Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
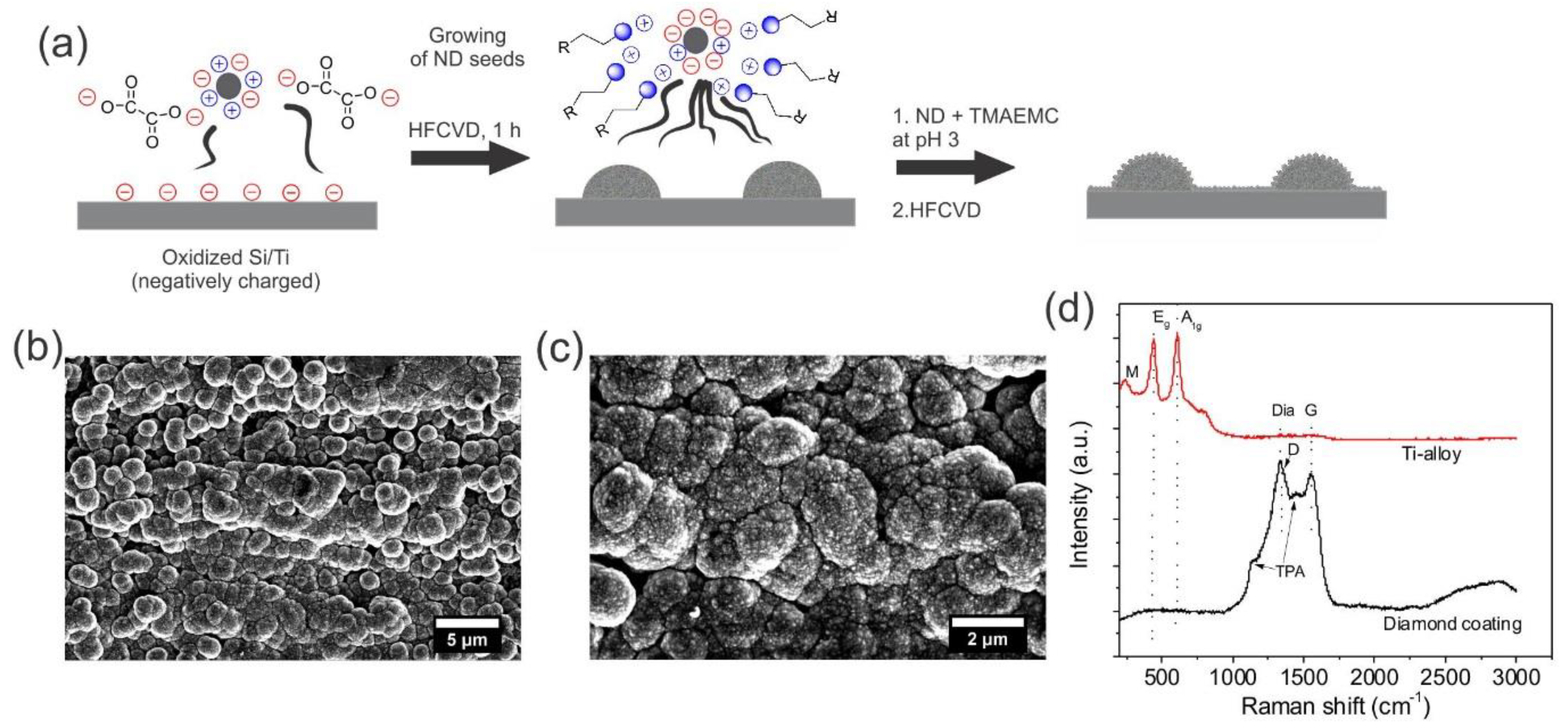
3.1. Synthesis of Hierarchical Rough Diamond Coating on Ti Alloy
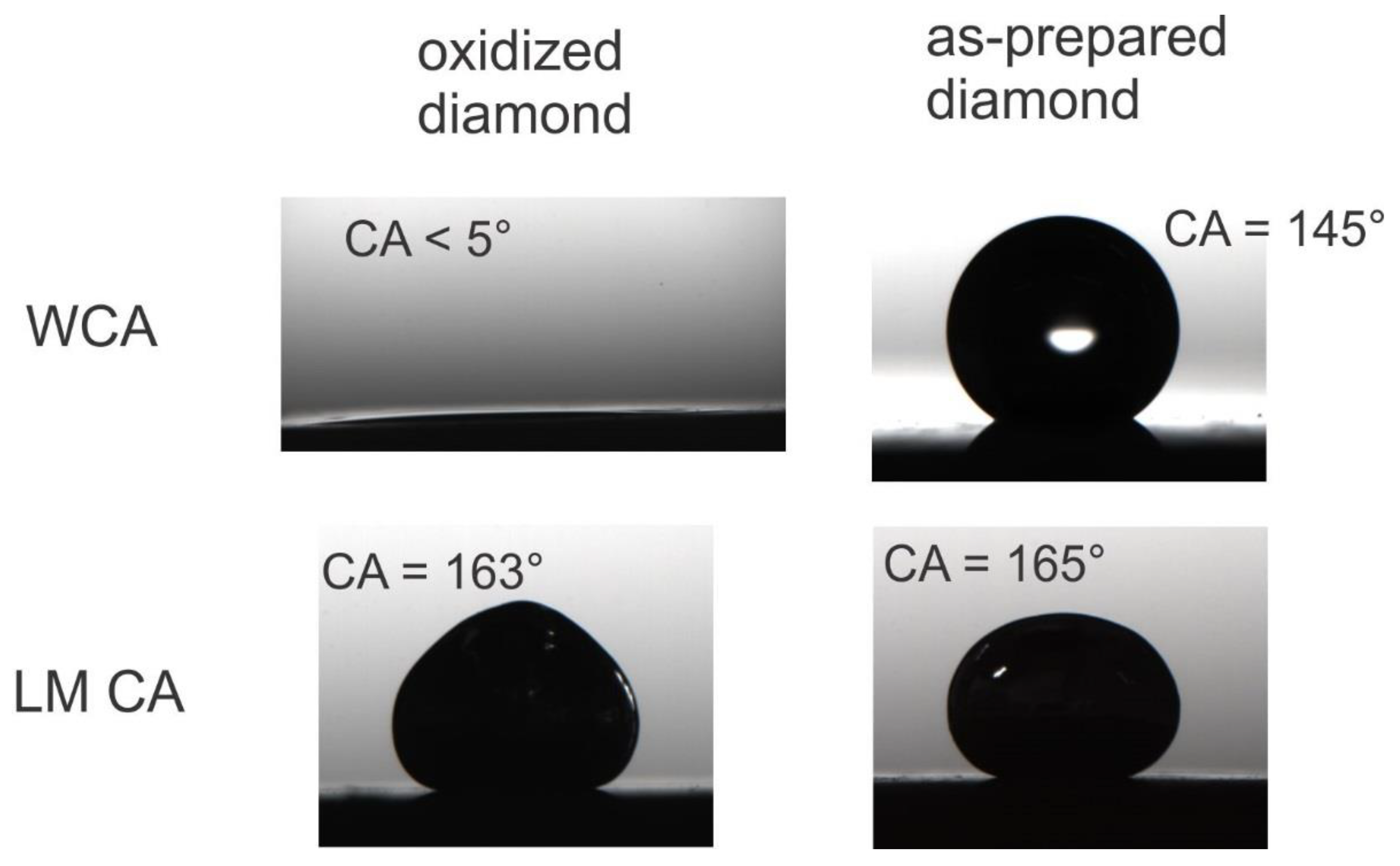
3.2. Wettability of the Diamond Coatings (Toward Liquid Metal)
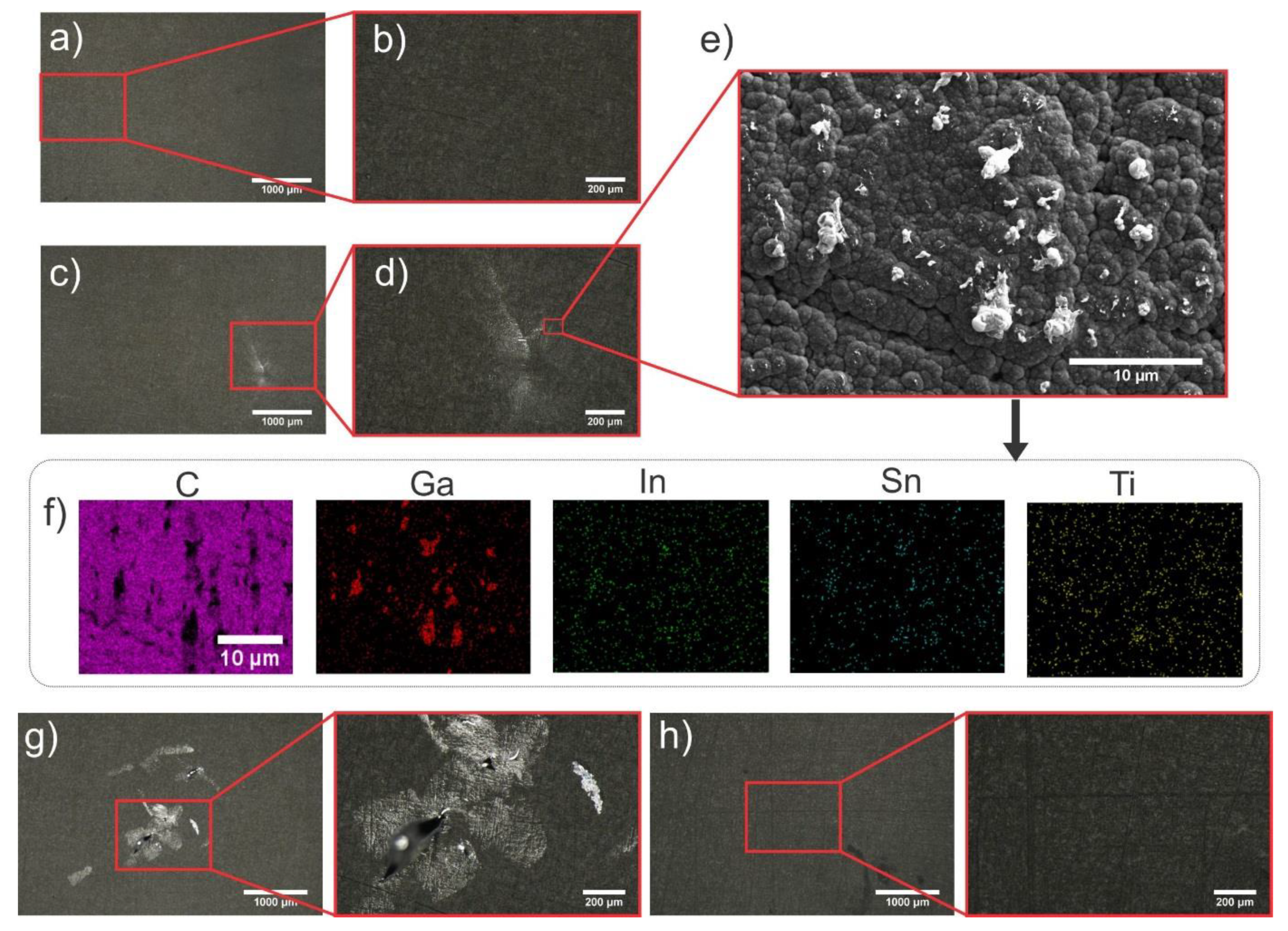
3.3. Comparison of Adhesion Resistance
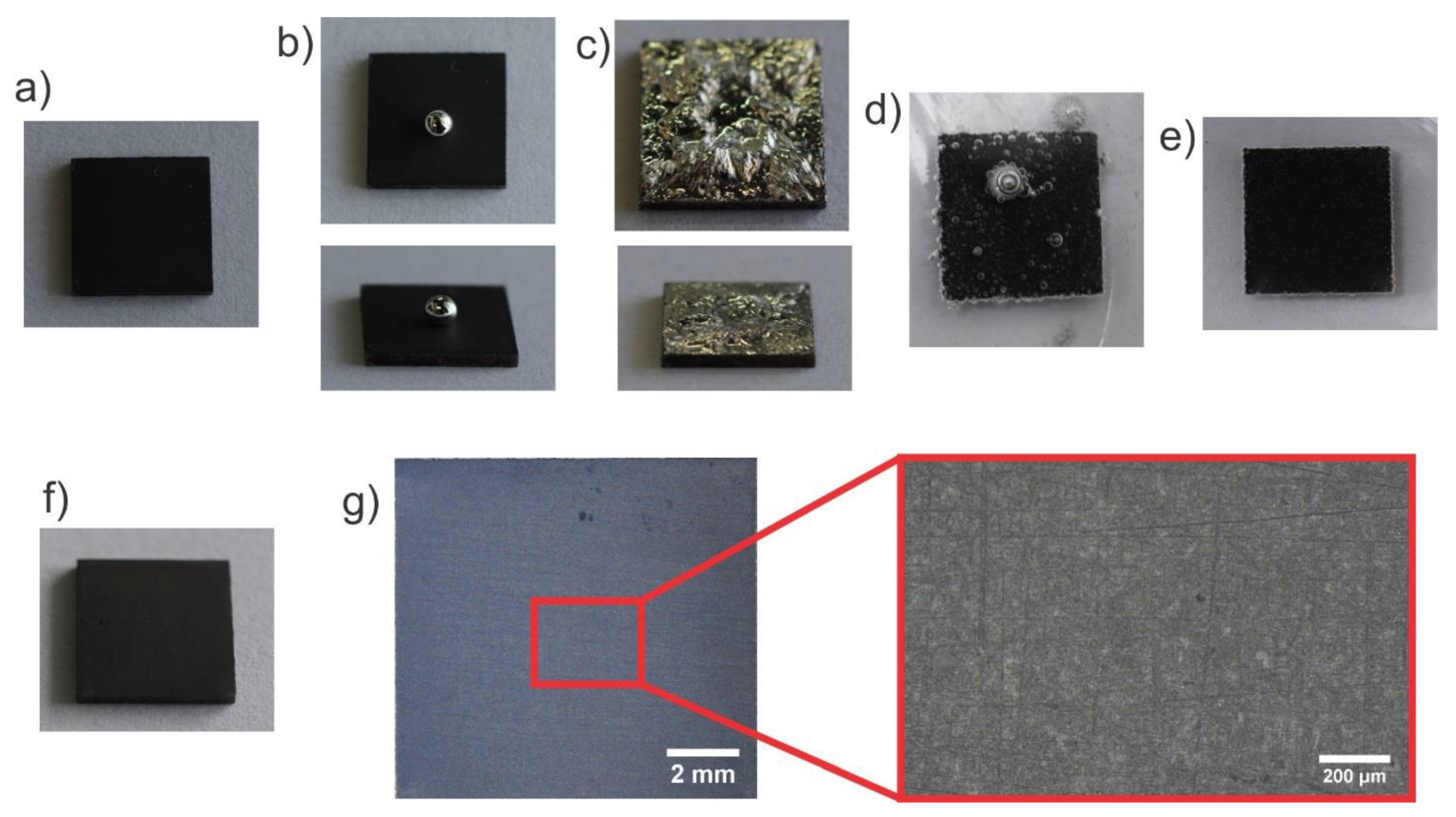
3.4. Application of the Liquid Metal Adhesion to the Hydrophilic Diamond Coating
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, S.W.; Jeong, Y.R.; Park, H.; Keum, K.; Lee, G.; Lee, Y.H.; Lee, H.; Kim, M.S.; Ha, J.S. A Flexible loudspeaker using the movement of liquid metal induced by electrochemically controlled interfacial tension. Small 2019, 15, 1905263. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, C.; Chi, J.; Chen, J.; Zhao, C.; Liu, H. A versatile approach for direct patterning of liquid metal using magnetic field. Adv. Funct. Mater. 2019, 29, 1901370. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Yang, X.-H.; Zhou, Y.-X.; Rao, W.; Gao, J.-Y.; Ding, Y.-J.; Shu, Q.-Q.; Liu, J. Experimental investigation of galinstan based minichannel cooling for high heat flux and large heat power thermal management. Energy Convers. Manag. 2019, 185, 248–258. [Google Scholar] [CrossRef]
- Cao, L.-X.; Yin, T.; Jin, M.-X.; He, Z.-Z. Flexible circulated-cooling liquid metal coil for induction heating. Appl. Therm. Eng. 2019, 162, 114260. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in Chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Diebold, A.V.; Watson, A.M.; Holcomb, S.; Tabor, C.; Mast, D.; Dickey, M.D.; Heikenfeld, J. Electrowetting-actuated liquid metal for RF applications. J. Micromech. Microeng. 2017, 27, 025010. [Google Scholar] [CrossRef]
