Effect of Pressure and Temperature on CO2/CH4 Competitive Adsorption on Kaolinite by Monte Carlo Simulations
Abstract
1. Introduction
2. Simulation Methods
2.1. Models
2.2. Interaction Potential Model
2.3. Simulation Details
3. Results and Discussion
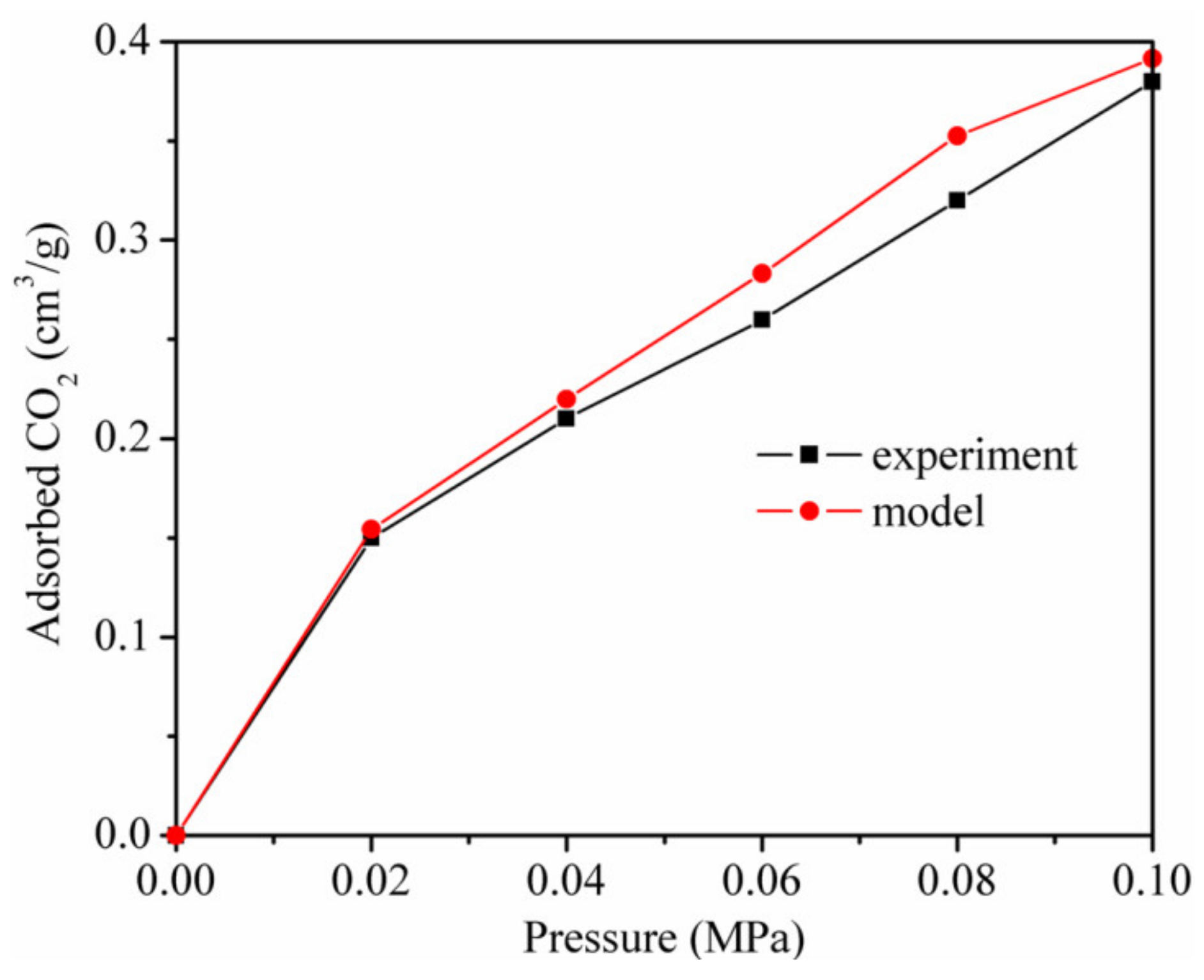
3.1. Validation
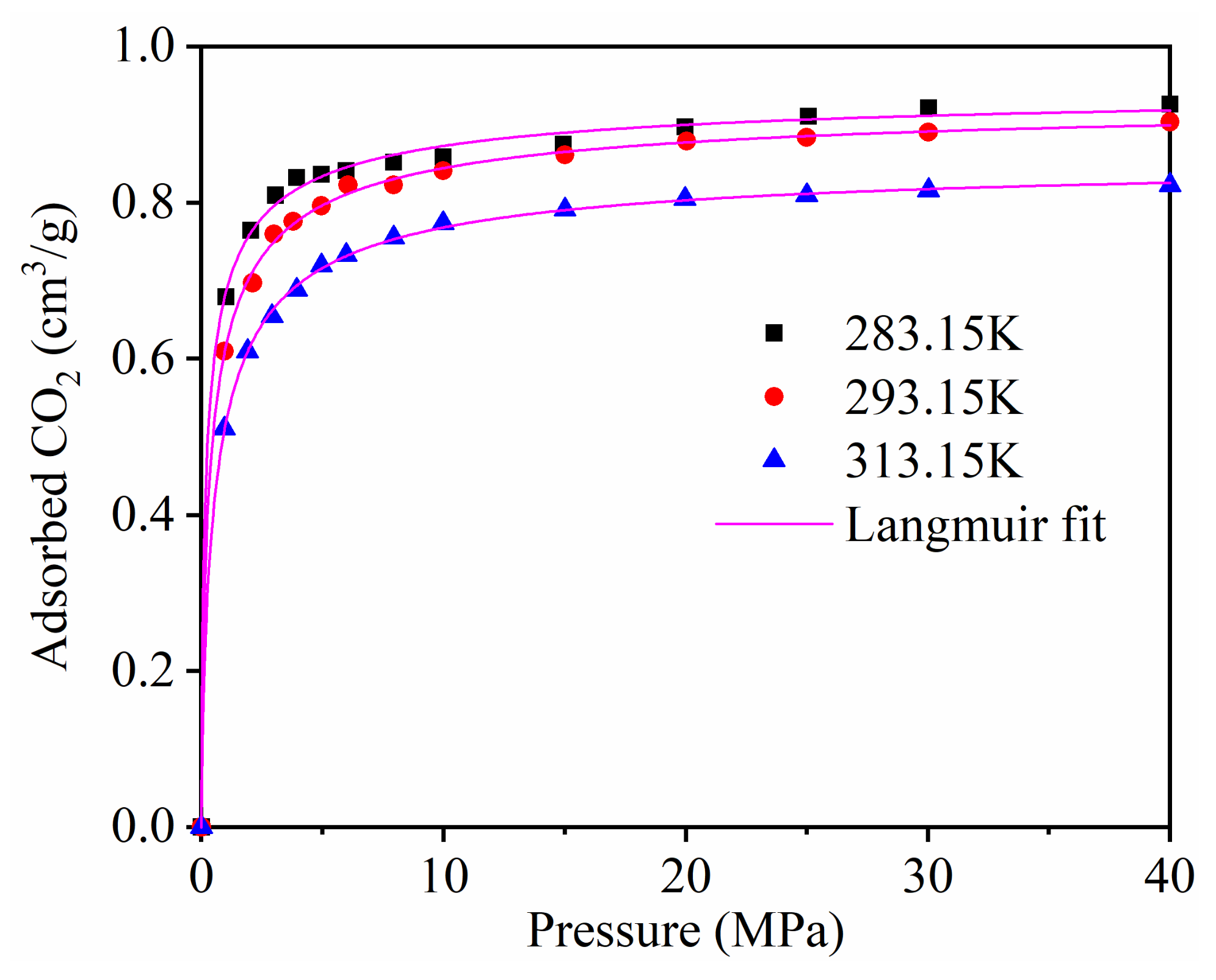
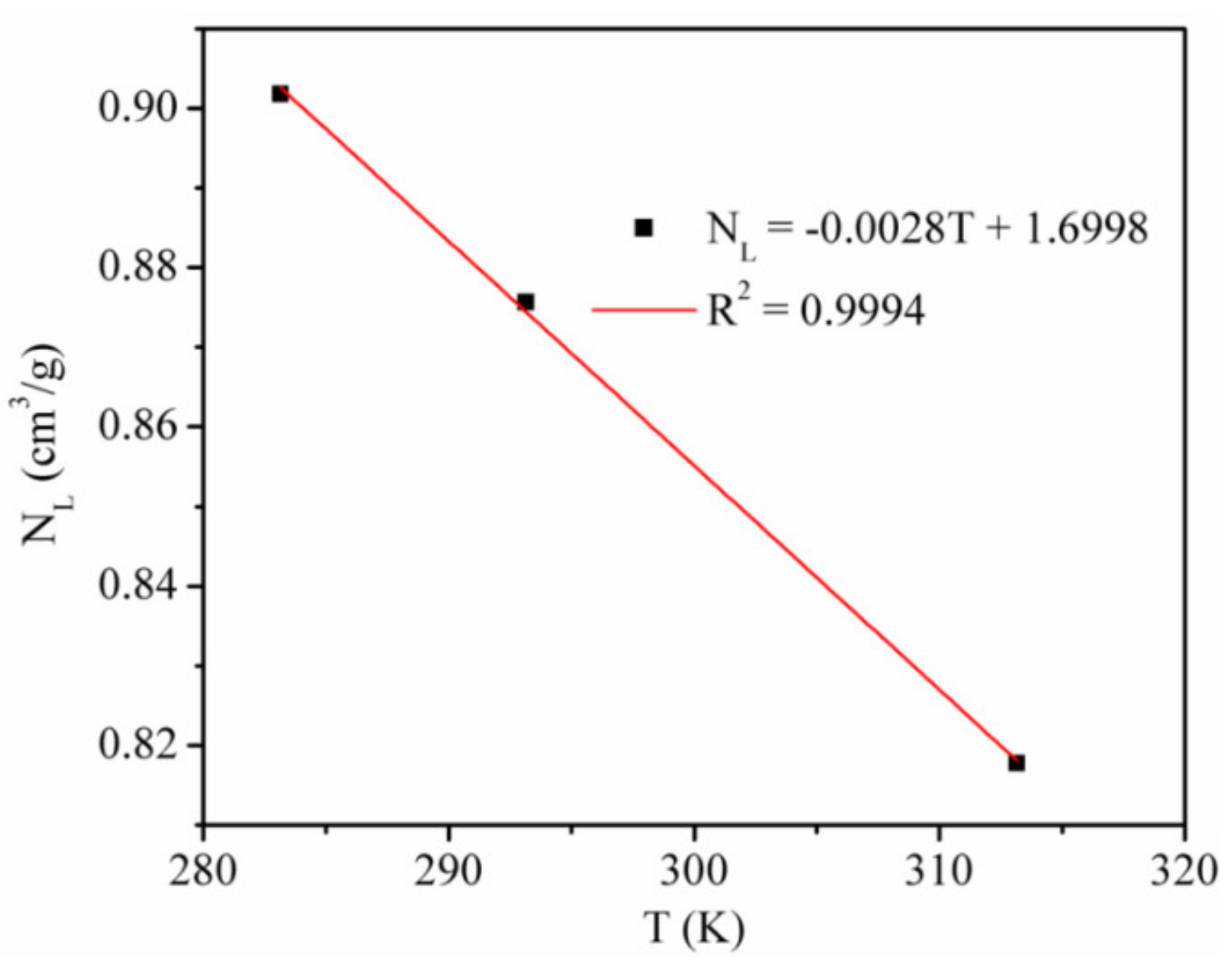
3.2. Single Component Adsorption
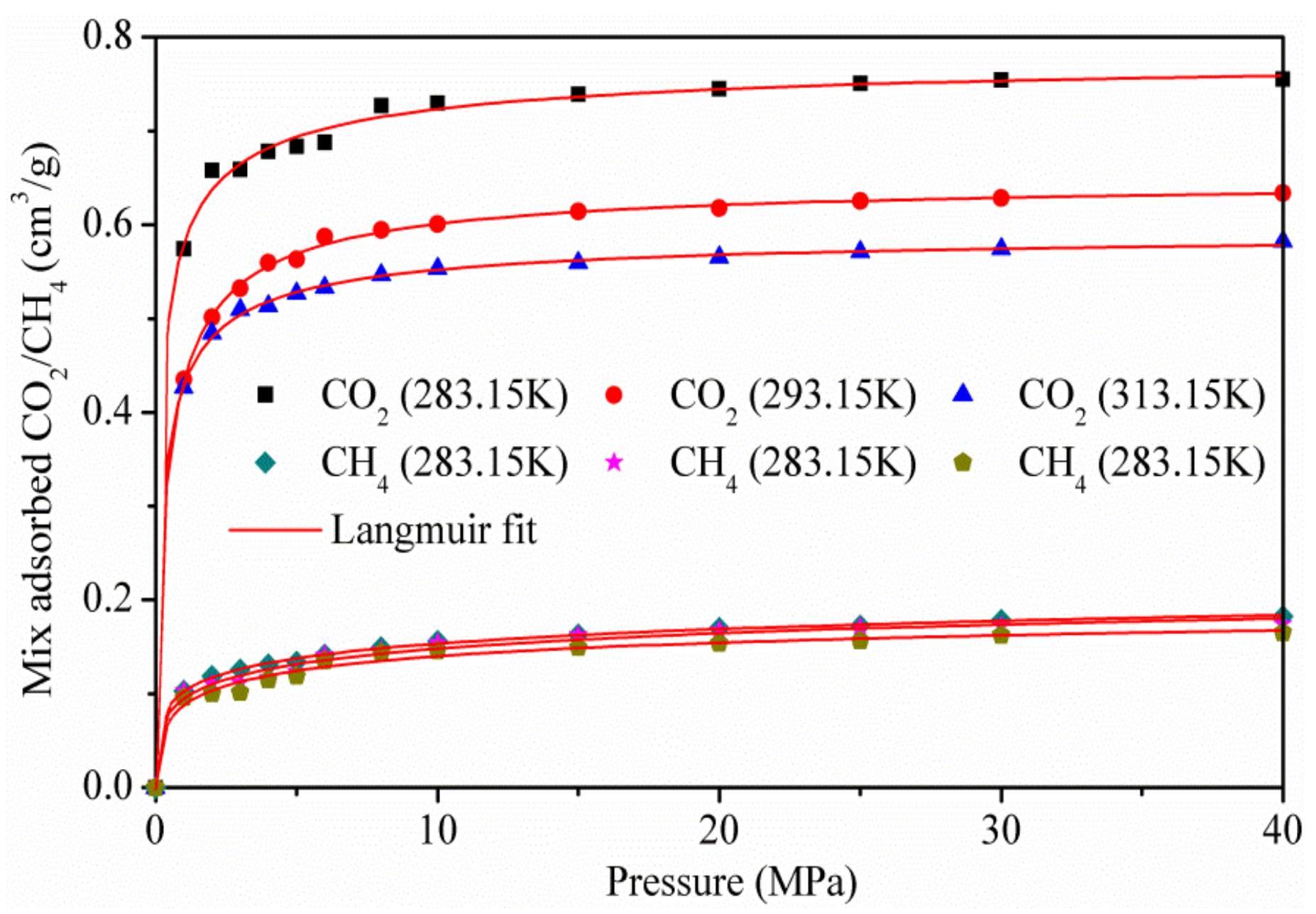
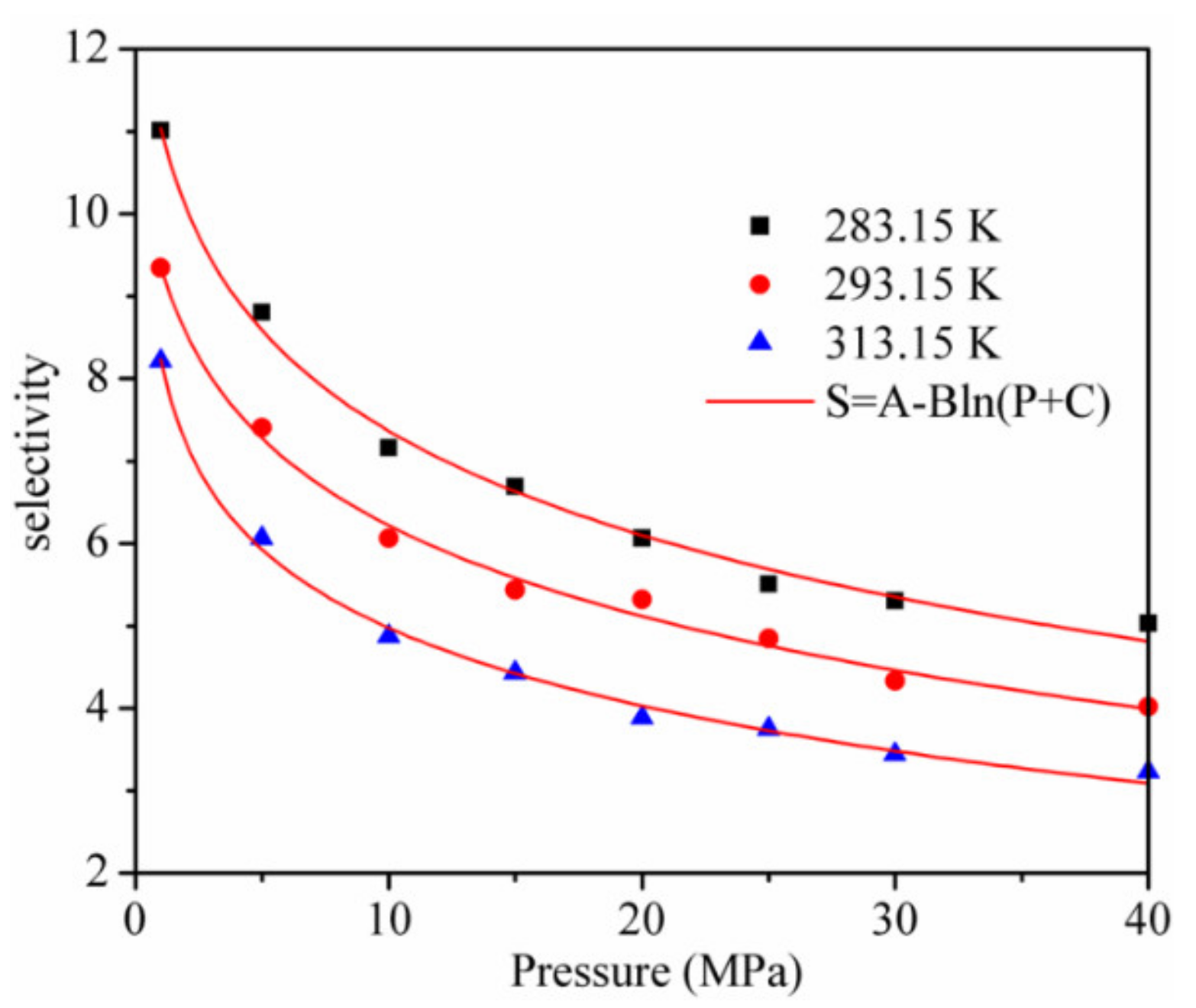
3.3. Adsorption of CO2/CH4 Mixtures
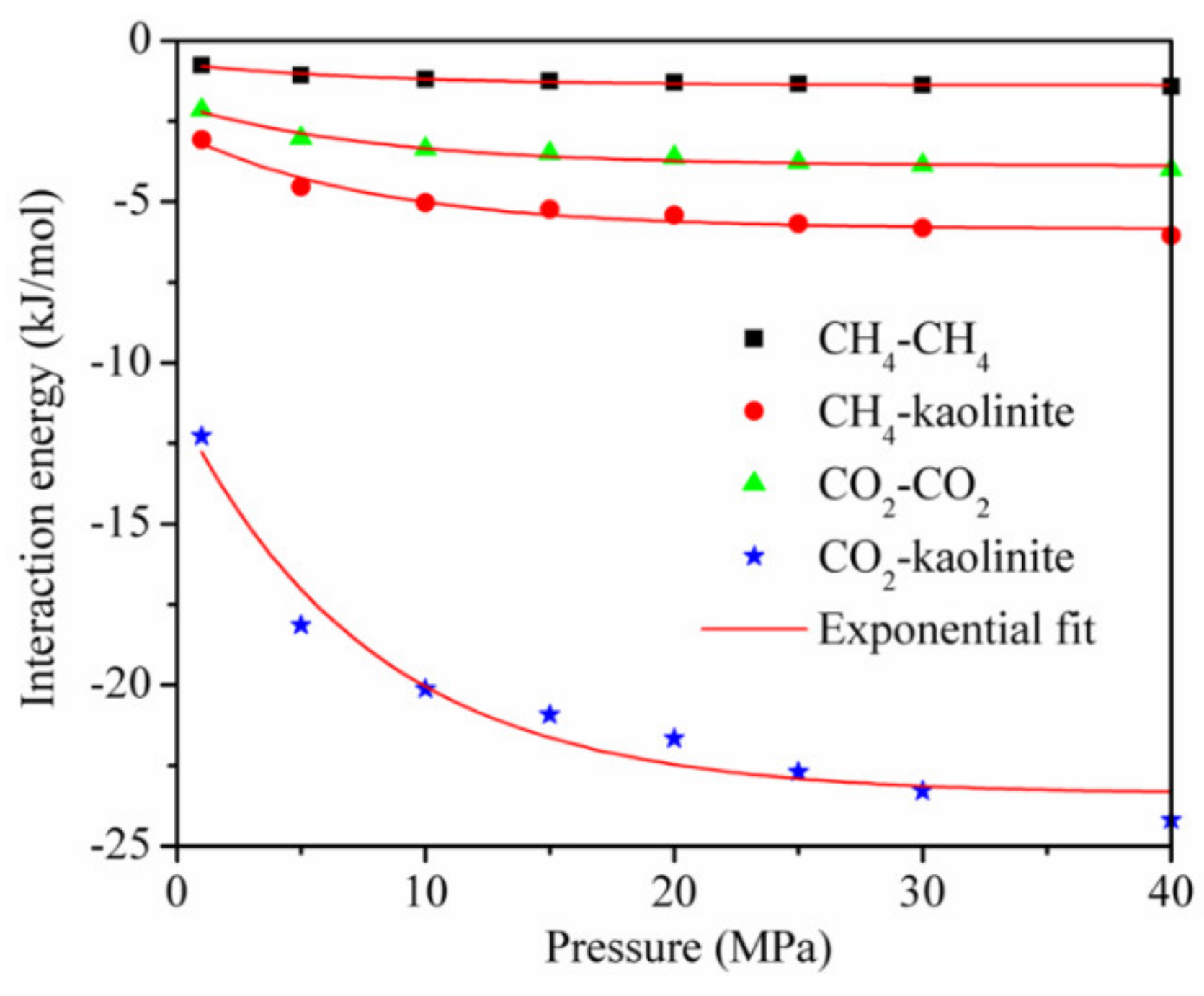
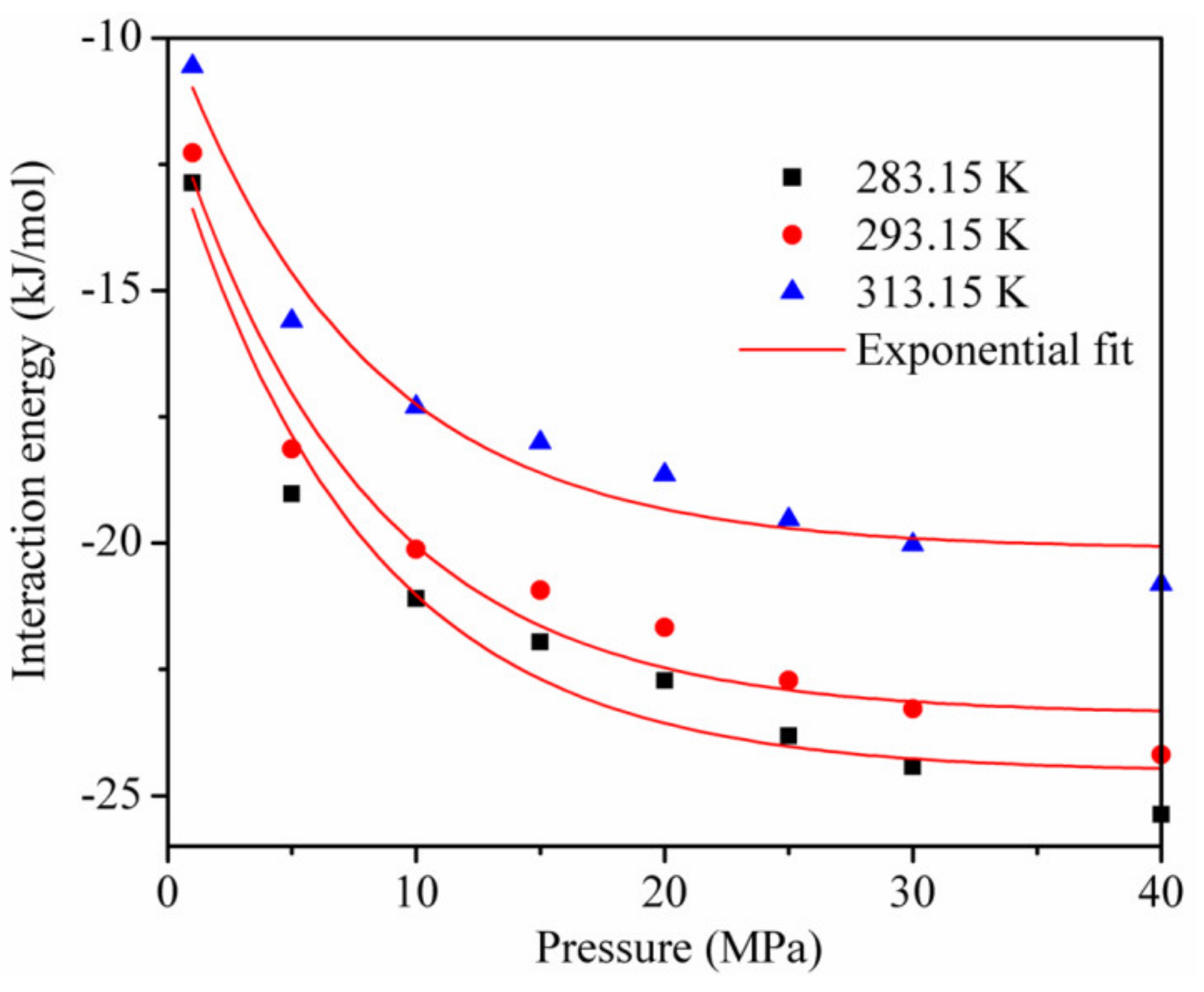
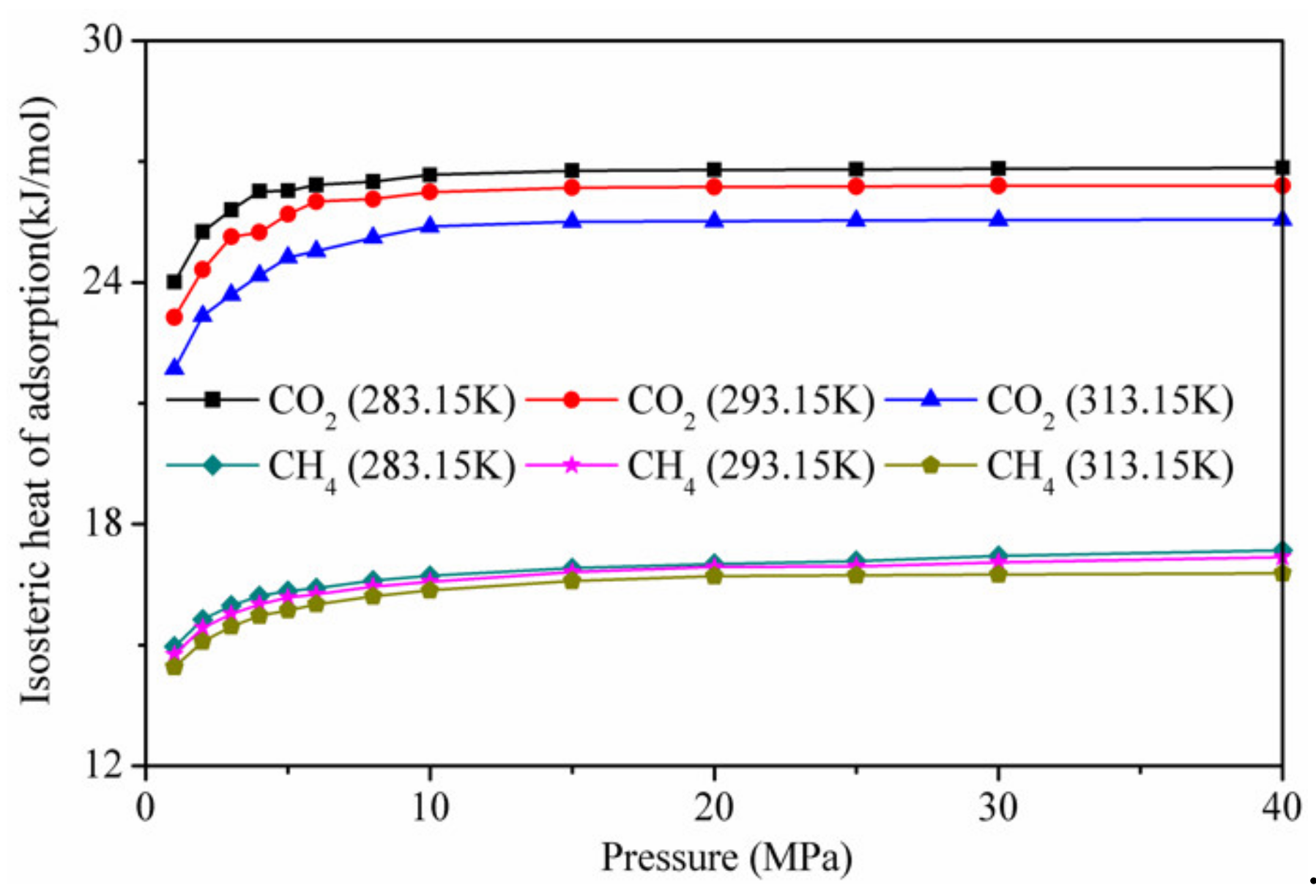
3.4. Interaction Energies and Isosteric Heat Analysis during CH4/CO2 Adsorption
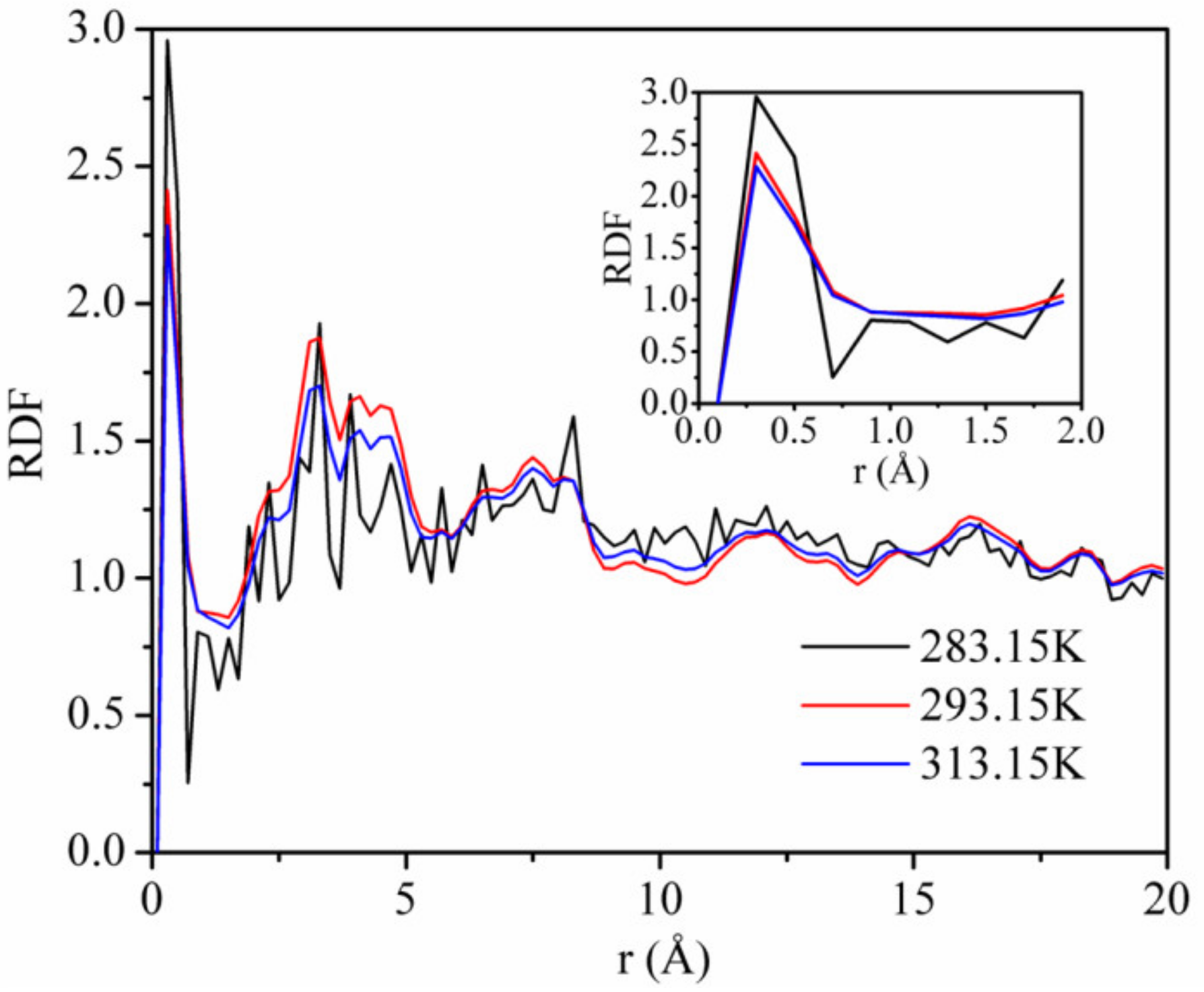
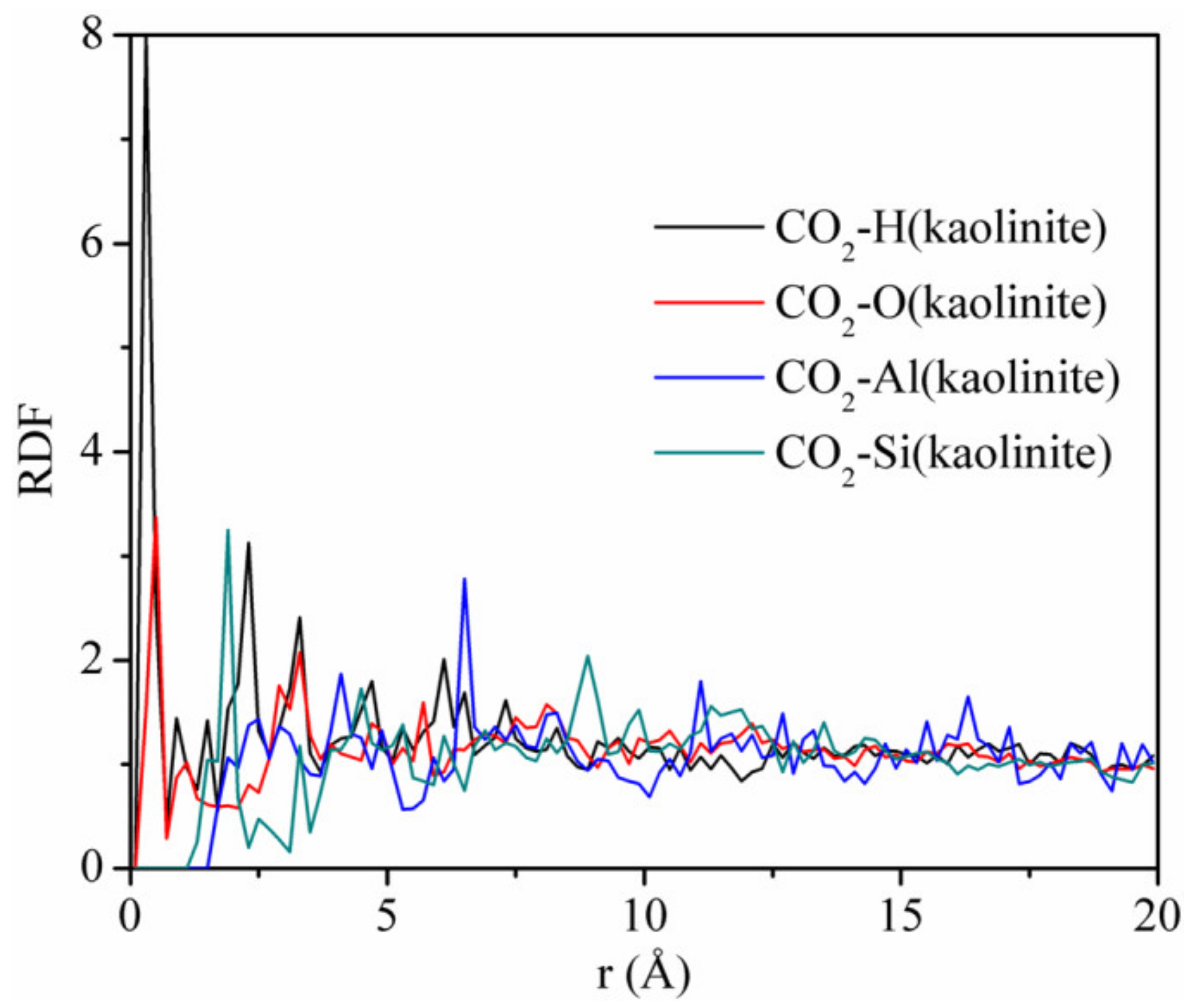
3.5. Radial Distribution Function
4. Conclusions
- (1)
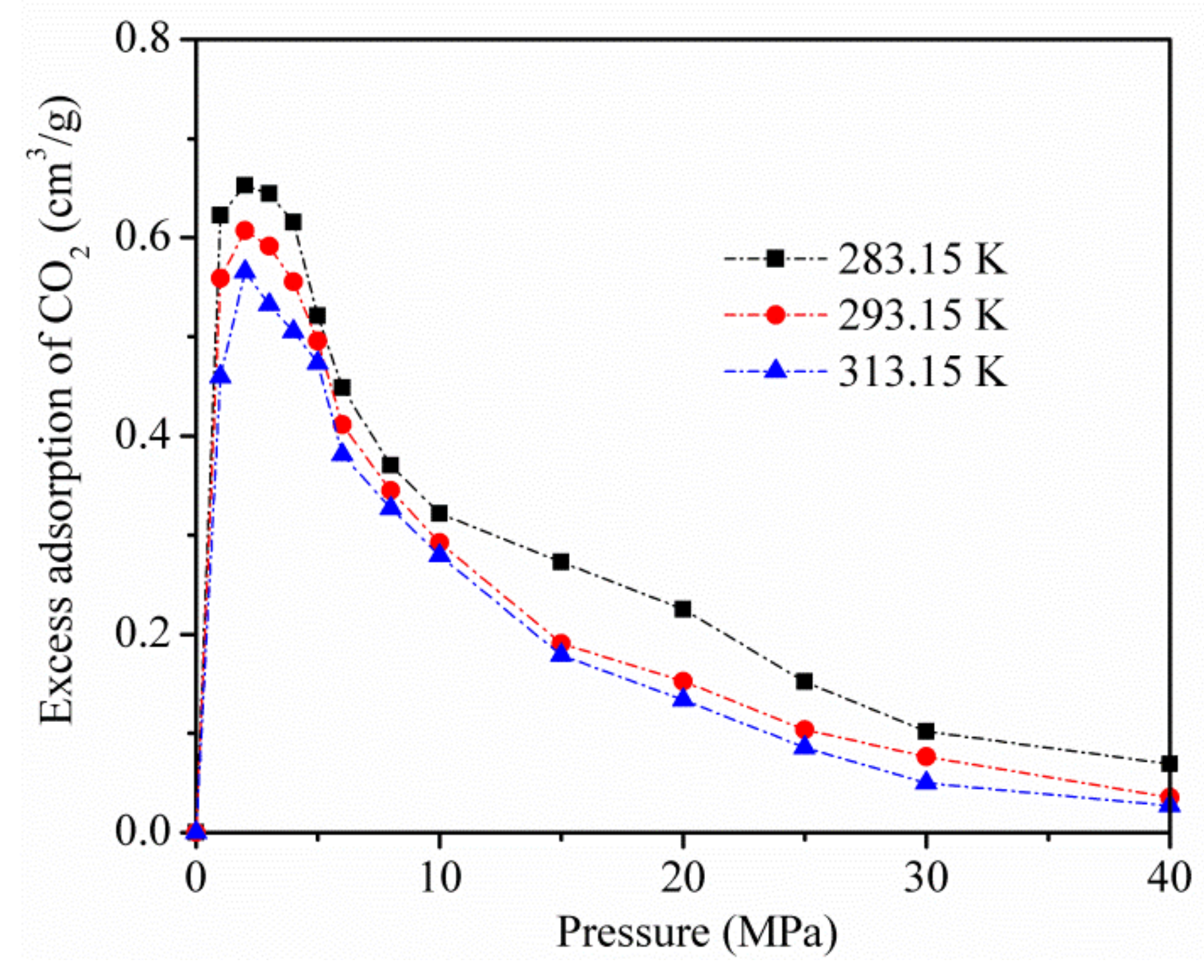
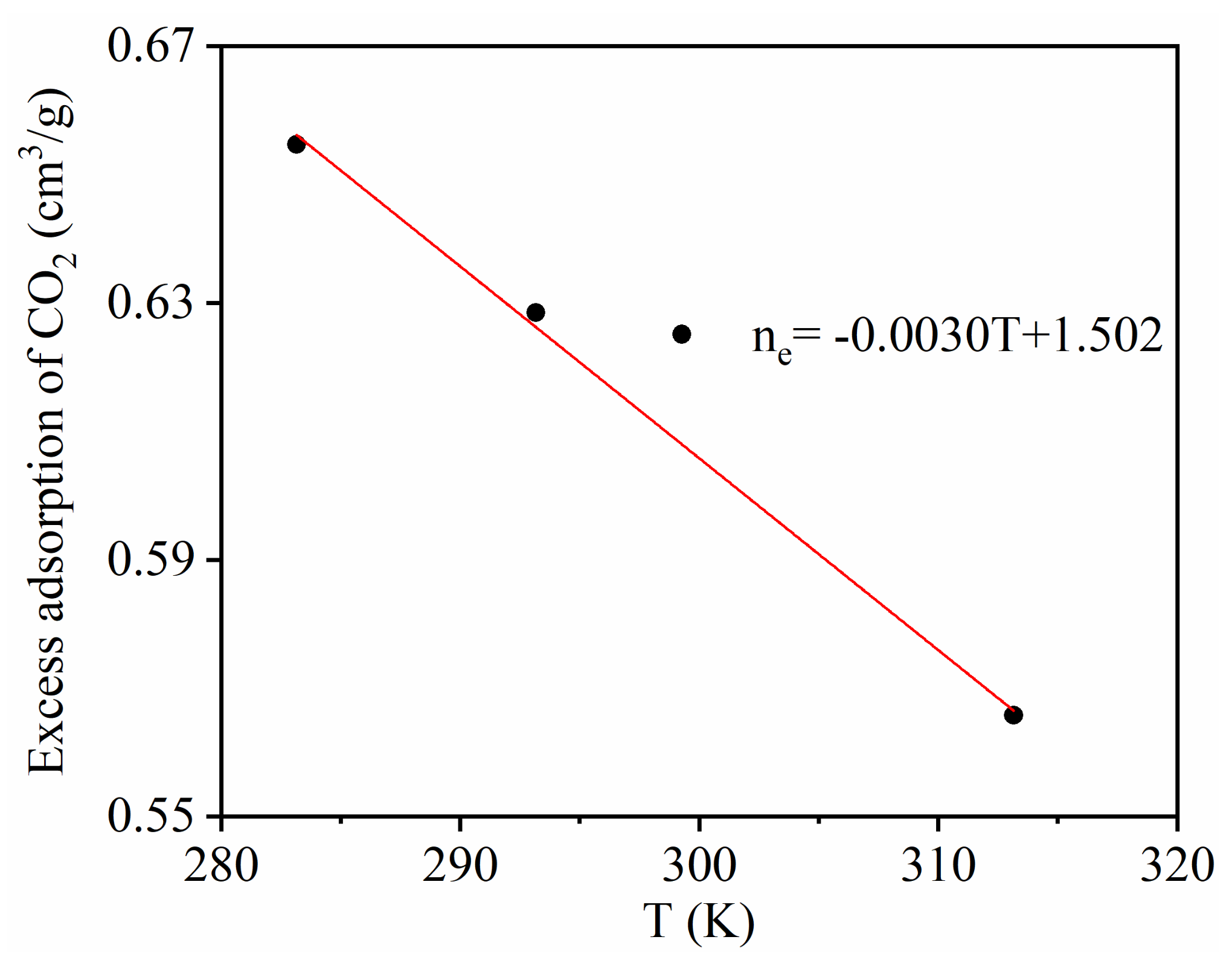
- The adsorption capacity of CO2 increased with an increase in the adsorption equilibrium pressure, and increased rapidly at low pressure. The adsorption amount of CO2 decreased as the temperature increased. Excessive adsorption at different temperatures increases to a maximum (about 3 MPa) as the pressure increases, and then decreases at a higher pressure;
- (2)
