High-Strength, Waterproof, Corrosion-Resistant Nano-Silica Carbon Nanotube Cementitious Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Materials Characterization Techniques
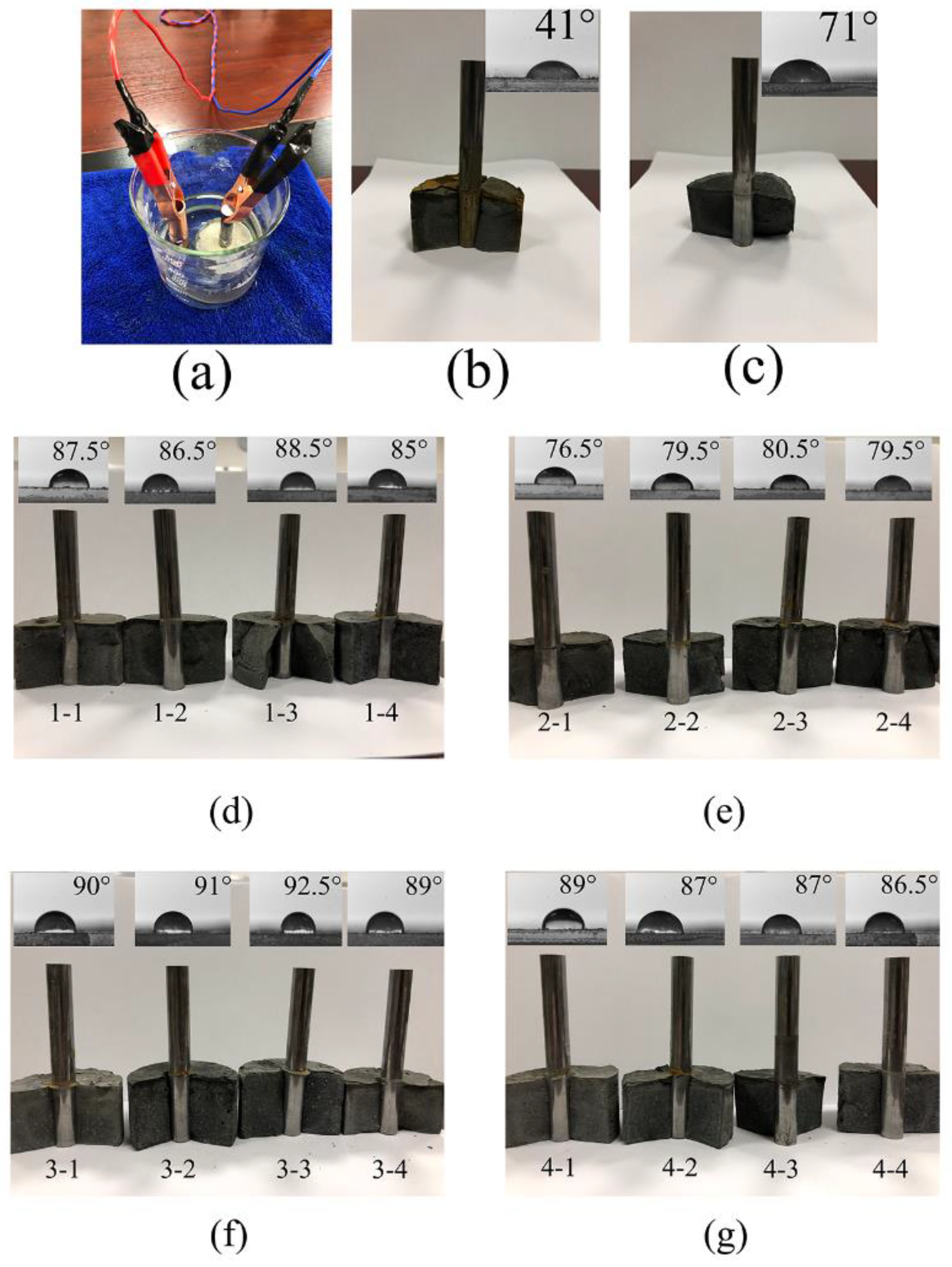
2.2.2. Anti-Freeze–Thaw Damage, Electrochemical Corrosion Test and Contact Angle Test
3. Results and Discussion
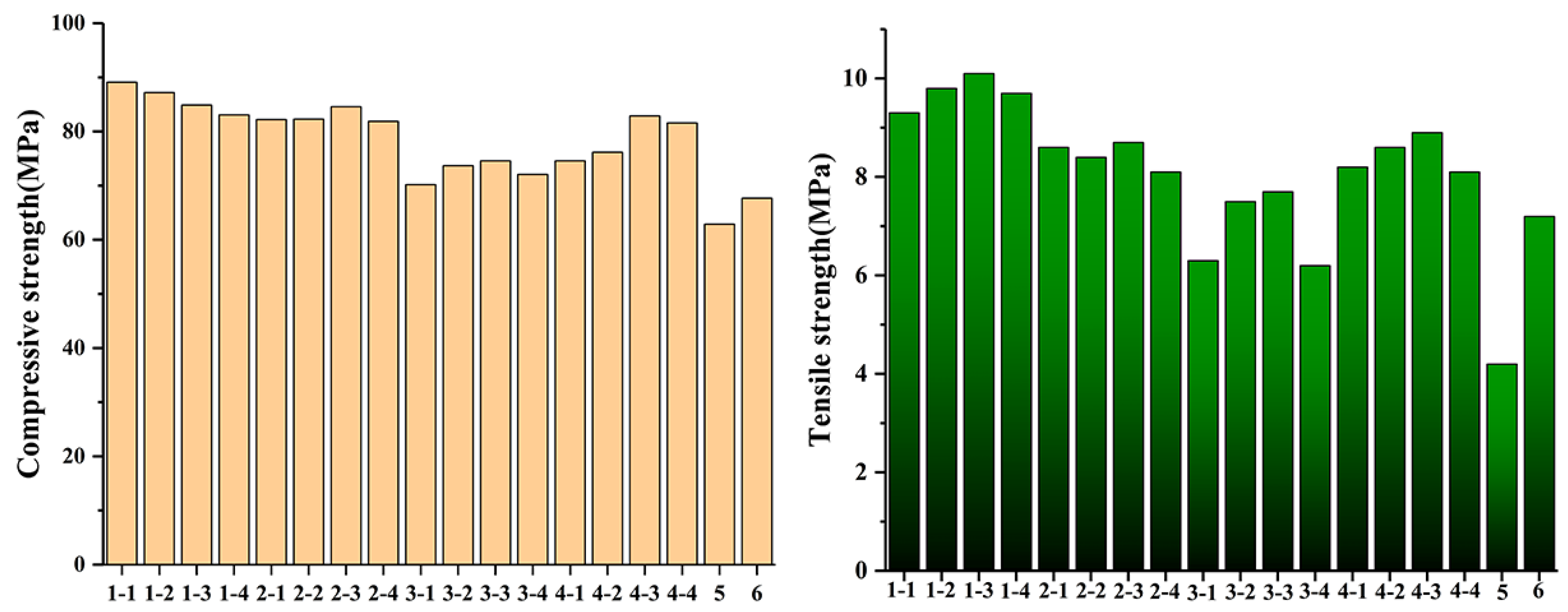
3.1. Mechanical Properties Analysis
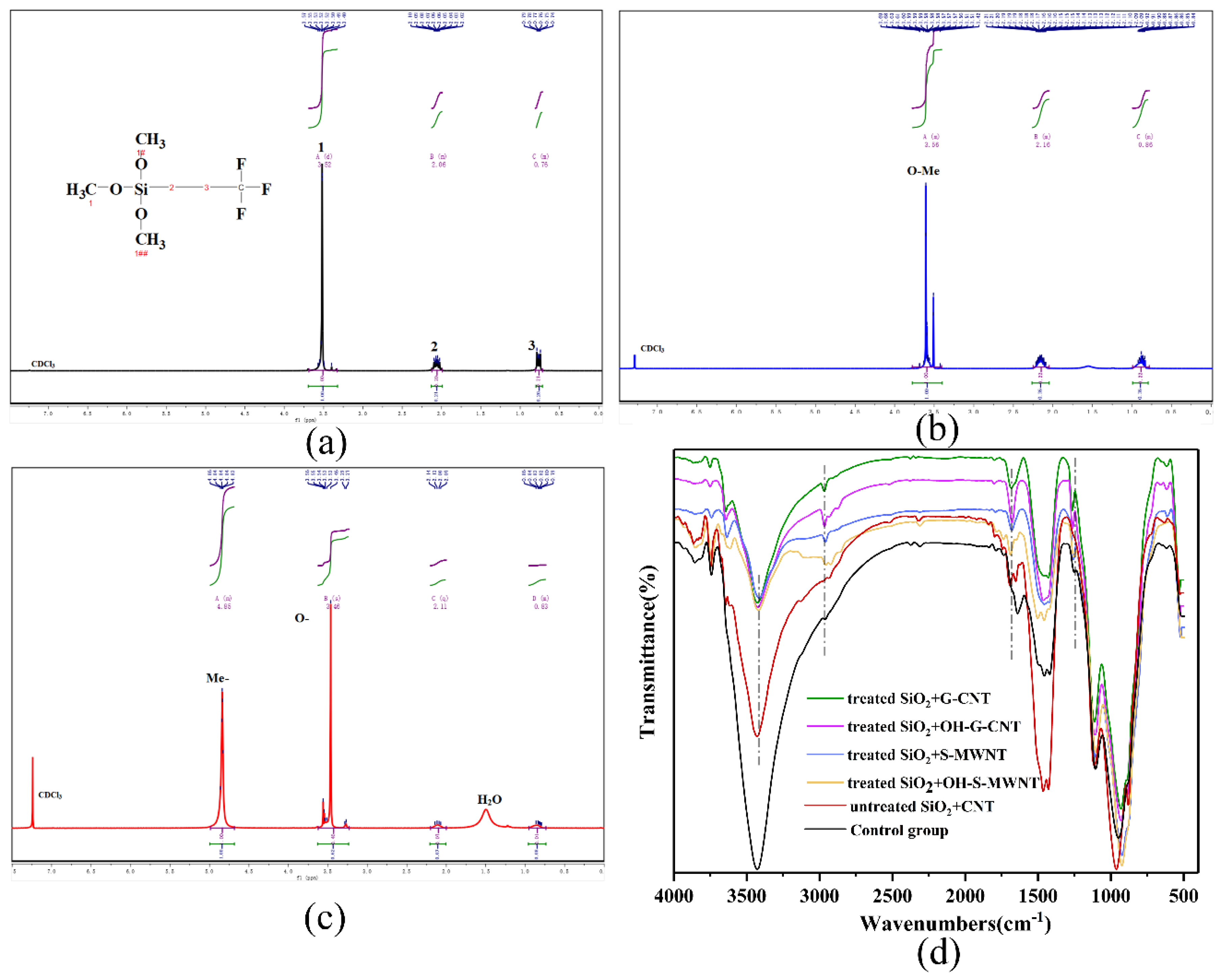
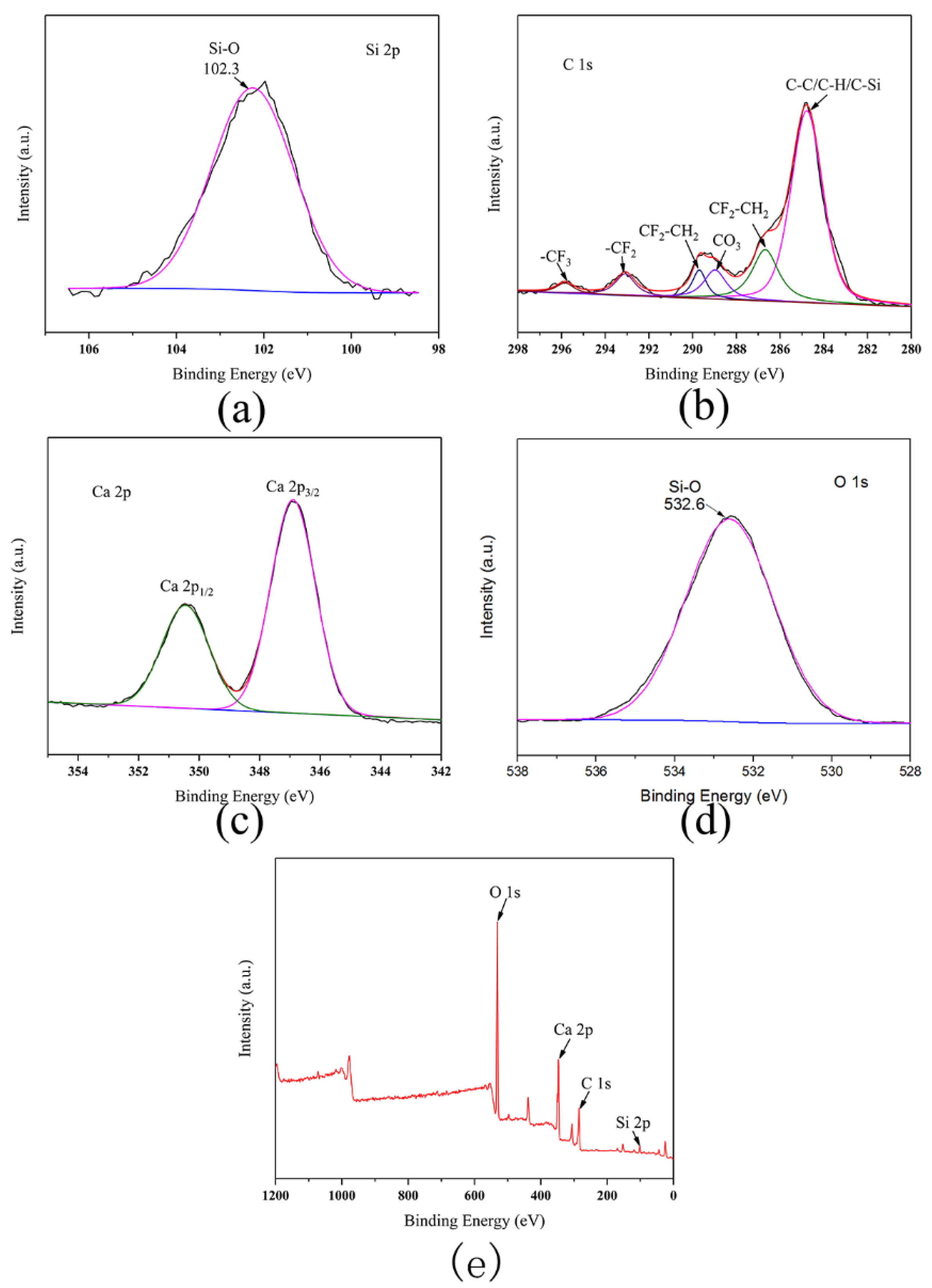
3.2. NMR, FTIR, and XPS Analysis
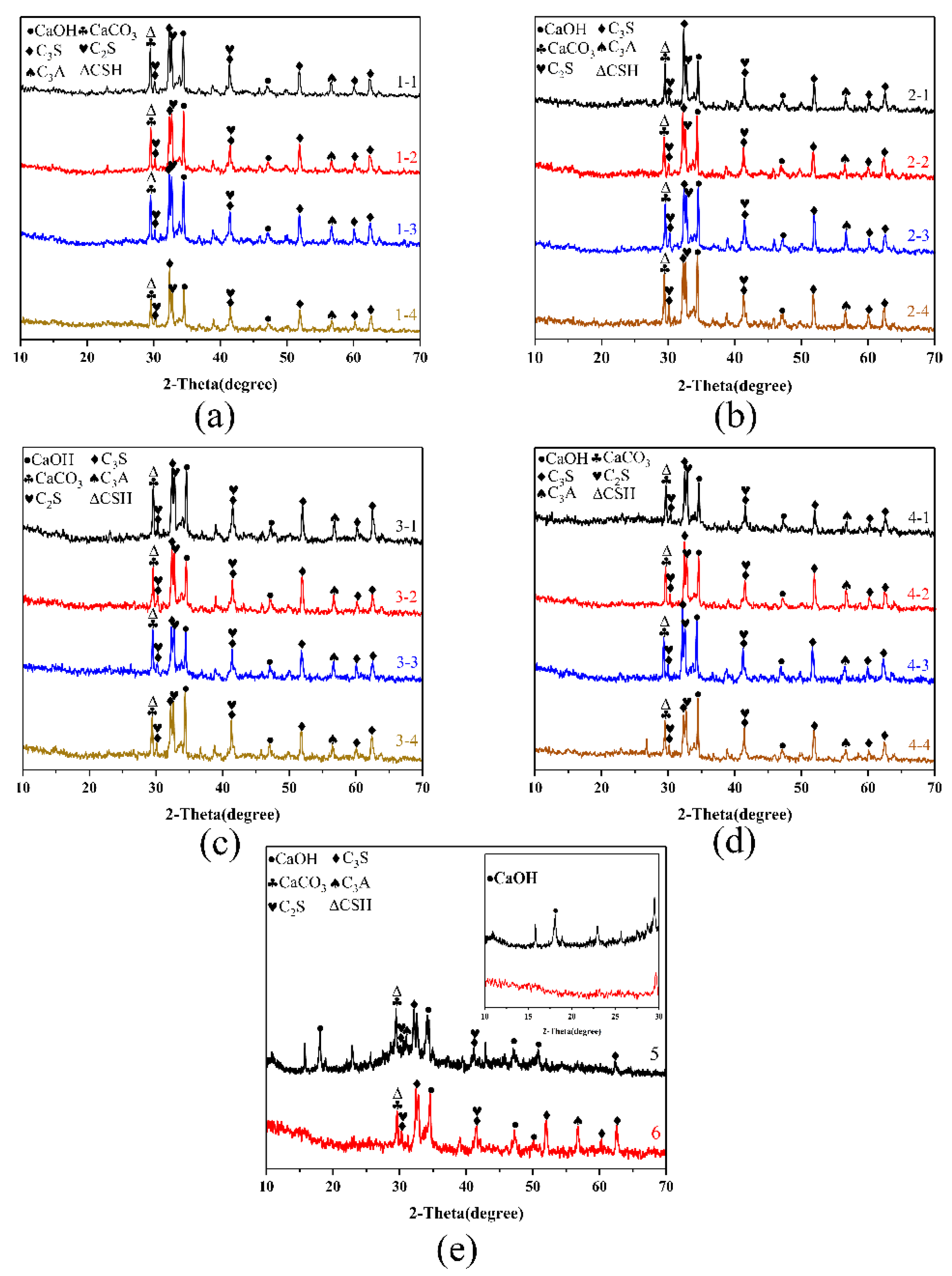
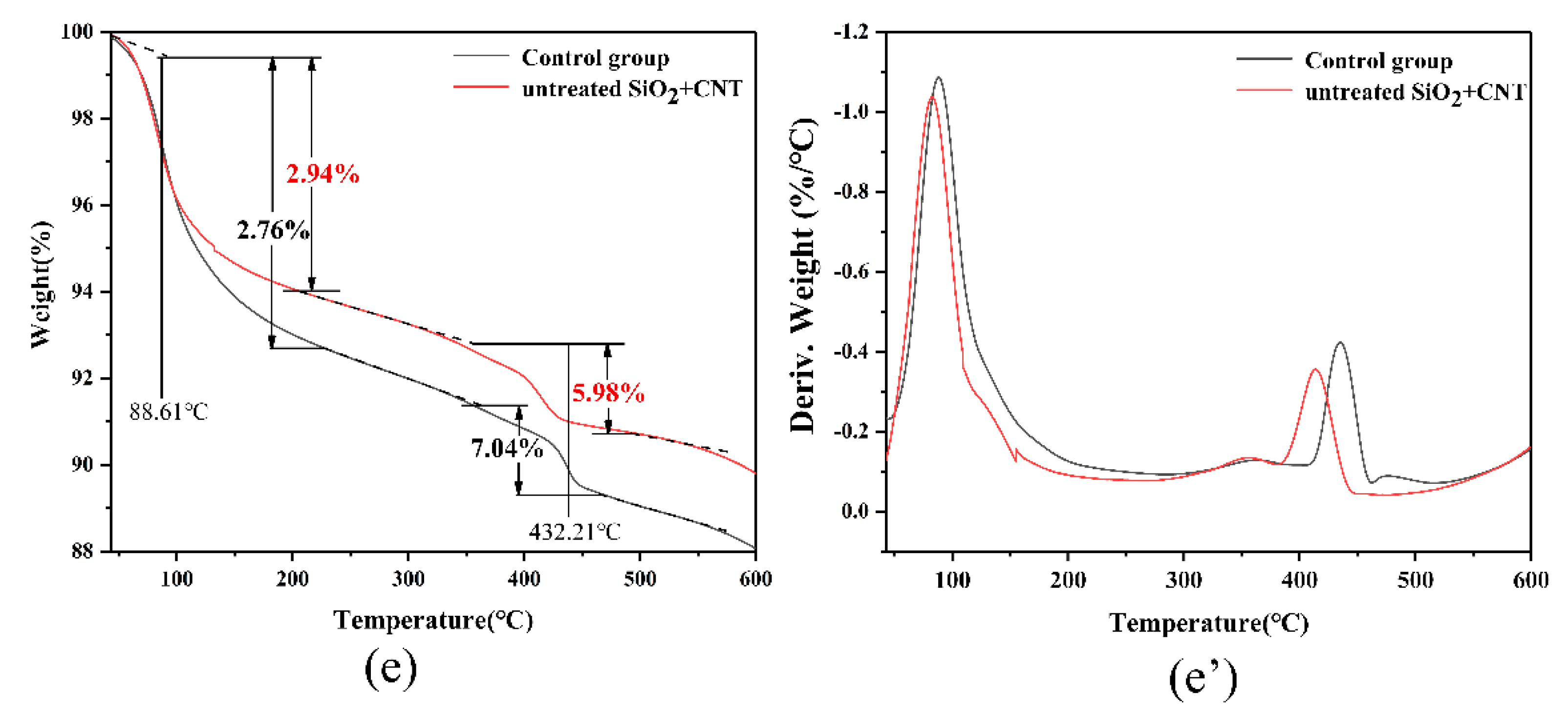
3.3. XRD, TG, and Isothermal Calorimetry Analysis
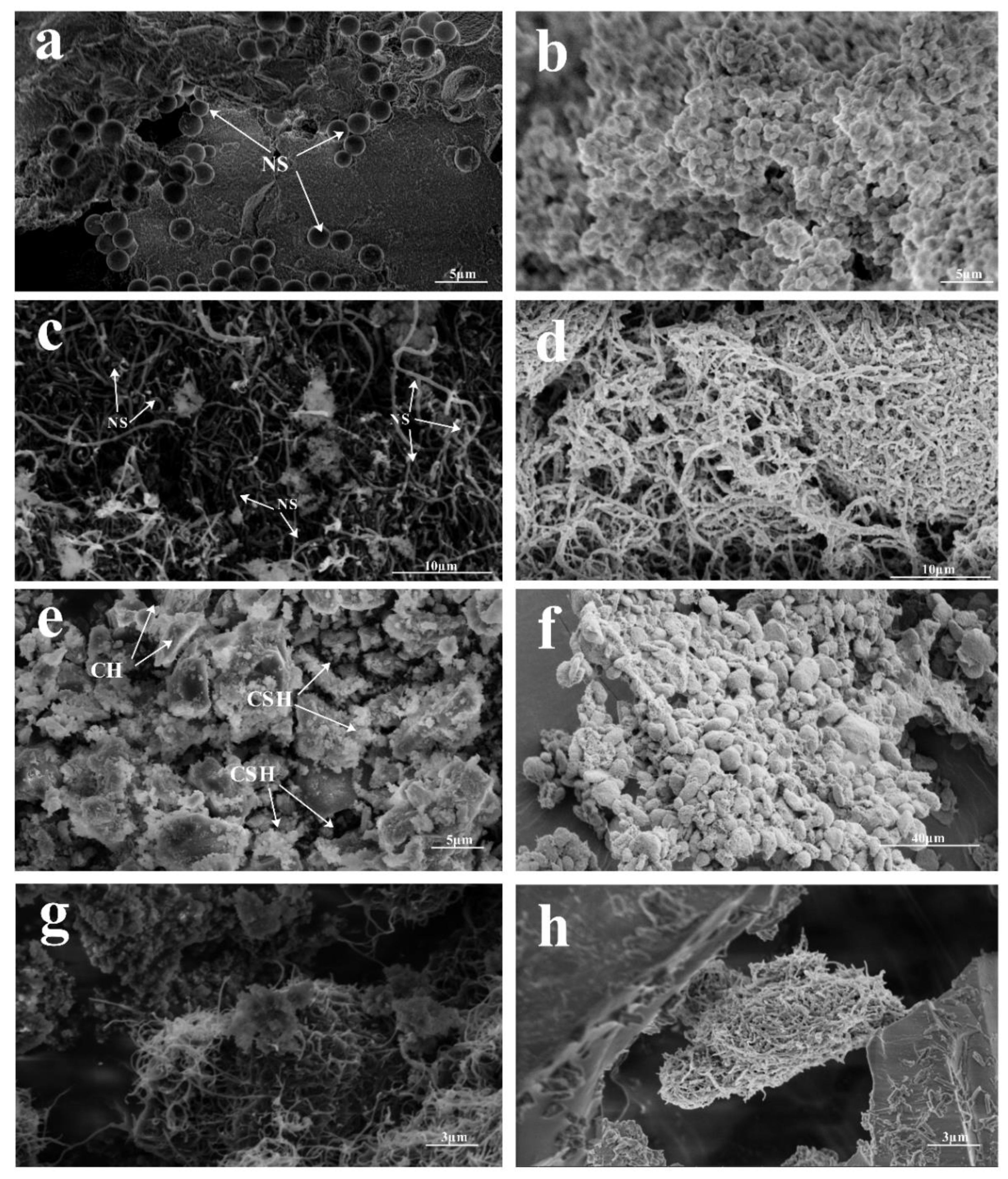
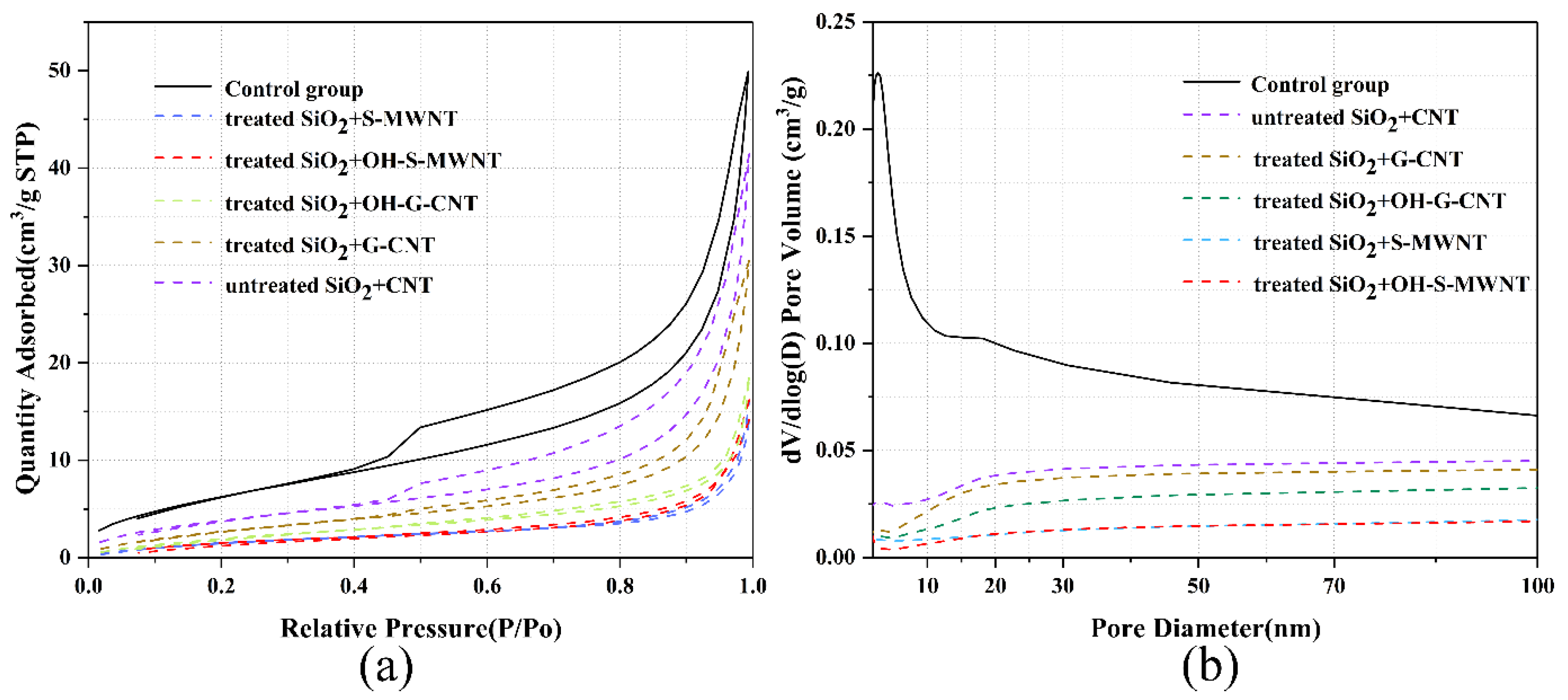
3.4. SEM-BET Analysis
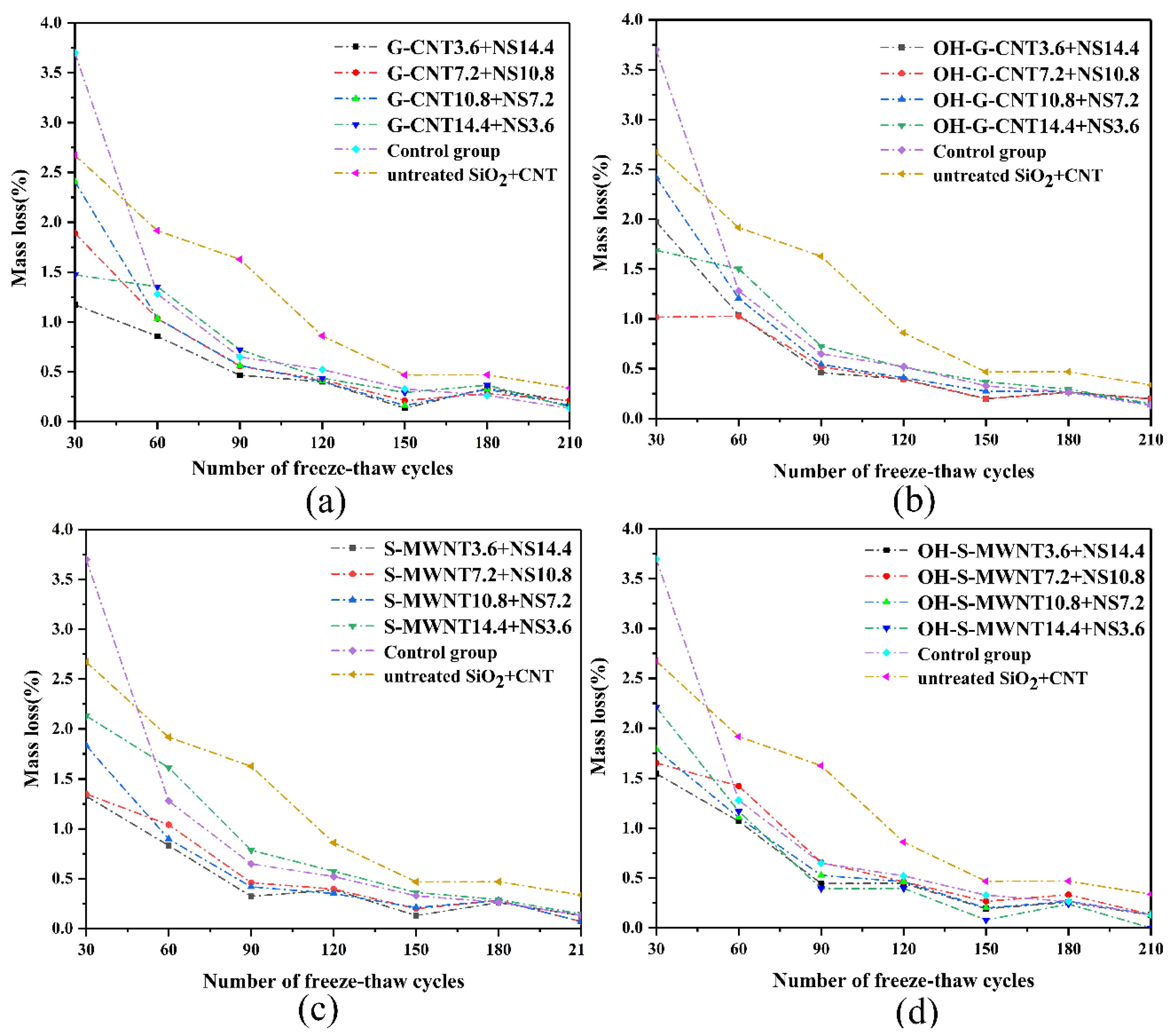
3.5. Anti-Freeze–Thaw Damage, Electrochemical Corrosion Test, and Contact Angle Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
Abbreviations
| BET | Brunauer–Emmett–Teller |
| CH | calcium hydroxide |
| C3A | tricalcium aluminate |
| CNTs | carbon nanotubes |
| CSH | calcium silicate hydrate |
| ClD3 | deuterated chloroform |
| C2S | dicalcium silicate |
| CSH | calcium silicate hydrate |
| C3S | tricalcium silicate |
| FTIR | Fourier-transform infrared |
| G-CNTs | graphitized CNTs |
| HMDZ | hexamethyldisilazane |
| NMR | nuclear magnetic resonance |
| NS | nano-silica |
| NS-CNT | nano-silica carbon nanotube |
| OH-G-CNTs | hydroxylated graphitized CNTs |
| SEM | scanning electron microscopy |
