3.1. Sample Preparation
For all NiO–YSZ dispersions prepared, the flow behavior was sufficient for the manufacturing of cellular ceramics following the Schwartzwalder process. After sintering, intensely green-colored foams were obtained (
Figure 1), whereas the color intensified with increasing NiO content. The green color is typical for NiO and the presence of Ni
2+ cations in an octahedral oxide ligand field. Therefore, no structural changes to the NiO phase by interaction with YSZ was assumed. After treatment with hydrogen-containing gas (98 vol.% Ar, 2 vol.% H
2), the color is lost and the specimens show a grey shade corresponding to a metallic Ni phase.
The overall mechanical stability of the obtained cellular structures is low and decreases with increasing NiO content. Consequently, all handling steps were performed with great care in order to avoid significant damage to the samples.
3.2. Sintering-Induced Shrinkage and Porosity Evolution
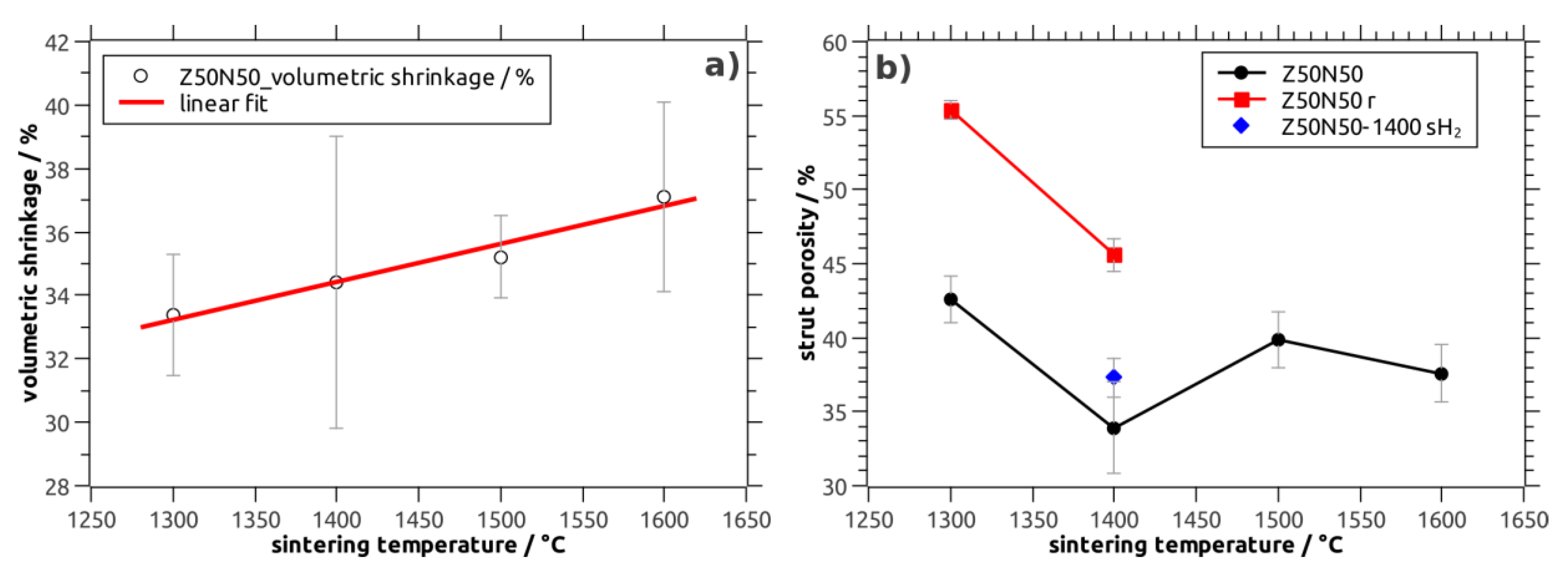
The volumetric shrinkage of the NiO–YSZ composite foams increases linearly from 33% after sintering at 1300 °C to 37% for specimens treated at 1600 °C (Z50N50 series;
Table 1,
Figure 2a). In addition, a clear correlation of the shrinkage behavior to the YSZ weight fraction in the material is found: The shrinkage increases linearly from 25 vol.% for the Z20N80-1400 samples to 50 vol.% for the Z80N20-1400 specimens, respectively. This indicates a significantly higher sintering activity of YSZ compared to the NiO powder, which has also been observed during the densification of NiO–YSZ SOFC anode materials, see references [
16,
17]. Conversely, the sintering of the YSZ powder should be considered as constrained in the composite foams; the low sintering activity and shrinkage of the NiO limits the compaction of the YSZ phase (
Figure 3). For the samples sintered directly in a reducing atmosphere an increased shrinkage of 39 vol.% is observed, which is significantly larger compared to the NiO–YSZ composites sintered in air at the same temperature (34 vol.%). This is a direct consequence of the volume decrease during the NiO → Ni conversion, which occurs at lower temperature and before the sintering of the YSZ powder starts. Consequently, the sintering of the YSZ phase is constrained to a lower extent resulting in a more pronounced shrinkage of the respective composite foams (
Figure 3). Furthermore, no additional shrinkage is observed during the hydrogen treatment of the already densified NiO–YSZ composite foams. This is a result of the reduction temperature of 850 °C, which is not sufficient for a further sintering of the material.
The total porosity of the NiO–YSZ and of the Ni–YSZ composite foams is predominantly affected by the Ni(O)-YSZ ratio in the strut material. Consequently, the total porosity is 90.1 vol.% for the Z80N20-1400 samples, 93.0 vol.% for the Z50N50-1400 series and 94.8 vol.% for the Z20N80-1400 foams. These data directly reflect the lower sintering activity of NiO compared to the YSZ powder. The influence of the sintering temperature on the total porosity is less pronounced, and it varies only by ±0.4 vol.% for the Z50N50-zzzz sample series. Nevertheless, a slight porosity decrease with increasing sintering temperature is observed. After the hydrogen treatment, the total porosity increases to 91.5 vol.% (Z80N20-1400r), 94.2 vol.% (Z50N50-1400r), 93.9 vol.% (Z50N50-1400sH2) and 96.1 vol.% (Z20N80-1400r), respectively. The course of the total porosity values is in good agreement with the tendency in the volumetric shrinkage of the respective samples. The cell size of the foams investigated within this work ranges between 2 mm and 3 mm being in accord to the 20 ppi polymer templates used in sample preparation.
