Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- Highly porous HA was prepared from aragonitic cuttlefish bone;
- There were four groups for each material: One group without HA particles added and three groups where the powders were modified by incorporation of 2, 5, and 10 wt% HA, respectively;
- Porous HA incorporated into the Fuji IX groups had a negative effect on CS, DTS, and FS;
- CS was significantly reduced in Fuji IX 2 wt% HA and Fuji IX 10 wt% HA groups, and FS in Fuji IX 5 wt% HA, compared to the Fuji IX group without HA particles added;
- The addition of HA particles in Fuji II had a positive impact on CS, DTS, and FS. Fuji II groups modified with 10 wt% HA showed the most favorable results with respect to FS.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Ngo, H.; Opsahl-Vital, S. Minimal intervention in cariology: The role of glass-ionomer cements in the preservation of tooth structures against caries. Br. Dent. J. 2014, 216, 561–565. [Google Scholar] [CrossRef]
- Peutzfeldt, A.; García-Godoy, F.; Asmussen, E. Surface hardness and wear of glass ionomers and compomers. Am. J. Dent. 1997, 10, 15–17. [Google Scholar] [PubMed]
- Wilson, A.D.; Kent, B.E. Surgical Cement. British Patent No. 1316129, 9 May 1973. [Google Scholar]
- Davidson, C.L. Advances in glass-ionomer cements. J. Appl. Oral. Sci. 2006, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Mauro, Y.; Nishigawa, G.; Suzuki, K.; Watts, D.C. Class I gap formation in highly viscous glass ionomer restorations: Delayed vs. immediate polishing. J. Oper. Dent. 2008, 33, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Šalinović, I.; Stunja, M.; Schauperl, Z.; Verzak, Ž.; Ivanišević Malčić, A.; Brzović Rajić, V. Mechanical Properties of High Viscosity Glass Ionomer and Glass Hybrid Restorative Materials. Acta Stomatol. Croat. 2019, 53, 125–131. [Google Scholar] [CrossRef]