| S-MWNTs | short arm CNTs |
| TEOS | tetraethyl orthosilicate |
| TG | Thermogravimetric |
| TMCS | Trimethylchlorosilane |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Zhang, L.; Kai, M.; Liew, K.M. Evaluation of microstructure and mechanical performance of CNT-reinforced cementitious composites at elevated temperatures. Compos. Part A Appl. Sci. Manuf. 2017, 95, 286–293. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; You, I.; Lee, S.-J. Electrical Properties of Cement-Based Composites with Carbon Nanotubes, Graphene, and Graphite Nanofibers. Sensors 2017, 17, 1064. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Khayat, K.H.; Shi, C. Effect of nano-SiO2 particles and curing time on development of fiber-matrix bond properties and microstructure of ultra-high strength concrete. Cem. Concr. Res. 2017, 95, 247–256. [Google Scholar] [CrossRef]
- Wu, X.-L.; Shi, Y.; Zhong, S.; Lin, H.; Chen, J. Facile synthesis of Fe3O4-graphene@mesoporous SiO2 nanocomposites for efficient removal of Methylene Blue. Appl. Surf. Sci. 2016, 378, 80–86. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Liew, K.M. Multiscale simulation of mechanical properties and microstructure of CNT-reinforced cement-based composites. Comput. Methods Appl. Mech. Eng. 2017, 319, 393–413. [Google Scholar] [CrossRef]
- Tafesse, M.; Kim, H.-K. The role of carbon nanotube on hydration kinetics and shrinkage of cement composite. Compos. Part B Eng. 2019, 169, 55–64. [Google Scholar] [CrossRef]