- The decreased logarithmically with increasing pressure, and the was lower with a higher temperature at the same pressure;
- (3)
- The interaction energy between the adsorbent and adsorbate was dominant, and that between CO2 and CO2 and between CH4 and CH4 accounted for less than 20% of the total interaction energy, respectively, during the adsorption of the CO2/CH4 binary mixture. The isothermal adsorption heat of CO2 was higher than that of CH4, indicating that the affinity of kaolinite to CO2 was higher than that of CH4;
- (4)
- The strong adsorption sites of CO2 on kaolinite are hydrogen, oxygen, and silicon atoms, respectively. CO2 is not only physically adsorbed on kaolinite, but also displays chemical adsorption;
- (5)
- By considering the results of adsorption, the capture and sequestration of CO2 and enhanced CS-EGR should be carried out at low temperatures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keith, D.W. Why capture CO2 from the atmosphere? Science 2009, 325, 1654. [Google Scholar] [CrossRef]
- Lashof, D.A.; Ahuja, D.R. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 mineral carbonation of oil shale ash. J. Hazard. Mater. 2010, 174, 209. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.K.; Shareef, F.H. Using natural clays and spent bleaching clay as cheap adsorbent for the removal of phenol in aqueous media. Int. J. Basic Appl. Sci. 2013, 13, 88–93. [Google Scholar]
