Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane
Abstract
1. Introduction
2. Materials and Methods
2.1. Set for Corrosion of AISI 1010 Steel in Sulfolane
2.2. Electrochemical Tests of AISI 1010 Steel
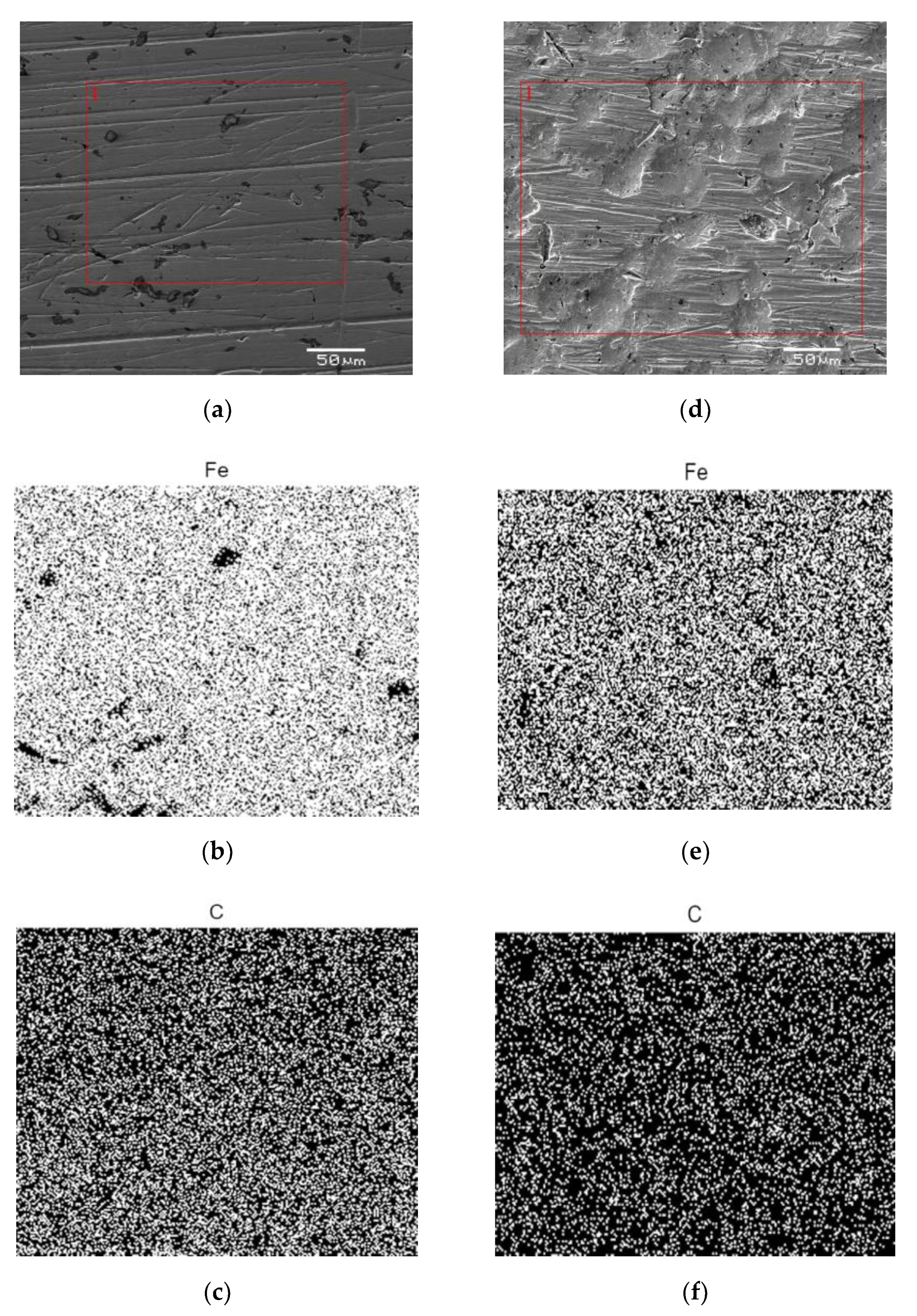
2.3. Surface Analysis of AISI 1010 Steel