- Mitra, S.B. Adhesion to dentin and physical properties of a light-cured glass-ionomer liner/base. J. Dent. Res. 1991, 70, 72–74. [Google Scholar] [CrossRef]
- Kerby, R.E.; Bleiholder, R.F. Physical properties of stainless-steel and silver-reinforced glass-ionomer cements. J. Dent. Res. 1991, 70, 1358–1361. [Google Scholar] [CrossRef]
- Gu, Y.W.; Yap, A.U.; Cheang, P.; Khor, K.A. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC). Biomaterials 2005, 26, 713–720. [Google Scholar] [CrossRef]
- Boyd, D.; Towler, M.R. The processing, mechanical properties and bioactivity of zinc-based glass ionomer cements. J. Mater. Sci. Mater. Med. 2005, 16, 843–850. [Google Scholar] [CrossRef]
- Lohbauer, U.; Walker, J.; Nikolaenko, S.; Werner, J.; Clare, A.; Petschelt, A.; Greil, P. Reactive fibre reinforced glass ionomer cements. Biomaterials 2003, 24, 2901–2907. [Google Scholar] [CrossRef]
- Menezes-Silva, R.; de Oliveira, B.M.B.; Fernandes, P.H.M.; Shimohara, L.Y.; Pereira, F.V.; Borges, A.F.S.; Buzalaf, M.A.R.; Pascotto, R.C.; Sidhu, S.K.; de Lima Navarro, M.F. Effects of the reinforced cellulose nanocrystals on glass-ionomer cement. Dent. Mater. 2019, 35, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Poosti, M.; Ramazanzadeh, B.; Zebarjad, M.; Javadzadeh, P.; Naderinasab, M.; Shakeri, M.T. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur. J. Orthod. 2013, 35, 676–679. [Google Scholar] [CrossRef]
- Alatawi, R.A.S.; Elsayed, N.H.; Mohamed, W.S. Influence of hydroxyapatite nanoparticles on the properties of glass ionomer cement. J. Mater. Res. Technol. 2019, 344–349. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabrić, D.; Guclu, Z.A.; Miletić, I.; Coleman, N.J. Assessment of the Impact of the Addition of Nanoparticles on the Properties of Glass-Ionomer Cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Chay, P.L. Development of HA-GIC Composites; Honors Year Project Nanyang Technological University: Singapore, 2000. [Google Scholar]