- Bharambe, V.T.; Ma, J.; Dickey, M.D.; Adams, J.J. Planar, Multifunctional 3D Printed Antennas Using Liquid Metal Parasitics. IEEE Access 2019, 7, 134245–134255. [Google Scholar] [CrossRef]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, O.B.; Majidi, C. EGaIn–Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Jeong, Y.R.; Kim, J.; Xie, Z.; Xue, Y.; Won, S.M.; Lee, G.; Jin, S.W.; Hong, S.Y.; Feng, X.; Huang, Y.; et al. A skin-attachable, stretchable integrated system based on liquid GaInSn for wireless human motion monitoring with multi-site sensing capabilities. NPG Asia Mater. 2017, 9, e443. [Google Scholar] [CrossRef]
- Hirsch, A.; Michaud, H.O.; Gerratt, A.P.; de Mulatier, S.; Lacour, S.P. Intrinsically stretchable biphasic (solid–liquid) thin metal films. Adv. Mater. 2016, 28, 4507–4512. [Google Scholar] [CrossRef]
- Kramer, R.K.; Majidi, C.; Wood, R.J. Masked deposition of Gallium-Indium alloys for liquid-Embedded elastomer conductors. Adv. Funct. Mater. 2013, 23, 5292–5296. [Google Scholar] [CrossRef]
- Li, G.; Lee, D.-W. An advanced selective liquid-metal plating technique for stretchable biosensor applications. Lab Chip 2017, 17, 3415–3421. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.G.; Old, C.F. Liquid metal embrittlement. J. Mater. Sci. 1979, 14, 1–18. [Google Scholar] [CrossRef]
- Sivan, V.; Tang, S.-Y.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-zadeh, K.; Mitchell, A. Liquid Metal Marbles. Adv. Funct. Mater. 2013, 23, 144–152. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Baek, S.-Y.; Song, H.-J.; Lee, J.I.; Kang, G.-H. Sliding interconnection for flexible electronics with a solution-processed diffusion barrier against a corrosive liquid metal. Adv. Electron. Mater. 2019, 5, 1900314. [Google Scholar] [CrossRef]
