In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
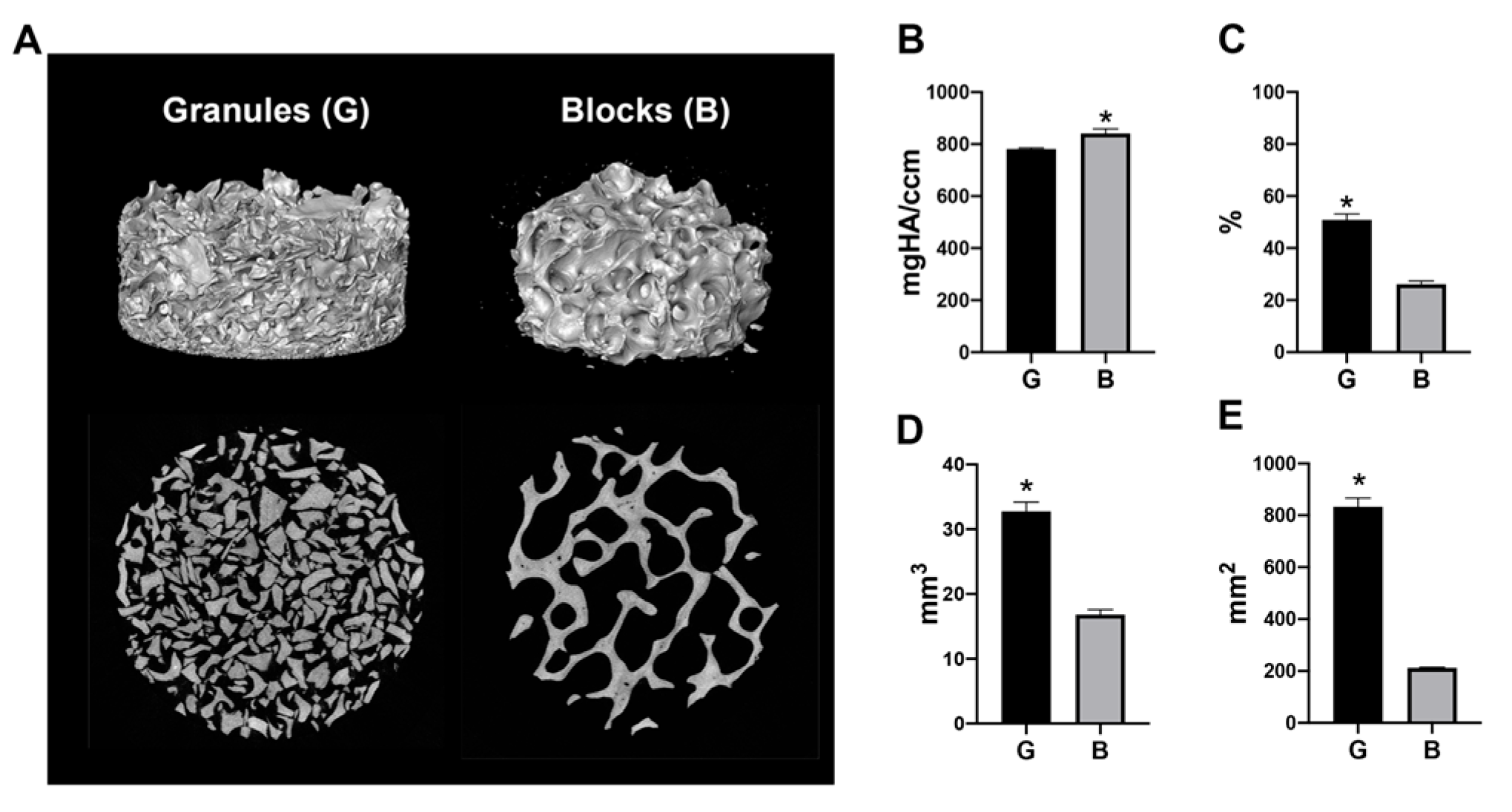
2.2. Micro-CT Analysis
2.3. Cell Culture
2.4. Cell Viability
2.5. Real-Time PCR Analysis
2.6. Undecalcified Frozen Section Preparation and Staining
2.7. Conditioned Media Experiment
2.8. Statistical Analysis
3. Results
3.1. Micro-CT Analysis
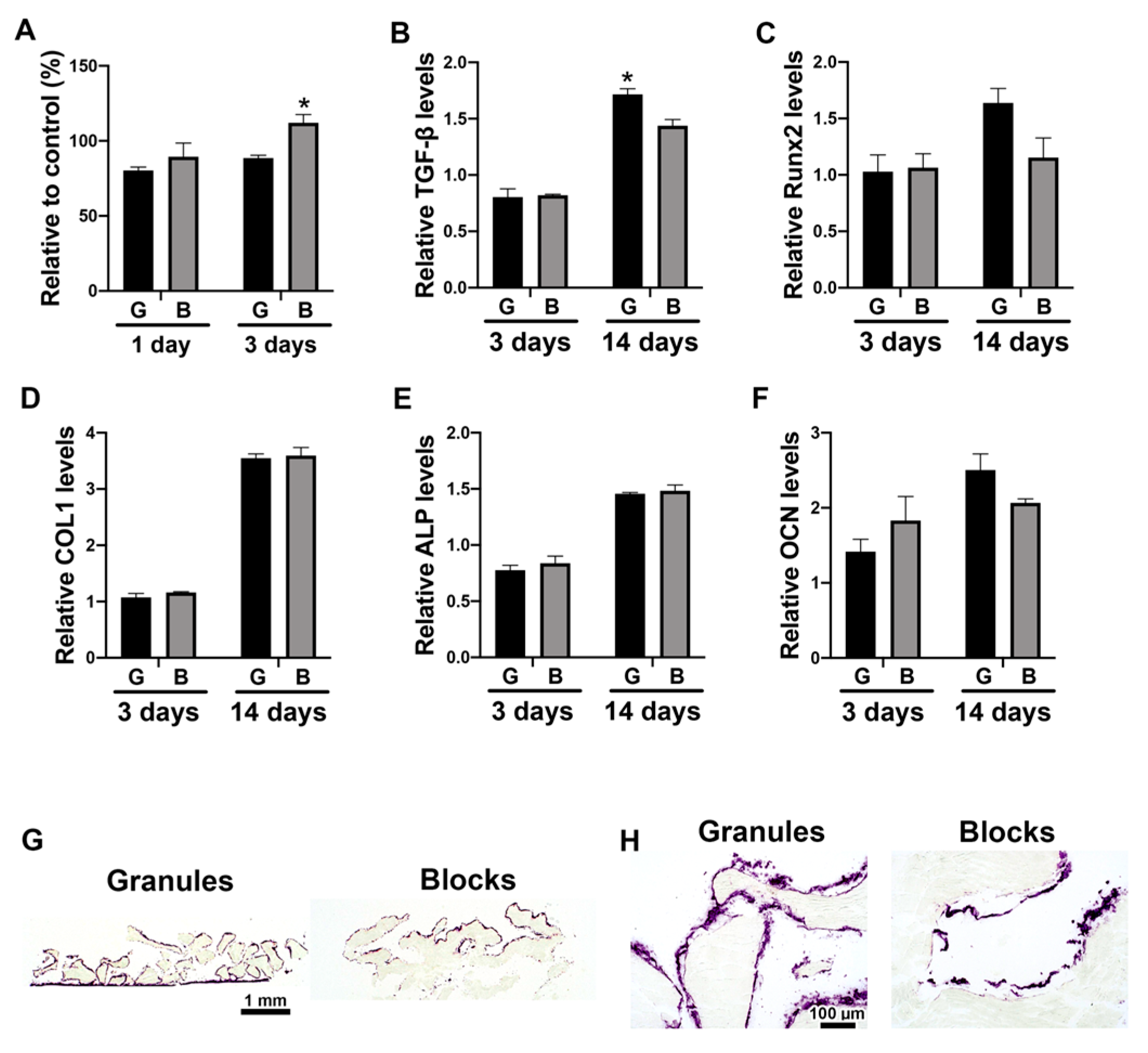
3.2. The Direct Effects of DBBM Shapes on Osteoblast Differentiation
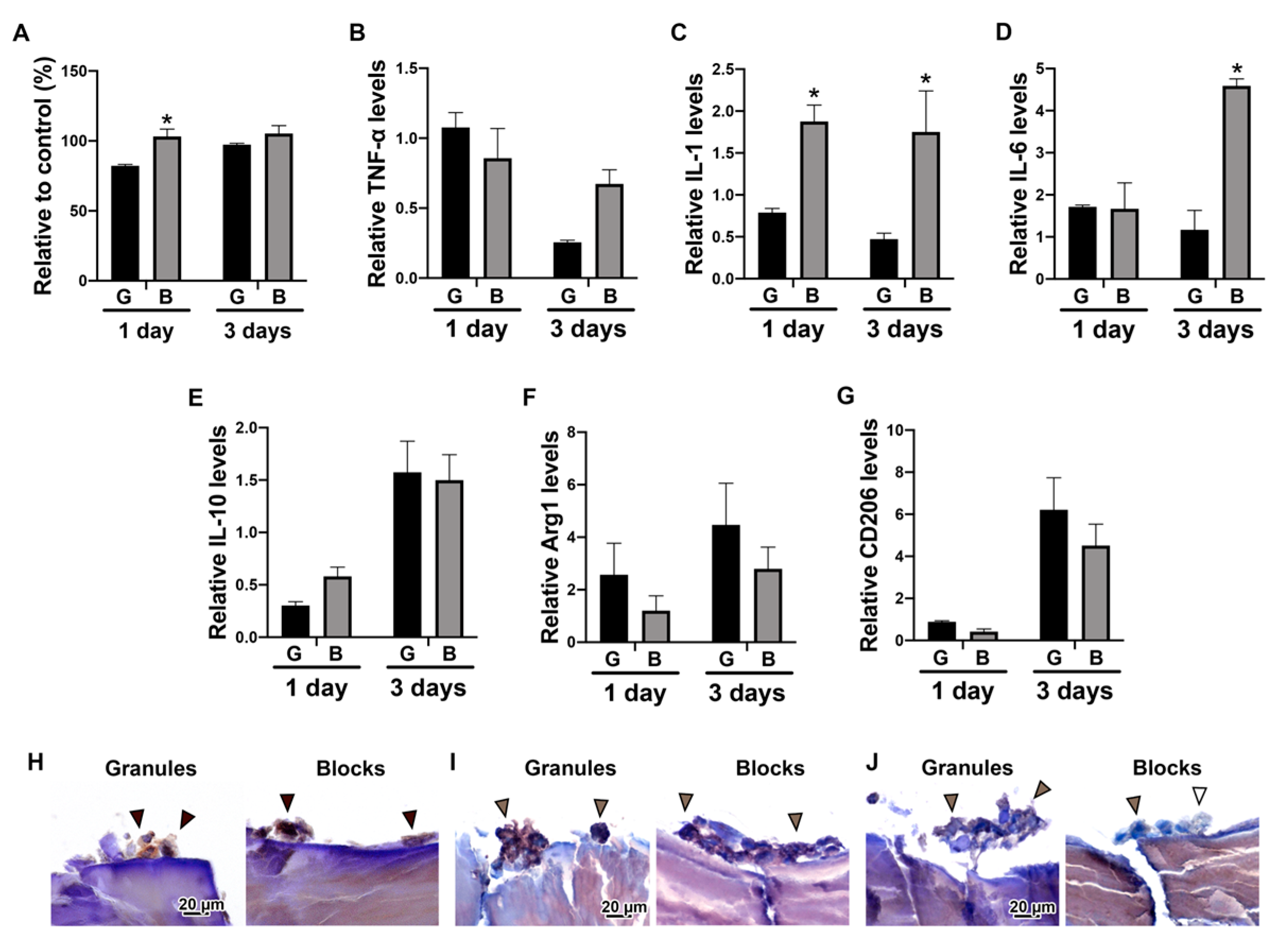
3.3. The Effects of DBBM Shapes on Macrophage Polarization
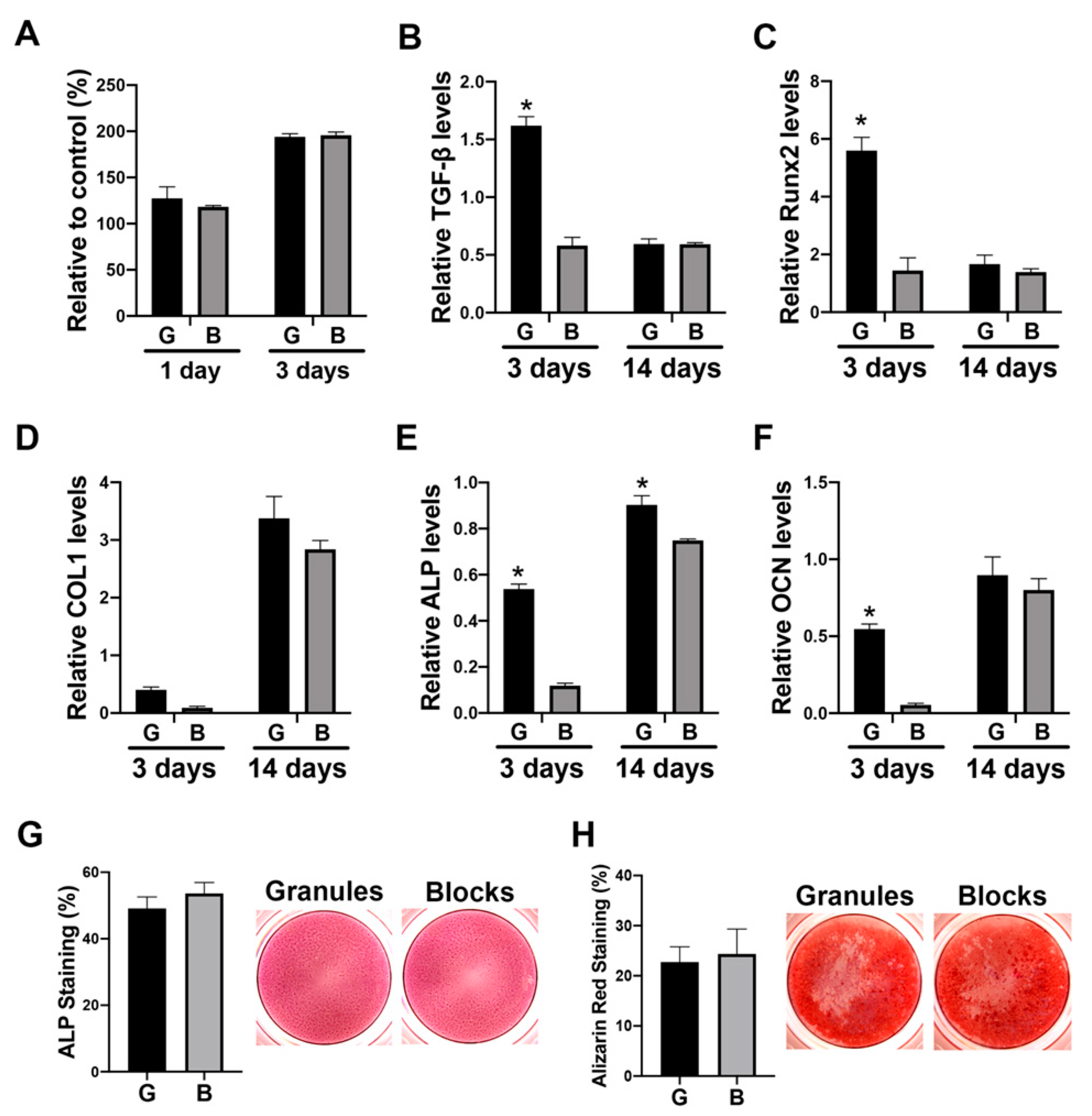
3.4. The Paracrine Effects of Macrophage Conditioned Media Cultured on DBBM on Osteoblast Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Gierloff, M.; Hedderich, J.; Açil, Y.; Wiltfang, J.; Terheyden, H. Biocompatibility of bone graft substitutes: Effects on survival and proliferation of porcine multilineage stem cells in vitro. Folia Morphol. 2011, 70, 154–160. [Google Scholar]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Doering, H.; Schmidt, T.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin. Oral Implant. Res 2013, 24, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Darby, I. Periodontal materials. Aust. Dent. J. 2011, 56, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Naef, R.; Schärer, P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int. J. Oral Maxillofac. Implant. 1997, 12, 844–852. [Google Scholar]
