. First steps to the molecular structure optimization of polycarboxylate ether superplasticizers: Mastering fluidity and retardation. Cem. Concr. Res. 2019, 115, 116–123. [Google Scholar] [CrossRef]
- Stroh, J.; Schlegel, M.-C.; Schmidt, W.; Thi, Y.N.; Meng, B.; Emmerling, F. Time-resolved in situ investigation of Portland cement hydration influenced by chemical admixtures. Constr. Build. Mater. 2016, 106, 18–26. [Google Scholar] [CrossRef]
- Donnet, M.; Aimable, A.; Lemaître, J.; Bowen, P. Contribution of aggregation to the growth mechanism of seeded calcium carbonate precipitation in the presence of polyacrylic acid. J. Phys. Chem. B 2010, 114, 12058–12067. [Google Scholar] [CrossRef][Green Version]
- Abile, R.; Russo, A.; Limone, C.; Montagnaro, F. Impact of the charge density on the behaviour of polycarboxylate ethers as cement dispersants. Constr. Build. Mater. 2018, 180, 477–490. [Google Scholar] [CrossRef]
- Keller, H.; Plank, J. Mineralisation of CaCO3 in the presence of polycarboxylate comb polymers. Cem. Concr. Res. 2013, 54, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Wang, Z.; Cui, S.; Wang, Y. Influence of Monomer Ratios on Molecular Weight Properties and Dispersing Effectiveness in Polycarboxylate Superplasticizers. Materials 2020, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Schutter, G.D. Different Effects of NSF and PCE Superplasticizer on Adsorption, Dynamic Yield Stress and Thixotropy of Cement Pastes. Materials 2018, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Flatt, R.J.; Houst, Y.F. A simplified view on chemical effects perturbing the actionof superplasticizers. Cem. Concr. Res. 2001, 31, 1169–1176. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Taheri, A.; Deng, J.; Ke, B. Effects of Superplasticizer on the Hydration, Consistency, and Strength Development of Cemented Paste Backfill. Minerals 2018, 8, 381. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Yoshioka, K.; Tazawa, E.-I.; Kawai, K.; Enohata, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Sakai, E.; Yamada, K.; Ohta, A. Molecular Structure and Dispersion-Adsorption Mechanisms of Comb-Type Superplasticizers Used in Japan. J. Adv. Concr. Technol. 2003, 1, 16–25. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of polycarboxylate-based superplasticizers with cements containing different C3A amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Multi-method approach to study influence of superplasticizers on cement suspensions. Cem. Concr. Res. 2011, 41, 1058–1066. [Google Scholar] [CrossRef]