- Oh, E.; Kim, T.; Yoon, J.; Lee, S.; Kim, D.; Lee, B.; Byun, J.; Cho, H.; Ha, J.; Hong, Y. Highly reliable liquid metal–solid metal contacts with a corrugated single-walled carbon nanotube diffusion barrier for stretchable Electronics. Adv. Funct. Mater. 2018, 28, 1806014. [Google Scholar] [CrossRef]
- Andrews, J.B.; Mondal, K.; Neumann, T.V.; Cardenas, J.A.; Wang, J.; Parekh, D.P.; Lin, Y.; Ballentine, P.; Dickey, M.D.; Franklin, A.D. Patterned liquid metal contacts for printed carbon nanotube transistors. ACS Nano 2018, 12, 5482–5488. [Google Scholar] [CrossRef]
- Ahlberg, P.; Jeong, S.H.; Jiao, M.; Wu, Z.; Jansson, U.; Zhang, S.; Zhang, Z. Graphene as a diffusion barrier in galinstan-solid metal contacts. IEEE Trans. Electron Devices 2014, 61, 2996–3000. [Google Scholar] [CrossRef]
- Kramer, R.K.; Boley, J.W.; Stone, H.A.; Weaver, J.C.; Wood, R.J. Effect of microtextured surface topography on the wetting behavior of eutectic gallium–indium alloys. Langmuir 2014, 30, 533–539. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, J.B. Surface modification with gallium coating as nonwetting surfaces for gallium-based liquid metal droplet manipulation. ACS Appl. Mater. Interfaces 2019, 11, 35488–35495. [Google Scholar] [CrossRef]
- Kim, D.; Lee, D.; Choi, W.; Lee, J. A Super-lyophobic 3-D PDMS channel as a novel microfluidic platform to manipulate oxidized galinstan. J. Microelectromech. Syst. 2013, 22, 1267–1275. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Xing, S.-T.; Zheng, R.-M.; Liu, S.-Q.; Deng, Z.-F.; Liu, H.-Z.; Wang, P.-P.; Liu, L. Interface design for enhancing the wettability of liquid metal to polyacrylate for intrinsically soft electronics. J. Mater. Chem. C 2018, 6, 6755–6763. [Google Scholar] [CrossRef]
