Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents
Abstract
1. Introduction
2. Materials, Experimental and Methods
2.1. Raw Materials
2.2. Iron(III) Oxide Modification Experiments
2.3. Adsorption Experiments
2.4. Analytical Methods
2.4.1. Fluid-Phase Characterization
2.4.2. Solid-Phase Characterization
3. Results and Discussion
3.1. Mineralogical Composition of the Adsorbents
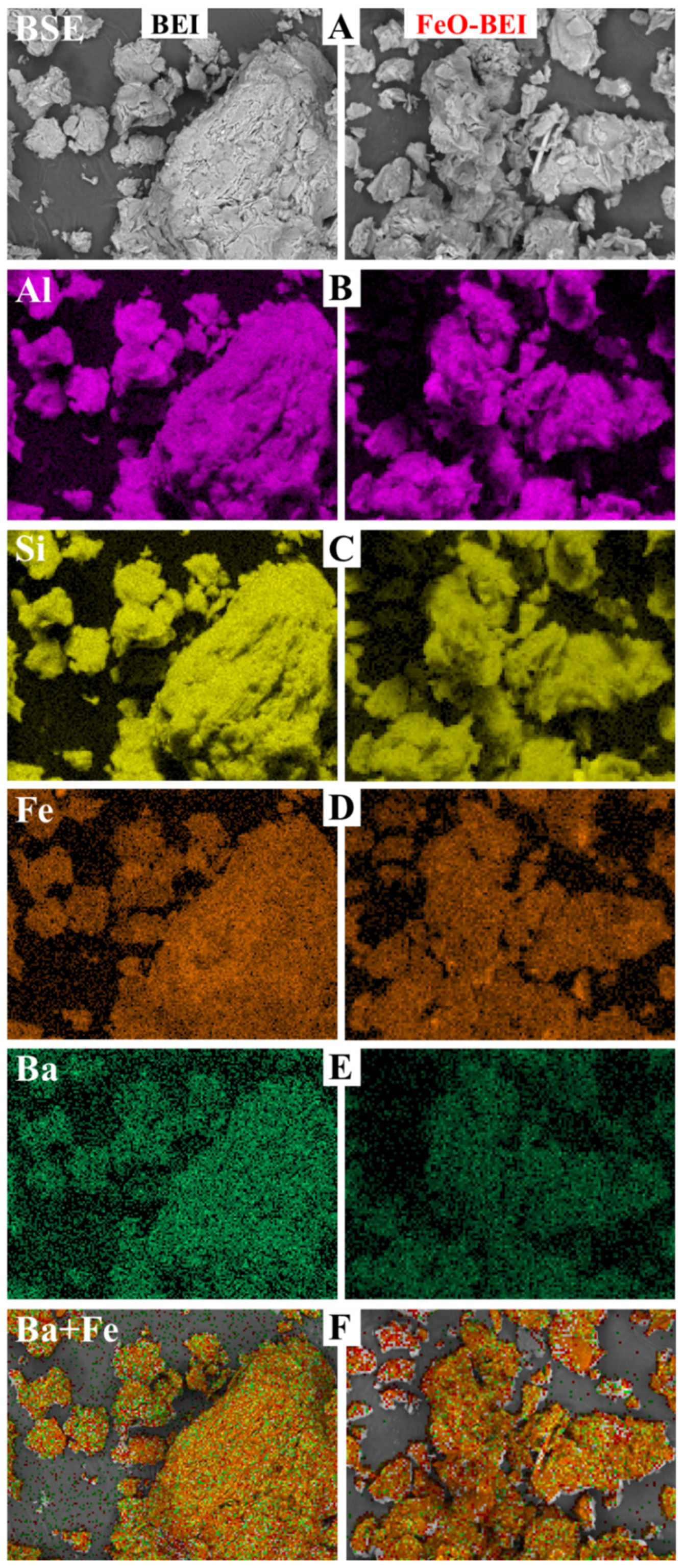
3.2. Geochemical and Microstructural Characterization of the Adsorbents
3.3. Adsorption Studies
3.3.1. Aqueous Speciation of Barium
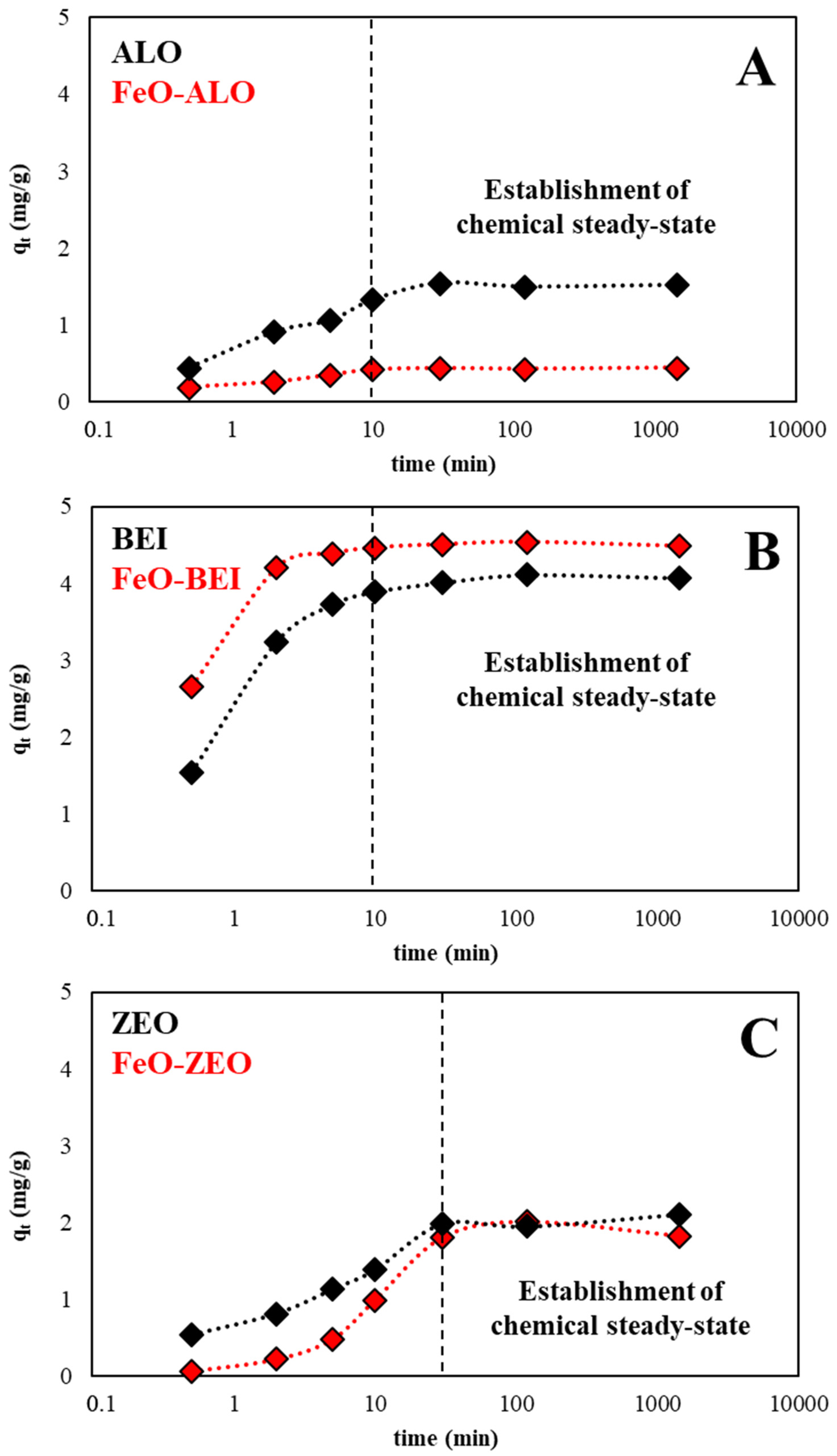
3.3.2. Kinetics Experiments
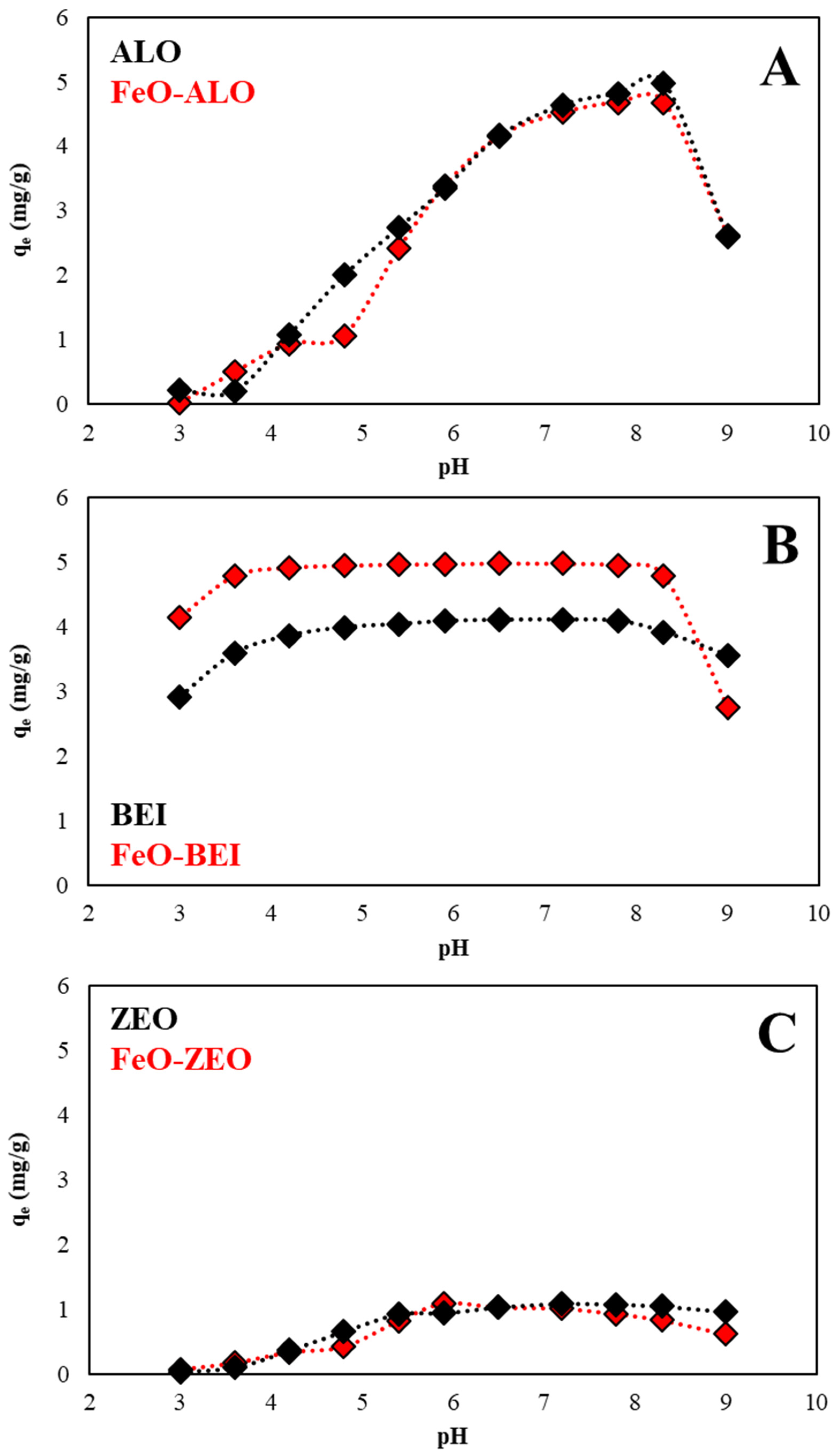
3.3.3. Effect of Solution pH on Ba2+ Removal
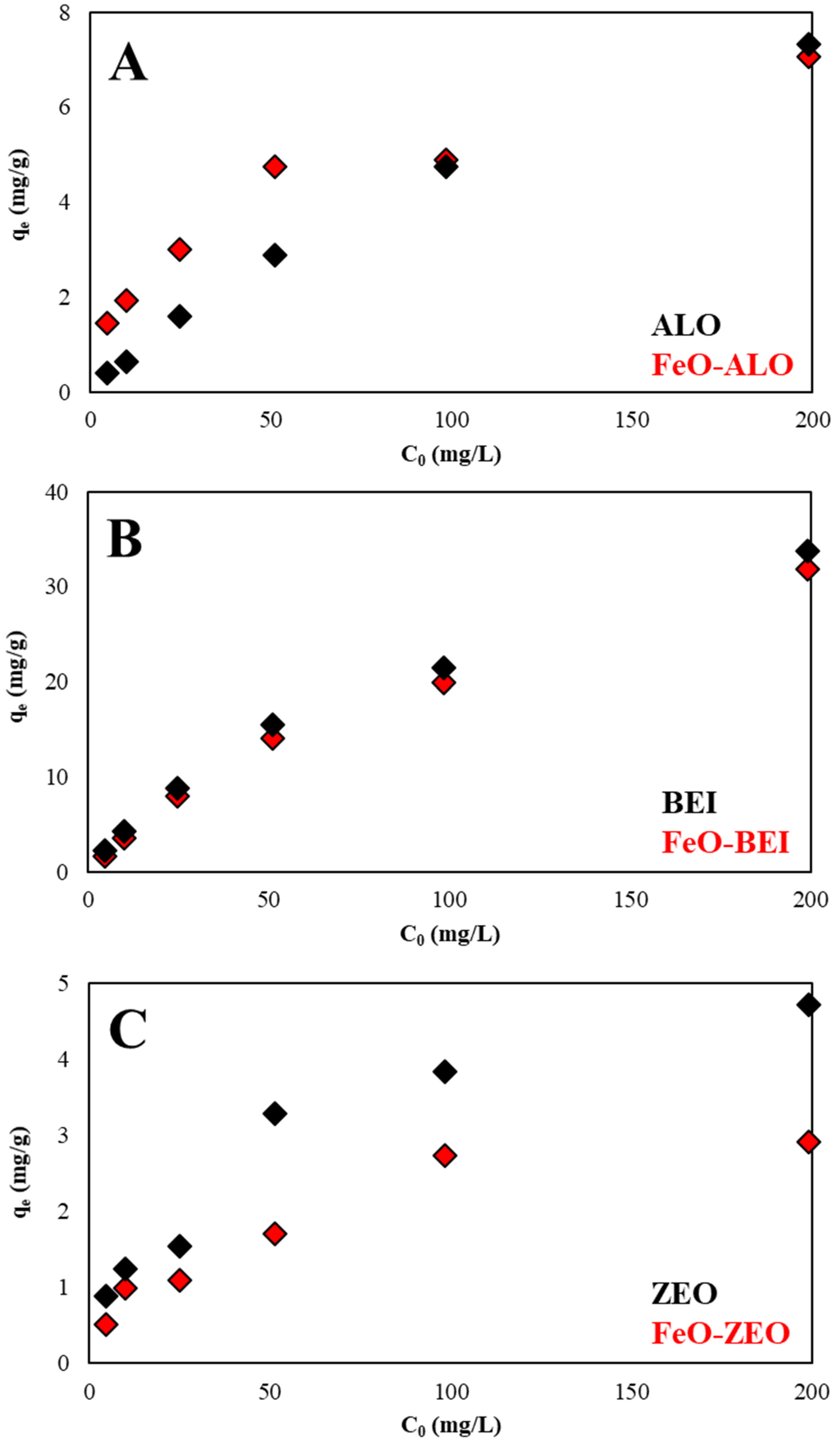
3.3.4. Effect of Initial Ba2+ Concentration
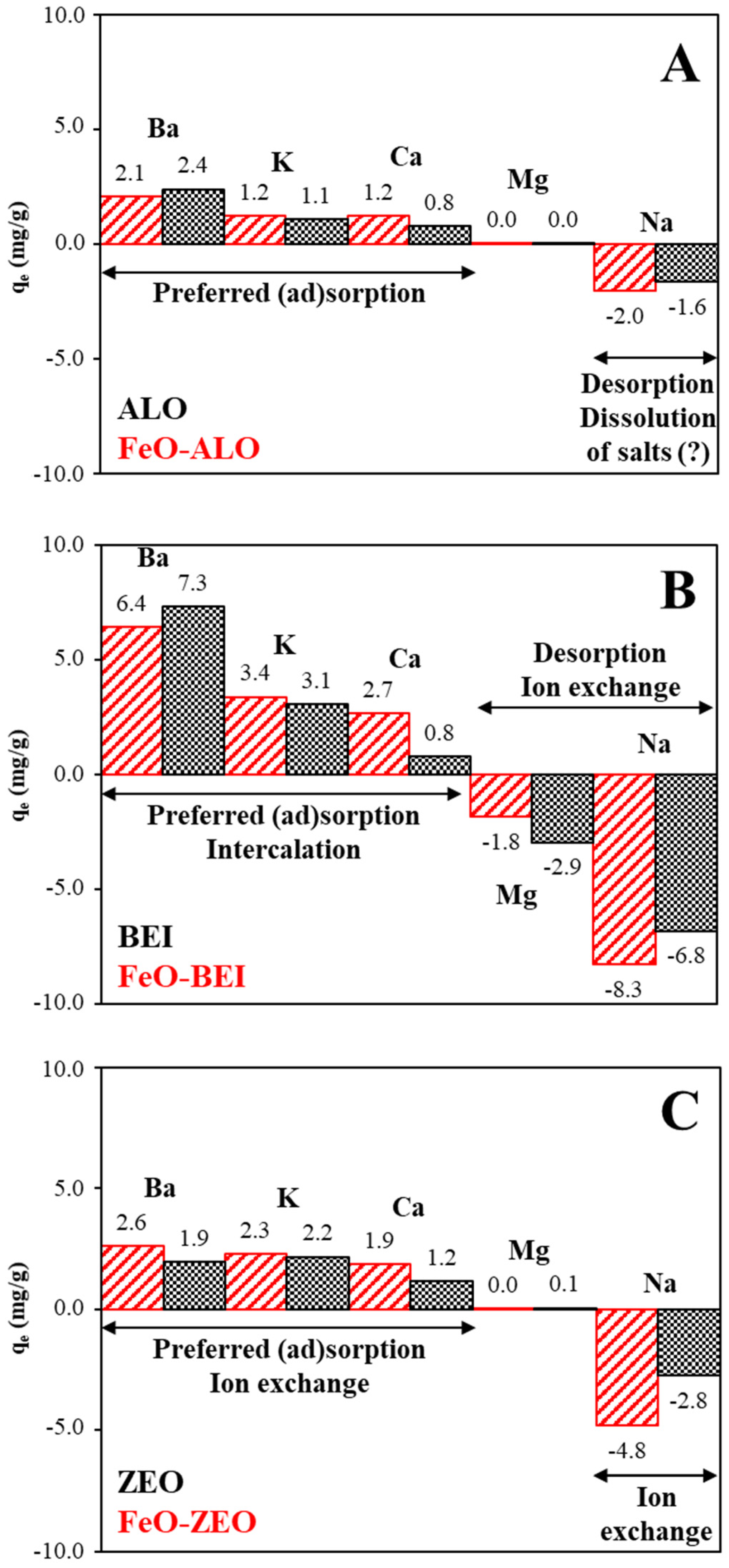
3.3.5. Effect of Competing Cations
3.3.6. Adsorption Isotherms
3.3.7. Thermodynamics
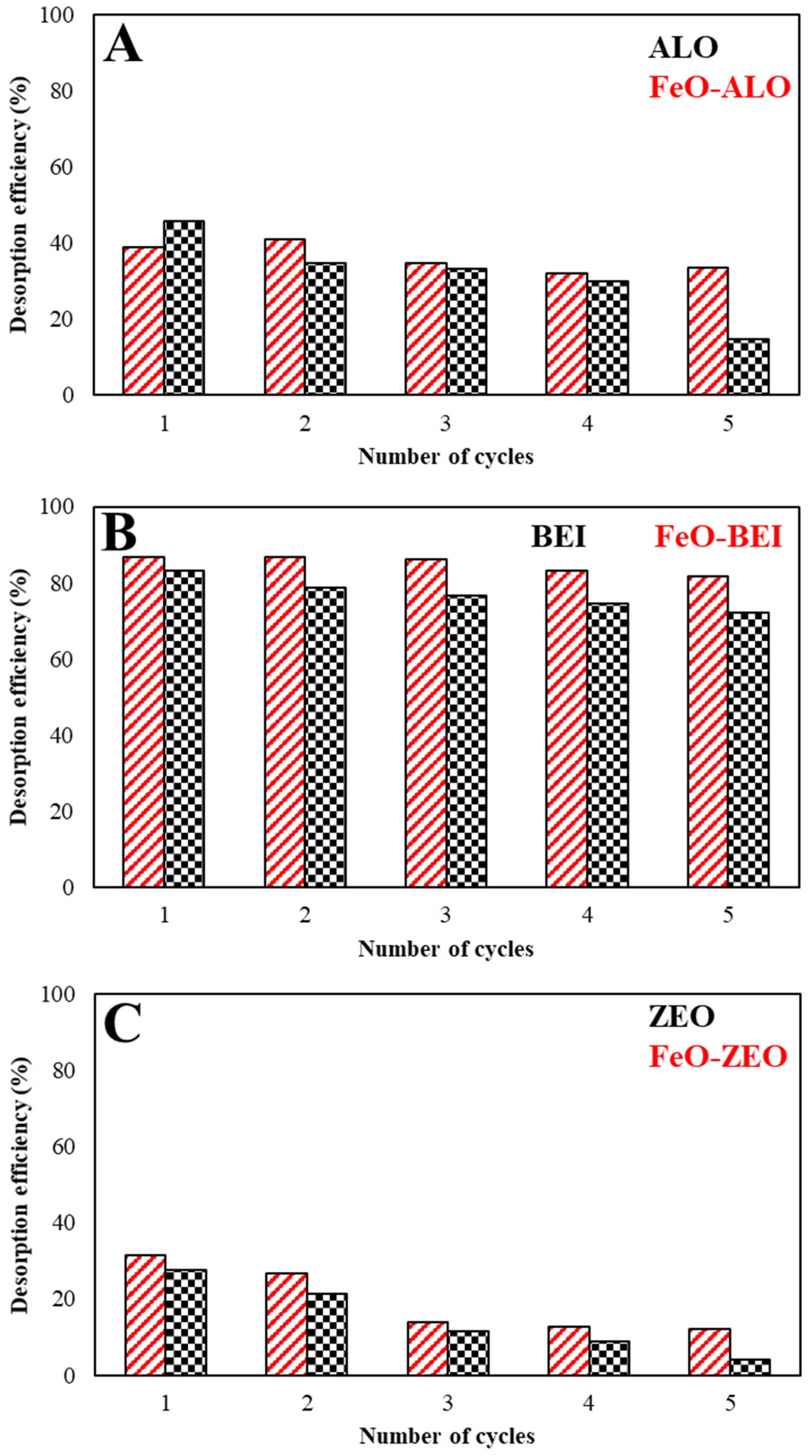
3.3.8. Regenerability
3.3.9. Uptake Mechanism(s)
3.3.10. Cost Factor
3.4. Comparison of Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bacquart, T.; Frisbie, S.; Mitchell, E.; Grigg, L.; Cole, C.; Small, C.; Sarkar, B. Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: Arsenic, manganese, fluoride, iron, and uranium. Sci. Total. Environ. 2015, 517, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Malik, Q.A.; Khan, M.S. Effect on Human Health due to Drinking Water Contaminated with Heavy Metals. J. Pollut. Eff. Cont. 2016, 5, 1000179. [Google Scholar]
- Chabukdhara, M.; Gupta, S.K.; Kotecha, Y.; Nema, A.K. Groundwater quality in Ghaziabad district, Uttar Pradesh, India: Multivariate and health risk assessment. Chemosphere 2017, 179, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, A.; Landler, A.; Mittermayr, F.; Letofsky-Papst, I.; Steindl, F.; Galan, I.; Dietzel, M. Removal of heavy metals (Co, Cr, and Zn) during calcium–aluminium–silicate–hydrate and trioctahedral smectite formation. J. Mater. Sci. 2019, 54, 9331–9351. [Google Scholar] [CrossRef]
- Araissi, M.; Ayed, I.; Elaloui, E.; Moussaoui, Y. Removal of barium and strontium from aqueous solution using zeolite 4A. Water Sci. Technol. 2016, 77, 1628–1636. [Google Scholar] [CrossRef]
- Dhingra, N.; Singh, N.S.; Parween, T.; Sharma, R. Heavy Metal Remediation by Natural Adsorbents. In Modern Age Waste Water Problems; Oves, M., Ansari, M., Zain Khan, M., Shahadat, M., M.I. Ismail, I., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, B97, 219–243. [Google Scholar] [CrossRef]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bhatnagar, A.; et al. Clay-polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef]