- Habbaba, A.; Plank, J. Interaction Between Polycarboxylate Superplasticizers and Amorphous Ground Granulated Blast Furnace Slag. J. Am. Ceram. Soc. 2010, 93, 2857–2863. [Google Scholar] [CrossRef]
- Meier, M.R.; Napharatsamee, T.; Plank, J. Dispersing performance of superplasticizers admixed to aged cement. Constr. Build. Mater. 2017, 139, 232–240. [Google Scholar] [CrossRef]
- Arend, J.; Wetzel, A.; Middendorf, B. In-situ investigation of superplasticizers: From fluorescence microscopy to concrete rheology. Cem. Concr. Res. 2018, 113, 178–185. [Google Scholar] [CrossRef]
- Arend, J.; Wetzel, A.; Middendorf, B. Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC. Materials 2020, 13, 1057. [Google Scholar] [CrossRef]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and submolecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Toward molecular design. J. Am. Ceram. Soc. 2017, 100, 817–841. [Google Scholar] [CrossRef]
- Cook, R.; Ma, H.; Kumar, A. Mechanism of tricalcium silicate hydration in the presence of polycarboxylate polymers. SN Appl. Sci. 2019, 1, 145. [Google Scholar] [CrossRef]
- Hirata, T.; Ye, J.; Branicio, P.; Zheng, J.; Lange, A.; Plank, J.; Sullivan, M. Adsorbed Conformations of PCE Superplasticizers in Cement Pore Solution Unraveled by Molecular Dynamics Simulations. Sci. Rep. 2017, 7, 16599. [Google Scholar] [CrossRef]
- Chuang, P.-H.; Tseng, Y.-H.; Fang, Y.; Gui, M.; Ma, X.; Luo, J. Effect of Side Chain Length on Polycarboxylate Superplasticizer in Aqueous Solution: A Computational Study. Polymers 2019, 11, 346. [Google Scholar] [CrossRef]
- Uddin, K.M.S.; Middendorf, B. Reactivity of Different Crystalline Surfaces of C3S During Early Hydration by the Atomistic Approach. Materials 2019, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Hirata, T.; Plank, J. Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem. Concr. Res. 2014, 60, 45–50. [Google Scholar] [CrossRef]
- Lange, A.; Plank, J. Optimization of comb-shaped polycarboxylate cement dispersants to achieve fast-flowing mortar and concrete. J. Appl. Polym. Sci. 2015, 132, 42529. [Google Scholar] [CrossRef]
- Meng, W.; Lunkad, P.; Kumar, A.; Khayat, K. Influence of Silica Fume and Polycarboxylate Ether Dispersant on Hydration Mechanisms of Cement. J. Phys. Chem. C 2016, 120, 26814–26823. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef]
