Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes
Abstract
1. Introduction
- (i)
- the type of biodegradable polymers;
- (ii)
- the type of composition: binary or ternary compositions; and
- (iii)
- the amount of SWCN
2. Experimental
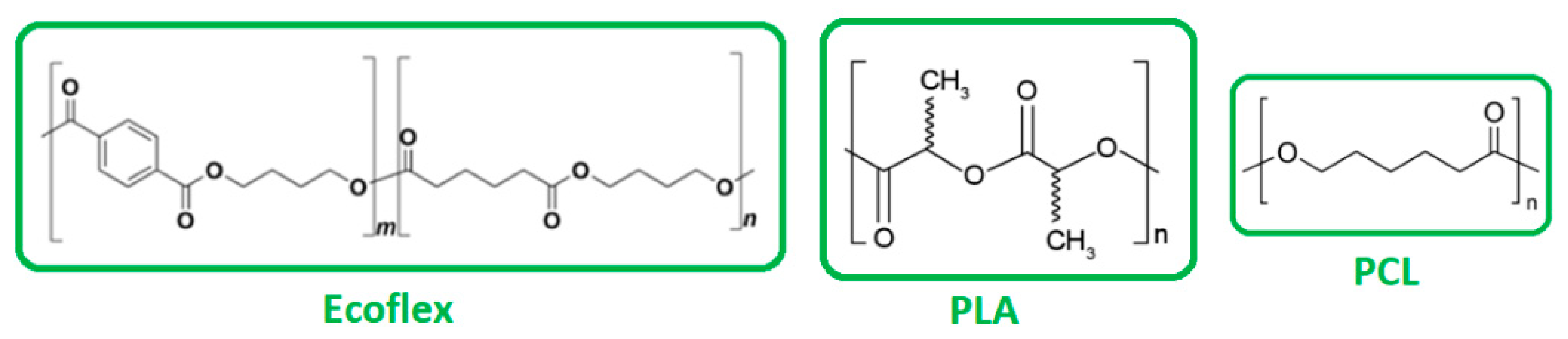
2.1. Materials