- Baldermann, A.; Warr, L.N.; Letofsky-Papst, I.; Mavromatis, V. Substantial iron sequestration during green-clay authigenesis in modern deep-sea sediments. Nat. Geosci. 2015, 8, 885–889. [Google Scholar] [CrossRef]
- Baldermann, A.; Grießbacher, A.C.; Baldermann, C.; Purgstaller, B.; Letofsky-Papst, I.; Kaufhold, S.; Dietzel, M. Removal of barium, cobalt, strontium, and zinc from solution by natural and synthetic allophane adsorbents. Geosciences 2018, 8, 309. [Google Scholar] [CrossRef]
- Abu-Danso, E.; Peräniemi, S.; Leiviskä, T.; Kim, T.Y.; Tripathi, K.M.; Bhatnagar, A. Synthesis of clay-cellulose biocomposite for the removal of toxic metal ions from aqueous medium. J. Hazard. Mater. 2020, 381, 120871. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, S.; Khameneh, E.S.; Rezazadeh, F.; Hosseini, M.H. Heavy metal removing by modified bentonite and study of catalytic activity. J. Mol. Struct. 2020, 1199, 126989. [Google Scholar] [CrossRef]
- Clark, C.J.; McBride, M.B. Chemisorption of Cu(II) and Co(II) on allophane and imogolite. Clays Clay Miner. 1984, 32, 300–310. [Google Scholar] [CrossRef]
- Parfitt, R.L. Allophane in New Zealand—A review. Aust. J. Soil Res. 1990, 28, 343–360. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, C.-P.; Mimura, H.; Zhang, X.; Wei, Y. Stable solidification of silica-based ammonium molybdophosphate by allophane: Application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J. Hazard. Mater. 2018, 341, 46–54. [Google Scholar] [CrossRef]
- Eylem, C.; Erten, H.N.; Görktürk, H. Sorption-Desorption Behaviour of Barium on Clays. J. Environ. Radioactivity 1990, 11, 183–200. [Google Scholar] [CrossRef]
- Chávez, M.L.; de Pablo, L.; García, T.A. Adsorption of Ba2+ by Ca-exchange clinoptilolite tuff and montmorillonite clay. J. Hazard. Mater. 2010, 175, 216–223. [Google Scholar] [CrossRef]
- Purdey, M. Chronic barium intoxication disrupts sulphated proteoglycan synthesis: A hypothesis for the origins of multiple sclerosis. Med. Hypotheses 2004, 62, 746–754. [Google Scholar] [CrossRef]
- Charbonnier, Q.; Moynier, F.; Bouchez, J. Barium isotope cosmochemistry and geochemistry. Sci. Bull. 2018, 63, 385–394. [Google Scholar] [CrossRef]