- Plank, J.; Keller, H.; Andres, P.R.; Dai, Z. Novel organo-mineral phases obtained by intercalation of maleic anhydride–allyl ether copolymers into layered calcium aluminum hydrates. Inorganica Chim. Acta 2006, 359, 4901–4908. [Google Scholar] [CrossRef]
- Ng, S.; Plank, J. Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem. Concr. Res. 2012, 42, 847–854. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem. Concr. Res. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Pott, U.; Jakob, C.; Jansen, D.; Neubauer, J.; Stephan, D. Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior. Materials 2020, 13, 977. [Google Scholar] [CrossRef]
- Yio, M.H.N.; Mac, M.J.; Wong, H.S.; Buenfeld, N.R. 3D imaging of cement-based materials at submicron resolution by combining laser scanning confocal microscopy with serial sectioning. J. Microsc. 2015, 258, 151–169. [Google Scholar] [CrossRef]
- Yio, M.H.N.; Wong, H.S.; Buenfeld, N.R. Fluorescence laser scanning confocal microscopy for real-time imaging of early cement hydration. In Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherlands, 17–19 June 2015; pp. 269–277. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1990; ISBN 0-7277-2592-0. [Google Scholar]
- Bensted, J.; Barnes, P. Structure and Performance of Cements, 2nd ed.; Spon Press: London, UK, 2002; ISBN 0203477782. [Google Scholar]
- Plank, J.; Pöllmann, K.; Zouaoui, N.; Andres, P.R.; Schaefer, C. Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly(ethylene glycol) side chains. Cem. Concr. Res. 2008, 38, 1210–1216. [Google Scholar] [CrossRef]
- Stecher, J.; Plank, J. Novel concrete superplasticizers based on phosphate esters. Cem. Concr. Res. 2019, 119, 36–43. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Impact of Molecular Structure on Zeta Potential and Adsorbed Conformation of.ALPHA.-Allyl-.OMEGA.-Methoxypolyethylene Glycol-Maleic Anhydride Superplasticizers. J. Adv. Concr. Technol. 2006, 4, 233–239. [Google Scholar] [CrossRef]
- Conte, T.; Plank, J. Impact of molecular structure and composition of polycarboxylate comb polymers on the flow properties of alkali-activated slag. Cem. Concr. Res. 2019, 116, 95–101. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Baueregger, S.; Perello, M.; Plank, J. Role of PVOH and kaolin on colloidal stability of liquid and powder EVA and SB latexes in cement pore solution. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 145–153. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- Plank, J.; Schröfl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. J. Adv. Concr. Technol. 2009, 5–12. [Google Scholar] [CrossRef]