Regarding the porosity inside the ceramic strut material of the NiO–YSZ composite foams (Z50N50 series), a tendency towards lower values is observed with increasing sintering temperature (
Table 1,
Figure 2b). This is in good accord to the shrinkage behavior and the course of the total porosity of the respective sample series. The specimens treated at 1400 °C show the lowest strut porosity with 34 vol.%—in analogy to results obtained for the sintering of NiO–YSZ SOFC anode materials [
18]. However, for the Z50N50-14000 specimens the standard deviation for the strut porosity as well as the shrinkage is higher compared to the other sample series. Therefore, the porosity data for the Z50N50-1400 samples has to be critically scrutinized.
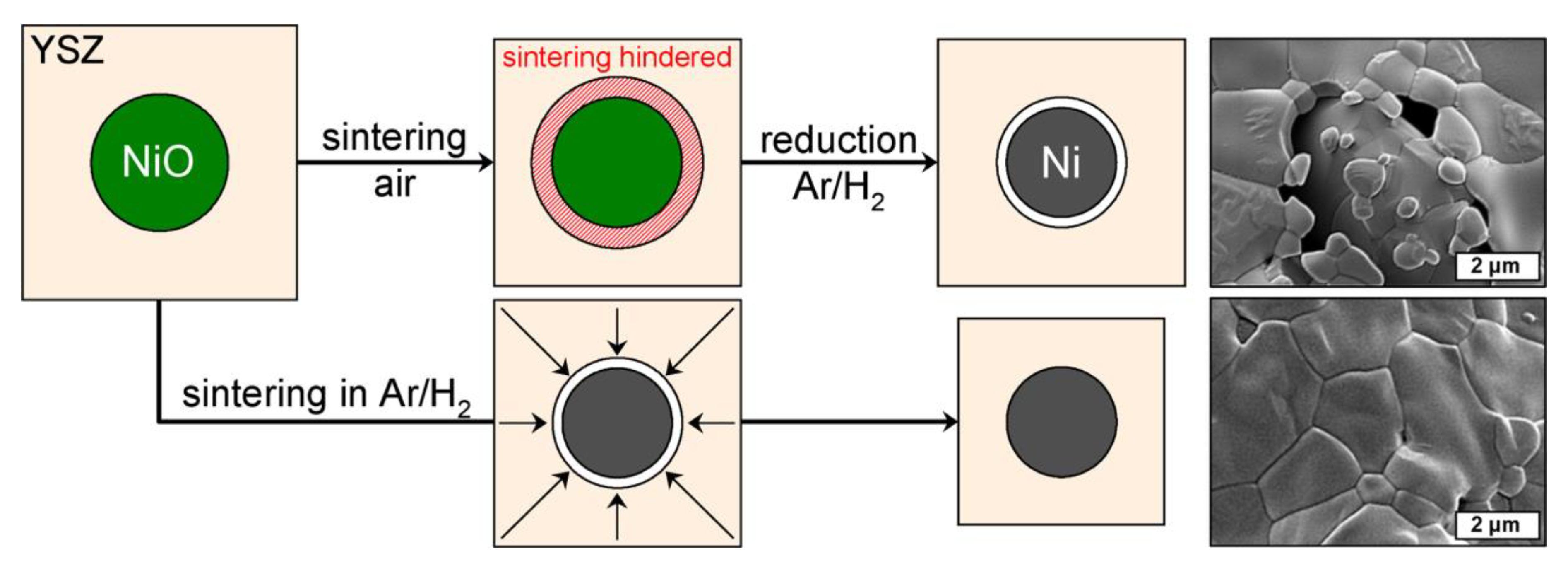
After the hydrogen treatment of the NiO–YSZ composites the strut porosity in the resulting Ni–YSZ foams increases significantly by 12 vol.% (Z50N50 series). The course of the strut porosity is in analogy to the results of the NiO–YSZ samples (
Table 1). The drastic increase of the strut porosity during the reduction process can be attributed to the volume decrease during the conversion of NiO (ρ = 6.72 g∙cm
−3) to Ni (ρ = 8.91 g∙cm
−3), which represents a volume reduction of 33 vol.% for the Ni-phase. For the specimens which were sintered first and reduced subsequently, the formation of voids between the YSZ matrix and the Ni grains is observed in electron micrographs (
Figure 3). This is the result of the above mentioned volume decrease for the Ni-phase and the reduction temperature of 850 °C, which is too low for an ongoing sintering of the YSZ matrix.
If the sintering is performed directly in an atmosphere of Ar/H
2, the strut porosity is significantly lower with 37 vol.% for the Z50N50-1400sH
2 specimens compared to 46 vol.% for the Z50N50-1400r series, which was sintered and reduced in two subsequent steps. If the heat treatment is performed in hydrogen-containing atmosphere, the onset of the NiO reduction is at significantly lower temperature (approximately 400 °C for NiO–YSZ composites, reference [
19]) compared to the densification processes in the YSZ matrix beginning not below 1000 °C [
20]. Consequently, the reduction is finished before the YSZ densification begins and the volumetric shrinkage arising from the NiO → Ni conversion can be compensated by an increased densification of the YSZ matrix. This results in the higher volumetric shrinkage described above as well as a lower strut porosity in these samples.