- Holmboe, M.; Bourg, I.C. Molecular Dynamics Simulations of Water and Sodium Diffusion in Smectite Interlayer Nanopores as a Function of Pore Size and Temperature. Chem. Soc. Rev. 2017, 42, 3628–3646. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Liu, H.; Li, T.; Tan, D.; Yuan, W.; He, H. High-pressure adsorption of methane on montmorillonite, kaolinite and illite. Appl. Clay Sci. 2013, 85, 25–30. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Clennell, M.B.; Dewhurst, D.N.; Pervukhina, M. Molecular simulation of CO2–CH4 competitive adsorption and induced coal swelling. Fuel 2015, 160, 309–317. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Zhang, W.; Cheng, Z.; Wu, X.; Qin, H. Effect of organic type and moisture on CO 2/CH 4 competitive adsorption in kerogen with implications for CO 2 sequestration and enhanced CH 4 recovery. Appl. Energy 2018, 210, 28–43. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Zheng, Q.; Pan, Z.; Guo, Q. Evaluation of geological features for deep coalbed methane reservoirs in the Dacheng Salient, Jizhong Depression, China. Int. J. Coal Geol. 2014, 133, 60–71. [Google Scholar] [CrossRef]
- Rother, G.; Ilton, E.S.; Wallacher, D.; Hauss, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G.; et al. CO2 Adsorption to Sub-Single Hydration Layer Montmorillonite Clay Studied by Excess Sorption and Neutron Diffraction. Environ. Sci. Technol. 2013, 47, 205–211. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4. [Google Scholar] [CrossRef]
- Yang, N.; Liu, S.; Yang, X. Molecular simulation of preferential adsorption of CO2 over CH4 in Na-montmorillonite clay material. Appl. Surf. Sci. 2015, 356, 1262–1271. [Google Scholar] [CrossRef]
- Jin, Z.; Firoozabadi, A. Methane and carbon dioxide adsorption in clay-like slit pores by Monte Carlo simulations. Fluid Phase Equilibria 2013, 360, 456–465. [Google Scholar] [CrossRef]
- Benazzouz, B.K.; Zaoui, A.; Belonoshko, A.B. Determination of the melting temperature of kaolinite by means of the Z-method. Am. Mineral. 2013, 98, 1881–1885. [Google Scholar] [CrossRef]
- Zhang, B.; Kai, W.; Kang, T.; Kang, G.; Zhao, G. Effect of the basal spacing on CH4 diffusion in kaolinite. Chem. Phys. Lett. 2019, 732, 136639. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld Refinement of Non-Hydrogen Atomic Positions in Kaolinite. Clays Clay Miner. 1989, 37, 289–296. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Monte Carlo simulations of methane adsorption on kaolinite as a function of pore size. J. Nat. Gas Sci. Eng. 2018, 49, 410–416. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Effect of water on methane adsorption on the kaolinite (001) surface based on molecular simulations. Appl. Surf. Sci. 2018, 439. [Google Scholar] [CrossRef]