- Stewart, A.; Schlosser, B.; Douglas, E.P. Surface Modification of Cured Cement Pastes by Silane Coupling Agents. ACS Appl. Mater. Interfaces 2013, 5, 1218–1225. [Google Scholar] [CrossRef]
- Song, J.; Zhao, D.; Han, Z.; Xu, W.; Lu, Y.; Liu, X.; Liu, B.; Carmalt, C.J.; Deng, X.; Parkin, I.P. Super-robust superhydrophobic concrete. J. Mater. Chem. A 2017, 5, 14542–14550. [Google Scholar] [CrossRef]
- Sikora, P.; Elrahman, M.A.; Chung, S.-Y.; Cendrowski, K.; Mijowska, E.; Stephan, D. Mechanical and microstructural properties of cement pastes containing carbon nanotubes and carbon nanotube-silica core-shell structures, exposed to elevated temperature. Cem. Concr. Compos. 2019, 95, 193–204. [Google Scholar] [CrossRef]
- Shao, Q.; Zheng, K.; Zhou, X.; Zhou, J.; Zeng, X. Enhancement of nano-alumina on long-term strength of Portland cement and the relation to its influences on compositional and microstructural aspects. Cem. Concr. Compos. 2019, 98, 39–48. [Google Scholar] [CrossRef]
- Seo, D.; Yun, T.S. NOx removal rate of photocatalytic cementitious materials with TiO2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Sardon, H.; Irusta, L.; Fernández-Berridi, M.J.; Lansalot, M.; Bourgeat-Lami, E. Synthesis of room temperature self-curable waterborne hybrid polyurethanes functionalized with (3-aminopropyl)triethoxysilane (APTES). Polymer 2010, 51, 5051–5057. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, Y.; Liu, G.; She, W. Interface Properties of Nanosilica-Modified Waterborne Epoxy Cement Repairing System. ACS Appl. Mater. Interfaces 2018, 10, 21696–21711. [Google Scholar] [CrossRef]