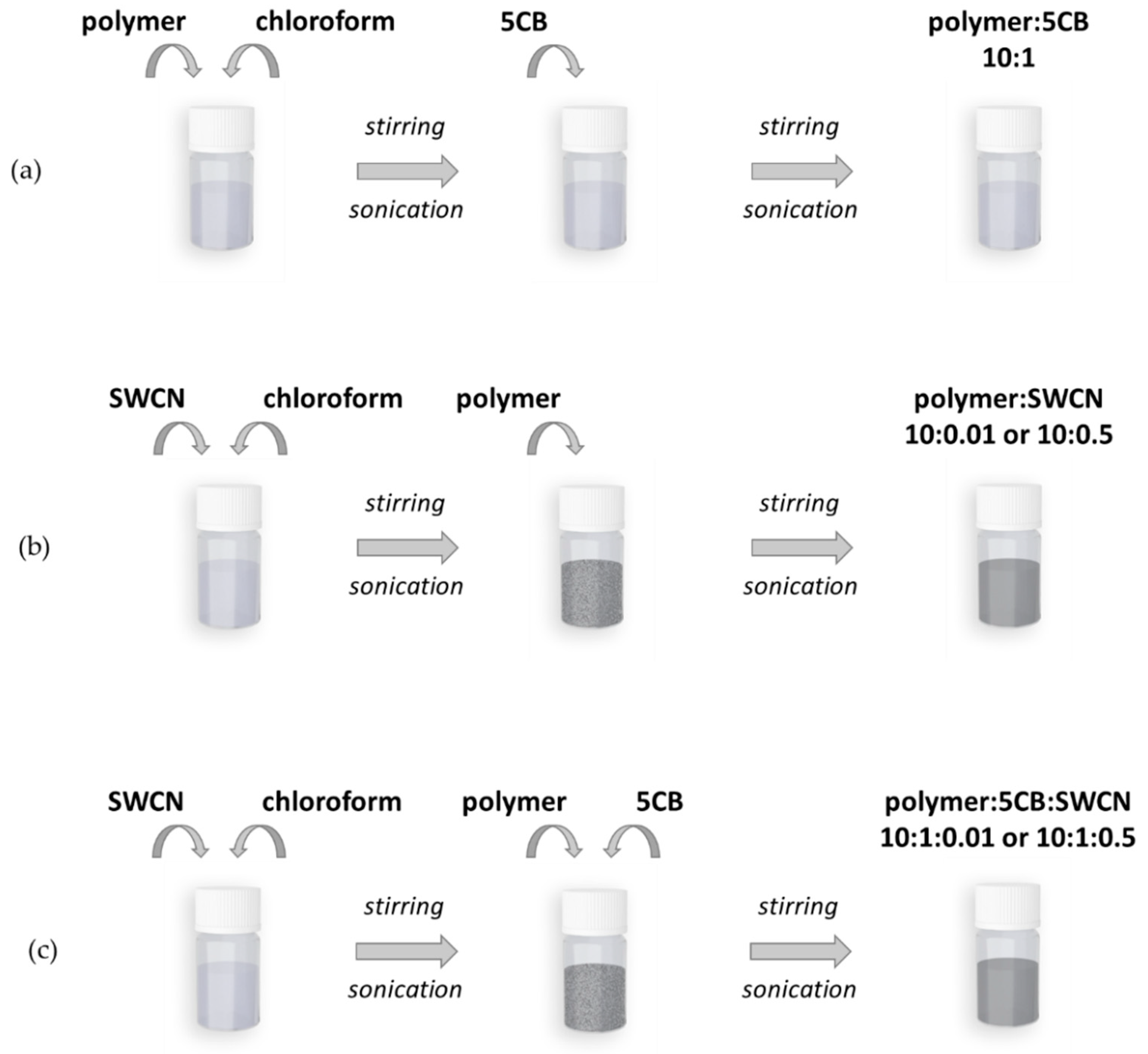
2.1.1. Preparation of Solutions
2.1.2. Preparation of Hybrid Layers
2.2. Characterization of Methods
3. Results and Discussion
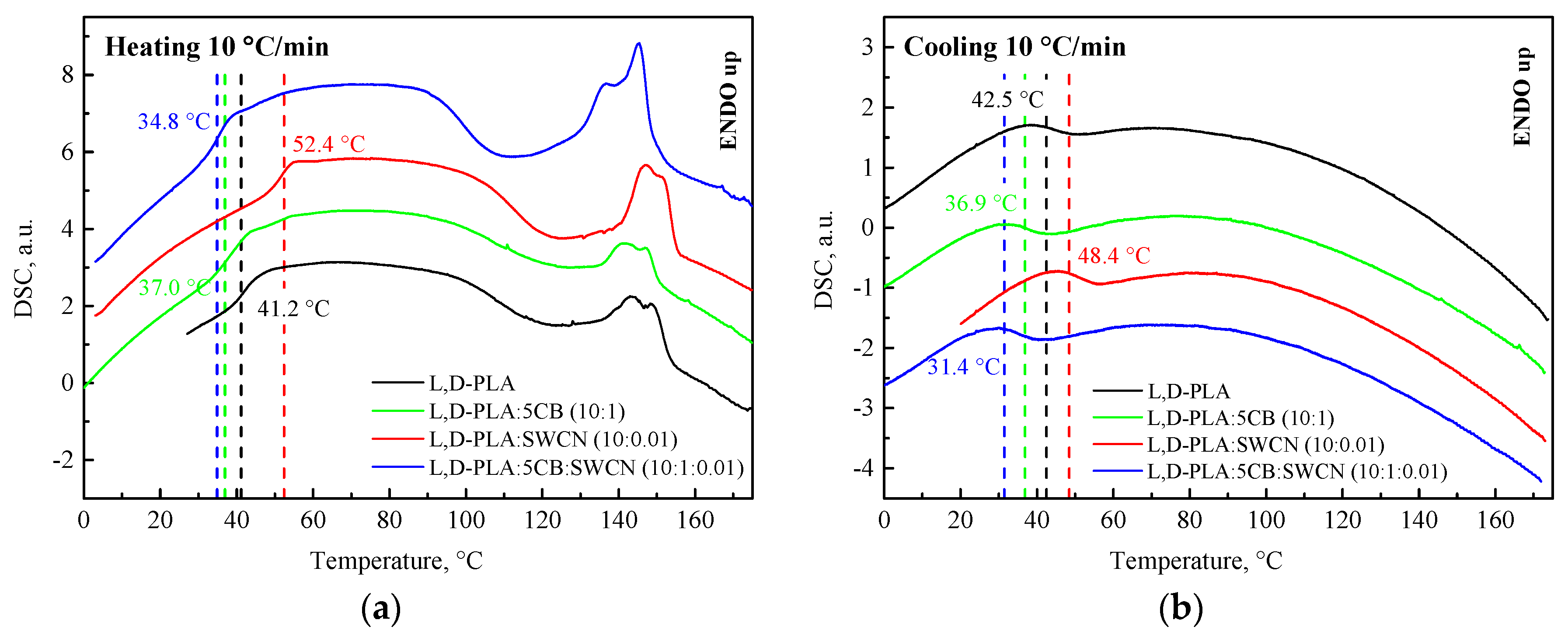
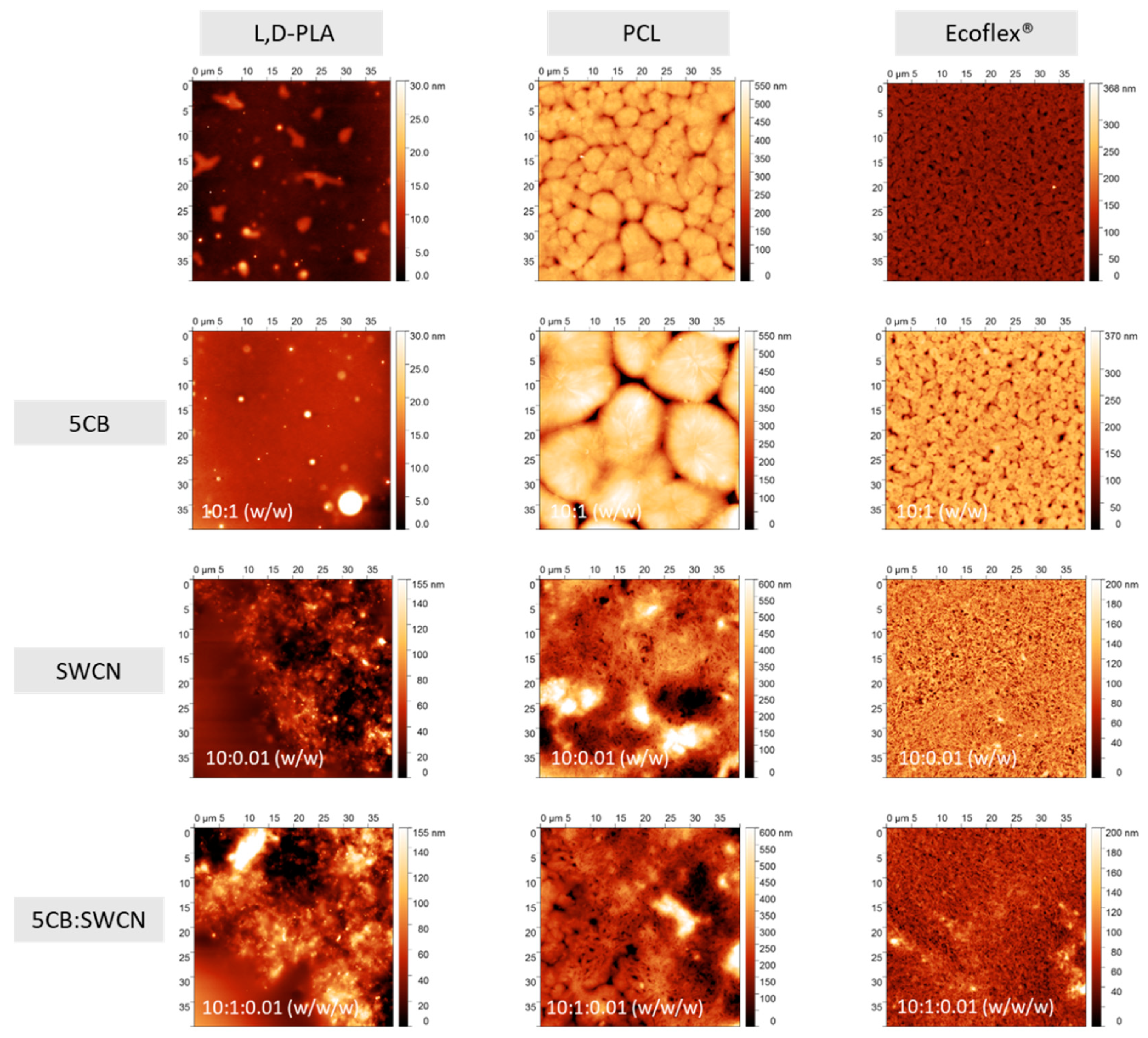
3.1. Thermal and Microscopic Studies