- Schröfl, C.; Gruber, M.; Plank, J. Preferential adsorption of polycarboxylate superplasticizers on cement and silica fume in ultra-high performance concrete (UHPC). Cem. Concr. Res. 2012, 42, 1401–1408. [Google Scholar] [CrossRef]
- Pirazzoli, I.; Alesiani, M.; Capuani, S.; Maraviglia, B.; Giorgi, R.; Ridi, F.; Baglioni, P. The influence of superplasticizers on the first steps of tricalcium silicate hydration studied by NMR techniques. Magn. Reson. Imaging 2005, 23, 277–284. [Google Scholar] [CrossRef]
- Caruso, F.; Mantellato, S.; Palacios, M.; Flatt, R.J. ICP-OES method for the characterization of cement pore solutions and their modification by polycarboxylate-based superplasticizers. Cem. Concr. Res. 2017, 91, 52–60. [Google Scholar] [CrossRef]
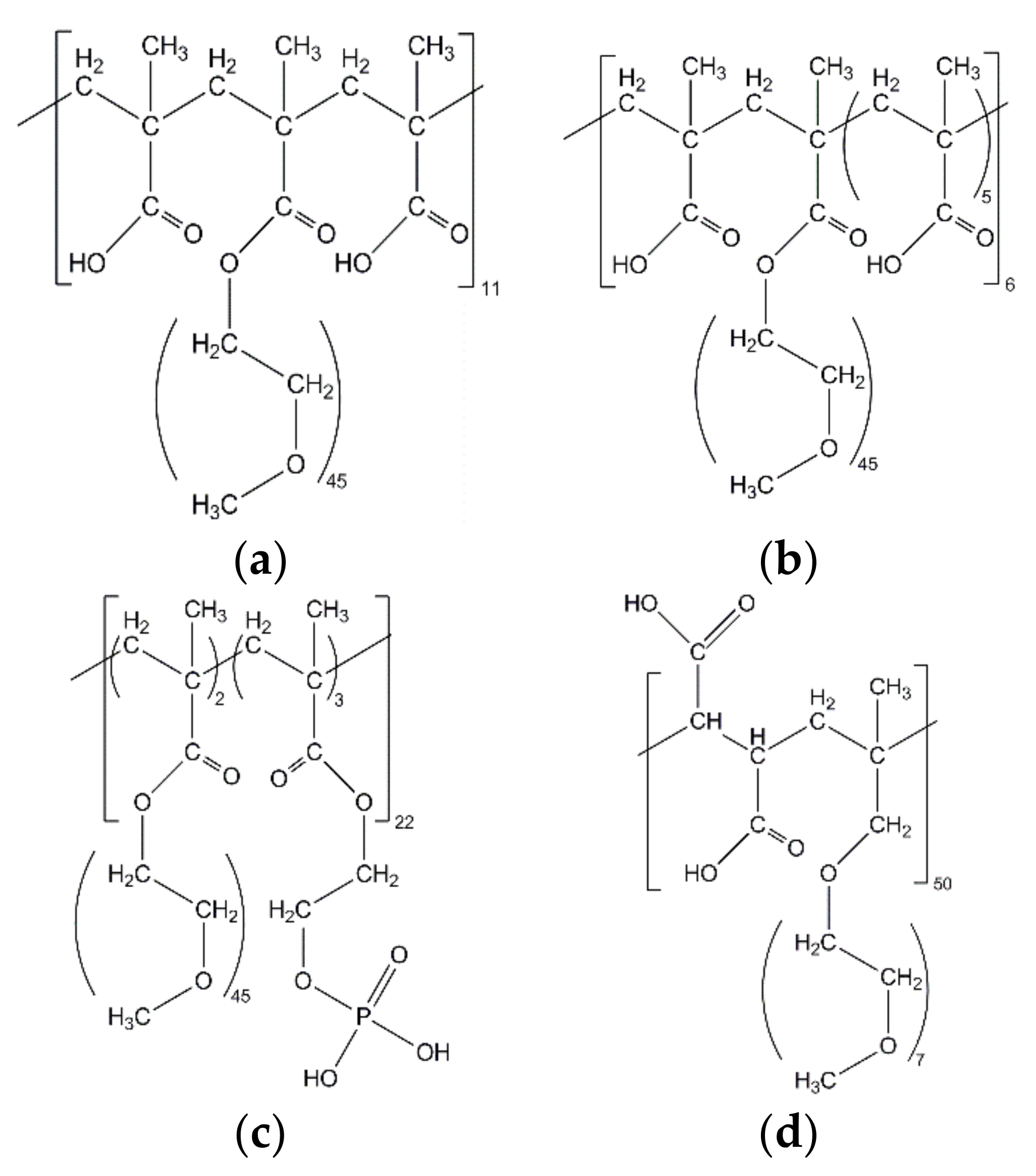
| Oxide Content [wt%] | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 | SO3 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Silica fume | 95.6 | 0.1 | 0.1 | 0.5 | 0.3 | 0.7 | 0.2 | 0.1 | 0.1 | 0.9 | 1.4 |
| OPC | 21.5 | 3.7 | 4.3 | 64.3 | 0.9 | 0.4 | 0.3 | 0.2 | 2.4 | 0.3 | 1.7 |
| GGBFS | 35.8 | 11.7 | 0.4 | 43.8 | 5.8 | 0.4 | 0.2 | – | 0.3 | 0.8 | 0.8 |
| Phase | C3S | C2S | C3A | C4AF | Gypsum and Hemihydrate |
|---|---|---|---|---|---|
| Content [%] | 61.3 | 15.9 | 3.9 | 13.9 | 3.3 |
| SP | %bwob | SP Conc. [wt%] | SP Total Mass [g] | C3S [g] | Silica Fume [g] | Water [g] | Syn. PS [g] |
|---|---|---|---|---|---|---|---|
| – | – | – | – | 1.5 | – | – | 0.60 |
| PC2 | 0.2 | 38 | 0.008 | 1.5 | – | – | 0.37 |
| PC2 | 0.5 | 38 | 0.020 | 1.5 | – | – | 0.36 |
| PC2 | 1.2 | 38 | 0.048 | 1.5 | – | – | 0.35 |
| PC6 | 0.5 | 40 | 0.019 | 1.5 | – | – | 0.36 |
| PC6 | 1.2 | 40 | 0.045 | 1.5 | – | – | 0.35 |
| Phos3 | 0.5 | 33 | 0.023 | 1.5 | – | – | 0.36 |
| Phos3 | 1.2 | 33 | 0.055 | 1.5 | – | – | 0.34 |
| APEG | 0.5 | 35 | 0.021 | 1.5 | – | – | 0.36 |
| APEG | 1.2 | 35 | 0.051 | 1.5 | – | – | 0.34 |
| – | – | – | – | 1.5 | – | 0.60 | – |
| PC2 | 0.5 | 38 | 0.020 | 1.5 | – | 0.36 | – |
| PC2 | 1.2 | 38 | 0.048 | 1.5 | – | 0.35 | – |
| PC6 | 0.5 | 40 | 0.019 | 1.5 | – | 0.36 | – |
| PC6 | 1.2 | 40 | 0.045 | 1.5 | – | 0.35 | – |
| Phos3 | 0.5 | 33 | 0.023 | 1.5 | – | 0.36 | – |
| Phos3 | 1.2 | 33 | 0.055 | 1.5 | – | 0.34 | – |