- Baeza-Alvarado, M.D.; Olguín, M.T. Surfactant-modified clinoptilolite-rich tuff to remove barium (Ba2+) and fulvic acid from mono- and bi-component aqueous media. Micropor. Mesopor. Mat. 2011, 139, 81–86. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Pepe, F.; de Gennaro, B.; Aprea, P.; Caputo, D. Natural zeolites for heavy metals removal from aqueous solutions: Modeling of the fixed bed Ba2+/Na+ ion-exchange process using a mixed phillipsite/chabazite-rich tuff. Chem. Eng. J. 2013, 219, 37–42. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Liu, D.; Zhong, C. Radioactive Barium Ion Trap Based on Metal-Organic Framework for Efficient and Irreversible Removal of Barium from Nuclear Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 8527–8535. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Baragaňo, D.; Alonso, J.; Gallego, J.R.; Lobo, M.C.; Gil-Díaz, M. Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 2020, 238, 124624. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Voigt, M.; Pearce, C.R.; Baldermann, A.; Oelkers, E.H. Stable and radiogenic strontium isotope fractionation during hydrothermal seawater-basalt interaction. Geochim. Cosmochim. Acta 2018, 240, 131–151. [Google Scholar] [CrossRef]
- Baldermann, A.; Abdullayev, E.; Taghiyeva, Y.; Alasgarov, A.; Javad-Zada, Z. Sediment petrography, mineralogy and geochemistry of the Miocene Islam Dağ Section (Eastern Azerbaijan): Implications for the evolution of sediment provenance, palaeo-environment and (post-)depositional alteration patterns. Sedimentology 2020, 67, 152–172. [Google Scholar] [CrossRef]
- Richoz, S.; Baldermann, A.; Frauwallner, A.; Harzhauser, M.; Daxner-Höck, G.; Klammer, D.; Piller, W.E. Geochemistry and mineralogy of the Oligo-Miocene sediments of the Valley of Lakes, Mongolia. Palaeobio. Palaeoenv. 2017, 97, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Mavromatis, V.; Immenhauser, A.; Buhl, D.; Purgstaller, B.; Baldermann, A.; Dietzel, M. Effect of organic ligands on Mg partitioning and Mg isotope fractionation during low-temperature precipitation of calcite in the absence of growth rate effects. Geochim. Cosmochim. Acta. 2017, 207, 139–153. [Google Scholar] [CrossRef]
- Levard, C.; Doelsch, E.; Basile-Doelsch, I.; Abidin, Z.; Miche, H.; Masion, A.; Rose, J.; Borschneck, D.; Bottero, J.-Y. Structure and distribution of allophanes, imogolite and proto-imogolite in volcanic soils. Geoderma 2012, 183–184, 100–108. [Google Scholar] [CrossRef]
- Baldermann, A.; Dohrmann, R.; Kaufhold, S.; Nickel, C.; Letofsky-Papst, I.; Dietzel, M. The Fe-Mg-saponite solid solution series—A hydrothermal synthesis study. Clay Miner. 2014, 49, 391–415. [Google Scholar] [CrossRef]
- Roy, J.; Bandyopadhyay, N.; Das, S.; Maitra, S. Studies on the Formation of Mullite from Diphasic Al2O3-SiO2 Gel by Fourier Transform Infrared Spectroscopy. Iran. J. Chem. Chem. Eng. 2011, 30, 65–71. [Google Scholar]