- Yap, A.U.J.; Pek, Y.S.; Kumar, R.A.; Cheang, P.; Khor, K.A. Experimental studies on a new bioactive material: HAIonomer cements. Biomaterials 2002, 23, 955–962. [Google Scholar] [CrossRef]
- Nishimura, T.; Shinonaga, Y.; Abe, Y.; Kawai, S.; Arita, K. Porous hydroxyapatite can improve strength and bioactive functions of glass ionomer cement. Nano Biomed. 2014, 6, 53–62. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef]
- Rogina, A.; Antunović, M.; Milovac, D. Biomimetic design of bone substitutes based on cuttlefish bone-derived hydroxyapatite and biodegradable polymers. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 197–204. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef]
- Yamamura, H.; da Silva, V.H.P.; Ruiz, P.L.M.; Ussui, V.; Lazar, D.R.R.; Renno, A.C.M.; Ribeiro, D.A. Physico-chemical characterization and biocompatibility of hydroxyapatite derived from fish waste. J. Mech. Behav. Biomed. Mater. 2018, 80, 137–142. [Google Scholar] [CrossRef]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Shinonaga, Y.; Abe, Y.; Harada, K.; Arita, K. Influence of Porous Spherical-Shaped Hydroxyapatite on Mechanical Strength and Bioactive Function of Conventional Glass Ionomer Cement. Materials 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1:2007 Dentistry—Water-based cements—Part 1: Powder/liquid acid-base cements; ISO: Geneva, Switzerland, 2017.
- ISO 9917-2:2017 Dentistry—Water-based cements—Part 2: Resin-modified cements; ISO: Geneva, Switzerland, 2017.
- American National Standard/American Dental Association Standard No. 27-2016—Polymer-based Restorative Matreials; American Dental Association: Chicago, IL, USA, 2016.