3. Results and Discussion
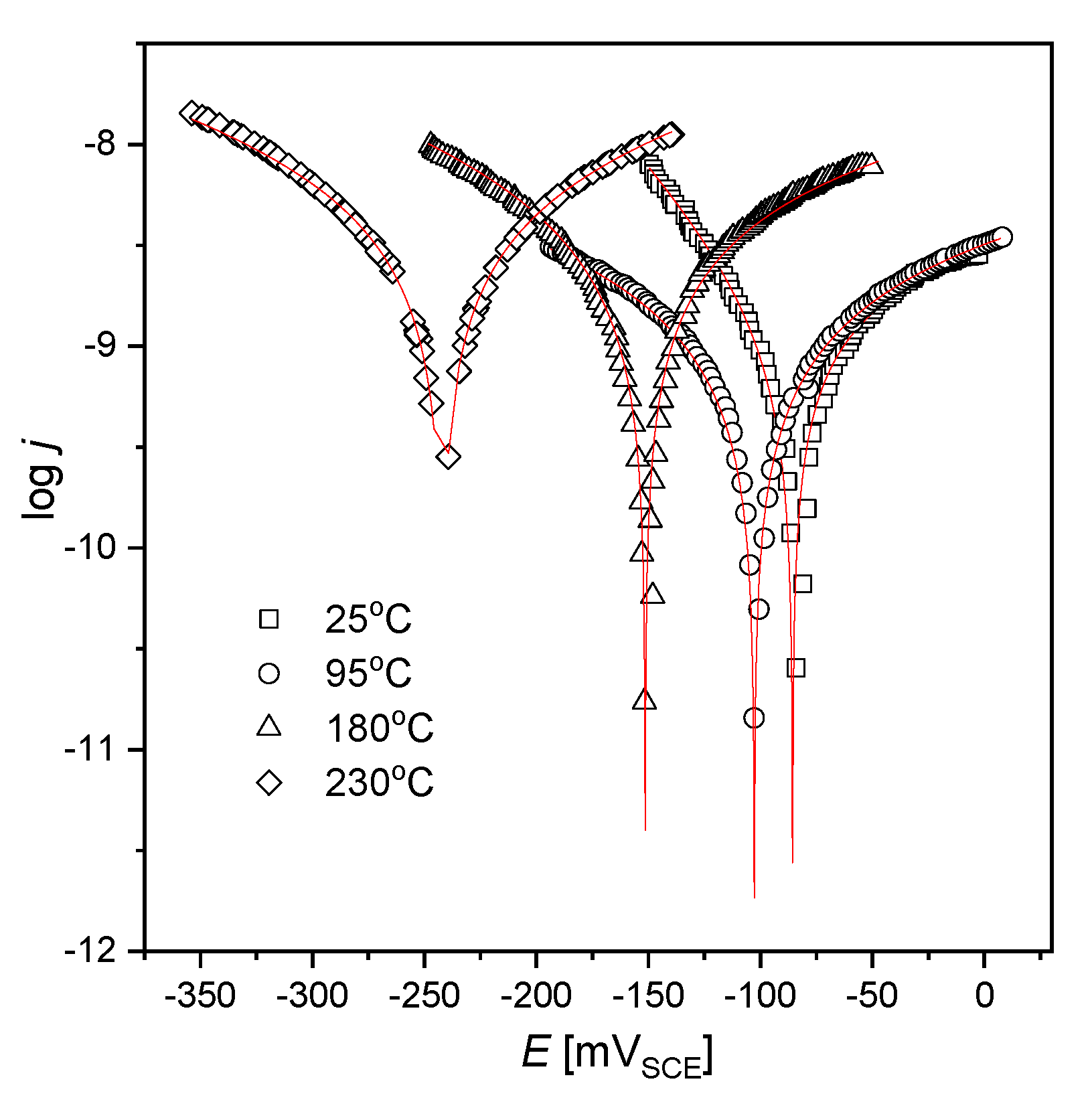
3.1. Corrosion Resistance Analysis of AISI 1010 Steel
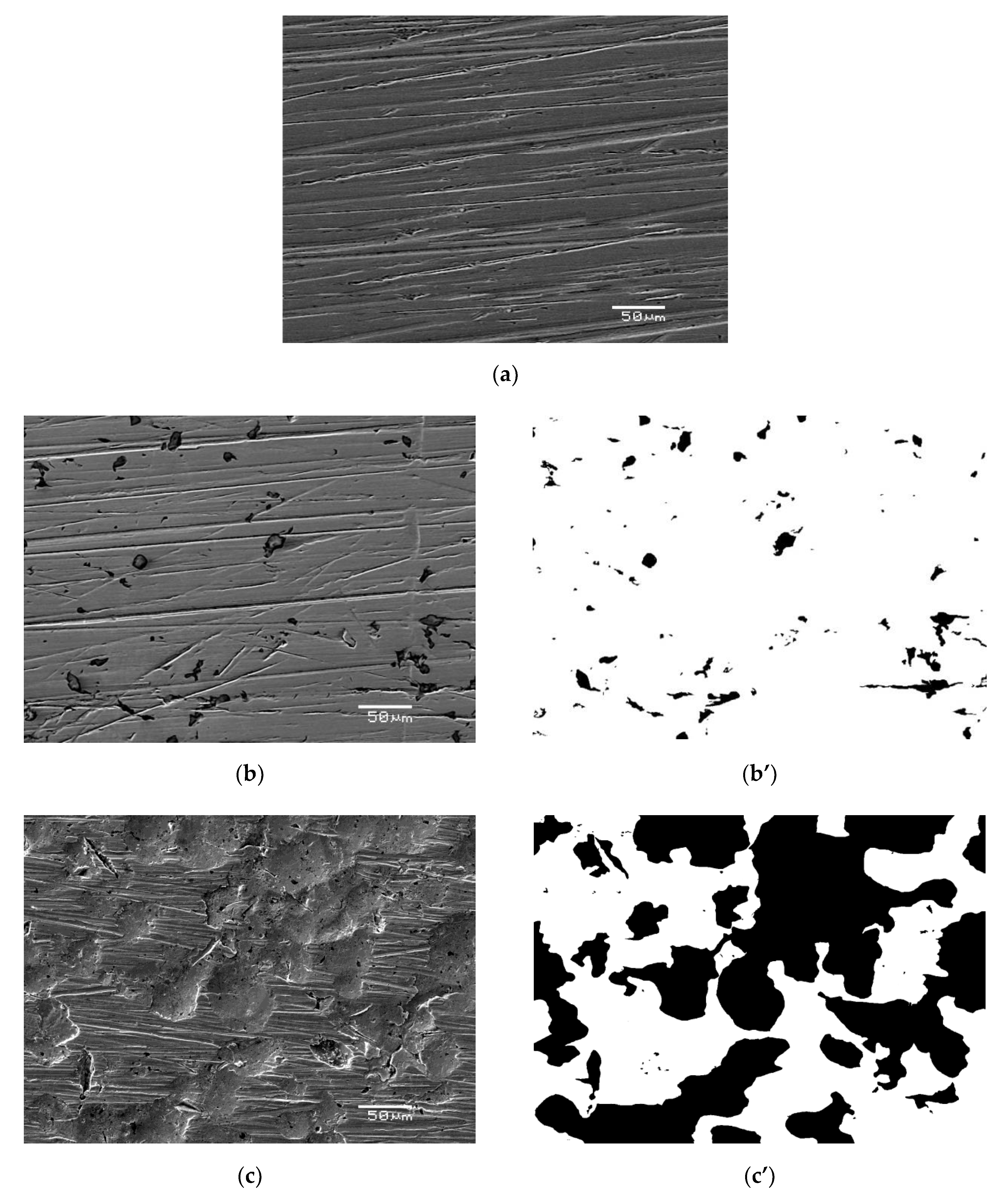
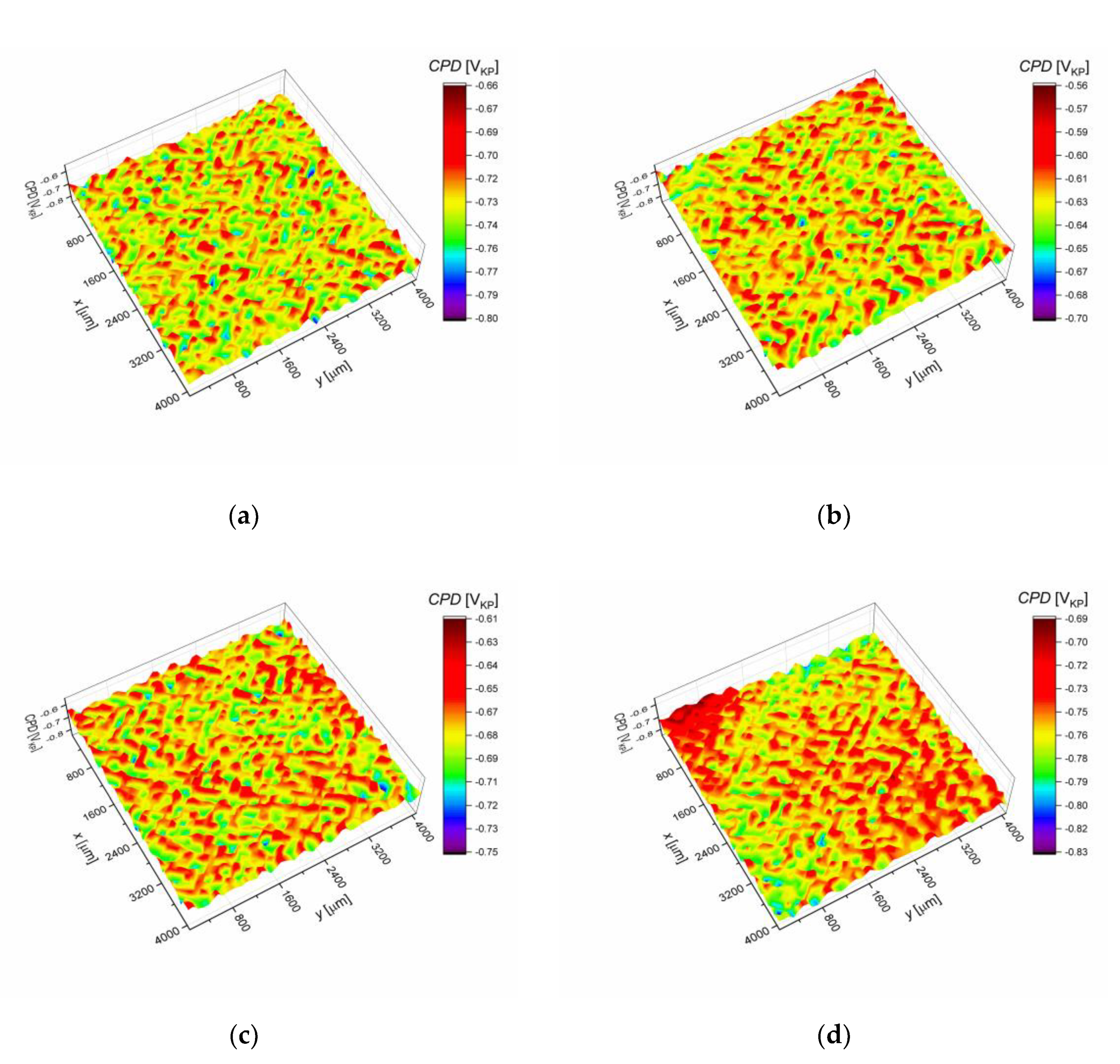
3.2. Surface Analysis of AISI 1010 Steel
4. Conclusions
- Thermodynamic tendency for the corrosion process, as well as the corrosion rate of the AISI 1010 steel, increase with increased sulfolane temperature. In particular, an increase in the sulfolane temperature from 25 to 230 °C results in a decrease in corrosion potential value by ca. 175 mV (Figure 1, Table 1) and, simultaneously, a 2.8-fold increase in the corrosion rate (Figure 3, Table 2).
- The corrosion product layer formed on the surface of the AISI 1010 steel electrodes in sulfolane is a physical barrier between the corrosive environment and the material and partially protects the alloy against further corrosion attack.