- Biase, E.D.; Sarkisov, L. Systematic development of predictive molecular models of high surface area activated carbons for adsorption applications. Carbon 2013, 64, 262–280. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; Alavi, S.; Ripmeester, J.A.; Englezos, P. Evolution of methane during gas hydrate dissociation. Fluid Phase Equilibria 2013, 358, 114–120. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve and Critical Properties as Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Sharma, A.; Namsani, S.; Singh, J.K. Molecular simulation of shale gas adsorption and diffusion in inorganic nanopores. Mol. Simul. 2015, 41, 414–422. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Zhang, J.; Burke, N.; Zhang, S.; Liu, K.; Pervukhina, M. Thermodynamic analysis of molecular simulations of CO 2 and CH 4 adsorption in FAU zeolites. Chem. Eng. Sci. 2014, 113, 54–61. [Google Scholar] [CrossRef]
- Rutkai, G.; Kristóf, T. Molecular simulation study of intercalation of small molecules in kaolinite. Chem. Phys. Lett. 2008, 462, 269–274. [Google Scholar] [CrossRef]
- Yeh, I.C.; Berkowitz, M.L. Ewald summation for systems with slab geometry. J. Chem. Phys. 1999, 111, 3155–3162. [Google Scholar] [CrossRef]
- Crozier, P.S.; Rowley, R.L.; Spohr, E.; Henderson, D. Comparison of charged sheets and corrected 3D Ewald calculations of long-range forces in slab geometry electrolyte systems with solvent molecules. J. Chem. Phys. 2000, 112, 9253–9257. [Google Scholar] [CrossRef][Green Version]
- Fisk, S.; Widom, B. Structure and Free Energy of the Interface between Fluid Phases in Equilibrium near the Critical Point. J. Chem. Phys. 1969, 50, 3219–3227. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 2004, 21, 1087–1092. [Google Scholar] [CrossRef]
- Hirotani, A.; Mizukami, K.; Miura, R.; Takaba, H.; Miya, T.; Fahmi, A.; Stirling, A.; Kubo, M.; Miyamoto, A. Grand canonical Monte Carlo simulation of the adsorption of CO 2 on silicalite and NaZSM-5. Appl. Surf. Sci. 1997, 120, 81–84. [Google Scholar] [CrossRef]
- Accelrys, I. Materials Studio; Accelrys Software Inc: San Diego, CA, USA, 2010. [Google Scholar]
- Liu, X.Q.; He, X.; Qiu, N.X.; Yang, X.; Tian, Z.Y.; Li, M.J.; Xue, Y. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 2016, 389, 894–905. [Google Scholar] [CrossRef]
- Fan, W.; Chakraborty, A. Investigation of the interaction of polar molecules on graphite surface: Prediction of isosteric heat of adsorption at zero surface coverage. J. Phys. Chem. C 2016, 120, 23490–23499. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Pan, H.; And, J.A.R.; Balbuena, P.B. Examination of the Approximations Used in Determining the Isosteric Heat of Adsorption from the Clausius−Clapeyron Equation. Langmuir 2015, 14, 535–542. [Google Scholar] [CrossRef]
- Kong, X.P.; Wang, J. Copper(II) adsorption on the kaolinite(001) surface: Insights from first-principles calculations and molecular dynamics simulations. Appl. Surf. Sci. 2016, 389, 316–323. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, D.L. CO_2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Wang, T.; Tian, S.; Li, G.; Sheng, M. Selective adsorption of supercritical carbon dioxide and methane binary mixture in shale kerogen nanopores. J. Nat. Gas Sci. Eng. 2018, 50, 181–188. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Zeng, Q. Adsorption Behavior of Methane on Kaolinite. Ind. Eng. Chem. Res. 2017, 56, 6229–6238. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Molecular simulation of methane adsorption and its effect on kaolinite swelling as functions of pressure and temperature. Mol. Simul. 2018, 44, 789–796. [Google Scholar] [CrossRef]
| Molecule | Element | σ/nm | ||
|---|---|---|---|---|
| CO2 | C | 2.757 | +0.6512 | |
| O | 3.033 | −0.3256 | ||
| CH4 | C | 3.184 | 0.06069 | −0.1360 |
| H | 2.963 | 0.06618 | +0.0340 | |
| kaolinite | Si | 0.400 | 0.20934 | +1.1 |
| Al | 0.420 | 0.20934 | +1.45 | |