- Ma, Y.; Liu, Z.; Geng, A.; Vogt, T.; Lee, Y. Structural and spectroscopic studies of alkali-metal exchanged stilbites. Micropor. Mesopor. Mat. 2016, 224, 339–348. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Wat. Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Massiani, P.; Davidson, A.; Che, M. Role of silanol groups in the incorporation of V in β zeolite. J. Mol. Catal. A Chem. 2000, 155, 169–182. [Google Scholar] [CrossRef]
- Çelebi, O.; Üzüm, Ç.; Shahwan, T.; Erten, H.N. A radiotracer study of the adsorption behavior of aqueous Ba2+ ions on nanoparticles of zero-valent iron. J. Hazard. Mater. 2007, 148, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zou, W.; Wang, Y.; Zhu, L. Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: Discussion of adsorption isotherms and pH effect. J. Environ. Radioactivity 2007, 93, 127–143. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Opiso, E.; Sato, T.; Yoneda, T. Adsorption and co-precipitation behavior of arsenate, chromate, selenate and boric acid with synthetic allophane-like materials. J. Hazard. Mater. 2009, 170, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.R.; Kim, M.; Kim, J.-G.; Hong, S.-M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Najafabadi, H.H.; Irani, M.; Rad, L.R.; Haratameh, A.H.; Haririan, I. Removal of Cu2+, Pb2+ and Cr6+ from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent. RSC Adv. 2015, 5, 16532. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- WHO. Barium in Drinking-Water; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Jun, B.-M.; Park, C.M.; Heo, J.; Yoon, Y. Adsorption of Ba2+ and Sr2+ on Ti3C2Tx MXene in model fracking wastewater. J. Environ. Manage. 2020, 256, 109940. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Mahramanlioglu, M.; Kizilcikli, I.; Bicer, I.O. Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J. Fluorine Chem. 2002, 115, 41–47. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- El-Korashy, S.A.; Elwakeel, K.Z.; El-Hafeiz, A.A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb(II), Mn(VII) and Cr(VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Sajih, M.; Bryan, N.D.; Livens, F.R.; Vaughan, D.J.; Descostes, M.; Phrommavanh, V.; Nos, J.; Morris, K. Adsorption of radium and barium on goethite and ferrihydrite: A kinetic and surface complexation modelling study. Geochim. Cosmochim. Acta 2014, 146, 150–163. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Habib, M.A.; Bockris, J.O. Specific Adsorption of Ions. In Comprehensive Treatise of Electrochemistry; Bockris, J.O., Conway, B.E., Yeager, E., Eds.; Springer: Boston, MA, USA, 1980; pp. 135–219. [Google Scholar]