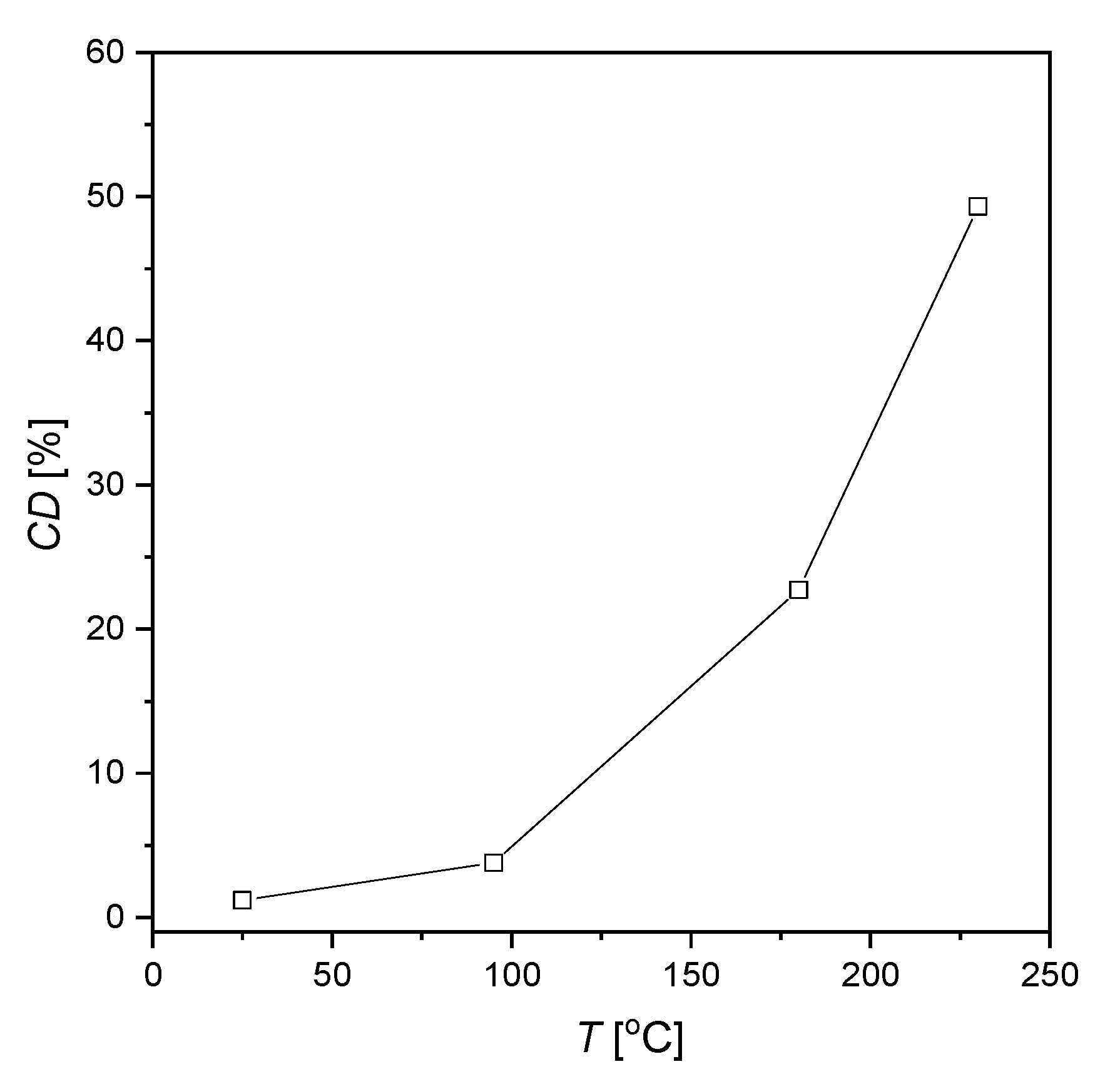
- An increase in the sulfolane temperature caused a gradual breakdown of the protective layer and an increase in the corrosion degree. It was found that the corrosion degree of AISI 1010 steel immersed in sulfolane doubles approximately every 42 °C (Figure 6).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curtis, L.; Rea, W.; Smith-Willis, P.; Fenyves, E.; Pan, Y. Adverse health effects of outdoor air pollutants. Environ. Int. 2006, 32, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Barro, R.; Regueiro, J.; Llompart, M.; Garcia-Jares, C. Analysis of industrial contaminants in indoor air: Part 1. Volatile organic compounds, carbonyl compounds, polycyclic aromatic hydrocarbons and polychlorinated biphenyls. J. Chromatogr. A 2009, 1216, 540–566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Dybal, P.; Sulowicz, S.; Kasperczyk, D.; Kus, S.; Barbusinski, K. Abatement robustness of volatile organic compounds using compact trickle-bed bioreactor: Biotreatment of styrene, ethanol and dimethyl sulfide mixture in contaminated airstream. Int. Biodeterior. Biodegr. 2017, 119, 316–328. [Google Scholar] [CrossRef]
- Wagh, R.B.; Gund, S.H.; Nagarkar, J.M. An eco-friendly oxidation of sulfide compounds. J. Chem. Sci. 2016, 128, 1321–1325. [Google Scholar] [CrossRef]
- Angaji, M.T.; Ghanbarabadi, H.; Gohari, F.K.Z. Optimizations of sulfolane concentration in propose Sulfinol-M solvent instead of MDEA solvent in the refineries of Sarakhs. J. Nat. Gas Sci. Eng. 2013, 15, 22–26. [Google Scholar] [CrossRef]
- Tilstam, U. Sulfolane: A versatile dipolar aprotic solvent. Org. Process Res. Dev. 2012, 16, 1273–1278. [Google Scholar] [CrossRef]
- Zaretskii, M.I.; Rusak, V.V.; Chartov, E.M. Sulfolane and dimethyl sulfoxide as extractants. Coke Chem. 2013, 56, 266–268. [Google Scholar] [CrossRef]
- Shah, S.M.; Wahba, M.; Yu, L.; Achari, G.; Habibi, H.R. Health impact assessment of sulfolane on embryonic development of zebrafish (Danio rerio). Toxics 2019, 7, 42. [Google Scholar] [CrossRef]
- Wang, S.; Wei, C.; Ding, W.; Zou, L.; Gong, Y.; Liu, Y.; Zang, L.; Xu, X. High-voltage sulfolane plasticized UV-curable gel polymer electrolyte. Polymers 2019, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Saint-Fort, R. Sulfolane attenuation by surface and subsurface soil matrices. J. Environ. Sci. Health A 2006, 41, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- Kasanke, C.P.; Leight, M.B. Factors limiting sulfolane biodegradation in contaminated subarctic aquifer substrate. PLoS ONE 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Gaylor, D.W.; Tachovsky, J.A.; Perry, C.; Caracostas, M.C.; Haws, L.C. Development of a chronic noncancer oral references dose and drinking water screening level for sulfolane using benchmark dose modelling. J. Appl. Toxicol. 