- Baldini, N.; De Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Wang, H.L. How to select replacement grafts for various periodontal and implant indications. Clin. Adv. Periodontics 2013, 3, 167–179. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Muñoz, F.; Sanz Martin, I.; Jung, R.E.; Hämmerle, C.H. Guided bone regeneration of peri-implant defects with particulated and block xenogenic bone substitutes. Clin. Oral Implant. Res. 2016, 27, 567–576. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Jung, R.E.; Sanz-Martín, I.; Unger, S.; Cantalapiedra, A.; Hämmerle, C.H.F.; Sanz, I.M. Guided bone regeneration with particulate vs. block xenogenic bone substitutes: A pilot cone beam computed tomographic investigation. Clin. Oral Implant. Res. 2017, 28, e262–e270. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Ferrari, D.; Sager, M.; Becker, J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: An immunohistochemical study in dogs. Clin. Oral Implant. Res. 2008, 19, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nakahara, K.; Haga-Tsujimura, M.; Iizuka, T.; Fujioka-Kobayashi, M.; Igarashi, K.; Saulacic, N. Comparison of three block bone substitutes for bone regeneration: Long-term observation in the beagle dog. Odontology 2018, 106, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Tanuma, Y.; Anada, T.; Honda, Y.; Kawai, T.; Kamakura, S.; Echigo, S.; Suzuki, O. Granule Size–Dependent Bone Regenerative Capacity of Octacalcium Phosphate in Collagen Matrix. Tissue Eng. Part A 2012, 18, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, F.; Torres, J.; Al-Abedalla, K.; Lopez-Cabarcos, E.; Alkhraisat, M.H.; Bassett, D.C.; Gbureck, U.; Barralet, J.E. Osseointegration of dental implants in 3D-printed synthetic onlay grafts customized according to bone metabolic activity in recipient site. Biomaterials 2014, 35, 5436–5445. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Ülgür, I.I.; Katagiri, H.; Vuignier, S.; Schaller, B. In vitro observation of macrophage polarization and gingival fibroblast behavior on three-dimensional xenogeneic collagen matrixes. J. Biomed. Mater. Res. Part A 2020, 108, 1408–1418. [Google Scholar] [CrossRef]
- Kawamoto, T.; Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot’s film method (2012). In Skeletal Development and Repair; Springer: Berlin/Heidelberg, Germany, 2014; pp. 149–164. [Google Scholar]
- Fujioka-Kobayashi, M.; Sawada, K.; Kobayashi, E.; Schaller, B.; Zhang, Y.; Miron, R.J. Osteogenic potential of rhBMP9 combined with a bovine-derived natural bone mineral scaffold compared to rhBMP2. Clin. Oral Implant. Res. 2017, 28, 381–387. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Mesa, F.; Carranza, N.; Juodzbalys, G.; Aguilar, M.; O′Valle, F. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2013, 15, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int. J. Oral Maxillofac. Implant. 1999, 14, 835–840. [Google Scholar]
- Miron, R.J.; Zohdi, H.; Fujioka-Kobayashi, M.; Bosshardt, D.D. Giant cells around bone biomaterials: Osteoclasts or multi-nucleated giant cells? Acta Biomater. 2016, 46, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells—New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O′Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Wang, C.; Wang, Y.; Tang, C.; Miron, R.J.; Zhang, Y. Deproteinized bovine bone matrix induces osteoblast differentiation via macrophage polarization. J. Biomed. Mater. Res. Part A 2018, 106, 1236–1246. [Google Scholar] [CrossRef]
- ISO: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Int. Organ. Stand. Geneva 2009, 1, 10993–10995.
| Gene | Primer Sequence (5′–3′) | |
|---|---|---|
| Forward | Reverse | |
| TNF-α | CAGCCTCTTCTCCTTCCTGAT | GCCAGAGGGCTGATTAGAGA |
| IL-1 | GGTTGAGTTTAAGCCAATCCA | TGCTGACCTAGGCTTGATGA |
| IL-6 | GAAAGGAGACATGTAACAAGAGT | GATTTTCACCAGGCAAGTCT |
| IL-10 | GAGGCTACGGCGCTGTCA | TCCACGGCCTTGCTCTTG |
| Arg-1 | ACGGAAGAATCAGCCTGGTG | GTCCACGTCTCTCAAGCCAA |
