Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite
Abstract
1. Introduction
2. Applications of Cellulose Derivatives/Hydroxyapatite Composite Membranes
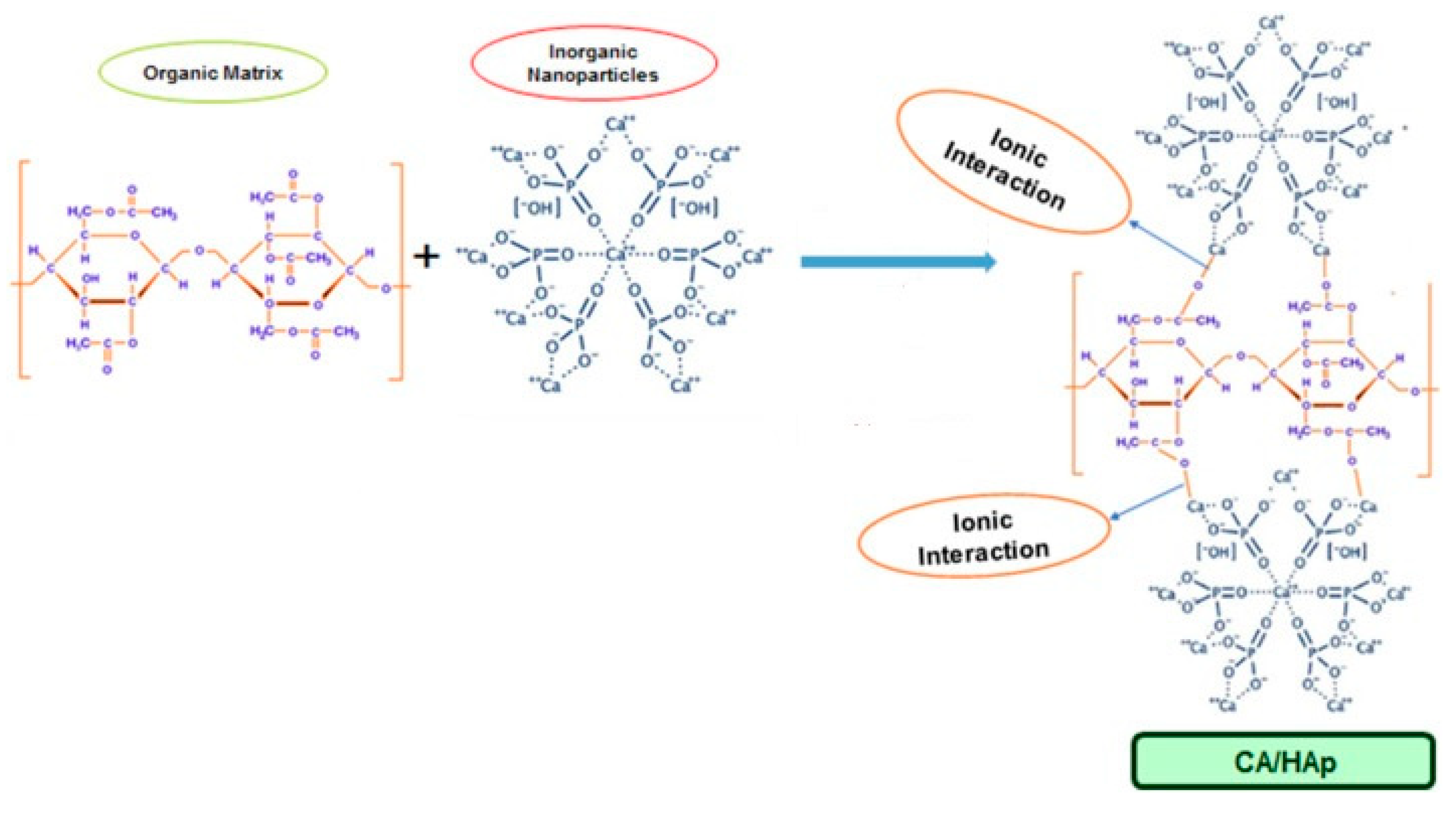
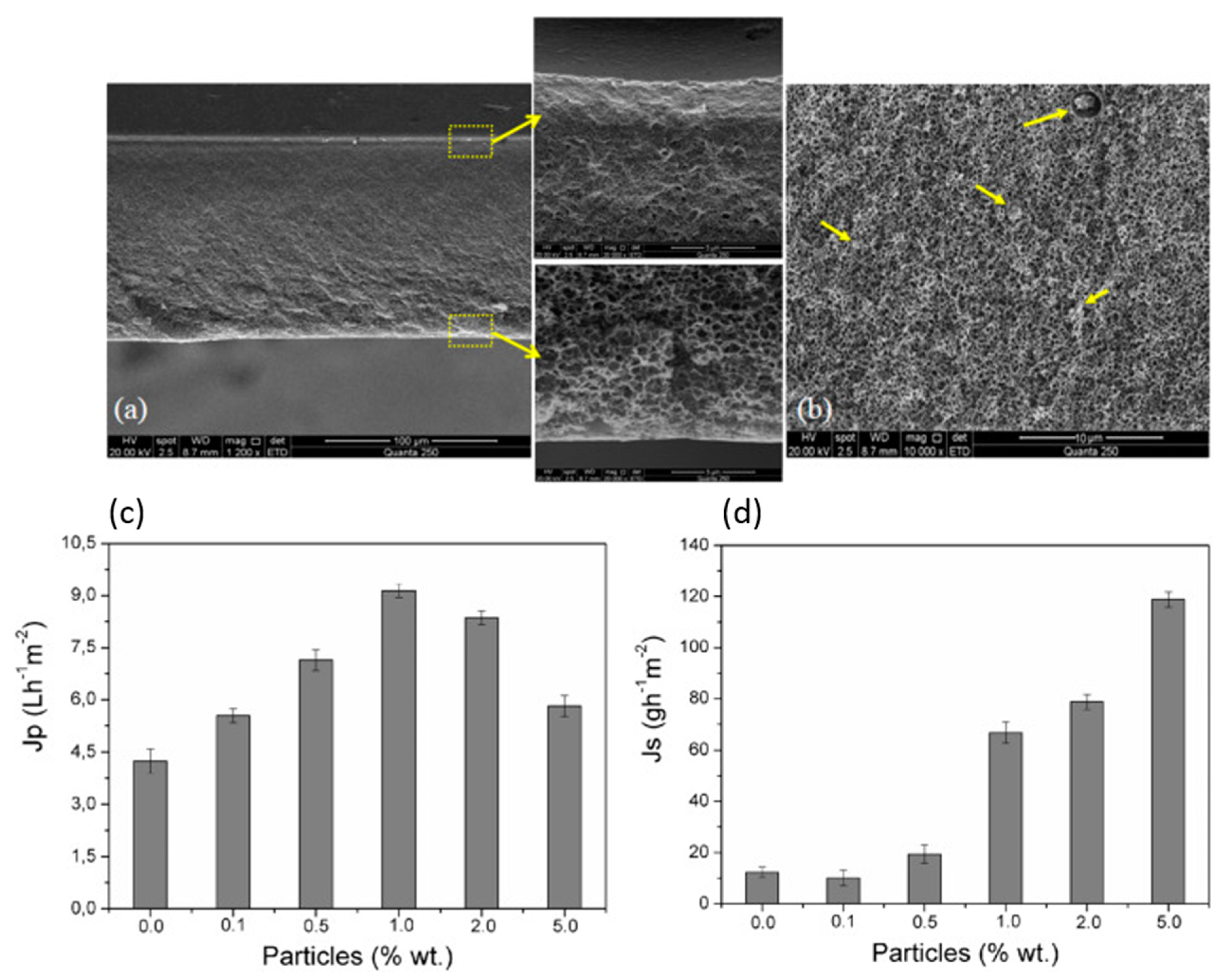
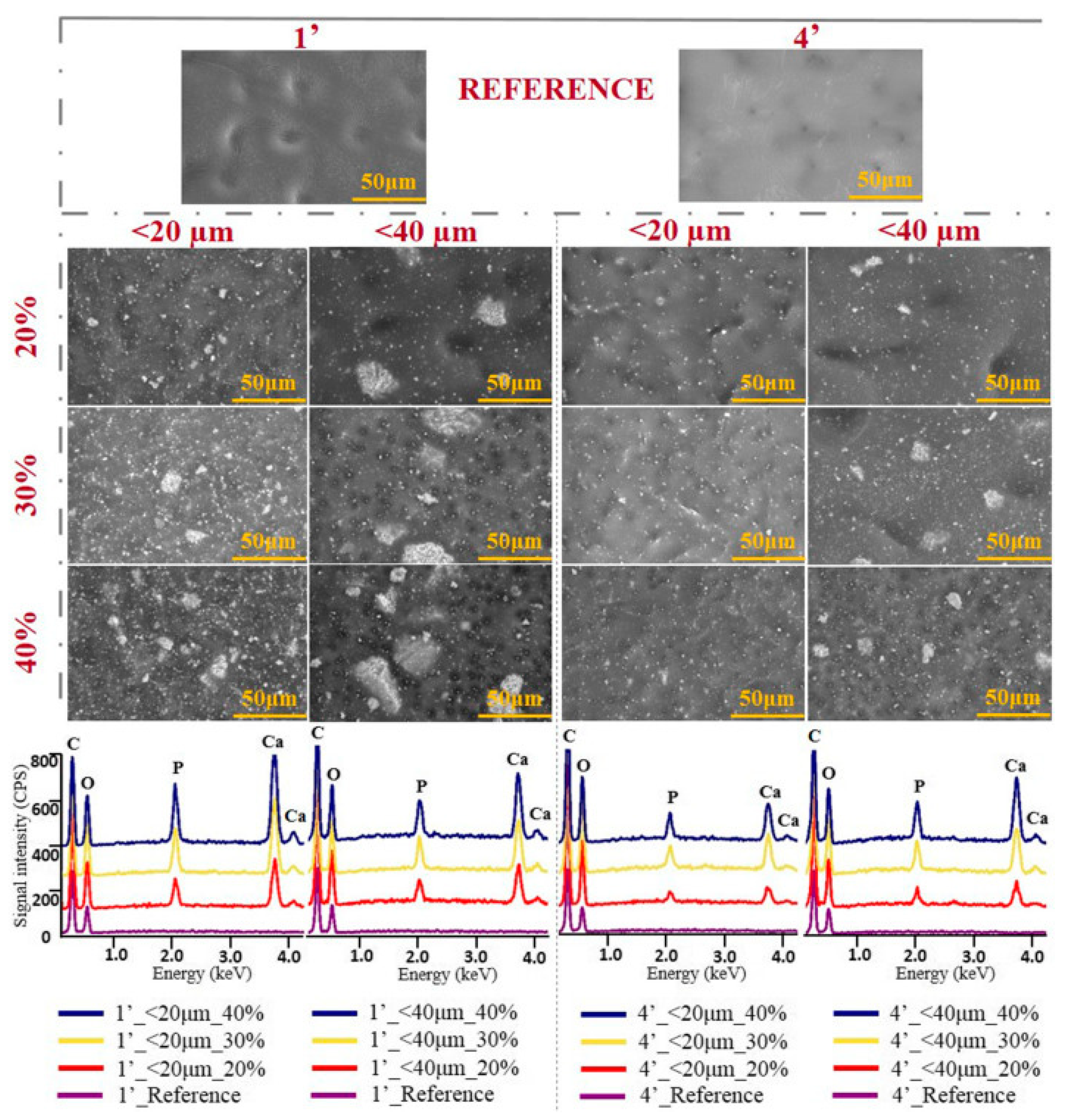
2.1. Water Purification
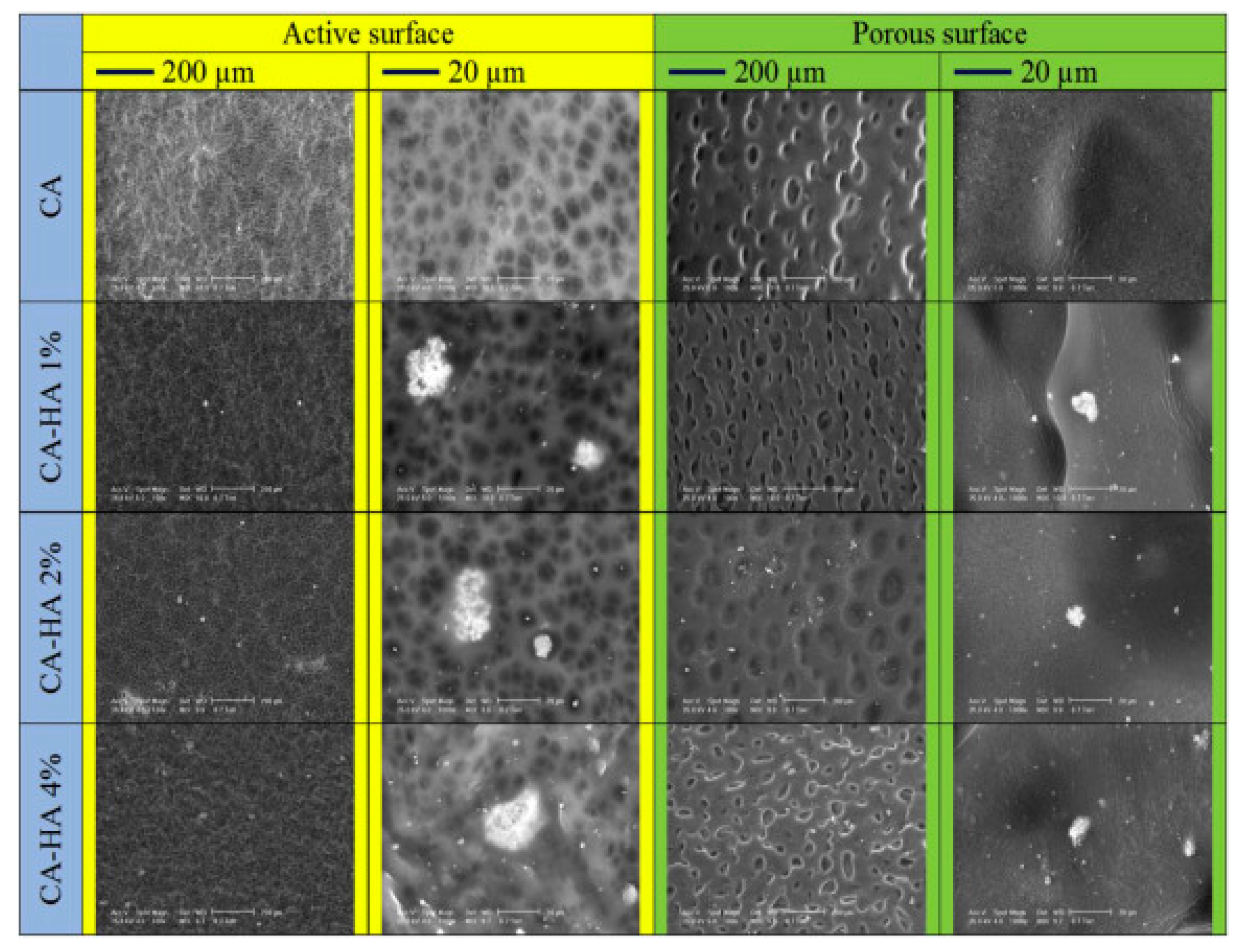
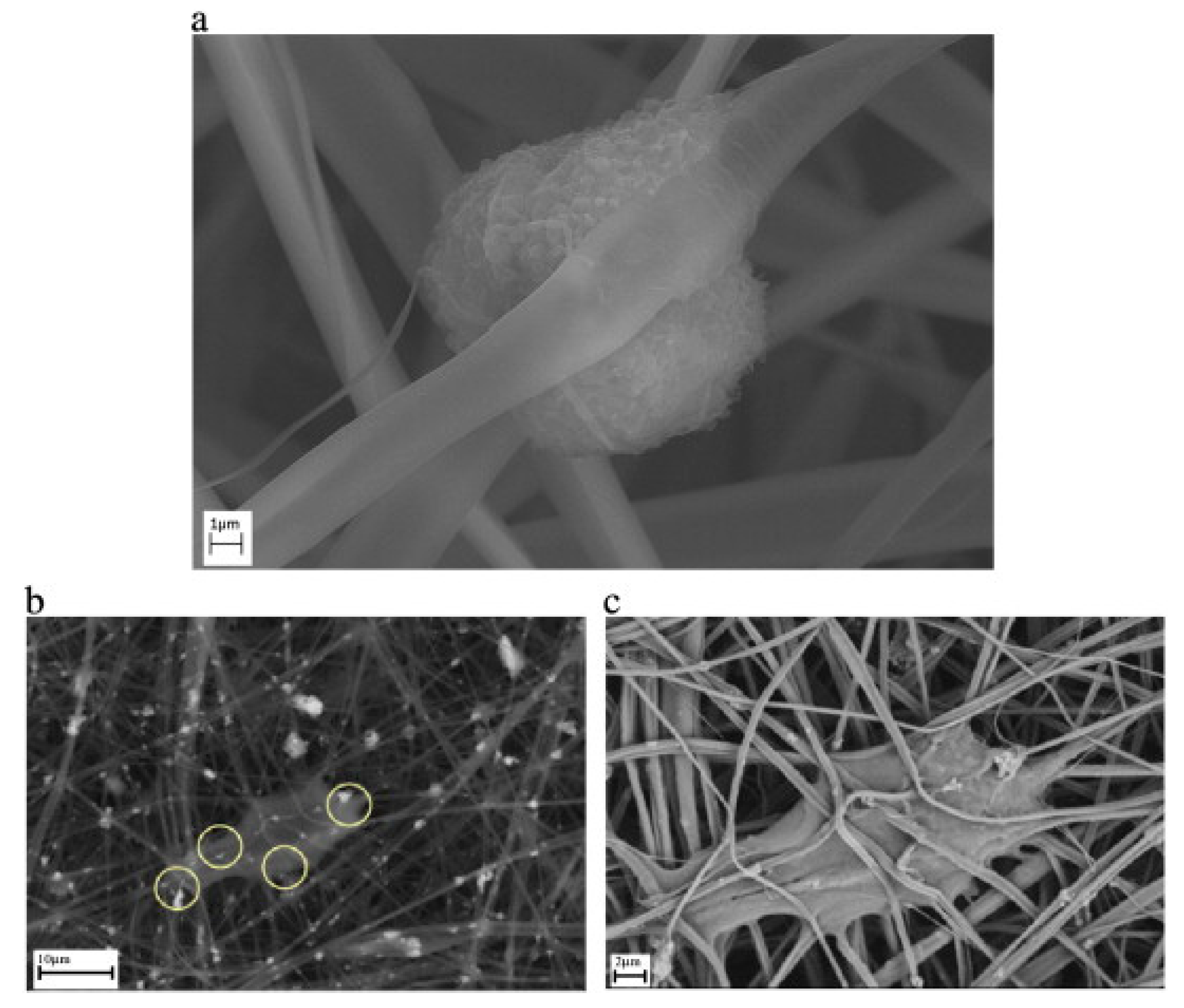
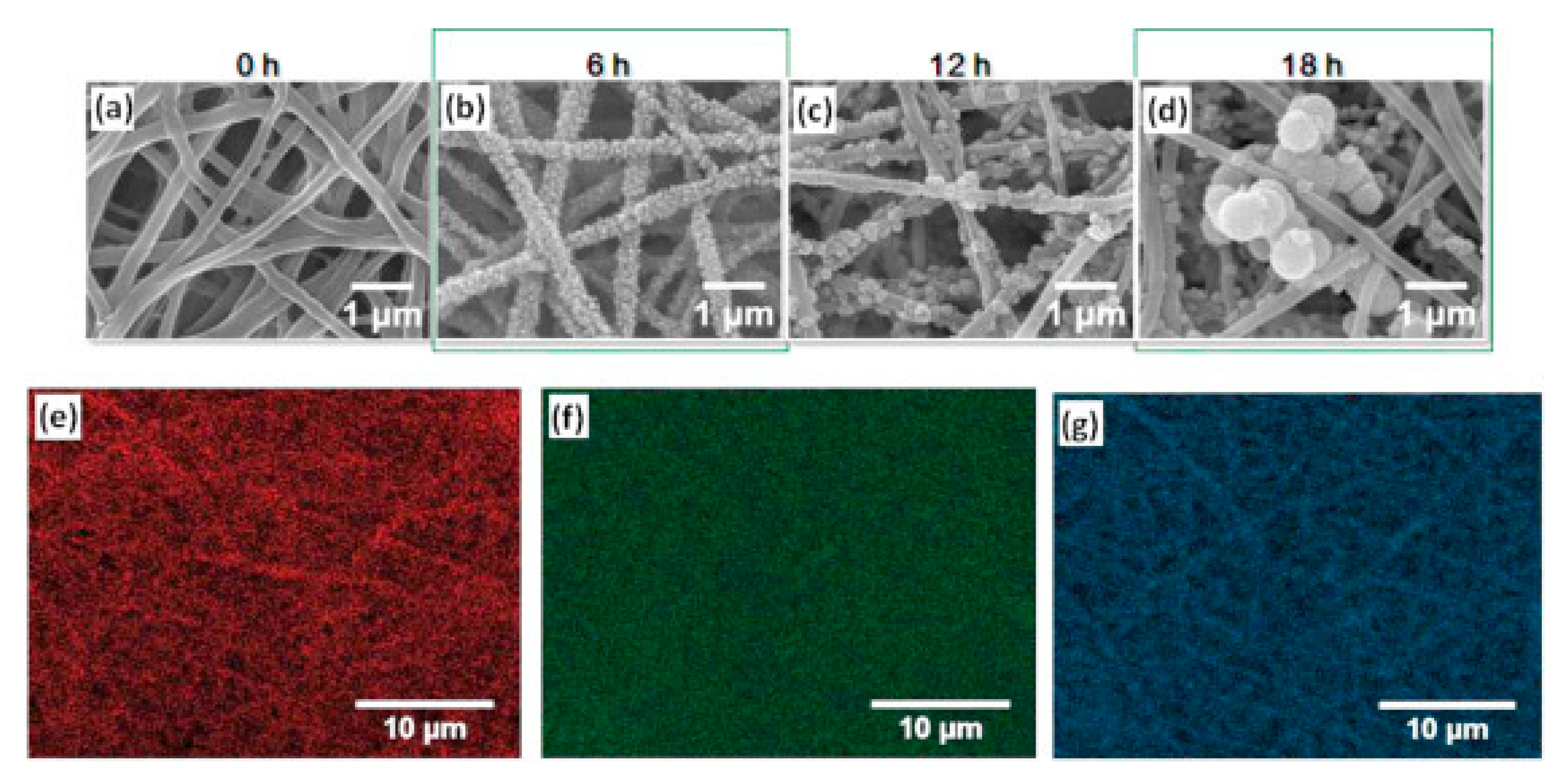
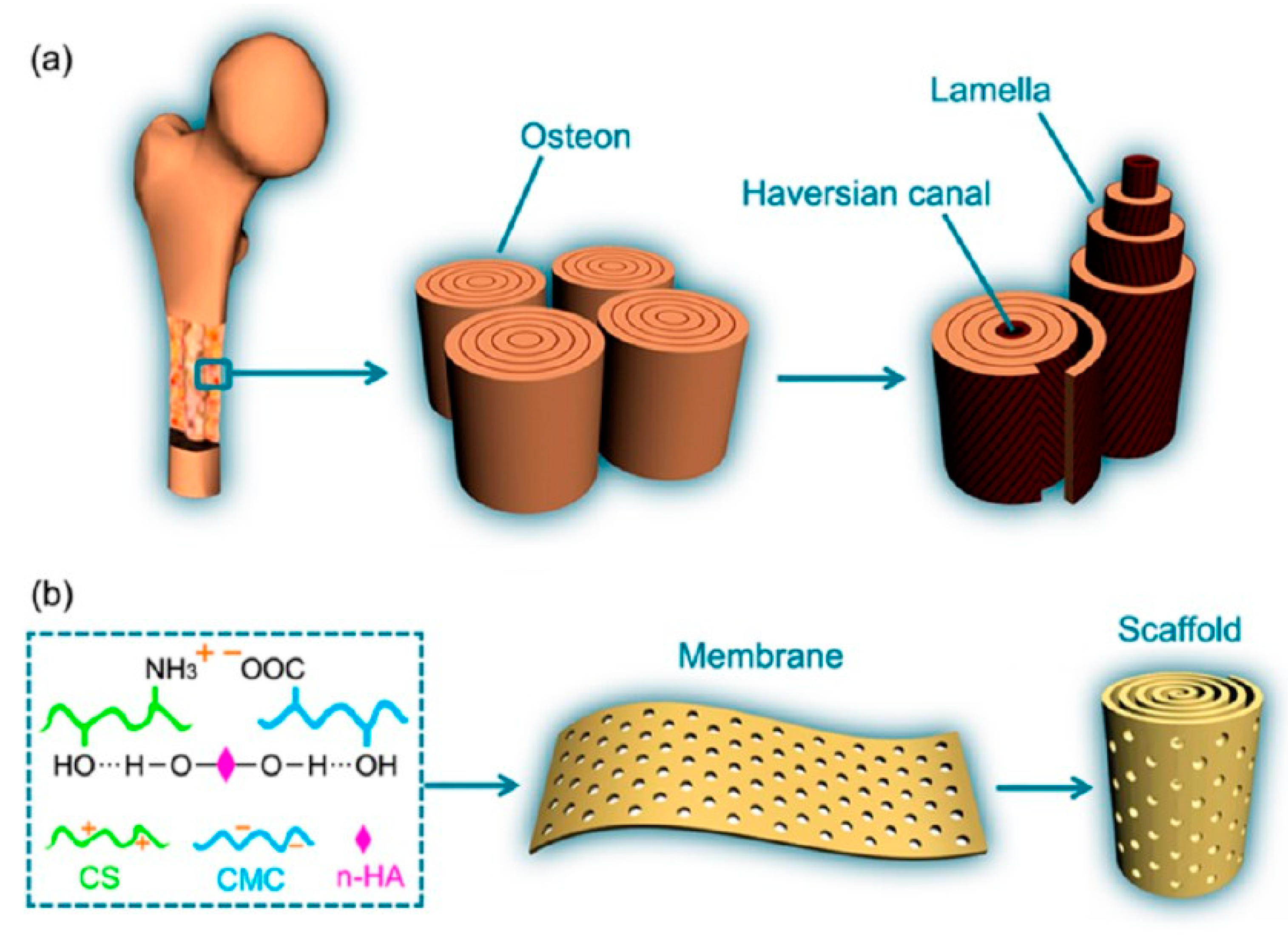
2.2. Bone Tissue Engineering
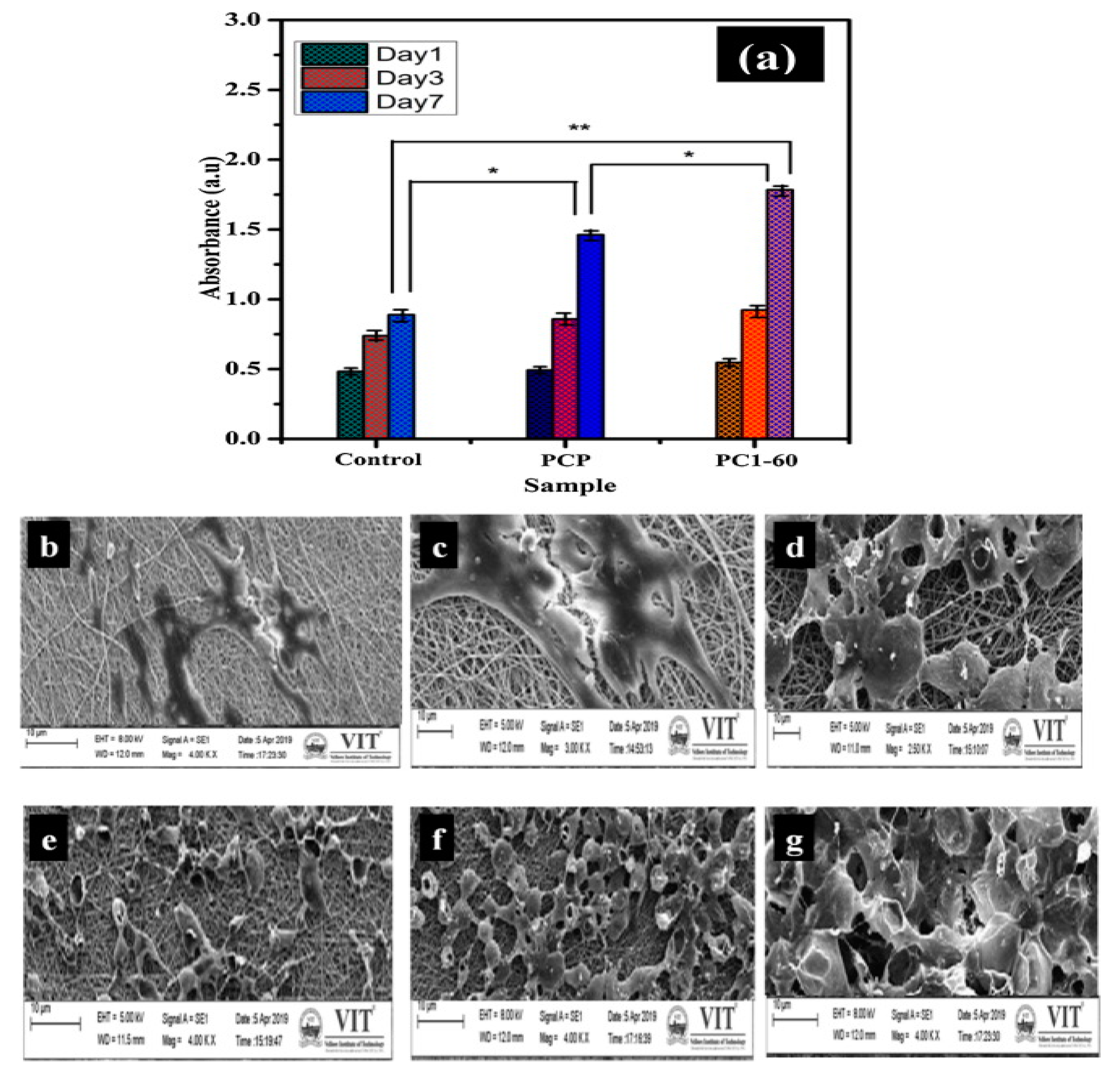
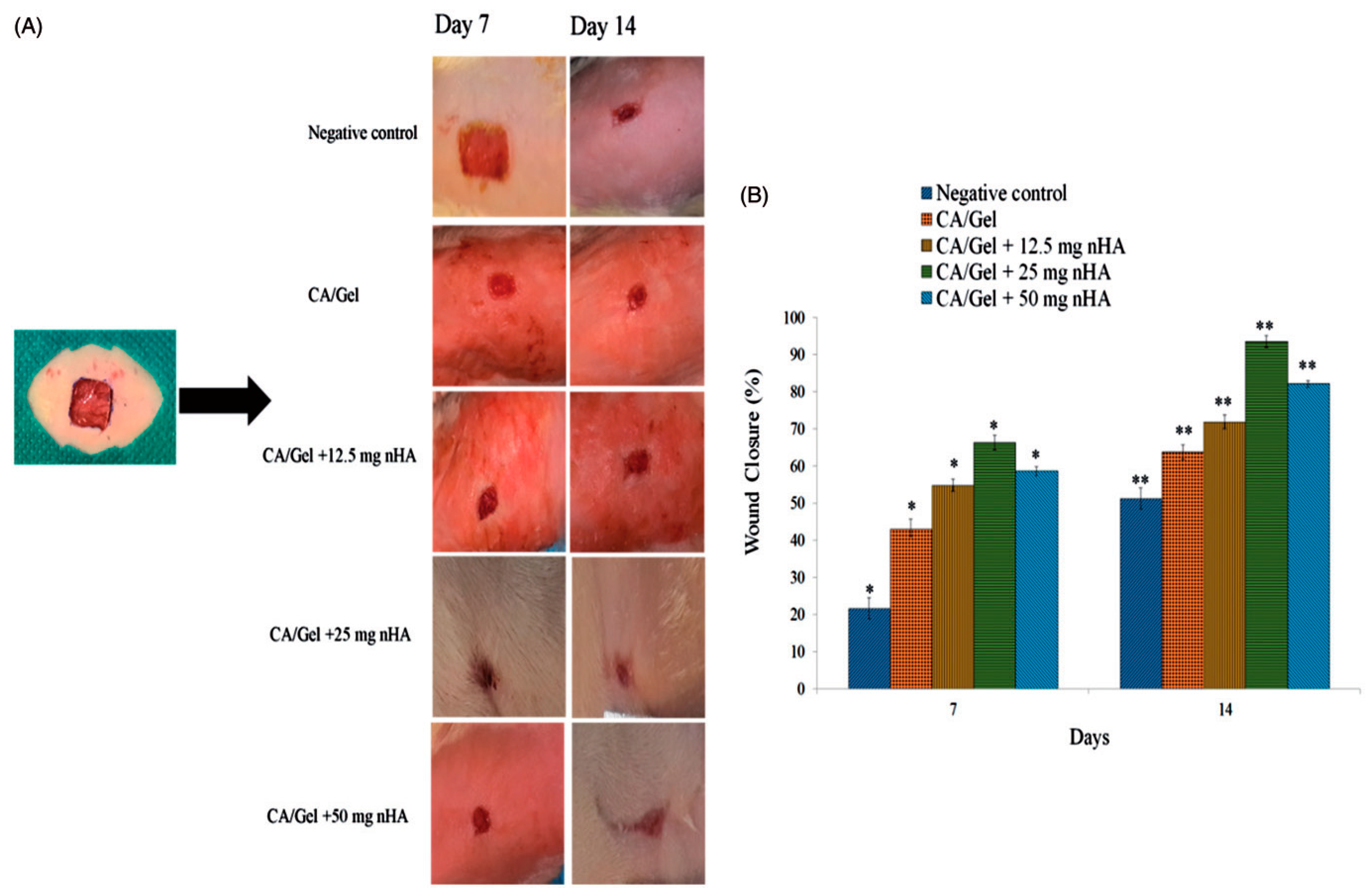
2.3. Wound Healing
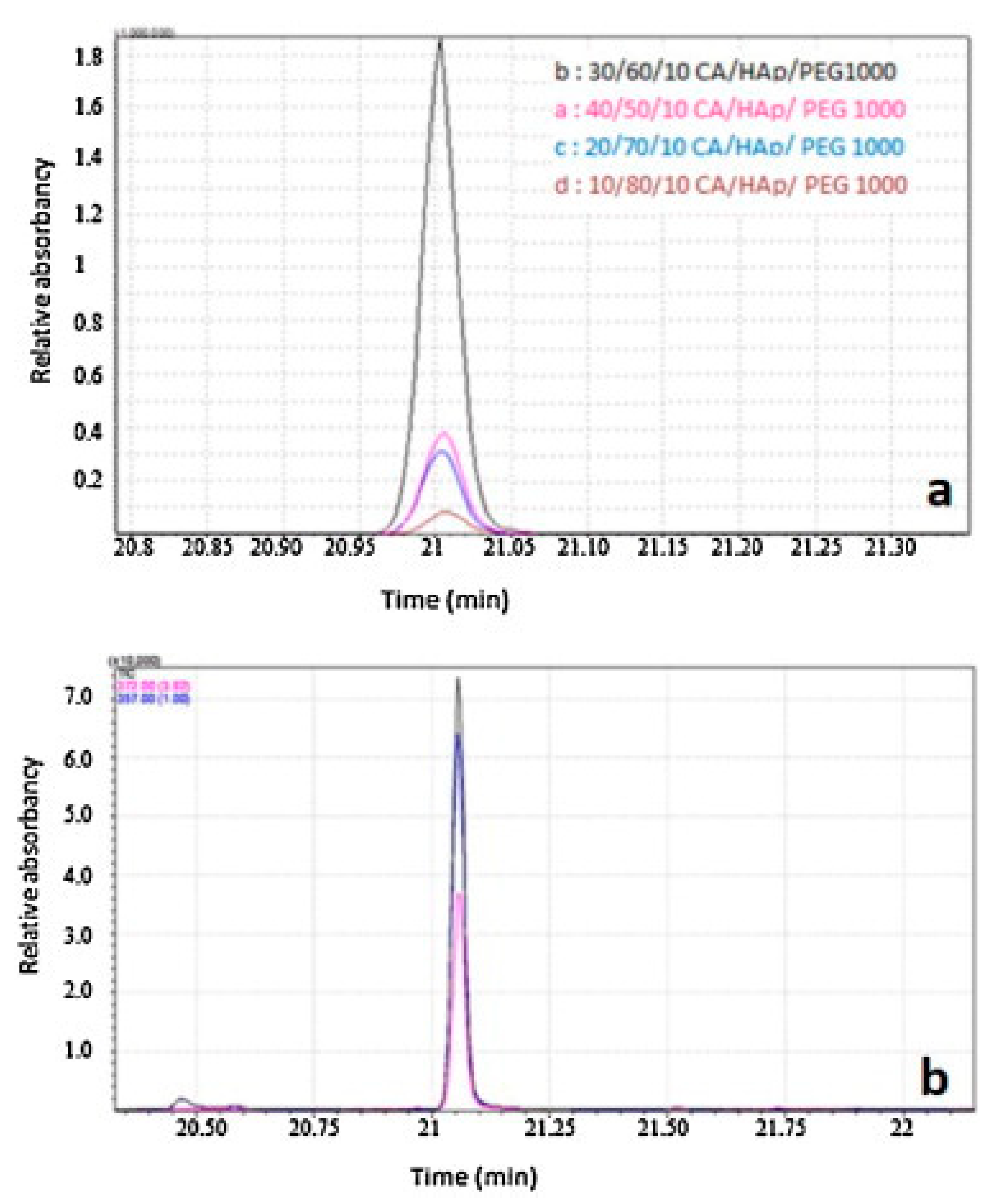
2.4. Controlled Drug Delivery
2.5. Hemodialysis
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley: Hoboken, NJ, USA, 1995. [Google Scholar]
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came About; American Chemical Society (ACS): Washington, DC, USA, 1981; Volume 153, pp. 1–9. [Google Scholar]
