Role of Natural Stone Wastes and Minerals in the Alkali Activation Process: A Review
Abstract
1. Introduction
2. Alkali-Activated Materials Based on Alumino-Silicate Minerals
2.1. Role of Raw Materials, Activators, and Curing Conditions
2.2. Properties of Fresh and Hardened Materials
2.2.1. Fresh Mixtures Properties
2.2.2. Mechanical Properties of Hardened Materials
2.2.3. Durability Properties of Hardened Materials
2.3. Role of Alumino-Silicate Minerals in the Alkali Activation Process
3. Alkali-Activated Materials Based on Carbonate Minerals
3.1. Role of Raw Materials, Activators, and Curing Conditions
3.2. Properties of Fresh and Hardened Materials
3.2.1. Fresh Mixture Properties
3.2.2. Mechanical Properties of Hardened Materials
3.2.3. Durability Properties of Hardened Materials
3.3. Role of Carbonate Fines in the Alkali Activation Process
4. Final Discussion on the Role of Alumino-Silicate and Carbonate Mineral Fines in the Alkali-Activation Process
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 29Si MAS-NMR | 29Si Magic Angle Spinning Nuclear Magnetic Resonance |
| C-A-H | Calcium Aluminate Hydrates |
| C-A-S-H | Calcium Aluminum Silicate Hydrate |
| N-A-S-H | Sodium Aluminum Silicate Hydrate |
| C-S-H | Calcium Silicate Hydrates |
| TGA/DTG | Thermo-Gravimetric Analysis coupled with Derivative Thermo-Gravimetric Analysis |
| EDX | Energy Dispersive X-ray Spectroscopy |
| FA | Fly Ash |
| FESEM | Field Emission Scanning Electron Microscopy |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| GBFS | Granulated Blast Furnace Slag |
| LS | Limestone |
| MK | Metakaolin |
| Ms | SiO2/Na2O molar ratio |
| RH | Relative Humidity |
| SEM | Scanning Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
| XRF | X-ray Fluorescence Spectroscopy |
| WBA | Wood Biomass Ash |
References
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Provis, J.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Davidovits, J. Why alkali-activated materials (AAM) are not geopolymers, Technical Paper #25. Geopolym. Inst. Lib. 2018. [Google Scholar] [CrossRef]
- Chen, X.; Sutrisno, A.; Zhu, L.; Struble, L.J. Setting and nanostructural evolution of metakaolin geopolymer. J. Am. Ceram. Soc. 2017, 100, 2285–2295. [Google Scholar] [CrossRef]
- Palmero, P.; Formia, A.; Antonaci, P.; Brini, S.; Tulliani, J.-M. Geopolymer technology for application-oriented dense and lightened materials. Elaboration and characterization. Ceram. Int. 2015, 41, 12967–12979. [Google Scholar] [CrossRef]
- Zain, H.; Abdullah, M.M.A.B.; Ariffin, N.; Bayuaji, R.; Hussin, K. Review on Various Types of Geopolymer Materials with the Environmental Impact Assessment. MATEC Web Conf. 2017, 97, 1021. [Google Scholar] [CrossRef]
- Ziegler, D.; Formia, A.; Tulliani, J.-M.; Palmero, P. Environmentally-Friendly Dense and Porous Geopolymers Using Fly Ash and Rice Husk Ash as Raw Materials. Materials 2016, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Habert, G.; Ouellet-Plamondon, C.M. Recent update on the environmental impact of geopolymers. RILEM Tech. Lett. 2016, 1, 17. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Cizer, Ö.; Mertens, G.; Veulemans, W.; Geysen, D.; Jones, P.T.; Vandewalle, L.; Van Balen, K.; Blanpain, B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cem. Concr. Res. 2012, 42, 778–788. [Google Scholar] [CrossRef]
- Nazari, A.; Sanjayan, J. Synthesis of geopolymer from industrial wastes. J. Clean. Prod. 2015, 99, 297–304. [Google Scholar] [CrossRef]
- Galetakis, M.; Soultana, A. A review on the utilisation of quarry and ornamental stone industry fine by-products in the construction sector. Constr. Build. Mater. 2016, 102, 769–781. [Google Scholar] [CrossRef]
- Mineral Commodity Summary; U.S. Geological Survey, U.S. Department of the Interior: Washington, DC, USA, 2018; p. 157. Available online: https://www.usgs.gov/centers/nmic/mineral-commodity-summaries (accessed on 2 March 2020).