3.3. Microstructure
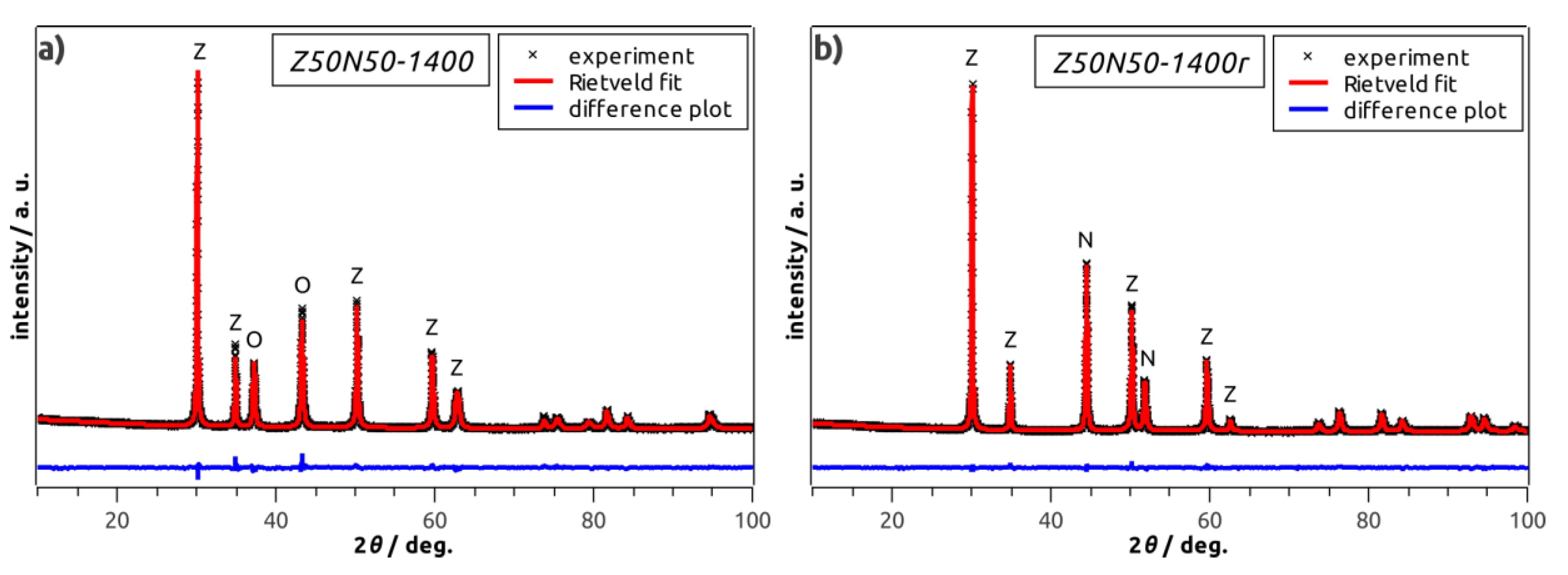
By powder XRD analysis, cubic YSZ and rhombohedral NiO were identified as only phases in all NiO–YSZ composite foams (
Table 1,
Figure 4a). The weight fractions of rhombohedral NiO and cubic YSZ are 50 wt.% each in the Z50N50 samples as determined by XRD and Rietveld analyses. No change in the composition and the crystal structure of the respective phases with increasing sintering temperature is observed. For the hydrogen-treated specimens of the Z
xxN
yy-
zzzzr series (see last four samples in
Table 1), cubic YSZ, face-centered cubic Ni and rhombohedral NiO were identified as constituents (
Figure 4b). However, except for the Z50N50-1300r sample, the NiO concentration is very low and does not exceed 2 wt.%. Consequently, the reduction procedure can be considered as almost quantitative.
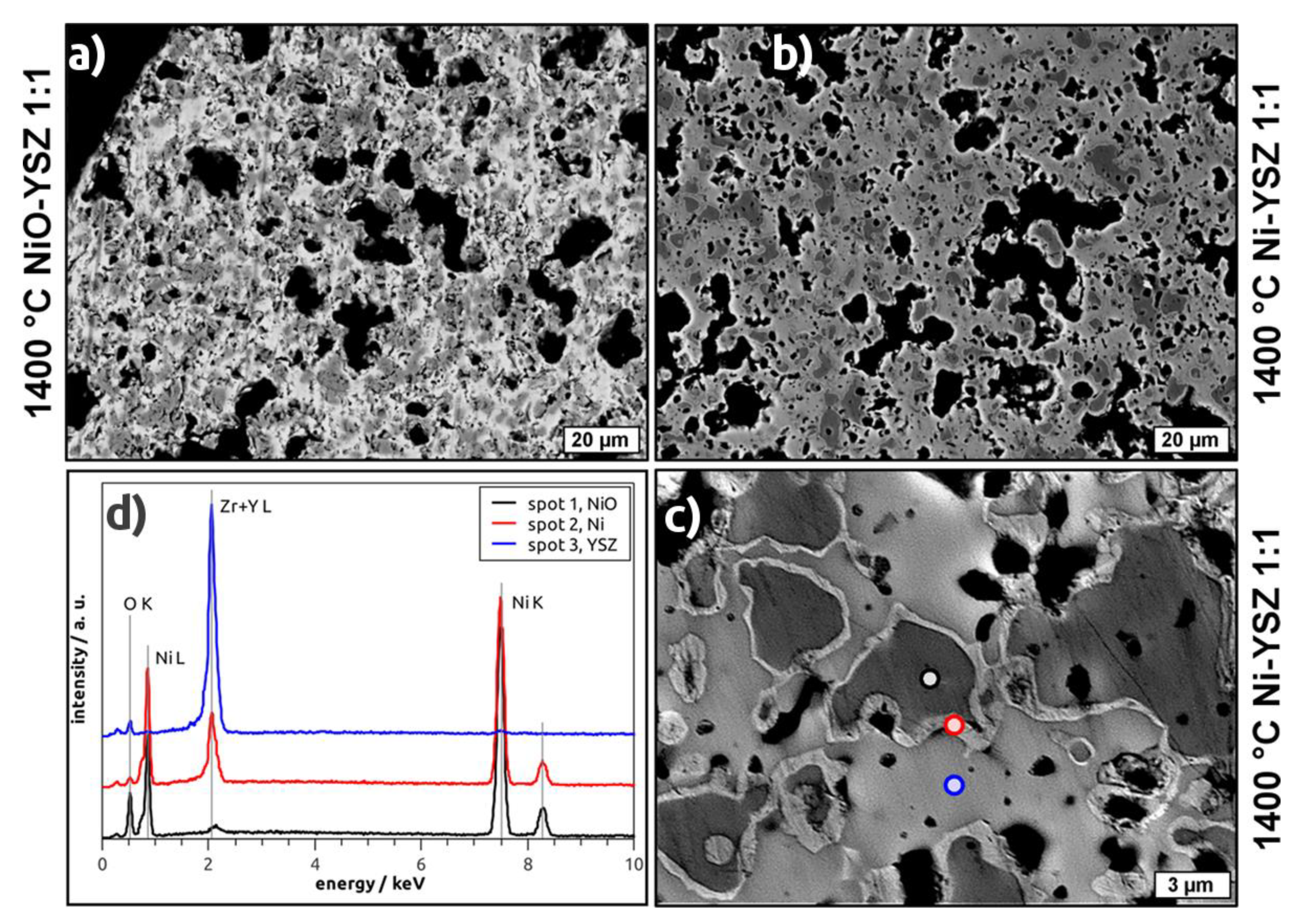
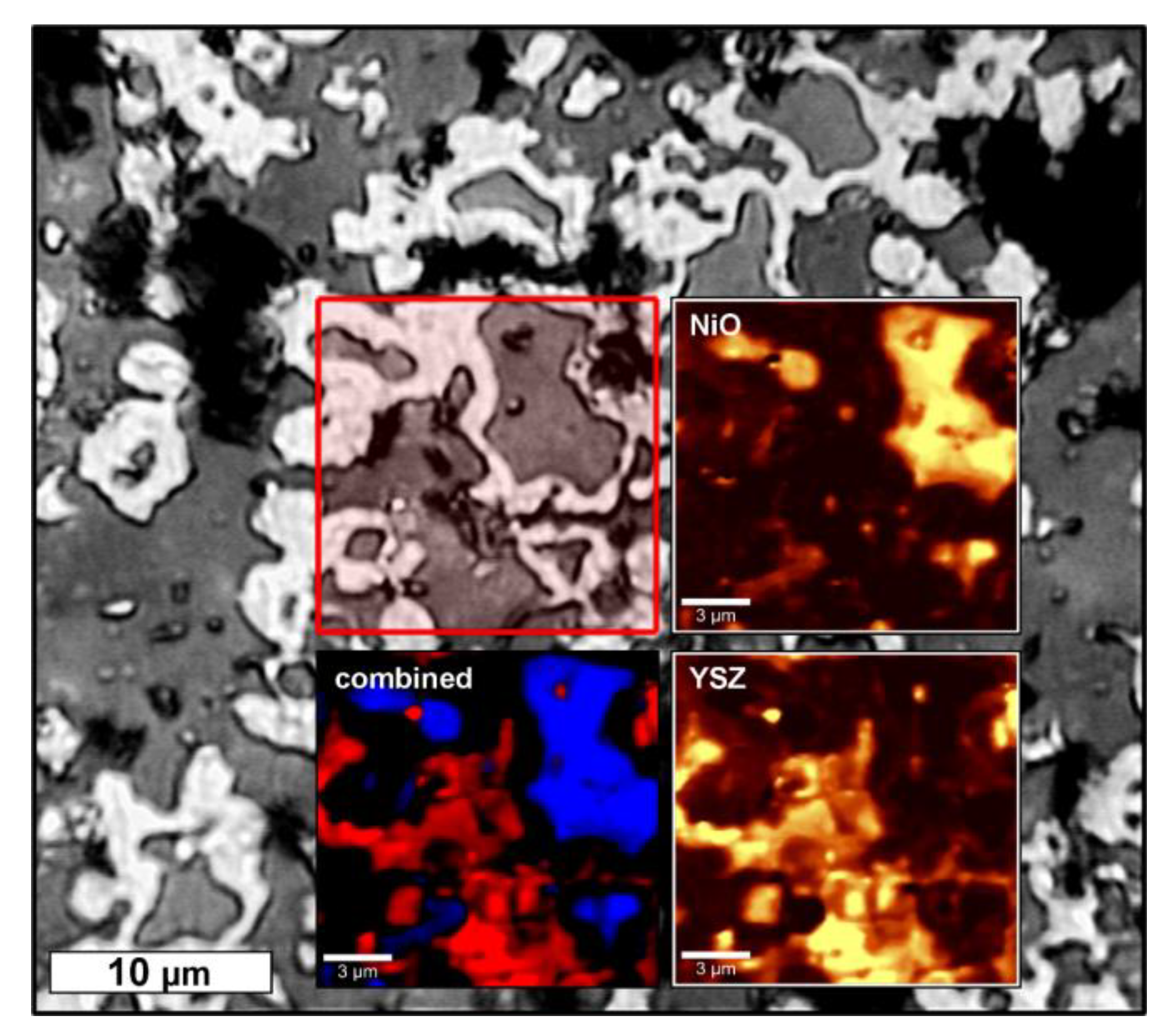
In BSE micrographs of NiO–YSZ as well as Ni–YSZ cellular composites the porous structure of the strut material becomes apparent (
Figure 5a,b). In addition, a microstructure consisting of two (NiO–YSZ) or three phases (Ni–YSZ) is observed with respect to the grey levels in the recorded micrographs. For the NiO–YSZ samples, these phases can be assigned to NiO and YSZ, respectively, which are homogeneously distributed in the microstructure forming grains with 2 µm to 10 µm in size (
Figure 5a). For the reduced samples, darker areas, surrounded by a brighter halo and embedded into a light grey matrix, are observed (
Figure 5b,c). The thickness of the halo is 0.2 µm to 0.8 µm. By EDS spectroscopy (
Figure 5d), these phases have been identified as a Ni-O compound (dark grey area), pure Ni (bright halo) and a Y/Zr-O compound (light grey area). Regarding the area of the respective phases in the micrographs of the reduced samples, the amount of Ni-O exceeds the fraction of Ni considerably.