- Tiwari, R.R.; Jin, J.; Freeman, B.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, F.; Liu, Q.; Zong, C.; Yang, W.; Cao, X.; Jin, W.; Xu, N. Microporous Polyamide Membranes for Molecular Sieving of Nitrogen from Volatile Organic Compounds. Angew. Chem. Int. Ed. 2017, 56, 5755–5759. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes 2018, 9, 3. [Google Scholar] [CrossRef]
- Voicu, Ş.I.; Dobrica, A.; Sava, S.; Ivan, A.; Naftanaila, L. Cationic surfactants-controlled geometry and dimensions of polymeric membrane pores. J. Optoelectron. Adv. Mater. 2012, 14, 923–928. [Google Scholar]
- Ionită, M.; Crica, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef]
- Voicu, Ş.I.; Pandele, A.; Tanasă, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Serbanescu, O.; Pandele, A.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 214, 204–212. [Google Scholar] [CrossRef]
- Thakur, V.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.; Voicu, S.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Agrawal, K.V.; Coronas, J. Ultrathin permselective membranes: The latent way for efficient gas separation. RSC Adv. 2020, 10, 12653–12670. [Google Scholar] [CrossRef]
- Stamatialis, D.; Papenburg, B.J.; Gironès, M.; Saiful, S.; Bettahalli, S.N.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef]
- Corobea, M.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I.; et al. Novel nanocomposite membranes from cellulose acetate and clay-silica nanowires. Polym. Adv. Technol. 2016, 27, 1586–1595. [Google Scholar] [CrossRef]
- Falkenhagen, D.; Strobl, W.; Hartmann, J.; Schrefl, A.; Linsberger, I.; Kellner, K.-H.; Aussenegg, F.; Leitner, A. Patient safety technology for microadsorbent systems in extracorporeal blood purification. Artif. Organs 2002, 26, 84–90. [Google Scholar] [CrossRef]