- Domingo, C.; Arcís, R.W.; López-Macipe, A.; Osorio, R.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Toledano, M. Dental composites reinforced with hydroxyapatite: Mechanical behaviour and absorption/elution characteristics. J. Biomed. Mater. Res. 2001, 56, 297–305. [Google Scholar] [CrossRef]
- Arita, K.; Yamamoto, A.; Shinonaga, Y.; Harada, K.; Abe, Y.; Nakagawa, K.; Sugiyama, S. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent. Mater. J. 2011, 30, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Sharafeddin, F.; Shoale, S.; Kowkabi, M. Effects of different percentages of microhydroxyapatite on microhardness of resin-modified glass-ionomer and zirconomer. J. Clin. Exp. Dent. 2017, 9, e805–e811. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, Y.K.; Choi, B.J.; Lee, J.H.; Choi, H.J.; Son, H.K.; Hwang, J.W.; Kim, S.O. Physical properties of resin-reinforced glass ionomer cement modified with micro and nano-hydroxyapatite. J. Nanosci. Nanotechnol. 2010, 10, 5270–5276. [Google Scholar] [CrossRef]
- Souza, P.P.; Aranha, A.M.; Hebling, J.; Giro, E.M.; Costa, C.A. In vitro cytotoxicity and in vivo biocompatibility of contemporary resin-modified glass-ionomer cements. Dent. Mater. 2006, 22, 838–844. [Google Scholar] [CrossRef]
- Goenka, S.; Balu, R.; Sampath Kumar, T.S. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J. Mech. Behav. Biomed. Mater. 2012, 7, 69–76. [Google Scholar] [CrossRef]
- Bresciani, E.; Barata, T.; Fagundes, T.C.; Adachi, A.; Terrin, M.M.; Navarro, M.F. Compressive and diametral tensile strength of glass ionomer cements. J. Appl. Oral. Sci. 2004, 12, 344–348. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial–hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluorapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Sharafeddin, F.; Feizi, N. Evaluation of the effect of adding micro-hydroxyapatite and nano-hydroxyapatite on the microleakage of conventional and resin-modified glass-ionomer Cl V restorations. J. Clin. Exp. Dent. 2017, 9, e242–e248. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int. J. Dent. 2013, 831976. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in nanotechnology for the treatment of osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 87–94. [Google Scholar] [CrossRef]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem. Cells. Int. 2016, 7230987. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Stem. Cells. Int. 2018, 2018, 6927401. [Google Scholar] [CrossRef]
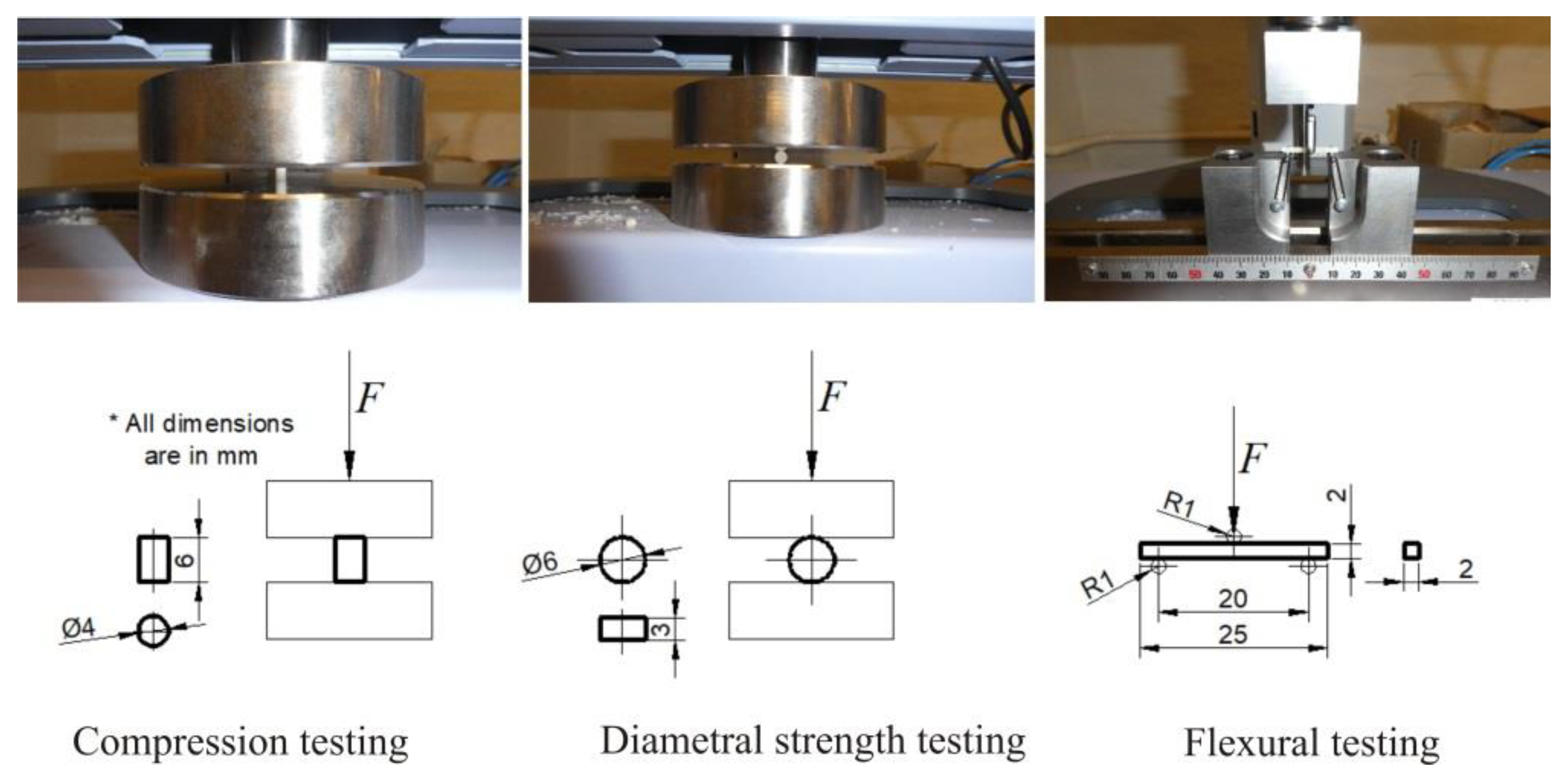
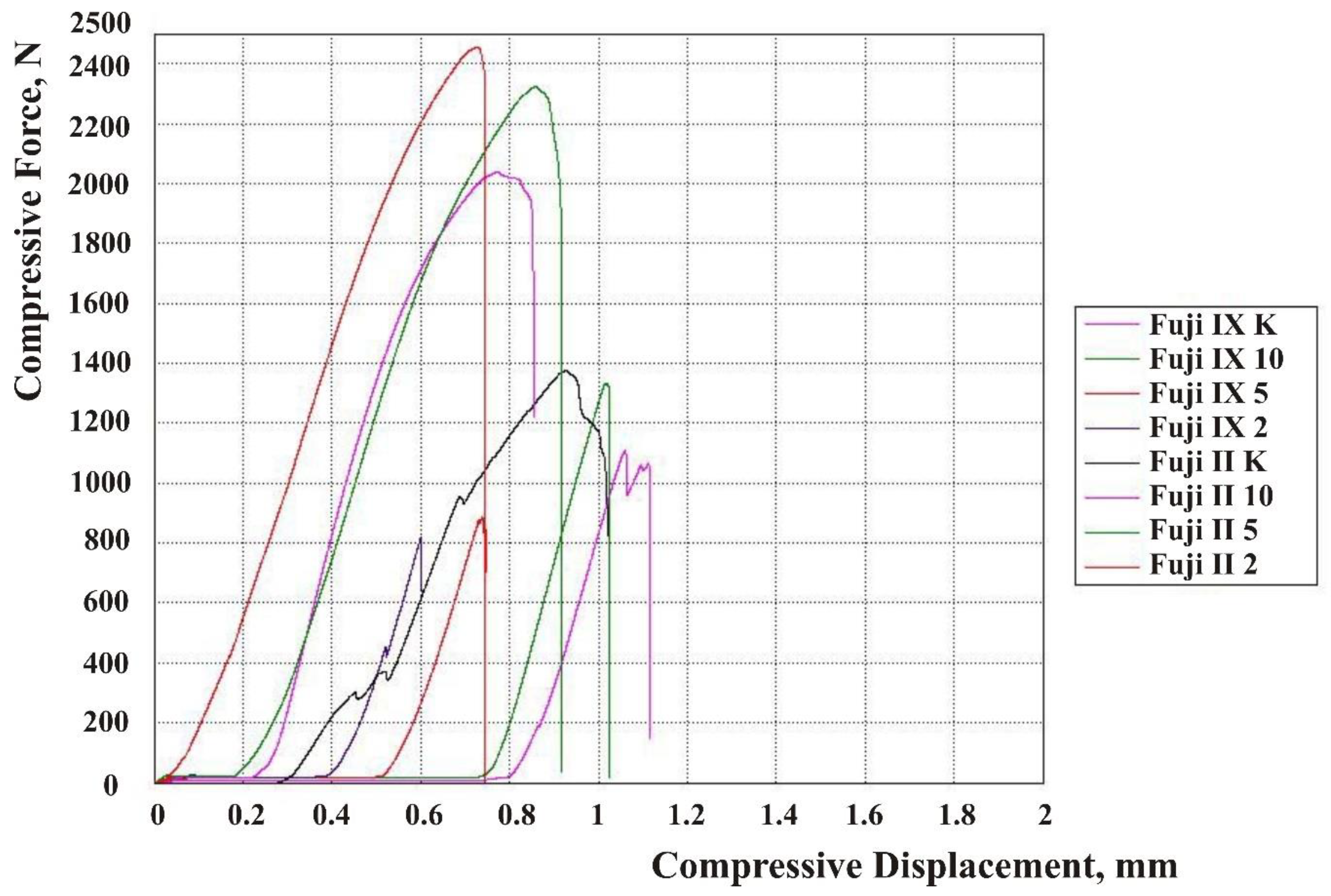

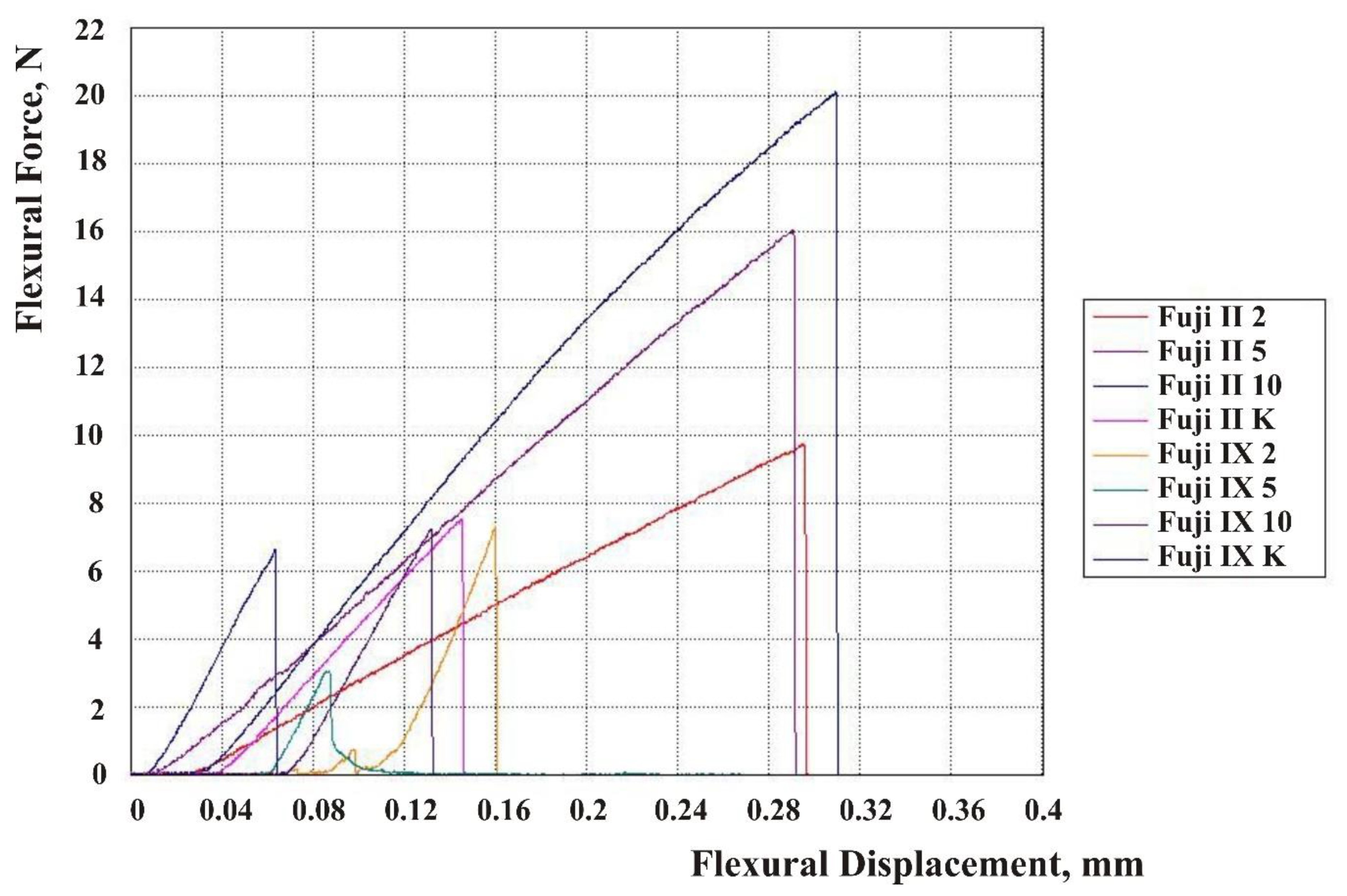
| Material | N | Mean | Standard Deviation | 95% CI Lower Bound | 95% CI Upper Bound |
|---|---|---|---|---|---|
| Fuji II 2 | 16 | 153.5 | 36.1 | 134.3 | 172.7 |
| Fuji II 5 | 17 | 158.3 | 25.5 | 145.2 | 171.4 |
| Fuji II 10 | 16 | 149.2 | 21.9 | 137.5 | 160.8 |
| Fuji II 0 | 16 | 141.5 | 26.5 | 127.4 | 155.6 |
| Fuji IX 2 | 17 | 80.4 | 31.6 | 64.1 | 96.6 |
| Fuji IX 5 | 18 | 92.3 | 37.4 | 73.6 | 110.9 |
| Fuji IX 10 | 16 | 82.8 | 20.9 | 71.7 | 93.9 |
| Fuji IX 0 | 18 | 111.3 | 31.2 | 95.8 | 126.8 |
| Group | N | Mean | Standard Deviation | 95% CI Lower Bound | 95% CI Upper Bound |
|---|---|---|---|---|---|
| Fuji II 2 | 15 | 13.8 | 3.7 | 11.7 | 15.8 |
| Fuji II 5 | 16 | 14.3 | 2.6 | 12.9 | 15.7 |
| Fuji II 10 | 16 | 12.3 | 3.1 | 10.6 | 14.0 |
| Fuji II 0 | 16 | 12.3 | 4.0 | 10.1 | 14.4 |
| Fuji IX 2 | 18 | 5.8 | 2.0 | 4.8 | 6.8 |
| Fuji IX 5 | 17 | 5.4 | 2.3 | 4.2 | 6.6 |
| Fuji IX 10 | 16 | 3.7 | 1.7 | 2.8 | 4.5 |
| Fuji IX 0 | 15 | 5.5 | 1.8 | 4.5 | 6.5 |
| Material | N | Mean | Standard Deviation | 95% CI Lower Bound | 95% CI Upper Bound |
|---|---|---|---|---|---|
| Fuji II 2 | 17 | 41.0 | 7.7 | 37.1 | 45.0 |
| Fuji II 5 | 17 | 41.4 | 8.4 | 37.1 | 45.7 |
| Fuji II 10 | 16 | 48.4 | 8.7 | 43.7 | 53.0 |