A further investigation of the Ni–YSZ composites by confocal Raman microscopy confirms the presence of NiO grains which are surrounded by a Raman-inactive metal phase (Ni) and are embedded into a YSZ matrix (
Figure 6). These results are in distinct opposition to the XRD investigations, which suggested an almost quantitative reduction of NiO to Ni.
An explanation for the ternary microstructure of the hydrogen-treated samples could be the formation of amorphous Ni-O intermediates during the interaction with hydrogen. These phases are then hidden for the XRD investigations. However, none of the collected XRD patterns show a pronounced broad maximum in the angular range between 20° and 40° usually observed when a significant amount of amorphous matter is present in the sample. Another possibility is the formation of a porous Ni phase containing traces of oxides, which is surrounded by dense Ni. The porous Ni then appears as darker regions in BSE micrographs surrounded by a brighter halo (dense Ni). This would be in agreement with the XRD results and also in accord to the grey color of the samples after reduction. In summary, these effects need further investigation and clarification. Nevertheless, the successful formation of a nickel phase in the microstructure of the foam struts has been demonstrated.
3.4. Compressive Strength
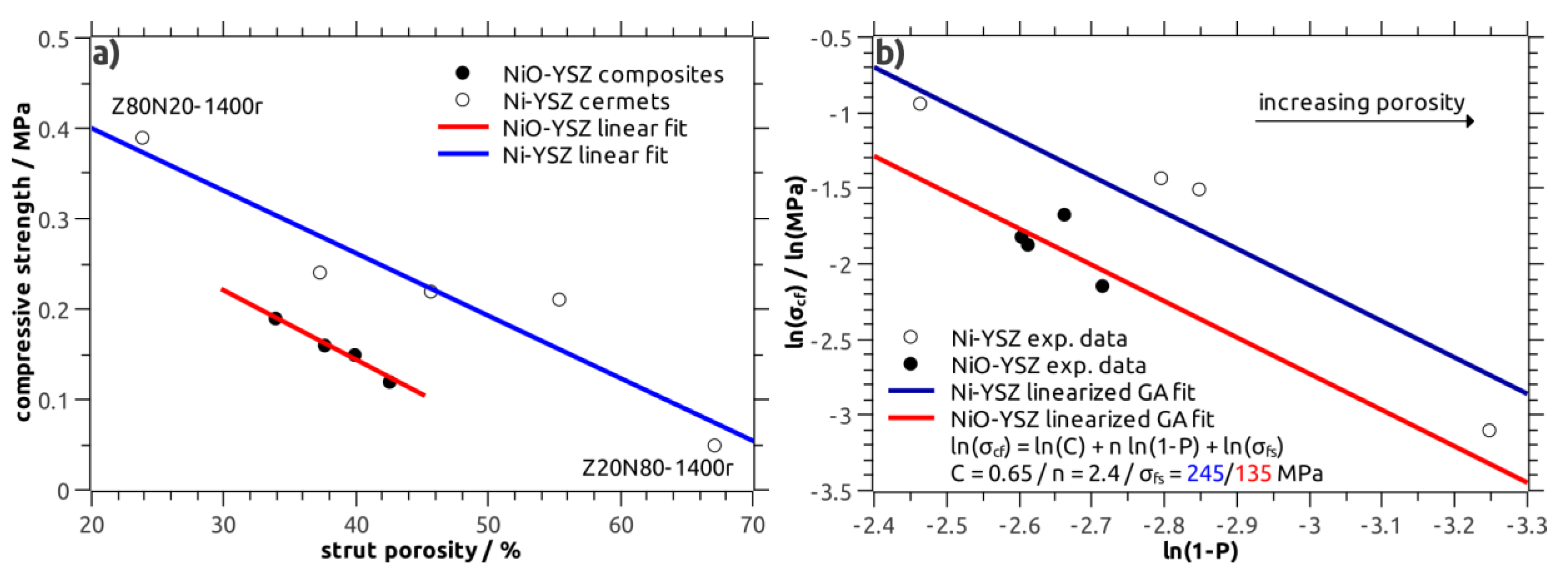
Both the NiO–YSZ and Ni–YSZ composite foams were characterized with respect to their compressive strength, the results are shown in
Figure 7a. The overall strength is very low with values in the range between 0.12 MPa and 0.19 MPa for NiO–YSZ and between 0.05 MPa and 0.39 MPa for the Ni–YSZ foams (
Table 2). The variation of the individual strength measurements within a sample series, as represented by the Weibull modulus m, is high with m ranging between 1.7 and 4.9, respectively. The low strength is a consequence of the high total porosity of the foams on the one hand, and the porous microstructure of the strut material on the other. Consequently, a clear correlation of the strength to the strut porosity of the respective foams is observed for the NiO–YSZ as well as the Ni–YSZ samples (
Figure 7a). Thus, the compressive strength is indirectly correlated to the process parameters “sintering temperature” and “Ni(O)-YSZ ratio”.
The correlation of the compressive strength to the total porosity of the NiO–YSZ and Ni–YSZ cellular composites has been modelled with the Gibson–Ashby relation for brittle, cellular structures (Equation (1), Reference [
21])
with
σcf being the average compressive strength of the cellular material,
ρrel its relative density (derived from the total porosity P as
ρrel = 1−P) and
σfs as bending strength of the bulk strut material. The parameter C is a constant of typically 0.65 for brittle cellular structures. The density exponent n is correlated to the defect concentration within the strut material; in the original Gibson–Ashby relation a value of n = 1.5 is suggested [
21]. However, for brittle cellular ceramics the density exponent is usually larger with values between 1.5 and 3, see references [
22,
23]. As the crushing behavior of both the NiO–YSZ composites and the Ni–YSZ cermets was typically of brittle nature without significant plastic deformation, the Gibson–Ashby model for brittle ceramic foams has been selected.