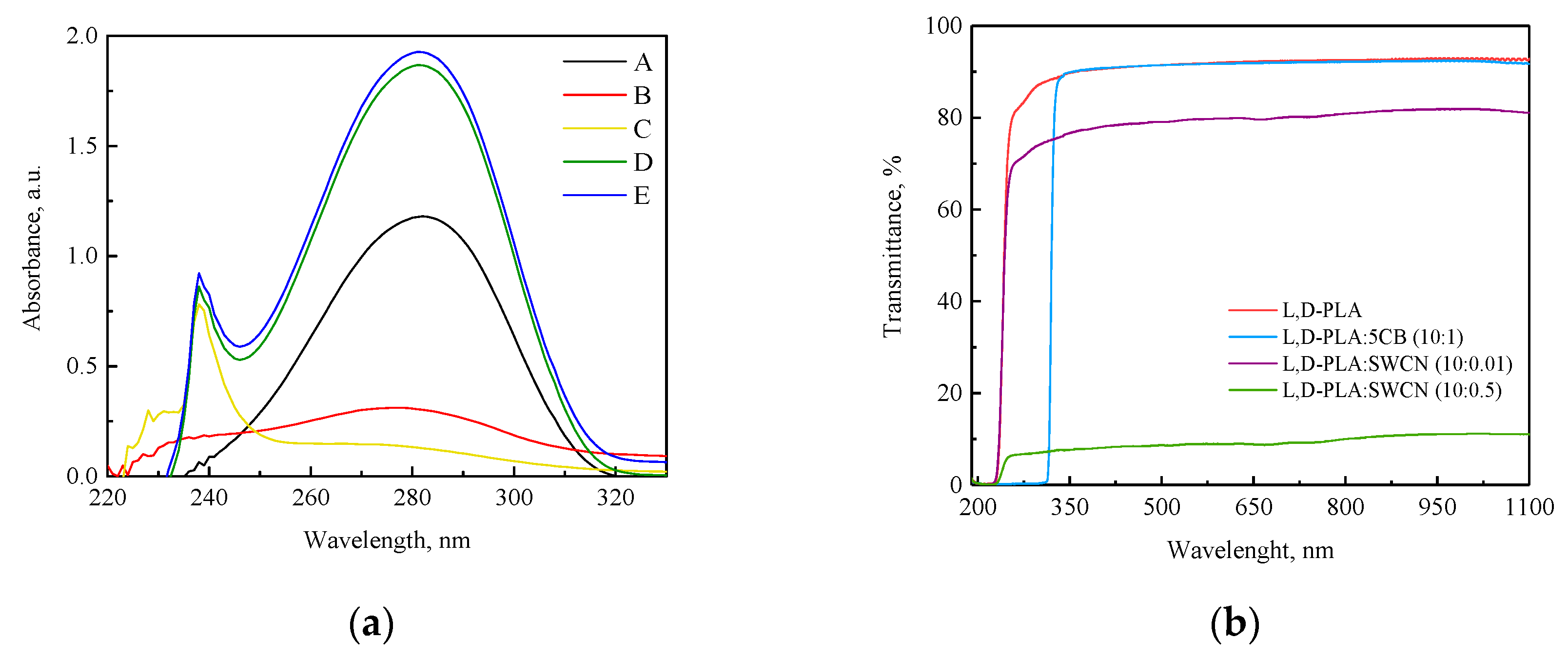
3.2. UV–Vis Studies
3.3. Statistic Angle Contact Measurement
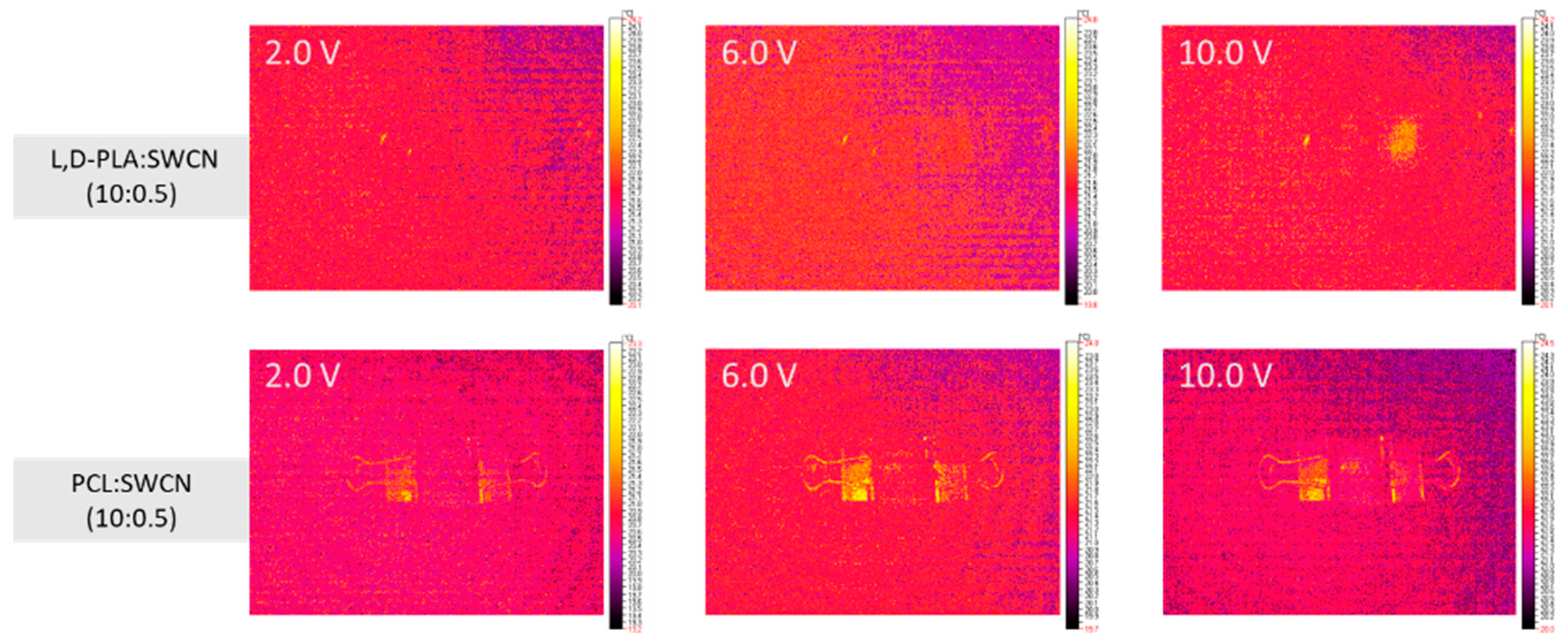
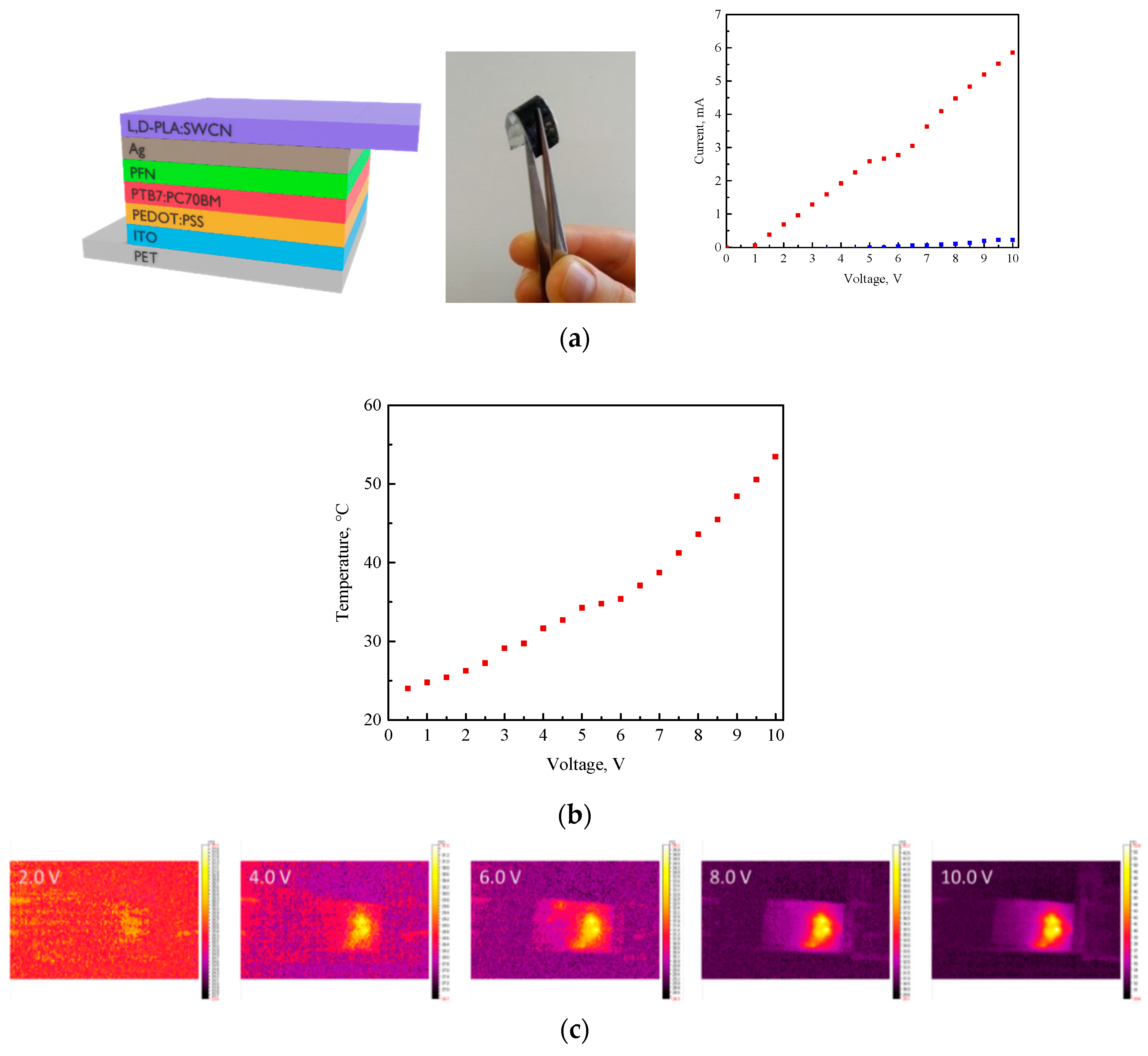
3.4. Infrared (IR) Thermography Study
4. Summary and Conclusions
- All investigated polymers and hybrid compositions exhibited a semi-crystalline nature with melting/crystallization and glass transition temperatures more or less visible. Addition of SWCN increased the Tg for L,D-PLA and Ecoflex®. In the case of L,D-PLA, the addition of both 5CB and SWCN decreased the Tg compared to the pure polymer and binary compositions. For the Ecoflex®:5CB:SWCN compositions, the Tg was almost identical as that for the pure polymer. The addition of 5CB to the polymer layer made it more homogeneous with a special track for SWCN and probably simplified the electron transfer in Ecoflex® due to the presence of SWCN.