- Creton, B.; Bougeard, D.; Smirnov, K.S.; Guilment, J.; Poncelet, O. Structural Model and Computer Modeling Study of Allophane. J. Phys. Chem. C 2008, 112, 358–364. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olsen, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–308. [Google Scholar]
- Dove, P.M.; Nix, C.J. The influence of the alkaline earth cations, magnesium, calcium, and barium on the dissolution kinetics of quartz. Geochim. Cosmochim. Acta 1997, 61, 3329–3340. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sust. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Mu, W.; Du, S.; Yu, Q.; Li, X.; Wei, H.; Yang, Y. Improving barium ion adsorption on two-dimensional titanium carbide by surface modification. Dalton Trans. 2018, 47, 8375–8381. [Google Scholar] [CrossRef]
- Kapashi, E.; Kapnisti, M.; Dafnomili, A.; Noli, F. Aloe Vera as an effective biosorbent for the removal of thorium and barium from aqueous solutions. J. Radioanal. Nucl. Chem. 2019, 321, 217–226. [Google Scholar] [CrossRef]
- Noli, F.; Kapnisti, M.; Buema, G.; Harja, M. Retention of barium and europium radionuclides from aqueous solutions on ash-based sorbents by application of radiochemical techniques. Appl. Radiat. Isot. 2016, 116, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Singh, V.K. Radiotracer Technique in Adsorption Study-XIII. Adsorption of Barium and Cesium Ions on Chromium(IV) Oxide Powder. Appl. Radiat. Isot. 1995, 46, 847–853. [Google Scholar] [CrossRef]
- Mishra, S.P.; Singh, V.K. Radiotracer Technique in Adsorption Study-XI. Adsorption of Barium and Cesium Ions on Hydrous Ceric Oxide. Appl. Radiat. Isot. 1995, 46, 75–81. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Ghaemi, A.; Ghassabzadeh, H.; Ghannadi-Maragheh, M. Removal of strontium and barium from aqueous solutions by adsorption onto expanded Perlite. Can. J Chem. Eng. 2011, 89, 1247–1254. [Google Scholar] [CrossRef]
- Faghihian, H.; Marageh, M.G.; Kazemian, H. The use of clinoptilolite and its sodium form for removal of radioactive cesium, and strontium from nuclear wastewater and Pb2+, Ni2+, Cd2+, Ba2+ from municipal wastewater. Appl. Radiat. Isot. 1999, 50, 655–660. [Google Scholar] [CrossRef]
- Younis, S.A.; Ghobashy, M.M.; Bassioni, G.; Gupta, A.K. Tailored functionalized polymer nanoparticles using gamma radiation for selected adsorption of barium and strontium in oilfield wastewater. Arab. J. Chem. 2020, 13, 3762–3774. [Google Scholar] [CrossRef]
- Kaveeshwar, A.R.; Kumar, P.S.; Revellame, E.D.; Gang, D.D.; Zappi, M.E.; Subramaniam, R. Adsorption properties and mechanism of barium (II) and strontium (II) removal from fracking wastewater using pecan shell based activated carbon. J. Clean. Prod. 2018, 193, 1–13. [Google Scholar] [CrossRef]