- Flendrig, L.M.; La Soe, J.W.; Jörning, G.G.; Steenbeek, A.; Karlsen, O.T.; Bovée, W.M.; Ladiges, N.C.; Velde, A.A.T.; Chamuleau, R.A. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J. Hepatol. 1997, 26, 1379–1392. [Google Scholar] [CrossRef]
- Shih, C.; Lee, K.-R.; Lai, J. 60Co γ-ray irradiation modified poly(4-methyli-pentene) membrane for oxygenator. Eur. Polym. J. 1994, 30, 629–634. [Google Scholar] [CrossRef]
- Sauer, I.M.; Neuhaus, P.; Gerlach, J.C. Concept for modular extracorporeal liver support for the treatment of acute hepatic failure. Metab. Brain Dis. 2002, 17, 477–484. [Google Scholar] [CrossRef]
- Leoni, L.; Boiarski, A.; Desai, T.A. Characterization of Nanoporous Membranes for Immunoisolation: Diffusion Properties and Tissue Effects. Biomed. Microdevices 2002, 4, 131–139. [Google Scholar] [CrossRef]
- Corobea, M.S.; Albu-Kaya, M.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Modification of titanium surface with collagen and doxycycline as a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 1–14. [Google Scholar] [CrossRef]
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V. Sericin Covalent Immobilization onto Cellulose Acetate Membrane for Biomedical Applications. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.E.; Radu, I.; Miculescu, F.; Voicu, S.I.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.; Stan, G.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Batalu, N.D.; Ciocoiu, R.C.; Voicu, S.I.; Stan, G.; Thakur, V. Synthesis and Characterization of Jellified Composites from Bovine Bone-Derived Hydroxyapatite and Starch as Precursors for Robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.I.; Ciocan, L.T.; Niculescu, M.; Corobea, M.; Rada, M.E.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Stan, G.; Miculescu, M.; Maidaniuc, A.; Cimpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Matei, E.; Pencea, I.; Csaki, I.; Machedon-Pisu, T.; Ciocan, L.T.; Voicu, S.I.; et al. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.-C.; Dascălu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.C.; Voicu, S.I.; Miculescu, M.; Thakur, V.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetraethylammonium hydroxide–carbamide solutions. Cellulose 2019, 27, 1933–1950. [Google Scholar] [CrossRef]
- Khiari, R.; Belgacem, M.N. Potential for using multiscale Posidonia oceanica waste. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 447–471. [Google Scholar]
- Alfassi, G.; Rein, D.M.; Shpigelman, A.; Cohen, Y.; Rein, D.M. Partially Acetylated Cellulose Dissolved in Aqueous Solution: Physical Properties and Enzymatic Hydrolysis. Polymers 2019, 11, 1734. [Google Scholar] [CrossRef]
- Global Industry Analysis. Cellulose Acetate (MCP-2035). Available online: https://www.strategyr.com/market-report-cellulose-acetate-forecasts-global-industry-analysts-inc.asp (accessed on 5 May 2020).