- Guo, R.; Tang, J.; Dong, S.; Lin, J.; Wang, H.; Liu, J.; Rao, W. One-step liquid metal transfer printing: Toward fabrication of flexible electronics on wide range of substrates. Adv. Mater. Technol. 2018, 3, 1800265. [Google Scholar] [CrossRef]
- Okouchi, M.; Yamaji, Y.; Yamauchi, K. Contact Angle of Poly(alkyl methacrylate)s and Effects of the Alkyl Group. Macromolecules 2006, 39, 1156–1159. [Google Scholar] [CrossRef]
- Eaker, C.B.; Joshipura, I.D.; Maxwell, L.R.; Heikenfeld, J.; Dickey, M.D. Electrowetting without external voltage using paint-on electrodes. Lab Chip 2017, 17, 1069–1075. [Google Scholar] [CrossRef]
- Larsen, R.J.; Dickey, M.D.; Whitesides, G.M.; Weitz, D.A. Viscoelastic properties of oxide-coated liquid metals. J. Rheol. 2009, 53, 1305–1326. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, S.; Peng, H.; Sing Kwok, H.; Zhou, X.; Chen, S. Selective wetting/dewetting for controllable patterning of liquid metal electrodes for all-printed device application. J. Mater. Chem. C 2017, 5, 12378–12383. [Google Scholar] [CrossRef]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different shades of oxide: From nanoscale wetting mechanisms to contact printing of gallium-based liquid metals. Langmuir 2014, 30, 6867–6877. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Ayers, H.R.; Castillo, G.A.; Ladd, C.; Tabor, C.E.; Adams, J.J.; Dickey, M.D. Patterning and reversible actuation of liquid gallium alloys by preventing adhesion on rough surfaces. ACS Appl. Mater. Interfaces 2018, 10, 44686–44695. [Google Scholar] [CrossRef]