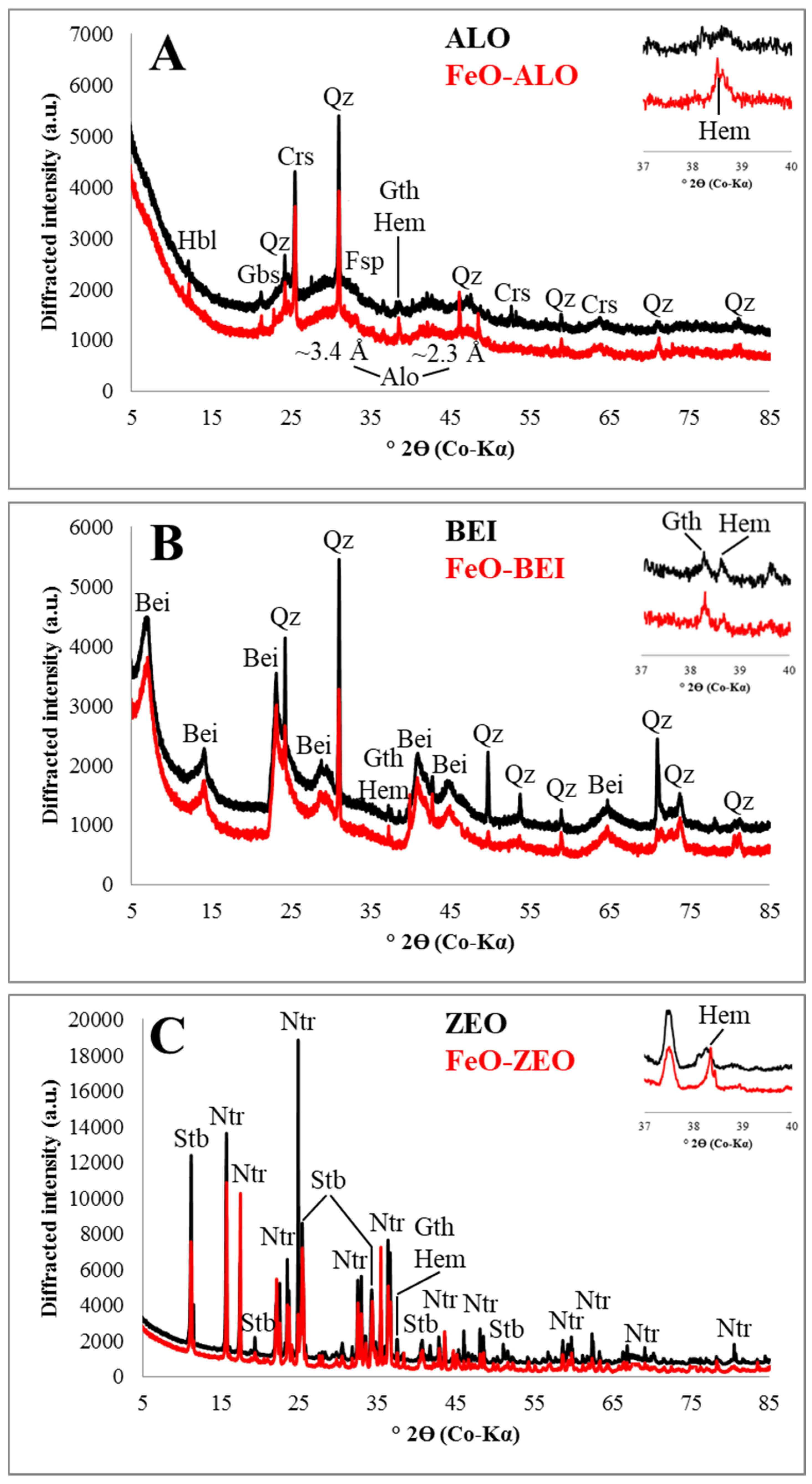
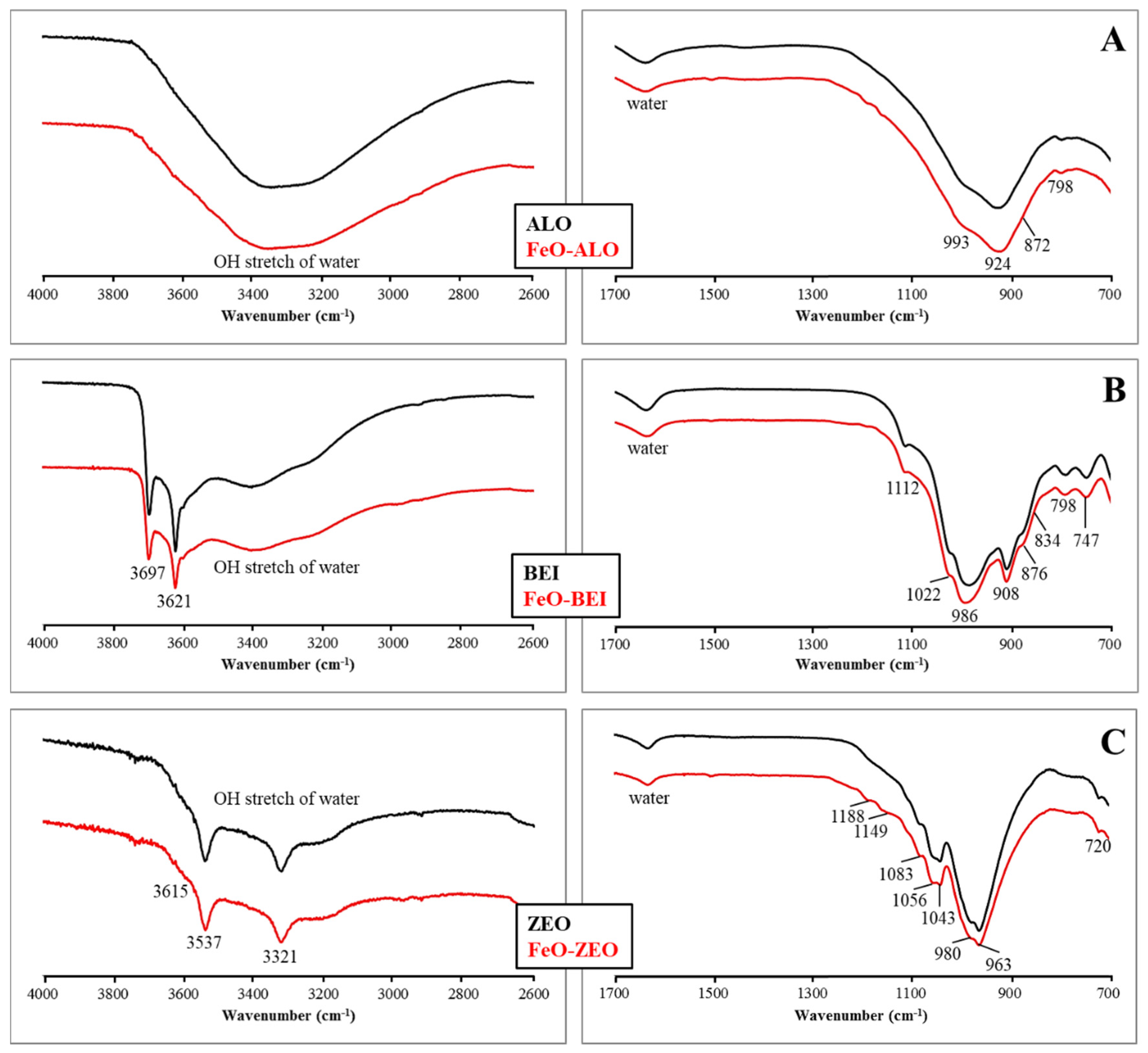
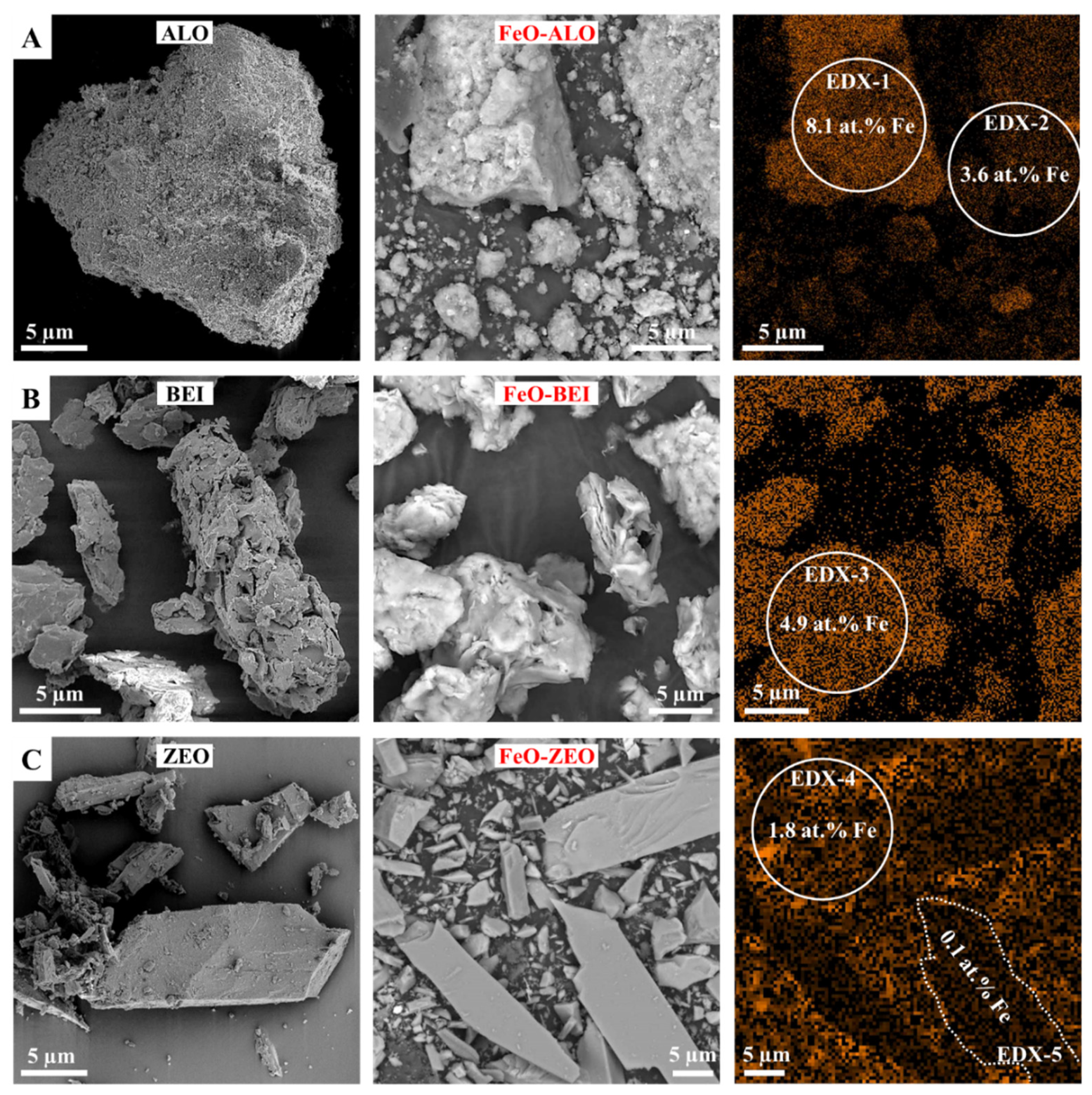

| Phase (wt%) | ALO | BEI | ZEO |
|---|---|---|---|
| Allophane | 70 | – | – |
| Beidellite | – | 91 | – |
| Clinochlore | 1 | – | – |
| Cristobalite | 4 | – | – |
| Feldspar | 1 | – | – |
| Quartz | 7 | 5 | – |
| Gibbsite | 4 | – | – |
| Goethite | 3 | – | – |
| Halloysite | 5 | – | – |
| Hematite | – | 4 | – |
| Hornblende | 4 | – | – |
| Illite | 1 | – | – |
| Natrolite | – | – | 74 |
| Stilbite | – | – | 26 |
| SUM | 100 | 100 | 100 |
| Comp. (wt%) | ALO | BEI | ZEO |
|---|---|---|---|
| LOI | 31.7 | 15.3 | 13.6 |
| Na2O | 0.2 | 0.1 | 10.8 |
| MgO | 0.8 | 5.2 | – |
| Al2O3 | 27.4 | 22.2 | 23.2 |
| SiO2 | 31.1 | 47.7 | 49.0 |
| P2O5 | 0.0 | 0.6 | – |
| K2O | 0.1 | 0.1 | 0.2 |
| CaO | 0.4 | 1.5 | 3.1 |
| TiO2 | 0.9 | 0.2 | – |
| MnO | 0.1 | – | – |
| Fe2O3 | 7.3 | 7.2 | – |
| SrO | – | – | 0.3 |
| SUM | 100.0 | 100.0 | 99.9 |
| Pseudo-First Order Parameters | ||||||
|---|---|---|---|---|---|---|
| Adsorbent | Equil. Time | qe† | Removal Efficiency | k | qe‡ | R2 |
| Material | (min) | (mg/g) | (%Removal) | (1/min) | (mg/g) | (-) |
| ALO | 10 | 1.5 | 30.5 | 0.185 | 1.2 | 0.940 |
| FeO-ALO | 5 | 0.4 | 8.8 | 0.308 | 0.8 | 0.981 |
| BEI | 10 | 4.0 | 80.6 | 0.787 | 3.6 | 0.983 |
| FeO-BEI | 5 | 4.5 | 89.6 | 1.266 | 3.6 | 0.970 |
| ZEO | 30 | 2.0 | 40.3 | 0.060 | 1.8 | 0.963 |
| FeO-ZEO | 30 | 1.9 | 37.6 | 0.065 | 2.1 | 0.999 |
| Langmuir Parameters | |||||
|---|---|---|---|---|---|
| Adsorbent | Temperature | Q0max | KL | R2 | RL |
| Material | (K) | (mg/g) | (L/mg) | (-) | (-) |
| ALO | 288 | 11.4 | 0.006 | 0.935 | 0.42–0.97 |
| ALO | 298 | 13.2 | 0.007 | 0.944 | 0.42–0.97 |
| ALO | 313 | 16.8 | 0.014 | 0.945 | 0.26–0.93 |
| FeO-ALO | 288 | 3.0 | 0.031 | 0.987 | 0.14–0.86 |
| FeO-ALO | 298 | 3.4 | 0.032 | 0.969 | 0.14–0.87 |
| FeO-ALO | 313 | 4.6 | 0.036 | 0.977 | 0.12–0.85 |
| BEI | 288 | 33.1 | 0.025 | 0.984 | 0.17–0.89 |
| BEI | 298 | 38.0 | 0.029 | 0.941 | 0.15–0.88 |
| BEI | 313 | 44.8 | 0.034 | 0.993 | 0.13–0.85 |
| FeO-BEI | 288 | 29.4 | 0.044 | 0.989 | 0.10–0.82 |
| FeO-BEI | 298 | 36.0 | 0.055 | 0.963 | 0.08–0.79 |
| FeO-BEI | 313 | 38.6 | 0.058 | 0.945 | 0.19–0.90 |
| ZEO | 288 | 5.6 | 0.044 | 0.988 | 0.10–0.81 |
| ZEO | 298 | 7.5 | 0.045 | 0.960 | 0.10–0.83 |
| ZEO | 313 | 9.8 | 0.051 | 0.983 | 0.09–0.80 |
| FeO-ZEO | 288 | 4.9 | 0.026 | 0.996 | 0.16–0.88 |
| FeO-ZEO | 298 | 5.4 | 0.032 | 0.966 | 0.14–0.87 |
| FeO-ZEO | 313 | 9.6 | 0.033 | 0.972 | 0.29–0.94 |