The modelling of the strength data obtained from the Ni–YSZ cermets was performed using the linearized Gibson–Ashby relation (Equation (2)) and a double-logarithmic compressive strength-relative density plot. This approach allows a simple linear regression analysis [
24].
For parameter C the literature value of 0.65 was used and the bending strength
σfs was set to 245 MPa in accord to literature data obtained for dense Ni–YSZ cermets [
25]. The density exponent n was refined as free parameter in order to fit the experimental data. A good approximation was obtained for a density exponent n of 2.4 (
Figure 7b), which is in good agreement to previous studies on the strength of cellular ceramics [
24,
26].
The strength data of the NiO–YSZ samples was modelled on a similar procedure; here, the parameters C and n were fixed to 0.65 and 2.4, respectively, and the bending strength
σfs was refined. For a
σfs of 135 MPa for the bulk NiO–YSZ material, which is in agreement to literature data, see reference [
27], a good approximation of the experimental strength of the NiO–YSZ foams was obtained (
Figure 7b). The Gibson–Ashby approach allows the comparison of the strength of NiO–YSZ and Ni–YSZ cellular composites separated from the severe influence of the sample porosity.
In summary, the compressive strength increases after the reduction of NiO to Ni amounts to 70%, on average, for the same sample porosity. In comparison, for Ni(O)-YSZ SOFC anode materials an increase in flexural strength by approximately 17% after reduction is reported [
25]. However, these data do not consider the increase in sample porosity due to the NiO → Ni conversion, but show at least the same general tendency.
3.5. Thermal and Electric Conductivity
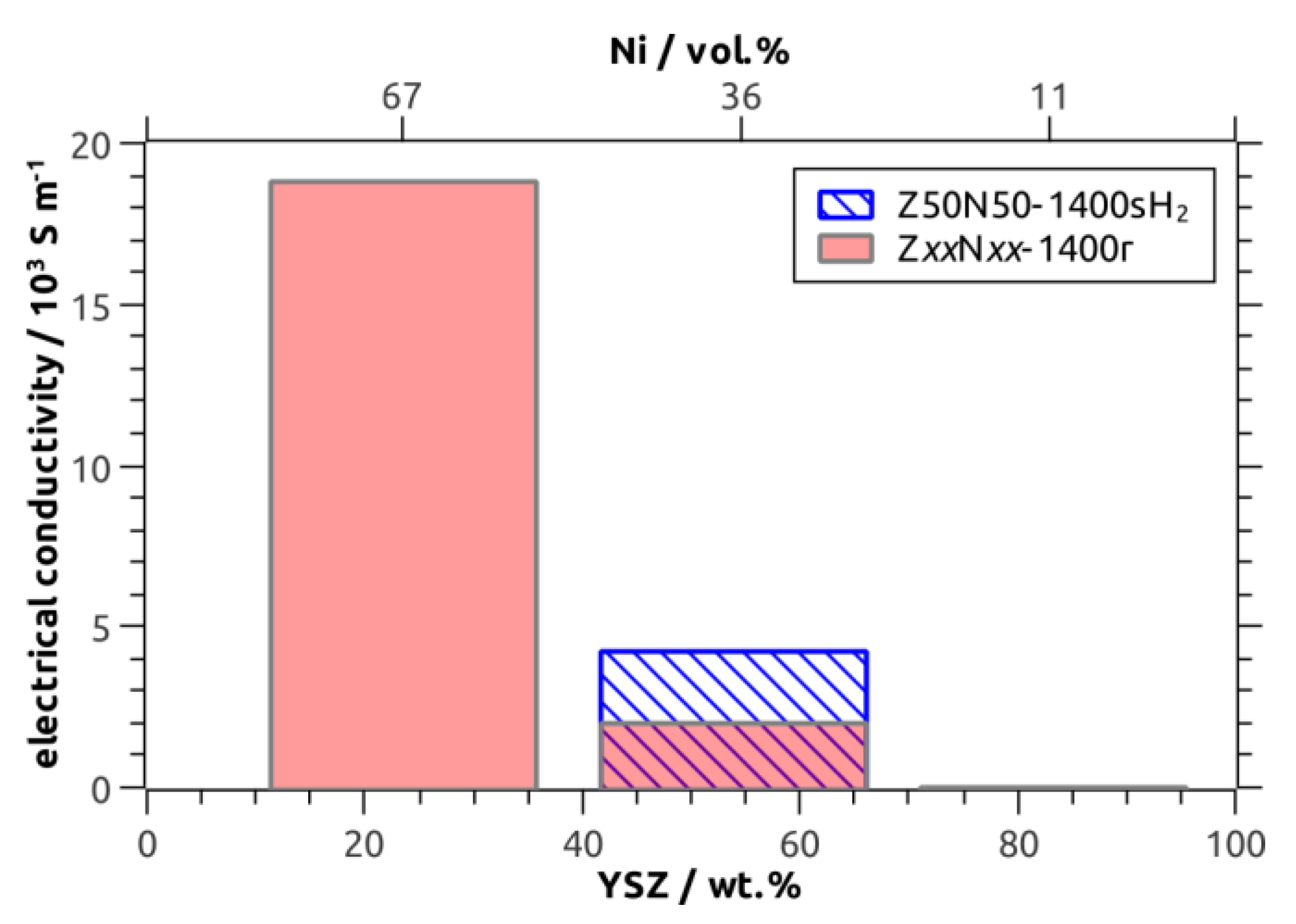
The room-temperature specific electric conductivity has been investigated for the Z
xxN
yy-1400r/sH
2 sample series as a function of the Ni fraction, which was 11 vol.% (Z80N20-1400r), 36/34 vol.% (Z50N50-1400r/sH