- Texture observations by POM showed that the admixture of SWCN had a dominant impact for morphology of the created hybrid layers of all studied polymers. On the other hand, no significant changes in the topography of hybrid layers with or without carbon nanotubes and 5CB were observed for all three polymers studied by the AFM method.
- No changes in the maximum of absorption bands of the investigated compositions were observed, only the hyperchromic effect with the increase in 5CB and SWCN content.
- The 5CB admixture did not change the transmittance of the investigated polymers while the addition of SWCN reduced it by approximately 15% in the case of L,D-PLA.
- It turns out that all investigated polymers were hydrophobic and it was not possible to form PEDOT:PSS layers from toluene or an aqueous solution. Moreover, for the Ecoflex® layers, with and without additives, a rapid sorption of the deposited water drop was observed.
- The resistance of the polymer:SWCN composition in a ratio of 10:0.01 w/w was above 100 MΩ, hence no change in the thermal images was noticed. The increase in SWCN content from 0.01 to 0.5 significantly improved the conductivity only in the case of the L,D-PLA:SWCN (10:0.5) composition, for which a current above 5 V was registered with a resistance of 3030.7 Ω.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable polymeric materials in degradable electronic devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pang, X. The assembly of C60 in semicrystalline PLLA matrix. Nano-Micro Lett. 2012, 4, 30–33. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, Z.; Chen, W.; Lu, D.; Yang, L.; Yang, R.; Zhu, H.; Tang, Z.; Gui, X. Direct patterning of carbon nanotube via stamp contact printing process for stretchable and sensitive sensing devices. Nano-Micro Lett. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Slabov, V.; Kopyl, S.; dos Santos, M.P.S.; Kholkin, A.L. Natural and eco-friendly materials for triboelectric energy harvesting. Nano-Micro Lett. 2020, 12, 42. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) based tear resistant and biodegradable flexible films by blown film extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef]
- Zeng, J.B.; Li, K.A.; Du, A.K. Compatibilization strategies in poly(lactic acid)-based blends. RSC Adv. 2015, 5, 32546–32565. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Harrison, I.; Andou, Y. Flexible graphene-based supercapacitors: A review. J. Phys. Chem. C 2016, 120, 4153–4172. [Google Scholar] [CrossRef]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes-Ecoflex nanocomposites. Nanotechnology 2015, 26, 375501. [Google Scholar] [CrossRef]
- Lee, J.W.; Xu, R.; Lee, S.; Jang, K.I.; Yang, Y.; Banks, A.; Yu, K.J.; Kim, J.; Xu, S.; Ma, S.; et al. Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. USA 2016, 113, 6131–6136. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, K.A.; Iwan, A.; Caballero-Briones, F.; Barceinas-Sanchez, J.; Przybył, W.; Januszko, A.; Miranda, J.B.; Espinosa-Ramirez, A.; Guerrero-Contreras, J. Optical and electrical properties of graphene oxide and reduced graphene oxide films deposited onto glass and Ecoflex® substrates towards organic solar cells. Adv. Mater. Lett. 2018, 9, 58–65. [Google Scholar] [CrossRef]
- Tsuji, H. Polylactides. In Biopolymers, Polyesters III—Application and Commercial Product; Doi, Y., Steinbüchel, A., Eds.; Wiley-Blackwell: New Jersey, NJ, USA, 2002; Volume 4, pp. 1–398. [Google Scholar]
- Fryń, P.; Bogdanowicz, K.A.; Górska, N.; Rysz, J.; Krysiak, P.; Marzec, M.; Marzec, M.; Iwan, A.; Januszko, A. Hybrid materials based on L,D-poly(lactic acid) and single-walled carbon nanotubes as flexible substrate for organic devices. Polymers 2018, 10, 1271. [Google Scholar] [CrossRef]
- Fryń, P.; Bogdanowicz, K.A.; Krysiak, P.; Marzec, M.; Iwan, A.; Januszko, A. Dielectric thermal and mechanical properties of L,D-poly(lactic acid) modified by 4′-pentyl-4 biphenylcarbonitrile and singlewalled carbon nanotube. Polymers 2019, 11, 1867. [Google Scholar] [CrossRef]