| O(surface) | 0.350 | 0.10467 | −0.55 | |
| O(apical) | 0.350 | 0.10467 | −0.75833 | |
| O(hydroxyl) | 0.350 | 0.10467 | −0.68333 | |
| H | 0.1098 | 0.0544284 | +0.2 |
| Temperature | Correlation Coefficient R2 | ||
|---|---|---|---|
| 283.15 K | 0.902 | 0.427 | 0.994 |
| 293.15 K | 0.876 | 0.478 | 0.997 |
| 313.15 K | 0.818 | 0.963 | 0.996 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.; Zhang, B.; Kang, T.; Guo, J.; Zhao, G. Effect of Pressure and Temperature on CO2/CH4 Competitive Adsorption on Kaolinite by Monte Carlo Simulations. Materials 2020, 13, 2851. https://doi.org/10.3390/ma13122851
Kang G, Zhang B, Kang T, Guo J, Zhao G. Effect of Pressure and Temperature on CO2/CH4 Competitive Adsorption on Kaolinite by Monte Carlo Simulations. Materials. 2020; 13(12):2851. https://doi.org/10.3390/ma13122851
Chicago/Turabian StyleKang, Guanxian, Bin Zhang, Tianhe Kang, Junqing Guo, and Guofei Zhao. 2020. "Effect of Pressure and Temperature on CO2/CH4 Competitive Adsorption on Kaolinite by Monte Carlo Simulations" Materials 13, no. 12: 2851. https://doi.org/10.3390/ma13122851
APA StyleKang, G., Zhang, B., Kang, T., Guo, J., & Zhao, G. (2020). Effect of Pressure and Temperature on CO2/CH4 Competitive Adsorption on Kaolinite by Monte Carlo Simulations. Materials, 13(12), 2851. https://doi.org/10.3390/ma13122851