- Zheng, Y.; He, Z.-Z.; Yang, J.; Liu, J. Personal electronics printing via tapping mode composite liquid metal ink delivery and adhesion mechanism. Sci. Rep. 2014, 4, 4588. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Pressured liquid metal screen printing for rapid manufacture of high resolution electronic patterns. RSC Adv. 2015, 5, 57686–57691. [Google Scholar] [CrossRef]
- Yunusa, M.; Amador, G.J.; Drotlef, D.-M.; Sitti, M. Wrinkling instability and adhesion of a highly bendable gallium oxide nanofilm encapsulating a liquid-gallium droplet. Nano Lett. 2018, 18, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Qiu, W.; Huang, K.; Feng, H.; Chu, S. Ultrastretchable liquid metal electrical conductors built-in cloth fiber networks for wearable electronics. ACS Appl. Mater. Interfaces 2020, 12, 7673–7678. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, C.; Chen, H.; Liu, X.; Li, H.; Ma, W.; Su, X.; Han, T. Super soft conductors based on liquid metal/cotton composites. J. Mater. Chem. C 2020, 8, 3551–3561. [Google Scholar] [CrossRef]
- Guo, R.; Sun, X.; Yao, S.; Duan, M.; Wang, H.; Liu, J.; Deng, Z. Semi-Liquid-Metal-(Ni-EGaIn)-Based Ultraconformable Electronic Tattoo. Adv. Mater. Technol. 2019, 4, 1900183. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X.; Li, J.; Guo, R.; Zhou, Y.; Liu, J. Gallium-Based Liquid Metal Amalgams: Transitional-State Metallic Mixtures (TransM2ixes) with Enhanced and Tunable Electrical, Thermal, and Mechanical Properties. ACS Appl. Mater. Interfaces 2017, 9, 35977–35987. [Google Scholar] [CrossRef]