- Cosi, M. The dimension stone sector: New perspectives on the global market and on the reporting of international mining standards. Eur. Geol. 2015, 39, 24–30. [Google Scholar]
- Liguori, V.; Rizzo, G.; Traverso, M. Marble quarrying: An energy and waste intensive activity in the production of building materials. Des. Nat. III Comp. Design Nat. Sci. Eng. 2008, 108, 197–207. [Google Scholar] [CrossRef]
- Mehta, P.; Mehta, V.K. Waste Generation and Minimization: A Study of Marble Mines of Rajsamand. Int. J. Inf. Futuristic Res. 2015, 2, 3049–3058. [Google Scholar]
- Aukour, F.J. Incorporation of marble sludge in industrial building eco-blocks or cement bricks formulation. Jordan J. Civ. Eng. 2009, 3, 58–65. [Google Scholar]
- Careddu, N.; Dino, G.A. Reuse of residual sludge from stone processing: Differences and similarities between sludge coming from carbonate and silicate stones—Italian experiences. Environ. Earth Sci. 2016, 75, 1075. [Google Scholar] [CrossRef]
- Dino, G.A.; Clemente, P.; Lasagna, M.; De Luca, D. Residual Sludge from Dimension Stones: Characterisation for their Exploitation in Civil and Environmental Applications. Energy Procedia 2013, 40, 507–514. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Fernandes, H.; Olhero, S.; Ferreira, J.M.F. Incorporation of wastes from granite rock cutting and polishing industries to produce roof tiles. J. Eur. Ceram. Soc. 2009, 29, 23–30. [Google Scholar] [CrossRef]
- Furcas, C.; Balletto, G. Converting waste from the dimension stone industry into sustainable environmental resources. Current trends, market opportunities and future outlooks. In Proceedings of the 28th International Conference on Solid Waste Technology and Management, Philadelphia, PA, USA, 10–13 March 2013. [Google Scholar]
- Allam, M.E.; Bakhoum, E.S.; Garas, G.L. Re-use of granite sludge in producing green concrete. J. Eng. Appl. Sci. 2014, 9, 2731–2737. [Google Scholar]
- Corinaldesi, V.; Moriconi, G.; Naik, T.R. Characterization of marble powder for its use in mortar and concrete. Constr. Build. Mater. 2010, 24, 113–117. [Google Scholar] [CrossRef]
- Ergün, A. Effects of the usage of diatomite and waste marble powder as partial replacement of cement on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Coppola, B.; Courard, L.; Michel, F.; Incarnato, L.; Scarfato, P.; Di Maio, L. Hygro-thermal and durability properties of a lightweight mortar made with foamed plastic waste aggregates. Constr. Build. Mater. 2018, 170, 200–206. [Google Scholar] [CrossRef]
- Coppola, B.; Courard, L.; Michel, F.; Incarnato, L.; Di Maio, L. Investigation on the use of foamed plastic waste as natural aggregates replacement in lightweight mortar. Compos. Part B Eng. 2016, 99, 75–83. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.M.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Reusing ceramic wastes in concrete. Constr. Build. Mater. 2010, 24, 832–838. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Falah, M.; Luukkonen, T.; Mazari, M.; Illikainen, M. Construction and Demolition Waste as Recycled Aggregates in Alkali-Activated Concretes. Materials 2019, 12, 4016. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Illikainen, M.; Falah, M.; Kinnunen, P.; Heiskanen, K. Geopolymers from mining tailings for more sustainable raw material supply. MATEC Web Conf. 2019, 274, 05001. [Google Scholar] [CrossRef]
- Kotwica, Ł.; Chorembała, M.; Kapeluszna, E.; Stępień, P.; Deja, J.; Illikainen, M.; Gołek, Ł. Influence of Calcined Mine Tailings on the Properties of Alkali Activated Slag Mortars. Key Eng. Mater. 2018, 761, 83–86. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Falah, M.; Illikainen, M. Assessment of mine tailings as precursors for alkali-activated materials for on-site applications. Constr. Build. Mater. 2020, 246, 118470. [Google Scholar] [CrossRef]
- Falah, M.; Ohenoja, K.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Improvement of mechanical strength of alkali-activated materials using micro low-alumina mine tailings. Constr. Build. Mater. 2020, 248, 118659. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K.; Brown, T.; Song, S. Effects of aluminates on the formation of geopolymers. Mater. Sci. Eng. B 2005, 117, 163–168. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J.; Sobrados, M.I. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Avila-López, U.; Almanza, J.; García, J.I.E. Investigation of novel waste glass and limestone binders using statistical methods. Constr. Build. Mater. 2015, 82, 296–303. [Google Scholar] [CrossRef]