2013, 33, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Waidyanatha, S.; Black, S.R.; Blystone, C.R.; Patel, P.R.; Watson, S.L.; Snyder, R.W.; Fennell, T.R. Disposition and metabolism of sulfolane in Harlan Sprague Dawley rats and B6C3F1/N mice and in vitro in hepatocytes from rats, mice, and humans. Xenobiotica 2020, 4, 442–453. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Dybal, P.; Kus, S.; Swietlicka, A.; Jampilek, J. Sulfolane: Magic extractor or bad actor? Pilot-scale study on solvent corrosion potential. Sustainability 2018, 10, 3677. [Google Scholar] [CrossRef]
- Silinski, M.A.R.; Uenoyama, T.; Cooper, S.D.; Fernando, R.A.; Robinson, V.G.; Waidyanatha, S. Development and validation of an analytical method for quantitation of sulfolane in rat and mouse plasma by GC–MS. J. Anal. Toxicol. 2019, 43, 477–481. [Google Scholar] [CrossRef]
- Yang, C.F.; Liu, S.H.; Su, Y.M.; Chen, Y.R.; Lin, C.W.; Lin, K.L. Bioremediation capability evaluation of benzene and sulfolane contaminated groundwater: Determination of bioremediation parameters. Sci. Total Environ. 2019, 648, 811–818. [Google Scholar] [CrossRef]
- Khan, M.F.; Yu, L.; Tay, J.H.; Achari, G. Coaggregation of bacterial communities in aerobic granulation and its application on the biodegradation of sulfolane. J. Hazard. Mater. 2019, 377, 206–214. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C.H. Mineralization of sulfolane in aqueous solutions by Ozone/CaO2 and Ozone/CaO with potential for field application. Chemosphere 2018, 197, 525–540. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C.H. Degradation of sulfolane using activated persulfate with UV and UV-Ozone. Water Res. 2017, 125, 325–331. [Google Scholar] [CrossRef]
- Brandão, M.; Yu, L.; García, C.; Achari, G. Advanced oxidation based treatment of soil wash water contaminated with sulfolane. Water 2019, 11, 2152. [Google Scholar] [CrossRef]
- Karimi, B.; Ghoreishi-Nezhad, M.; Clark, J.H. Selective oxidation of sulfides to sulfoxides using 30% hydrogen peroxide catalyzed with a recoverable silica-based tungstate interphase catalyst. Org. Lett. 2005, 7, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.F. Avoid sulfolane regeneration problems. Chem. Eng. Progress 2004, 100, 34–39. [Google Scholar]
- Bak, A.; Losiewicz, B.; Kozik, V.; Kubisztal, J.; Dybal, P.; Swietlicka, A.; Barbusinski, K.; Kus, S.; Howaniec, N.; Jampilek, J. Real-time corrosion monitoring of AISI 1010 carbon steel with metal surface mapping in sulfolane. Materials 2019, 12, 3276. [Google Scholar] [CrossRef] [PubMed]