2) and 67 vol.% (Z20N80-1400r), respectively. The Z80N20-1400r specimen showed no measurable electric conductivity (
Table 2,
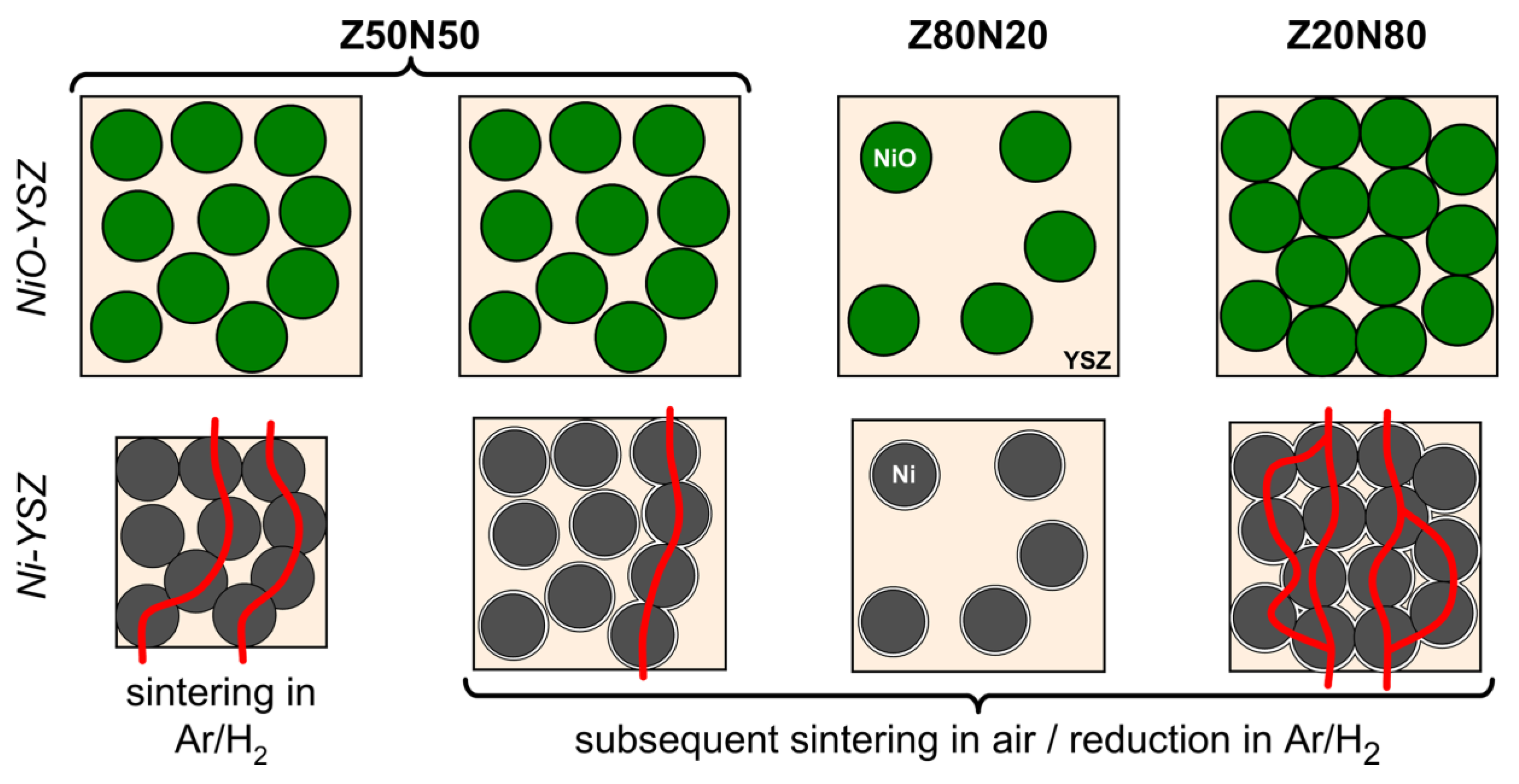
Figure 8). For this sample, the amount of metal in the microstructure is too low in order to form a percolating network and distinct conduction pathways (
Figure 9). The room temperature ionic conductivity of YSZ is too low and only relevant at higher temperature (≈ 80 S∙m
−1 at 1000 °C, reference [
28]). For the Ni–YSZ cermet system, a percolation threshold of Φ
p = 30 vol.% has been observed for compact samples [
29], and was confirmed by theoretical investigations (percolation theory: Φ
p = 32 vol.%) [
30]. This is in good agreement with the electric conductivity of 2.0∙10
3 S∙m
−1 measured for the Z50N50-1400r composite foam, in which the struts contain 36 vol.% Ni. Accordingly, the electric conductivity increases significantly to 18.8∙10
3 S∙m
−1 for the Z20N80-1400r samples containing 67 vol.% Ni in the strut material. This effect can be explained by an improved contacting between the increased number of Ni grains in the microstructure, which increases the number of possible conduction pathways (
Figure 9).
The sample Z50N50-1400sH
2, which was sintered directly in hydrogen-containing atmosphere, represents an exceptional position. The electric conductivity is 4.2 × 10
3 S∙m
−1 and more than doubled compared to the Z50N50-1400r specimen, which was reduced subsequent to the sintering process in air. For both composite foams the Ni content is similar (36 vol.% and 34 vol.%) and clearly above the percolation threshold. Consequently, the improved electric conductivity of the Z50N50-1400sH
2 composite is a consequence of the higher shrinkage and increased densification for these samples. By this, the Ni grains are compressed to each other, which improves the contacting between them and increases the number of conduction pathways (
Figure 9).