- Guo, R.; Yao, S.; Sun, X.; Liu, J. Semi-liquid metal and adhesion-selection enabled rolling and transfer (SMART) printing: A general method towards fast fabrication of flexible electronics. Sci. China Math. 2019, 62, 982–994. [Google Scholar] [CrossRef]
- Chang, H.; Guo, R.; Sun, Z.; Wang, H.; Hou, Y.; Wang, Q.; Rao, W.; Liu, J. Direct Writing and Repairable Paper Flexible Electronics Using Nickel–Liquid Metal Ink. Adv. Mater. Interfaces 2018, 5, 1800571. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Kolesnichenko, G.A. Investigation of the wetting of diamond and graphite by molten metals and alloys. Powder Metall. Met. Ceram. 1965, 3, 191–195. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Handschuh-Wang, S.; Zhang, S.; Li, X.; Chen, B.; Yang, Y.; Zhou, X.; Tang, Y. Adherent and low friction nanocrystalline diamond films via adsorbing organic molecules in self-assembly seeding process. Appl. Surf. Sci. 2018, 456, 75–82. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Macpherson, J.V.; Einaga, Y.; Zhao, H.; Zhao, G.; Swain, G.M.; Jiang, X. Conductive diamond: Synthesis, properties, and electrochemical applications. Chem. Soc. Rev. 2019, 48, 157–204. [Google Scholar] [CrossRef] [PubMed]
- Ekoi, E.J.; Stallard, C.; Reid, I.; Dowling, D.P. Tailoring oxide-layer formation on titanium substrates using microwave plasma treatments. Surf. Coat. Technol. 2017, 325, 299–307. [Google Scholar] [CrossRef]
- Kuzmany, H.; Pfeiffer, R.; Salk, N.; Günther, B. The mystery of the 1140 cm−1 Raman line in nanocrystalline diamond films. Carbon 2004, 42, 911–917. [Google Scholar] [CrossRef]
- Schügerl, F.B.; Kuzmany, H. Optical modes of trans-polyacetylene. J. Chem. Phys. 1981, 74, 953–958. [Google Scholar] [CrossRef]