- Ortega-Zavala, D.E.; Santana-Carrillo, J.L.; Burciaga-Díaz, O.; García, J.I.E. An initial study on alkali activated limestone binders. Cem. Concr. Res. 2019, 120, 267–278. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Yip, C.K.; Van Deventer, J.S.J. Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Coppola, B.; Palmero, P. Alkali-activation of aggregate fines from construction and demolition waste: Valorisation in view of road pavement subbase applications. J. Clean. Prod. 2019, 234, 71–84. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Firouz, R.M.; Illikainen, M. Drying shrinkage in alkali-activated binders–A critical review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Coppola, B.; Palmero, P.; Montanaro, L.; Tulliani, J.-M. Alkali-activation of marble sludge: Influence of curing conditions and waste glass addition. J. Eur. Ceram. Soc. 2019. [Google Scholar] [CrossRef]
- Palmero, P.; Formia, A.; Tulliani, J.-M.; Antonaci, P. Valorisation of alumino-silicate stone muds: From wastes to source materials for innovative alkali-activated materials. Cem. Concr. Compos. 2017, 83, 251–262. [Google Scholar] [CrossRef]
- Choi, S.J.; Jun, S.S.; Oh, J.E.; Monteiro, P.J.M. Properties of alkali-activated systems with stone powder sludge. J. Mater. Cycles Waste Manag. 2010, 12, 275–282. [Google Scholar] [CrossRef]
- Tchadjié, L.; Djobo, J.N.Y.; Ranjbar, N.; Tchakouté, H.; Kenne, B.; Elimbi, A.; Njopwouo, D. Potential of using granite waste as raw material for geopolymer synthesis. Ceram. Int. 2016, 42, 3046–3055. [Google Scholar] [CrossRef]
- Feng, D.; Provis, J.; Deventer, J.S.J. Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers. J. Am. Ceram. Soc. 2011, 95, 565–572. [Google Scholar] [CrossRef]
- Clausi, M.; Tarantino, S.C.; Magnani, L.L.; Riccardi, M.P.; Tedeschi, C.; Zema, M. Metakaolin as a precursor of materials for applications in Cultural Heritage: Geopolymer-based mortars with ornamental stone aggregates. Appl. Clay Sci. 2016, 132, 589–599. [Google Scholar] [CrossRef]
- Li, C.; Zhang, T.; Wang, L. Mechanical properties and microstructure of alkali activated Pisha sandstone geopolymer composites. Constr. Build. Mater. 2014, 68, 233–239. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Zhang, T.; Dong, J. Development of building material utilizing a low pozzolanic activity mineral. Constr. Build. Mater. 2016, 121, 300–309. [Google Scholar] [CrossRef]
- Hemra, K.; Aungkavattana, P. Effect of cordierite addition on compressive strength and thermal stability of metakaolin based geopolymer. Adv. Powder Technol. 2016, 27, 1021–1026. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.; Vasquez-Garcia, S.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.; Mohammed, M.S.; Flores, N. Cleaner production of one-part white geopolymer cement using pre-treated wood biomass ash and diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Yip, C.K.; Provis, J.; Lukey, G.C.; Van Deventer, J.S.J. Carbonate mineral addition to metakaolin-based geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.; Abo-El-Enein, S. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Aboulayt, A.; Riahi, M.; Touhami, M.O.; Hannache, H.; Gomina, M.; Moussa, R. Properties of metakaolin based geopolymer incorporating calcium carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]
- Kürklü, G.; Görhan, G. Investigation of usability of quarry dust waste in fly ash-based geopolymer adhesive mortar production. Constr. Build. Mater. 2019, 217, 498–506. [Google Scholar] [CrossRef]
- Ye, H.; Fu, C.; Yang, G. Influence of dolomite on the properties and microstructure of alkali-activated slag with and without pulverized fly ash. Cem. Concr. Compos. 2019, 103, 224–232. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Xiao, Q.G.; Song, F.; Xie, W.; Yu, L.C.; Huang, H.W.; Yi, S.J. Effects of alkali on one-part alkali-activated cement synthesized by calcining bentonite with dolomite and Na2CO3. Appl. Clay Sci. 2017, 139, 64–71. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Zhu, H.; Zhang, W.; Gao, Y.; Zhang, X.; Wu, Q. Effects of calcined dolomite addition on reaction kinetics of one-part sodium carbonate-activated slag cements. Constr. Build. Mater. 2019, 211, 329–336. [Google Scholar] [CrossRef]