In order to evaluate the electric conductivity data measured for the cellular Ni–YSZ composites against literature results for Ni–YSZ SOFC anode materials, the influence of the sample’s (cellular) porosity has to be considered. For this purpose, a simple model derived by Gibson and Ashby has been applied for the estimation of the bulk electric conductivity κ
B (Equation (3), Reference [
1])
with κ
cf as the electric conductivity of the composite foam and the total porosity P. From this relation a bulk electric conductivity of 7 × 10
4 S∙m
−1 (Z50N50-1400r), 14 × 10
4 S∙m
−1 (Z50N50-1400sH
2) and 104 × 10
4 S∙m
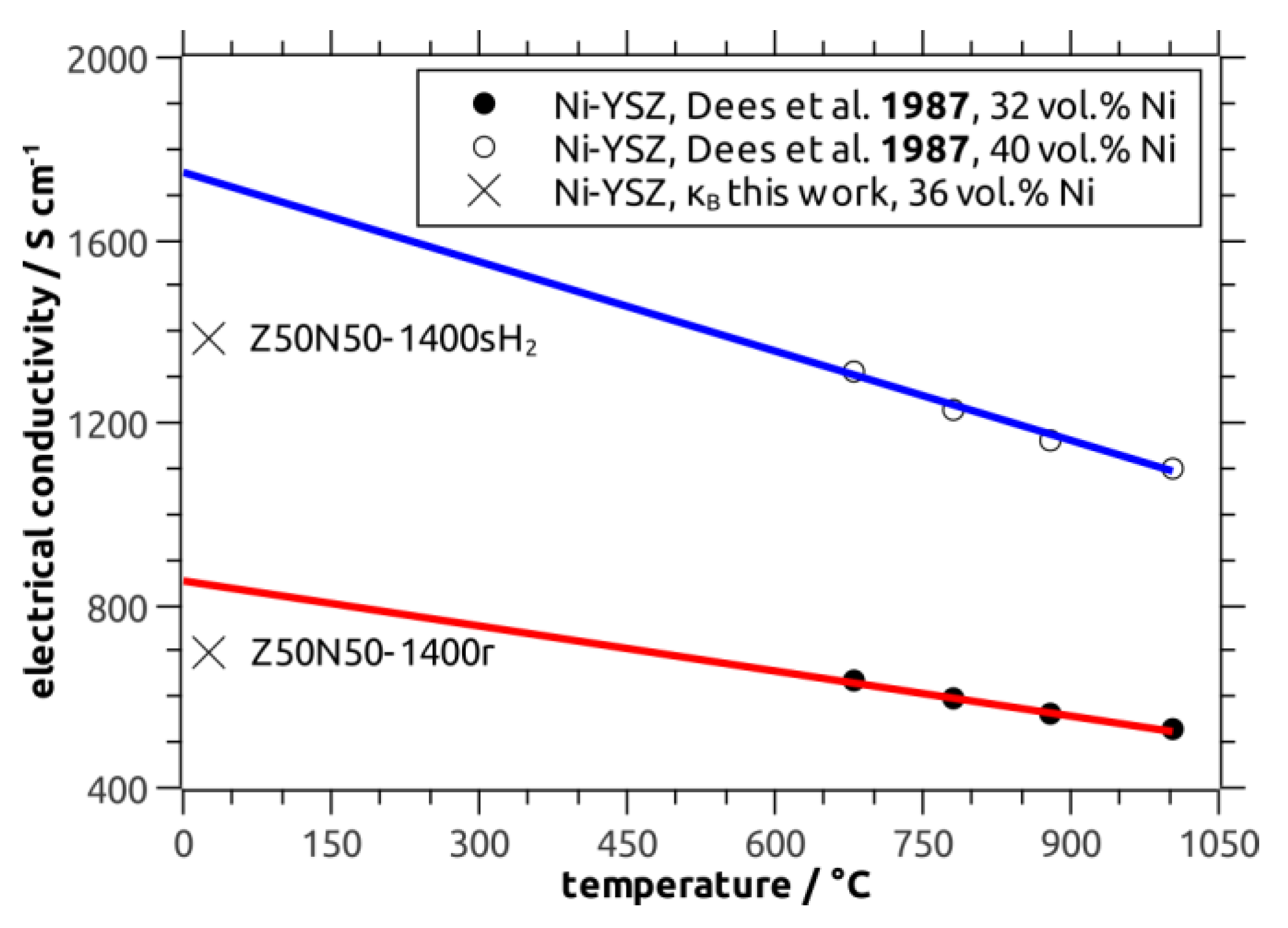
−1 (Z20N80-1400r), respectively, has been calculated. The κ
B values for the Z50N50 samples are in good agreement to data collected for Ni–YSZ cermets by Dees and coworkers (reference [
29]), which were extrapolated to room temperature (
Figure 10). The calculated bulk conductivity of the Z20N80-1400r sample is in the regime of typical metallic conductors (>10
6 S∙m
−1).
Nevertheless, it should be noted that the numbers obtained for the Ni–YSZ bulk electric conductivity should be interpreted with care. The Gibson–Ashby model in Equation (3) has been initially designed for the evaluation of the electric properties of pure metallic foams, not for the modelling of data collected from cellular ceramic-metal composite structures. Therefore, a homogeneous strut material is assumed and all porosity is considered as cellular voids (e.g., no strut porosity). Both is a good approximation of most of the typical metal foam structures but not the case for the materials investigated within this work. Nevertheless, the numbers give a conservative estimate of the electric conductivity of the (cellular) Ni–YSZ composites prepared within this study.
The thermal conductivity has only been investigated for a selected specimen of the Z50N50-1400 series which was measured before and after the chemical reduction of the NiO with hydrogen. For both samples, the thermal conductivity is almost equal with 0.23 W∙m
−1∙K
−1. Regarding the microstructure of the investigated samples, this is not unexpected, despite the better thermal conductivity of Ni (79 W∙m
−1∙K
−1) compared to NiO (35 W∙m
−1∙K
−1) [
31]. However, as discussed before, a significant increase in porosity (total and strut porosity) has been observed after the hydrogen treatment. These additional voids reduce the effective thermal conductivity of the cellular Ni–YSZ composites, regardless the hypothetically benefit from the higher conductivity of the Ni phase.