- López-Ríos, T.; Sandré, É.; Leclercq, S.; Sauvain, É. Polyacetylene in diamond films evidenced by surface enhanced raman scattering. Phys. Rev. Lett. 1996, 76, 4935–4938. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Liu, Y.; Li, X.; Liu, C.; Handschuh-Wang, S.; Xu, Y.; Zhao, Y.; Tang, Y. Robust biomimetic hierarchical diamond architecture with self-cleaning, antibacterial and antibiofouling surface. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Wang, T.; Handschuh-Wang, S.; Yang, Y.; Zhuang, H.; Schlemper, C.; Wesner, D.; Schönherr, H.; Zhang, W.; Jiang, X. Controlled surface chemistry of diamond/β-SiC composite films for preferential protein adsorption. Langmuir 2014, 30, 1089–1099. [Google Scholar] [CrossRef]
- Wang, T.; Handschuh-Wang, S.; Huang, L.; Zhang, L.; Jiang, X.; Kong, T.; Zhang, W.; Lee, C.-S.; Zhou, X.; Tang, Y. Controlling directional liquid motion on micro- and nanocrystalline diamond/β-SiC composite gradient films. Langmuir 2018, 34, 1419–1428. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Guo, P.; Lv, Q. Impact force of a low speed water droplet colliding on a solid surface. Int. J. Appl. Phys 2014, 116, 214903. [Google Scholar] [CrossRef]
- Richard, D.; Clanet, C.; Quéré, D. Contact time of a bouncing drop. Nature 2002, 417, 811. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Chen, Y.; Zhu, L.; Zhou, X. Analysis and transformations of room-temperature liquid metal interfaces—A closer look through interfacial tension. ChemPhysChem 2018, 19, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, A.; Daeneke, T.; Zavabeti, A.; Zhang, B.Y.; Orrell-Trigg, R.; Kalantar-Zadeh, K.; Wójcik, A.; Maziarz, W.; Gancarz, T. Investigation of the surface of Ga–Sn–Zn eutectic alloy by the characterisation of oxide nanofilms obtained by the touch-printing method. Nanomaterials 2019, 9, 235. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Chen, Y.; Zhu, L.; Gan, T.; Zhou, X. Electric actuation of liquid metal droplets in acidified aqueous electrolyte. Langmuir 2019, 35, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Ye, S.; Handschuh-Wang, S.; Zhou, X.; Gan, T.; Zhou, X. Liquid metal-based transient circuits for flexible and recyclable electronics. Adv. Funct. Mater. 2019, 29, 1808739. [Google Scholar] [CrossRef]
| Measurement Method | Oxidized Diamond (WCA ≤ 5°) | H-Terminated Diamond (WCA ≈ 150°) |
|---|---|---|
| Scroll off angle | 11.3 ± 1.5° | 8.5 ± 1.0° |
| Minimum height (speed, Weber number (We)) for adhesion of LM upon droplet impact | 15 cm (1.7 m/s, We 4.11) | 15 cm (1.7 m/s, We 4.11) |
| Number of deposition/removal cycles necessary for LM adhesion | 53.3 ± 2.5 | >100 |
| (static) contact angle | 163.4 ± 2.0° | 162.5 ± 2.0° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handschuh-Wang, S.; Zhu, L.; Wang, T. Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals? Materials 2020, 13, 2283. https://doi.org/10.3390/ma13102283
Handschuh-Wang S, Zhu L, Wang T. Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals? Materials. 2020; 13(10):2283. https://doi.org/10.3390/ma13102283
Chicago/Turabian StyleHandschuh-Wang, Stephan, Lifei Zhu, and Tao Wang. 2020. "Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals?" Materials 13, no. 10: 2283. https://doi.org/10.3390/ma13102283
APA StyleHandschuh-Wang, S., Zhu, L., & Wang, T. (2020). Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals? Materials, 13(10), 2283. https://doi.org/10.3390/ma13102283