| CD206 | GGGTTGCTATCACTCTCTATGC | TTTCTTGTCTGTTGCCGTAGTT |
| TGF-β1 | ACTACTACGCCAAGGAGGTCA | TGCTTGAACTTGTCATAGATTTCG |
| Runx 2 | TCTTAGAACAAATTCTGCCCTTT | TGCTTTGGTCTTGAAATCACA |
| COL1a2 | CCCAGCCAAGAACTGGTATAGG | GGCTGCCAGCATTGATAGTTTC |
| ALP | GACCTCCTCGGAAGACACTC | TGAAGGGCTTCTTGTCTGTG |
| OCN | AGCAAAGGTGCAGCCTTTGT | GCGCCTGGGTCTCTTCACT |
| GAPDH | AGCCACATCGCTCAGACA | GCCCAATACGACCAAATCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujioka-Kobayashi, M.; Marjanowski, S.D.; Kono, M.; Katagiri, H.; Miron, R.J.; Schaller, B. In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral. Materials 2020, 13, 2682. https://doi.org/10.3390/ma13122682
Fujioka-Kobayashi M, Marjanowski SD, Kono M, Katagiri H, Miron RJ, Schaller B. In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral. Materials. 2020; 13(12):2682. https://doi.org/10.3390/ma13122682
Chicago/Turabian StyleFujioka-Kobayashi, Masako, Simon D. Marjanowski, Michihide Kono, Hiroki Katagiri, Richard J. Miron, and Benoit Schaller. 2020. "In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral" Materials 13, no. 12: 2682. https://doi.org/10.3390/ma13122682
APA StyleFujioka-Kobayashi, M., Marjanowski, S. D., Kono, M., Katagiri, H., Miron, R. J., & Schaller, B. (2020). In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral. Materials, 13(12), 2682. https://doi.org/10.3390/ma13122682