- Maruthapandian, V.; Saraswathy, V.; Muralidharan, S. Development of solid state embeddable reference electrode for corrosion monitoring of steel in reinforced concrete structures. Cem. Concr. Compos. 2016, 74, 100–108. [Google Scholar] [CrossRef]
- Mahadik, D.; Rao, A.V.; Rao, A.P.; Wagh, P.B.; Ingale, S.V.; Gupta, S.C. Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J. Colloid Interface Sci. 2011, 356, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.M.; Kai, M.; Zhang, L. Carbon nanotube reinforced cementitious composites: An overview. Compos. Part A Appl. Sci. Manuf. 2016, 91, 301–323. [Google Scholar] [CrossRef]
- Li, W.-W.; Ji, W.; Isfahani, F.T.; Wang, Y.; Li, G.; Liu, Y.; Xing, F. Nano-Silica Sol-Gel and Carbon Nanotube Coupling Effect on the Performance of Cement-Based Materials. Nanomaterials 2017, 7, 185. [Google Scholar] [CrossRef]
- Li, Q.; Gao, X.; Xu, S. Multiple effects of nano-SiO2 and hybrid fibers on properties of high toughness fiber reinforced cementitious composites with high-volume fly ash. Cem. Concr. Compos. 2016, 72, 201–212. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, X.; Zhu, B.; Yu, J.; Ho, W.; Yu, J. Hierarchical NiO–SiO2 composite hollow microspheres with enhanced adsorption affinity towards Congo red in water. J. Colloid Interface Sci. 2016, 466, 238–246. [Google Scholar] [CrossRef]