| Dubinin-Radushkevich Parameters | |||||
|---|---|---|---|---|---|
| Adsorbent | Temperature | qDR | KDR | R2 | E |
| Material | (K) | (mg/g) | (mol2/kJ2) | (-) | (kJ/mol) |
| ALO | 288 | 5.1 | 3.94E-08 | 0.943 | 3.6 |
| ALO | 298 | 5.3 | 3.63E-08 | 0.923 | 3.7 |
| ALO | 313 | 9.2 | 2.77E-08 | 0.934 | 4.3 |
| FeO-ALO | 288 | 2.7 | 2.97E-08 | 0.983 | 4.1 |
| FeO-ALO | 298 | 2.5 | 2.00E-08 | 0.892 | 5.0 |
| FeO-ALO | 313 | 3.5 | 1.97E-08 | 0.891 | 5.0 |
| BEI | 288 | 22.2 | 2.31E-08 | 0.974 | 4.70 |
| BEI | 298 | 25.8 | 2.11E-08 | 0.979 | 4.9 |
| BEI | 313 | 35.7 | 2.29E-08 | 0.988 | 4.7 |
| FeO-BEI | 288 | 27.6 | 2.37E-08 | 0.980 | 4.6 |
| FeO-BEI | 298 | 22.9 | 1.09E-08 | 0.923 | 6.8 |
| FeO-BEI | 313 | 22.8 | 9.06E-09 | 0.878 | 7.4 |
| ZEO | 288 | 4.3 | 1.58E-08 | 0.902 | 5.6 |
| ZEO | 298 | 5.4 | 1.27E-08 | 0.871 | 6.3 |
| ZEO | 313 | 6.8 | 1.07E-08 | 0.729 | 6.8 |
| FeO-ZEO | 288 | 3.9 | 2.83E-08 | 0.985 | 4.2 |
| FeO-ZEO | 298 | 3.8 | 1.81E-08 | 0.850 | 5.3 |
| FeO-ZEO | 313 | 6.6 | 1.81E-08 | 0.815 | 5.3 |
| ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (kJ/mol·K) | |||
|---|---|---|---|---|---|
| Adsorbent | 288 K | 298 K | 313 K | ||
| ALO | −25.69 | −26.97 | −30.13 | 3.13 | 0.022 |
| FeO-ALO | −29.63 | −30.73 | −32.59 | 0.54 | 0.014 |
| BEI | −29.11 | −30.49 | −32.44 | 1.10 | 0.016 |
| FeO-BEI | −30.46 | −32.07 | −33.83 | 0.96 | 0.016 |
| ZEO | −30.46 | −31.60 | −33.49 | 0.54 | 0.015 |
| FeO-ZEO | −29.20 | −30.74 | −32.36 | 0.82 | 0.015 |
| Q0max | Surface Area/ | Conc. Range | Equil. Time | T | (Optimum) | ||
|---|---|---|---|---|---|---|---|
| Adsorbent | (mg/g) | Particle Size | of Pollutant | (h) | (°C) | PH | Reference |
| Alk-Ti3C2Tx | 46.5 | 76.4 m2/g | 50–500 mg/L | 24 | RT | 7.0–10.0 | [63] |
| Aloe Vera biosorbent | 107.5 | <125 µm | 5–200 mg/L | 1 | 20 | 5.0 | [64] |
| Ash (thermal power plant) | 15.1 | 7 m2/g | 50–1000 mg/L | 0.5 | 50 | 4.0–5.0 | [65] |
| Ca-clinoptilolite | 15.3 | <75 µm | 0.005–0.1 N | 168 | RT | – | [20] |
| Ca–montmorillonite | 15.3 | <75 µm | 0.005–0.1 N | 48 | RT | – | [20] |
| Chromium(IV) oxide | 0.1 | 100–150 µm | 0.01–1370 mg/L | 1.5 | 60 | 11.4 | [66] |
| Dolomite | 4.0 | 20 µm | 10–50 mg/L | 2 | 20 | 5.5 | [24] |
| Hydrous ceric oxide | 0.1 | 120 µm | 0.01–1370 mg/L | 1 | 60 | 11.4 | [67] |
| Expanded perlite | 2.5 | 20 µm | 5–50 mg/L | 1.5 | 20 | 6.0 | [68] |
| Kaolinite | 14.8 | <38 µm | 0.002–214 mg/L | 288 | RT | >8.0 | [19] |
| Mixed chlorite-illite | 16.7 | <38 µm | 0.002–214 mg/L | 144 | RT | >8.0 | [19] |
| MOF-based Ba traps | 131.1 | 0.4 m2/g | 10–1000 mg/L | 0.1 | 25 | 5.8 | [26] |