- Xu, J.; Wu, Z.; Wu, Q.; Kuang, Y. Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydr. Polym. 2020, 229, 115553. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A. Green synthesis of cellulose acetate from corncob: Physicochemical properties and assessment of environmental impacts. J. Clean. Prod. 2020, 260, 120865. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2010, 19, 152–165. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Dobos, A.M.; Filimon, A.; Bargan, A.; Zaltariov, M.-F. New approaches for the development of cellulose acetate/tetraethyl orthosilicate composite membranes: Rheological and microstructural analysis. J. Mol. Liq. 2020, 309, 113129. [Google Scholar] [CrossRef]
- Martin-Gil, V.; Ahmad, M.; Castro-Muñoz, R.; Fila, V. Economic Framework of Membrane Technologies for Natural Gas Applications. Sep. Purif. Rev. 2018, 48, 298–324. [Google Scholar] [CrossRef]
- Altunina, L.; Tikhonova, L.; Yarmukhametova, E. Method for Deriving Carboxymethyl Cellulose. Eurasian Chem. J. 2016, 3, 49. [Google Scholar] [CrossRef]
- Golbaghi, L.; Khamforoush, M.; Hatami, T. Carboxymethyl cellulose production from sugarcane bagasse with steam explosion pulping: Experimental, modeling, and optimization. Carbohydr. Polym. 2017, 174, 780–788. [Google Scholar] [CrossRef]
- Shui, T.; Feng, S.; Chen, G.; Li, A.; Yuan, Z.; Shui, H.; Kuboki, T.; Xu, C.C. Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk. Biomass Bioenergy 2017, 105, 51–58. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Jodeh, S.; Hamed, O.; Berrabah, M.; Jerdioui, S.; Salghi, R.; Akartasse, N.; Errich, A.; et al. Preparation and characterization of biodegradable nanocomposites derived from carboxymethyl cellulose and hydroxyapatite. Carbohydr. Polym. 2017, 167, 59–69. [Google Scholar] [CrossRef]
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245. [Google Scholar] [CrossRef]
- Chen, H. 5-Lignocellulose biorefinery product engineering. In Lignocellulose Biorefinery Engineering; Chen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 125–165. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, G.; Dan, N.; Huang, Y.; Bai, Z.; Yang, C.; Dan, W. Preparation and characterization of dopamine–sodium carboxymethyl cellulose hydrogel. SN Appl. Sci. 2019, 1, 609. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. Int. J. Boil. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef]
- Ohta, S.; Nishiyama, T.; Sakoda, M.; Machioka, K.; Fuke, M.; Ichimura, S.; Inagaki, F.; Shimizu, A.; Hasegawa, K.; Kokudo, N.; et al. Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. J. Biosci. Bioeng. 2015, 119, 718–723. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef]
- Hayder, A.; Hussain, A.; Khan, A.N.; Waheed, H. Fabrication and characterization of cellulose acetate/hydroxyapatite composite membranes for the solute separations in Hemodialysis. Polym. Bull. 2017, 75, 1197–1210. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.; Carp, C.; Miculescu, M.; Voicu, S.; Thakur, V.; Serban, B. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef]
- Azzaoui, K.; Lamhamdi, A.; Mejdoubi, E.M.; Berrabah, M.; Hammouti, B.; Elidrissi, A.; Fouda, M.M.; Al-Deyab, S.S.; Lamhamdi, A. Synthesis and characterization of composite based on cellulose acetate and hydroxyapatite application to the absorption of harmful substances. Carbohydr. Polym. 2014, 111, 41–46. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ciobanu, O. High-performance ultrafiltration mixed-matrix membranes based on cellulose acetate and nanohydroxyapatite. Desalin. Water Treat. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ana-Maria, B.; Luca, C. Nystatin-loaded Cellulose Acetate/Hydroxyapatite Biocomposites. Revista de Chimie 2013, 64, 1426–1429. [Google Scholar]
- Ohland, A.L.; Salim, V.M.M.; Borges, C.P. Plasma functionalized hydroxyapatite incorporated in membranes for improved performance of osmotic processes. Desalination 2019, 452, 87–93. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 107–156. [Google Scholar]
- Jiang, H.; Zuo, Y.; Cheng, L.; Wang, H.; Gu, A.; Li, Y. A homogenous CS/NaCMC/n-HA polyelectrolyte complex membrane prepared by gradual electrostatic assembling. J. Mater. Sci. Mater. Electron. 2010, 22, 289–297. [Google Scholar] [CrossRef]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US1975504A, 2 October 1934. [Google Scholar]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.I.; Elkady, M.F.; Elkawi, M.A.A.; Hamad, H.A. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Boil. Macromol. 2020, 151, 1299–1313. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, V.; Sumathi, S. Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications. Int. J. Boil. Macromol. 2019, 145, 1018–1030. [Google Scholar] [CrossRef]
- El-Newehy, M.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. J. Macromol. Sci. Part A 2016, 53, 566–573. [Google Scholar] [CrossRef]
- Shi, D.; Wang, F.; Lan, T.; Zhang, Y.; Shao, Z. Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 2016, 23, 1899–1909. [Google Scholar] [CrossRef]
- Gašparič, P.; Kurecic, M.; Kargl, R.J.; Maver, U.; Gradišnik, L.; Hribernik, S.; Kleinschek, K.S.; Smole, M.S. Nanofibrous polysaccharide hydroxyapatite composites with biocompatibility against human osteoblasts. Carbohydr. Polym. 2017, 177, 388–396. [Google Scholar] [CrossRef]
- Raghavan, P.; Nageswaran, S.; Thakur, V.; Ahn, J.-H. Electrospinning of Cellulose: Process and Applications. In Nanocellulose Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2014; pp. 311–340. [Google Scholar]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Fragal, E.H.; Cellet, T.S.; Fragal, V.H.; Companhoni, M.V.; Nakamura, T.U.; Muniz, E.C.; Silva, R.; Rubira, A.F. Hybrid materials for bone tissue engineering from biomimetic growth of hydroxiapatite on cellulose nanowhiskers. Carbohydr. Polym. 2016, 152, 734–746. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surf. B Biointerfaces 2018, 163, 394–401. [Google Scholar] [CrossRef]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Ma, B.; Han, J.; Zhang, S.; Liu, F.; Wang, S.; Duan, J.; Sang, Y.; Jiang, H.; Li, N.; Ge, S.; et al. Hydroxyapatite nanobelt/polylactic acid Janus membrane with osteoinduction/barrier dual functions for precise bone defect repair. Acta Biomater. 2018, 71, 108–117. [Google Scholar] [CrossRef]