- Lavergne, F.; Belhadi, R.; Carriat, J.; Ben Fraj, A. Effect of nano-silica particles on the hydration, the rheology and the strength development of a blended cement paste. Cem. Concr. Compos. 2019, 95, 42–55. [Google Scholar] [CrossRef]
- Kim, G.; Yoon, H.; Lee, H. Autogenous shrinkage and electrical characteristics of cement pastes and mortars with carbon nanotube and carbon fiber. Constr. Build. Mater. 2018, 177, 428–435. [Google Scholar] [CrossRef]
- John, E.; Matschei, T.; Stephan, D. Nucleation seeding with calcium silicate hydrate—A review. Cem. Concr. Res. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Reales, O.A.M.; Filho, R.D.T. A review on the chemical, mechanical and microstructural characterization of carbon nanotubes-cement based composites. Constr. Build. Mater. 2017, 154, 697–710. [Google Scholar] [CrossRef]
- Gabrielli, L.; Poveda, A.; Jones, J.R.; Jimenez-Barbero, J.; Russo, L.; Nicotra, F.; Cipolla, L. Epoxide Opening versus Silica Condensation during Sol–Gel Hybrid Biomaterial Synthesis. Chem. A Eur. J. 2013, 19, 7856–7864. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Boudissa, N.; Fernandez-Jimenez, A.; Palomo, A. Use of clays in alkaline hybrid cement preparation. The role of bentonites. Mater. Lett. 2018, 233, 134–137. [Google Scholar] [CrossRef]
- El-Gamal, S.M.; Hashem, F.; Amin, M. Influence of carbon nanotubes, nanosilica and nanometakaolin on some morphological-mechanical properties of oil well cement pastes subjected to elevated water curing temperature and regular room air curing temperature. Constr. Build. Mater. 2017, 146, 531–546. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Bragança, M.O.; Portella, K.F.; Bonato, M.M.; Alberti, E.; Marino, C.E.B. Performance of Portland cement concretes with 1% nano-Fe3O4 addition: Electrochemical stability under chloride and sulfate environments. Constr. Build. Mater. 2016, 117, 152–162. [Google Scholar] [CrossRef]
- Wang, R.; Zhuo, D.; Weng, Z.; Wu, L.; Cheng, X.; Zhou, Y.; Wang, J.; Xuan, B. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J. Mater. Chem. A 2015, 3, 9826–9836. [Google Scholar] [CrossRef]
- Pradhan, B.; Srivastava, S.K. Synergistic effect of three-dimensional multi-walled carbon nanotube-graphene nanofiller in enhancing the mechanical and thermal properties of high-performance silicone rubber. Polym. Int. 2014, 63, 1219–1228. [Google Scholar] [CrossRef]
- Monfared, R.M.; Ayatollahi, M.R.; Barbaz Isfahani, R. Synergistic effects of hybrid MWCNT/nanosilica on the tensile and tribological properties of woven carbon fabric epoxy composites. Theor. Appl. Fract. Mech. 2018, 96, 272–284. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Li, P.; Chen, D.; Xu, Y.; Feng, Y.; Yu, Y.; Zhang, H. Effect of characteristics of chemical combined of graphene oxide-nanosilica nanocomposite fillers on properties of cement-based materials. Constr. Build. Mater. 2019, 225, 745–753. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Moghimi Monfared, R.; Barbaz Isfahani, R. Experimental investigation on tribological properties of carbon fabric composites: Effects of carbon nanotubes and nano-silica. Proc. Inst. Mech. Eng. L 2017, 233, 874–884. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Barbaz Isfahani, R.; Moghimi Monfared, R. Effects of multi-walled carbon nanotube and nanosilica on tensile properties of woven carbon fabric-reinforced epoxy composites fabricated using VARIM. J. Compos. Mater. 2017, 51, 4177–4188. [Google Scholar] [CrossRef]


| Type of Carbon Nanotube | Diameter (nm) | Length (μm) | Purity | Specific Surface Area (m2/g) |
|---|---|---|---|---|
| Nano silica | 30 | — | 99.9% | >600 |
| 1: G-CNT | 8–15 | 50 | 99.9% | >233 |
| 2: OH-G-CNT | 8–15 | 50 | 99.9% | >233 |
| 3: S-MWNT | 10–20 | 0.5–2 | 99.9% | >233 |
| 4: OH-S-MWNT | 8–15 | 0.5–2 | 99.9% | >233 |
| Item | G oil Well Cementitious Composite Mass (%) | Ultrafine Portland Cementitious Composite Mass (%) |
|---|---|---|
| SiO2 | 22.10 | 19.20 |
| Al2O3 | 2.90 | 4.20 |
| Fe2O3 | 4.61 | 2.77 |
| CaO | 65.10 | 62.60 |
| MgO | 1.6 | 1.84 |
| SO3 | 2.2 | 4.41 |
| K2O | 0.35 | 0.91 |
| Na2O | 0.09 | 0.15 |
| LOI (loss on ignition) | 0.5 | 3.23 |
| Mineral composition according to Bogue calculation (%) | ||
| C3S | 64 | 62.9 |
| C2S | 13.6 | 14.2 |
| C3A | 1 | 1 |
| C4AF | 16 | 16.3 |
| Mixture | Ultrafine Portland Cementitious Composite (kg/m3) | Water (kg/m3) | Super-Plasticizer | Defoamer | CNT | Nano Silica | SCA |
|---|---|---|---|---|---|---|---|
| 1-1 | 900 | 342 | 9 | 1.8 | 3.6 | 14.4 | 13.5 |
| 1-2 | 900 | 342 | 9 | 1.8 | 7.2 | 10.8 | 13.5 |
| 1-3 | 900 | 342 | 9 | 1.8 | 10.8 | 7.2 | 13.5 |