- Mutahfar, F.; Aithan, G.; Iski, E.V.; Keller, M.W.; Shirazi, S.; Roberts, K.P. 31-Mechanistic modeling of erosion–corrosion for carbon steel. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Elsevier: London, UK, 2017; pp. 749–763. ISBN 9780081011058. [Google Scholar]
- Mingy, L.; Zhong, J.; Xujiang, S. Cause of equipment corrosion and counter measures in the sulfolane recycling system of aromatics extraction unit. Pet. Process Pe. 2005, 36, 30–33. [Google Scholar]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780521884075. [Google Scholar]
- Kubisztal, J.; Kubisztal, M.; Haneczok, G. Quantitative characterization of material surface – application to Ni + Mo electrolytic composite coatings. Mater. Charact. 2016, 122, 45–53. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Stach, S.; Haneczok, G. Corrosion resistance of anodic coatings studied by scanning microscopy and electrochemical methods. Surf. Coat. Tech. 2018, 350, 419–427. [Google Scholar] [CrossRef]
- Othmer, K. Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 9780471238966. [Google Scholar]



| Parameter | 25 °C | 95 °C | 180 °C | 230 °C |
|---|---|---|---|---|
| OCPav (mVSCE) | −81.2 | −103.8 | −152.6 | −255.7 |
| SE (mVSCE) | 0.7 | 0.5 | 0.3 | 1.2 |
| Temperature (°C) | Ecorr (mVSCE) | jcorr (nA·cm−2) | βa (mVSCE) | βc (mVSCE) | B (mVSCE) | Rp (MΩ·cm2) |
|---|---|---|---|---|---|---|
| 25 | −85.6(4) | 2.6(1) | 219(14) | 80(2) | 25 | 9.6 |
| 95 | −104.1(4) | 4.2(2) | 776(46) | 485(22) | 130 | 30.8 |
| 180 | −154.5(2) | 6.8(2) | 471(14) | 299(6) | 79 | 11.7 |
| 230 | −242.1(7) | 7.5(1) | 336(52) | 318(43) | 71 | 9.5 |
| Parameter | 25 °C | 95 °C | 180 °C | 230 °C |
|---|---|---|---|---|
| CPDav (mVKP) | −732.8(4) | −628.3(4) | −679.2(4) | −757.3(3) |
| σ (mVKP) | 19.6(5) | 17.5(5) | 18.4(5) | 19.3(6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubisztal, J.; Łosiewicz, B.; Dybal, P.; Kozik, V.; Bak, A. Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane. Materials 2020, 13, 2563. https://doi.org/10.3390/ma13112563
Kubisztal J, Łosiewicz B, Dybal P, Kozik V, Bak A. Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane. Materials. 2020; 13(11):2563. https://doi.org/10.3390/ma13112563
Chicago/Turabian StyleKubisztal, Julian, Bożena Łosiewicz, Paulina Dybal, Violetta Kozik, and Andrzej Bak. 2020. "Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane" Materials 13, no. 11: 2563. https://doi.org/10.3390/ma13112563
APA StyleKubisztal, J., Łosiewicz, B., Dybal, P., Kozik, V., & Bak, A. (2020). Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane. Materials, 13(11), 2563. https://doi.org/10.3390/ma13112563