- Domínguez, J.H.L.; Jiménez, H.T.; Cocoletzi, H.H.; Hernández, M.G.; Banda, J.A.M.; Nygren, H. Development and in vivo response of hydroxyapatite/whitlockite from chicken bones as bone substitute using a chitosan membrane for guided bone regeneration. Ceram. Int. 2018, 44, 22583–22591. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgor, E.; Yilgor, E. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Boil. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Ohland, A.L.; Salim, V.M.M.; Borges, C.P. Nanocomposite membranes for osmotic processes: Incorporation of functionalized hydroxyapatite in porous substrate and in selective layer. Desalination 2019, 463, 23–31. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Zaoui, S.; Berrabah, M.; Elidrissi, A.; Hammouti, B.; Fouda, M.M.; Al-Deyab, S.S. Structure and properties of hydroxyapatite/hydroxyethyl cellulose acetate composite films. Carbohydr. Polym. 2015, 115, 170–176. [Google Scholar] [CrossRef]
- Hellawell, J.M. Toxic substances in rivers and streams. Environ. Pollut. 1988, 50, 61–85. [Google Scholar] [CrossRef]
- El-Aziz, M.E.A.; Saber, E.; El-Khateeb, M. Preparation and characterization of CMC/HA-NPs/pulp nanocom-posites for the removal of heavy metal ions. KGK Rubberpoint 2019, 72, 36–41. [Google Scholar]
- Minh, D.P.; Rio, S.; Sharrock, P.; Sebei, H.; Lyczko, N.; Tran, N.D.; Raii, M.; Nzihou, A. Hydroxyapatite starting from calcium carbonate and orthophosphoric acid: Synthesis, characterization, and applications. J. Mater. Sci. 2014, 49, 4261–4269. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.; Elmore, D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef]
- Gouma, P.; Xue, R.; Goldbeck, C.; Perrotta, P.; Balázsi, C. Nano-hydroxyapatite—Cellulose acetate composites for growing of bone cells. Mater. Sci. Eng. C 2012, 32, 607–612. [Google Scholar] [CrossRef]
- Kwak, D.H.; Lee, E.J.; Kim, D.J. Bioactivity of cellulose acetate/hydroxyapatite nanoparticle composite fiber by an electro-spinning process. J. Nanosci. Nanotechnol. 2014, 14, 8464–8471. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, Y.; Li, B.; Chen, L. Hierarchical micro/submicrometer-scale structured scaffolds prepared via coaxial electrospinning for bone regeneration. J. Mater. Chem. B 2017, 5, 9219–9228. [Google Scholar] [CrossRef]
- Nisar, F.; Bin Khalid, U.; Akram, M.A.; Javed, S.; Mujahid, M. Fabrication of Cellulose Acetate/Cellulose-HA Composite Films for Bone Fixation. Key Eng. Mater. 2018, 778, 325–330. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Y.; Xue, H.; Dou, Y. Biomimetic Growth of Hydroxyapatite on Electrospun CA/PVP Core⁻Shell Nanofiber Membranes. Polymers 2018, 10, 1032. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Prabakaran, M.; Ke, M.; Gang, X.; Chung, I.-M.; Um, I.C.; Gopiraman, M.; Kim, I.S. Highly dispersed nanoscale hydroxyapatite on cellulose nanofibers for bone regeneration. Mater. Lett. 2016, 168, 56–61. [Google Scholar] [CrossRef]
- Ogiwara, T.; Katsumura, A.; Sugimura, K.; Teramoto, Y.; Nishio, Y. Calcium Phosphate Mineralization in Cellulose Derivative/Poly(acrylic acid) Composites Having a Chiral Nematic Mesomorphic Structure. Biomacromolecules 2015, 16, 3959–3969. [Google Scholar] [CrossRef]
- Qi, P.; Ohba, S.; Hara, Y.; Fuke, M.; Ogawa, T.; Ohta, S.; Ito, T. Fabrication of calcium phosphate-loaded carboxymethyl cellulose non-woven sheets for bone regeneration. Carbohydr. Polym. 2018, 189, 322–330. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Dascălu, C.-A.; Maidaniuc, A.; Pandele, A.M.; Voicu, S.I.; Machedon-Pisu, T.; Stan, G.E.; Cîmpean, A.; Mitran, V.; Antoniac, I.V.; Miculescu, F. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl. Surf. Sci. 2019, 494, 335–352. [Google Scholar] [CrossRef]
- Liuyun, J.; Yubao, L.; Chengdong, X. A novel composite membrane of chitosan-carboxymethyl cellulose polyelectrolyte complex membrane filled with nano-hydroxyapatite I. Preparation and properties. J. Mater. Sci. Mater. Electron. 2009, 20, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zuo, Y.; Zou, Q.; Wang, H.; Du, J.; Li, Y.; Yang, X. Biomimetic Spiral-Cylindrical Scaffold Based on Hybrid Chitosan/Cellulose/Nano-Hydroxyapatite Membrane for Bone Regeneration. ACS Appl. Mater. Interfaces 2013, 5, 12036–12044. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Wang, F.-J.; Yang, Y.Y.; Zhang, X.-Z.; Zhu, X.; Chung, T.-S.; Moochhala, S.; Chung, T.-S. Cellulose acetate membranes for transdermal delivery of scopolamine base. Mater. Sci. Eng. C 2002, 20, 93–100. [Google Scholar] [CrossRef]
- Ruggiero, R.; Carvalho, V.D.A.; Da Silva, L.G.; Magalhães, D.; Ferreira, J.A.; De Menezes, H.H.M.; De Melo, P.G.; Naves, M.M. Study of in vitro degradation of cellulose acetate membranes modified and incorporated with tetracycline for use as an adjuvant in periodontal reconstitution. Ind. Crop. Prod. 2015, 72, 2–6. [Google Scholar] [CrossRef]
- Aksungur, P.; Sungur, A.; Ünal, S.; Iskit, A.B.; Squier, C.A.; Şenel, S. Chitosan delivery systems for the treatment of oral mucositis: In vitro and in vivo studies. J. Control. Release 2004, 98, 269–279. [Google Scholar] [CrossRef]
- Abousamra, M.M.; Basha, M.; Awad, G.E.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymeric carrier for the treatment of topical candidiasis. J. Drug Deliv. Sci. Technol. 2019, 49, 365–374. [Google Scholar] [CrossRef]
- Vadakedath, S.; Kandi, V. Dialysis: A Review of the Mechanisms Underlying Complications in the Management of Chronic Renal Failure. Cureus 2017, 9, e1603. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, C.; Voicu, S.I.; Muhulet, A.; Nechifor, G.; Popescu, S.; Ungureanu, C.; Carja, A.; Miculescu, F.; Andronescu, E.; Pirvu, C. Production and characterization of cellulose acetate—Titanium dioxide nanotubes membrane fraxiparinized through polydopamine for clinical applications. Carbohydr. Polym. 2018, 181, 215–223. [Google Scholar] [CrossRef]
- Sunohara, T.; Masuda, T.; Kawanishi, H.; Takemoto, Y. Fundamental Characteristics of the Newly Developed ATA™ Membrane Dialyzer. Contrib. Nephrol. 2016, 189, 215–221. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. https://doi.org/10.3390/ma13112481
Oprea M, Voicu SI. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials. 2020; 13(11):2481. https://doi.org/10.3390/ma13112481
Chicago/Turabian StyleOprea, Madalina, and Stefan Ioan Voicu. 2020. "Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite" Materials 13, no. 11: 2481. https://doi.org/10.3390/ma13112481
APA StyleOprea, M., & Voicu, S. I. (2020). Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials, 13(11), 2481. https://doi.org/10.3390/ma13112481