| 1-4 | 900 | 342 | 9 | 1.8 | 14.4 | 3.6 | 13.5 |
| 2-1 | 900 | 342 | 9 | 1.8 | 3.6 | 14.4 | 13.5 |
| 2-2 | 900 | 342 | 9 | 1.8 | 7.2 | 10.8 | 13.5 |
| 2-3 | 900 | 342 | 9 | 1.8 | 10.8 | 7.2 | 13.5 |
| 2-4 | 900 | 342 | 9 | 1.8 | 14.4 | 3.6 | 13.5 |
| 3-1 | 900 | 342 | 9 | 1.8 | 3.6 | 14.4 | 13.5 |
| 3-2 | 900 | 342 | 9 | 1.8 | 7.2 | 10.8 | 13.5 |
| 3-3 | 900 | 342 | 9 | 1.8 | 10.8 | 7.2 | 13.5 |
| 3-4 | 900 | 342 | 9 | 1.8 | 14.4 | 3.6 | 13.5 |
| 4-1 | 900 | 342 | 9 | 1.8 | 3.6 | 14.4 | 13.5 |
| 4-2 | 900 | 342 | 9 | 1.8 | 7.2 | 10.8 | 13.5 |
| 4-3 | 900 | 342 | 9 | 1.8 | 10.8 | 7.2 | 13.5 |
| 4-4 | 900 | 342 | 9 | 1.8 | 14.4 | 3.6 | 13.5 |
| 5 | 900 | 342 | 9 | 1.8 | |||
| 6 | 900 | 342 | 9 | 1.8 | 10.8 | 7.2 | 13.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Shi, Y. High-Strength, Waterproof, Corrosion-Resistant Nano-Silica Carbon Nanotube Cementitious Composites. Materials 2020, 13, 3737. https://doi.org/10.3390/ma13173737
Li H, Shi Y. High-Strength, Waterproof, Corrosion-Resistant Nano-Silica Carbon Nanotube Cementitious Composites. Materials. 2020; 13(17):3737. https://doi.org/10.3390/ma13173737
Chicago/Turabian StyleLi, Hao, and Yongmin Shi. 2020. "High-Strength, Waterproof, Corrosion-Resistant Nano-Silica Carbon Nanotube Cementitious Composites" Materials 13, no. 17: 3737. https://doi.org/10.3390/ma13173737
APA StyleLi, H., & Shi, Y. (2020). High-Strength, Waterproof, Corrosion-Resistant Nano-Silica Carbon Nanotube Cementitious Composites. Materials, 13(17), 3737. https://doi.org/10.3390/ma13173737