| APEG | 0.5 | 35 | 0.021 | 1.5 | – | 0.36 | – |
| APEG | 1.2 | 35 | 0.051 | 1.5 | – | 0.34 | – |
| – | – | – | – | 1.275 | 0.225 | – | 0.60 |
| PC2 | 0.5 | 38 | 0.020 | 1.275 | 0.225 | – | 0.36 |
| PC2 | 1.2 | 38 | 0.048 | 1.275 | 0.225 | – | 0.35 |
| PC6 | 0.5 | 40 | 0.019 | 1.275 | 0.225 | – | 0.36 |
| PC6 | 1.2 | 40 | 0.045 | 1.275 | 0.225 | – | 0.35 |
| Phos3 | 0.5 | 33 | 0.023 | 1.275 | 0.225 | – | 0.36 |
| Phos3 | 1.2 | 33 | 0.055 | 1.275 | 0.225 | – | 0.34 |
| APEG | 0.5 | 35 | 0.021 | 1.275 | 0.225 | – | 0.36 |
| APEG | 1.2 | 35 | 0.051 | 1.275 | 0.225 | – | 0.34 |
| – | – | – | – | 1.275 | 0.225 | 0.60 | – |
| PC2 | 0.5 | 38 | 0.020 | 1.275 | 0.225 | 0.36 | – |
| PC2 | 1.2 | 38 | 0.048 | 1.275 | 0.225 | 0.35 | – |
| PC6 | 0.5 | 40 | 0.019 | 1.275 | 0.225 | 0.36 | – |
| PC6 | 1.2 | 40 | 0.045 | 1.275 | 0.225 | 0.35 | – |
| Phos3 | 0.5 | 33 | 0.023 | 1.275 | 0.225 | 0.36 | – |
| Phos3 | 1.2 | 33 | 0.055 | 1.275 | 0.225 | 0.34 | – |
| APEG | 0.5 | 35 | 0.021 | 1.275 | 0.225 | 0.36 | – |
| APEG | 1.2 | 35 | 0.051 | 1.275 | 0.225 | 0.34 | – |
| SP | %bwob | SP Conc. [wt%] | SP Total Mass [g] | OPC [g] | GGBFS [g] | Water [g] |
|---|---|---|---|---|---|---|
| PC2 | 1.2 | 38 | 22.2 | 700 | – | 161.2 |
| PC6 | 1.2 | 40 | 21.2 | 700 | – | 162.2 |
| Phos3 | 1.2 | 33 | 25.5 | 700 | – | 158.0 |
| APEG | 1.2 | 35 | 24.0 | 700 | – | 159.4 |
| PC2 | 1.2 | 38 | 22.2 | 350 | 350 | 161.2 |
| PC6 | 1.2 | 40 | 21.2 | 350 | 350 | 162.2 |
| Phos3 | 1.2 | 33 | 25.5 | 350 | 350 | 158.0 |
| APEG | 1.2 | 35 | 24.0 | 350 | 350 | 159.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arend, J.; Wetzel, A.; Middendorf, B. Fluorescence Microscopy of Superplasticizers in Cementitious Systems: Applications and Challenges. Materials 2020, 13, 3733. https://doi.org/10.3390/ma13173733
Arend J, Wetzel A, Middendorf B. Fluorescence Microscopy of Superplasticizers in Cementitious Systems: Applications and Challenges. Materials. 2020; 13(17):3733. https://doi.org/10.3390/ma13173733
Chicago/Turabian StyleArend, Johannes, Alexander Wetzel, and Bernhard Middendorf. 2020. "Fluorescence Microscopy of Superplasticizers in Cementitious Systems: Applications and Challenges" Materials 13, no. 17: 3733. https://doi.org/10.3390/ma13173733
APA StyleArend, J., Wetzel, A., & Middendorf, B. (2020). Fluorescence Microscopy of Superplasticizers in Cementitious Systems: Applications and Challenges. Materials, 13(17), 3733. https://doi.org/10.3390/ma13173733