- Ma, P.; Lv, P.; Xu, P.; Du, M.; Zhu, H.; Dong, W.; Chen, M. Design of bio-based conductive and fast crystallizing nanocomposites with controllable distribution of multiwalled carbon nanotubes via interfacial stereocomplexation. Chem. Eng. J. 2018, 336, 223–232. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Jing, J.; Yang, L. Preparation and 3D-printing of highly conductive polylactic acid/carbon nanotube nanocomposites: Via local enrichment strategy. RSC Adv. 2019, 9, 29980–29986. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Following the morphological and thermal properties of PLA/PEO blends containing carbon nanotubes (CNTs) during hydrolytic degradation. Compos. Part B Eng. 2019, 175, 107132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, F.; Leng, J.; Pei, S.; Wang, L.; Jia, X.; Cotton, C.; Sun, B.; Chou, T.W. Microstructural design for enhanced shape memory behavior of 4D printed composites based on carbon nanotube/polylactic acid filament. Compos. Sci. Technol. 2019, 181, 107692. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test. 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Zhou, X.; Liu, J.; Li, Y.; Yang, M.; Yuan, Q.; Zhang, W. Effects of carbon nanotube on the thermal, mechanical, and electrical properties of PLA/CNT printed parts in the FDM process. Synth. Met. 2019, 253, 122–130. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation behavior in vitro of carbon nanotubes (CNTs)/poly(lactic acid) (PLA) composite suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef]
- Li, M.Q.; Wu, J.M.; Song, F.; Li, D.D.; Wang, X.L.; Chen, L.; Wang, Y.Z. Flexible and electro-induced shape memory poly(Lactic Acid)-based material constructed by inserting a main-chain liquid crystalline and selective localization of carbon nanotubes. Compos. Sci. Technol. 2019, 173, 1–6. [Google Scholar] [CrossRef]
- Babu, S.S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Enhanced antimicrobial performance of cloisite 30B/poly (ε-caprolactone) over cloisite 30B/poly (L-lactic acid) as evidenced by structural features. Appl. Clay Sci. 2018, 153, 198–204. [Google Scholar] [CrossRef]
- Urquijo, J.; Dagréou, S.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Morphology and properties of electrically and rheologically percolated PLA/PCL/CNT nanocomposites. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Gopinathan, J.; Pillai, M.M.; Elakkiya, V.; Selvakumar, R.; Bhattacharyya, A. Carbon nanofillers incorporated electrically conducting poly ε-caprolactone nanocomposite films and their biocompatibility studies using MG-63 cell line. Polym. Bull. 2016, 73, 1037–1053. [Google Scholar] [CrossRef]
- Babu, S.S.; Mathew, S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Antimicrobial, antibiofilm, and microbial barrier properties of poly (ε-caprolactone)/cloisite 30B thin films. 3 Biotech. 2016, 6, 249. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Atmaca, H. Prechondrogenic ATDC5 cell response to graphene/multi-walled carbon nanotube-containing porous polycaprolactone biocomposite scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1154–1166. [Google Scholar] [CrossRef]
- Gopinathan, J.; Pillai, M.M.; Sahanand, K.S.; Rai, B.K.D.; Selvakumar, R.; Bhattacharyya, A. Synergistic effect of electrical conductivity and biomolecules on human meniscal cell attachment, growth, and proliferation in poly-ε-caprolactone nanocomposite scaffolds. Biomed. Mater. 2017, 12, 65001. [Google Scholar] [CrossRef]
- Fortunati, E.; D’Angelo, F.; Martino, S.; Orlacchio, A.; Kenny, J.M.; Armentano, I. Carbon nanotubes and silver nanoparticles for multifunctional conductive biopolymer composites. Carbon 2011, 49, 2370–2379. [Google Scholar] [CrossRef]