| Fuji II 0 | 16 | 36.3 | 10.8 | 30.5 | 42.0 |
| Fuji IX 2 | 11 | 12.9 | 3.1 | 10.8 | 15.0 |
| Fuji IX 5 | 14 | 11.0 | 3.0 | 9.3 | 12.7 |
| Fuji IX 10 | 16 | 13.7 | 3.4 | 11.9 | 15.5 |
| Fuji IX 0 | 12 | 15.6 | 5.0 | 12.4 | 18.7 |
| Factor | CS | DTS | FS |
|---|---|---|---|
| Material | <0.0001 | <0.0001 | <0.0001 |
| HA | 0.38 | 0.06 | 0.14 |
| Material * HA | 0.01 | 0.15 | 0.0003 |
| Material | CS | DTS | FS | |
|---|---|---|---|---|
| Fuji II 2 | 153.5 | 13.8 | 41.0 | - |
| Fuji II 5 | 158.3 | 14.3 | 41.4 | - |
| Fuji II 10 | 149.2 | 12.3 | 48.4 | a |
| Fuji II 0 | 141.5 | 12.3 | 36.3 | a |
| p * | 0.37 | - | 0.004 | - |
| Material | CS | DTS | FS | ||
|---|---|---|---|---|---|
| Fuji IX 2 | 80.4 | a | 5.8 | 12.9 | - |
| Fuji IX 5 | 92.3 | - | 5.4 | 11.0 | a |
| Fuji IX 10 | 82.8 | b | 3.7 | 13.7 | - |
| Fuji IX 0 | 111.3 | ab | 5.5 | 15.6 | a |
| p * | 0.019 | - | - | 0.023 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilić-Prcić, M.; Rajić, V.B.; Ivanišević, A.; Pilipović, A.; Gurgan, S.; Miletić, I. Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite. Materials 2020, 13, 3542. https://doi.org/10.3390/ma13163542
Bilić-Prcić M, Rajić VB, Ivanišević A, Pilipović A, Gurgan S, Miletić I. Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite. Materials. 2020; 13(16):3542. https://doi.org/10.3390/ma13163542
Chicago/Turabian StyleBilić-Prcić, Maja, Valentina Brzović Rajić, Ana Ivanišević, Ana Pilipović, Sevil Gurgan, and Ivana Miletić. 2020. "Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite" Materials 13, no. 16: 3542. https://doi.org/10.3390/ma13163542
APA StyleBilić-Prcić, M., Rajić, V. B., Ivanišević, A., Pilipović, A., Gurgan, S., & Miletić, I. (2020). Mechanical Properties of Glass Ionomer Cements after Incorporation of Marine Derived Hydroxyapatite. Materials, 13(16), 3542. https://doi.org/10.3390/ma13163542