| Montmorillonite | 20.8 | <38 µm | 0.002–214 mg/L | 192 | RT | >8.0 | [19] |
| Ti3C2Tx Mxene | 175.1 | 10 m2/g | 0.2–10 g/L | 1 | 40 | 7.0–9.0 | [50] |
| Natural allophane | 10.6 | 294 m2/g | 1–100 mg/L | 0.2 | 25 | 8.5 | [13] |
| Natural clinoptilolite | 41.1 | 224–500 µm | 0.1 N | 72 | 25 | – | [69] |
| Na-clinoptilolite | 109.6 | 224–500 µm | 0.1 N | 73 | 26 | – | [69] |
| Nano-polymer SAB | 57.7 | 4.3 m2/g | 10–500 mg/L | 8 | 20 | 8.0 | [70] |
| Nano-polymer SASB | 210.4 | 5.9 m2/g | 10–500 mg/L | 6 | 20 | 8.0 | [70] |
| Nanoscale zero valent iron | 22.6 | 20–100 nm | 0.1–137 mg/L | 4 | 25 | – | [42] |
| Pecan shell act. carbon | 3.3 | 0.4 m2/g | 25–100 mg/L | 0.5 | 70 | 6.0–10.0 | [71] |
| Synthetic allophane-1 | 38.6 | 358 m2/g | 1–100 mg/L | 0.2 | 25 | 8.5 | [13] |
| Synthetic allophane-2 | 17.2 | 370 m2/g | 1–100 mg/L | 0.2 | 25 | 8.5 | [13] |
| Ti3C2Tx | 12.0 | 9.8 m2/g | 50–500 mg/L | 24 | RT | 7.0–10.0 | [63] |
| Zeolite-rich tuff | 230.2 | 0.3–0.6 mm | 50–500 mg/L | 0.5 | 22 | 7.7 | [25] |
| Zeolite Z70-4 | 17.0 | 25 m2/g | 50–1000 mg/L | 0.5 | 50 | 4.0–5.0 | [65] |
| Zeolite Z90-4 | 119.0 | 40 m2/g | 50–1000 mg/L | 0.5 | 50 | 4.0–5.0 | [65] |
| Zeolite Z90-15 | 117.7 | 122 m2/g | 50–1000 mg/L | 0.5 | 50 | 4.0–5.0 | [65] |
| ALO | 16.8 | 368.5 m2/g | 5–200 mg/L | 0.2 | 40 | 7.5–8.5 | TS |
| FeO-ALO | 4.6 | 342.1 m2/g | 5–200 mg/L | 0.1 | 40 | 7.5–8.5 | TS |
| BEI | 44.8 | 50.3 m2/g | 5–200 mg/L | 0.2 | 40 | 7.5–8.5 | TS |
| FeO-BEI | 38.6 | 50.1 m2/g | 5–200 mg/L | 0.1 | 40 | 7.5–8.5 | TS |
| ZEO | 9.8 | 2.3 m2/g | 5–200 mg/L | 0.5 | 40 | 7.5–8.5 | TS |
| FeO-ZEO | 9.6 | 2.6 m2/g | 5–200 mg/L | 0.5 | 40 | 7.5–8.5 | TS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldermann, A.; Fleischhacker, Y.; Schmidthaler, S.; Wester, K.; Nachtnebel, M.; Eichinger, S. Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents. Materials 2020, 13, 2582. https://doi.org/10.3390/ma13112582
Baldermann A, Fleischhacker Y, Schmidthaler S, Wester K, Nachtnebel M, Eichinger S. Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents. Materials. 2020; 13(11):2582. https://doi.org/10.3390/ma13112582
Chicago/Turabian StyleBaldermann, Andre, Yvonne Fleischhacker, Silke Schmidthaler, Katharina Wester, Manfred Nachtnebel, and Stefanie Eichinger. 2020. "Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents" Materials 13, no. 11: 2582. https://doi.org/10.3390/ma13112582
APA StyleBaldermann, A., Fleischhacker, Y., Schmidthaler, S., Wester, K., Nachtnebel, M., & Eichinger, S. (2020). Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents. Materials, 13(11), 2582. https://doi.org/10.3390/ma13112582