- Dottori, M.; Armentano, I.; Fortunati, E.; Kenny, J.M. Production and properties of solvent-cast poly(ε-caprolactone) composites with carbon nanostructures. J. Appl. Polym. Sci. 2011, 119, 3544–3552. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon nanotube thin films: Fabrication, properties, and applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wei, J.; Wang, K.; Wu, D. Applications of carbon materials in photovoltaic solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1461–1470. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Po, R.; Carbonera, C.; Bernardi, A.; Tinti, F.; Camaioni, N. Polymer- and carbon-based electrodes for polymer solar cells: Toward low-cost, continuous fabrication over large area. Sol. Energy Mater. Sol. Cells. 2012, 100, 97–114. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef]
- Cai, W.; Gong, X.; Cao, Y. Polymer solar cells: Recent development and possible routes for improvement in the performance. Sol. Energy Mater. Sol. Cells 2010, 94, 114–127. [Google Scholar] [CrossRef]
- Jewłoszewicz, B.; Bogdanowicz, K.; Przybył, W.; Iwan, A.; Plebankiewicz, I. PEDOT:PSS in Water and Toluene for Organic Devices—Technical Approach. Polymers 2020, 12, 565. [Google Scholar] [CrossRef]





| Code | Phase Transitions (°C) | |||
|---|---|---|---|---|
| Heating | Cooling | |||
| L,D-PLA | 41.16 (0.35) | 137.04 (8.85) | 42.45 (0.26) | - |
| PCL | - | 52.69 (61.50) | - | 35.80 (−68.104) |
| Ecoflex® | - | - | 119.82 (0.54) | - |
| L,D-PLA:5CB | 36.95 (0.29) | 135.23 (5.15) | 36.88 (0.25) | - |
| PCL:5CB | - | 44.98 (63.21) | - | 31.42 (−64.490) |
| Ecoflex®:5CB | - | - | 105.38 (0.76) | - |
| L,D-PLA:SWCN | 52.41 (0.38) | 140.96 (12.12) | 48.42 (0.34) | - |
| PCL:SWCN | - | 53.51 (64.06) | - | 45.47 (−63.175) |
| Ecoflex®:SWCN | - | - | 126.14 (0.30) | - |
| L,D-PLA:5CB:SWCN | 34.84 (0.28) | 132.48 (10.95) | 31.45(0.31) | - |
| PCL:5CB:SWCN | - | 46.25 (62.06) | - | 40.24 (−61.151) |
| Ecoflex®:5CB:SWCN | - | - | 119.72 (0.24) | - |
| Code | Surface Statistics * | |
|---|---|---|
| Sa (nm) | Sq (nm) | |
| L,D-PLA | 1.27 | 2.12 |
| PCL | 31.26 | 43.78 |
| Ecoflex® | 12.74 | 17.28 |
| L,D-PLA:5CB | 0.80 | 1.44 |
| PCL:5CB | 84.20 | 109.40 |
| Ecoflex®:5CB | 25.13 | 35.59 |
| L,D-PLA:SWCN | 13.79 | 18.63 |
| PCL:SWCN | 56.38 | 75.13 |
| Ecoflex®:SWCN | 16.97 | 22.09 |
| L,D-PLA:5CB:SWCN | 21.56 | 28.18 |
| PCL:5CB:SWCN | 42.70 | 55.39 |
| Ecoflex®:5CB:SWCN | 11.29 | 14.91 |
| Sample | Average Contact Angle, (°) | Image |
|---|---|---|
| L,D-PLA | 112.9 ± 1.5 | 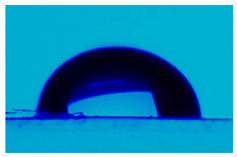 |
| PCL | 108.1 ± 0.4 | 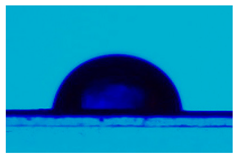 |
| Ecoflex® | 115.3 ± 2.5 | 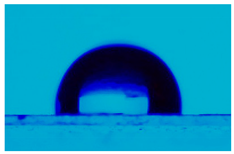 |
| L,D-PLA:5CB (10:1) | 111.9 ± 1.6 |  |
| PCL:5CB (10:1) | 103.7 ± 0.8 | 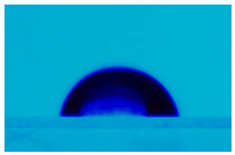 |
| Ecoflex®:5CB (10:1) | 116.0 ± 0.6 | 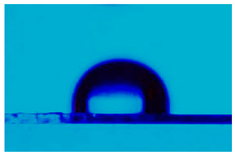 |
| L,D-PLA:SWCN (10:0.01) | 105.9 ± 0.2 | 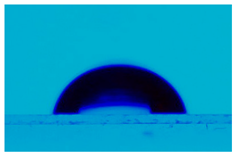 |
| PCL:SWCN (10:0.01) | 110.4 ± 0.9 | 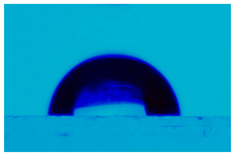 |
| Ecoflex®:SWCN (10:0.01) | 111.9 ± 0.9 |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fryń, P.; Jewłoszewicz, B.; Bogdanowicz, K.A.; Przybył, W.; Gonciarz, A.; Pich, R.; Marzec, M.; Iwan, A. Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes. Materials 2020, 13, 2480. https://doi.org/10.3390/ma13112480
Fryń P, Jewłoszewicz B, Bogdanowicz KA, Przybył W, Gonciarz A, Pich R, Marzec M, Iwan A. Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes. Materials. 2020; 13(11):2480. https://doi.org/10.3390/ma13112480
Chicago/Turabian StyleFryń, Patryk, Beata Jewłoszewicz, Krzysztof Artur Bogdanowicz, Wojciech Przybył, Agnieszka Gonciarz, Robert Pich, Monika Marzec, and Agnieszka Iwan. 2020. "Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes" Materials 13, no. 11: 2480. https://doi.org/10.3390/ma13112480
APA StyleFryń, P., Jewłoszewicz, B., Bogdanowicz, K. A., Przybył, W., Gonciarz, A., Pich, R., Marzec, M., & Iwan, A. (2020). Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes. Materials, 13(11), 2480. https://doi.org/10.3390/ma13112480