- Cohen, E.; Alva, P.; Bar-Nes, G. Dolomite-based quarry-dust as a substitute for fly-ash geopolymers and cement pastes. J. Clean. Prod. 2019, 235, 910–919. [Google Scholar] [CrossRef]
- Tekin, I. Properties of NaOH activated geopolymer with marble, travertine and volcanic tuff wastes. Constr. Build. Mater. 2016, 127, 607–617. [Google Scholar] [CrossRef]
- Valentini, L.; Contessi, S.; Dalconi, M.C.; Zorzi, F.; Garbin, E. Alkali-activated calcined smectite clay blended with waste calcium carbonate as a low-carbon binder. J. Clean. Prod. 2018, 184, 41–49. [Google Scholar] [CrossRef]
- Salihoğlu, N.K.; Salihoğlu, G. Marble Sludge Recycling by Using Geopolymerization Technology. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018019. [Google Scholar] [CrossRef]
- Thakur, A.K.; Pappu, A.; Thakur, V. Synthesis and characterization of new class of geopolymer hybrid composite materials from industrial wastes. J. Clean. Prod. 2019, 230, 11–20. [Google Scholar] [CrossRef]
- Cwirzen, A.; Provis, J.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Brouwers, H. Properties of alkali activated slag–fly ash blends with limestone addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.; Brouwers, H.J.H. Assessing the chemical involvement of limestone powder in sodium carbonate activated slag. Mater. Struct. 2017, 50, 136. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.; Dainese, E.; Brouwers, H. Autogenous and drying shrinkage of sodium carbonate activated slag altered by limestone powder incorporation. Constr. Build. Mater. 2017, 153, 459–468. [Google Scholar] [CrossRef]
- Bayiha, B.N.; Billong, N.; Yamb, E.; Kaze, R.C.; Nzengwa, R. Effect of limestone dosages on some properties of geopolymer from thermally activated halloysite. Constr. Build. Mater. 2019, 217, 28–35. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R.; Naumkina, N.I.; Khuzin, A.; Osin, Y.N. Influence of limestone content, fineness, and composition on the properties and microstructure of alkali-activated slag cement. Cem. Concr. Compos. 2016, 72, 268–274. [Google Scholar] [CrossRef]
- El-Habaak, G.; Askalany, M.; Abdel-Hakeem, M.S. Building up and Characterization of Calcined Marl-Based Geopolymeric Cement. Infrastructures 2018, 3, 22. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R.; Morozov, V.; Gaifullin, A.R.; Potapova, L.; Gubaidullina, A.M.; Osin, Y.N. Marl-based geopolymers incorporated with limestone: A feasibility study. J. Non-Cryst. Solids 2018, 492, 1–10. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R.; Morozov, V.; Potapova, L.; Osin, Y.N. Marl as a supplementary material to alkali-activated blended cements. Eur. J. Environ. Civ. Eng. 2019, 1–18. [Google Scholar] [CrossRef]
- Clausi, M.; Fernández-Jiménez, A.; Palomo, A.; Tarantino, S.C.; Zema, M. Reuse of waste sandstone sludge via alkali activation in matrices of fly ash and metakaolin. Constr. Build. Mater. 2018, 172, 212–223. [Google Scholar] [CrossRef]
- Wang, A.; Liu, H.; Hao, X.; Wang, Y.; Liu, X.; Li, Z. Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines. Mineral 2019, 9, 48. [Google Scholar] [CrossRef]
- Ceruttin, M.; Palmero, P.; Zerbinatti, M.; Tulliani, J.-M.; Antonaci, P.; Formia, A.; Marian, M. Building Material Obtained from an Alkaline Activation of Sawing Sludge of Stone Materials and Process for Producing such Building Material. European Patent EP3371125, 12 September 2018. [Google Scholar]
- Ceruttin, M.; Palmero, P.; Zerbinatti, M.; Tulliani, J.-M.; Antonaci, P.; Formia, A.; Marian, M. Building Material Obtained from an Alkaline Activation of Sawing Sludge of Stone Materials and Process for Producing such Building Material. Patent Cooperation Treaty Application WO2017056122, 6 April 2017. [Google Scholar]
- Adesanya, E.; Ohenoja, K.; Luukkonen, T.; Kinnunen, P.; Illikainen, M. One-part geopolymer cement from slag and pretreated paper sludge. J. Clean. Prod. 2018, 185, 168–175. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Luukkonen, T.; Mastali, M.; Kinnunen, P.; Illikainen, M. Development of One-Part Alkali-Activated Ceramic/Slag Binders Containing Recycled Ceramic Aggregates. J. Mater. Civ. Eng. 2019, 31, 04018386. [Google Scholar] [CrossRef]
- Coppola, B.; Di Maio, L.; Scarfato, P.; Incarnato, L. Use of polypropylene fibers coated with nano-silica particles into a cementitious mortar. AIP Conf. Proc. 2015, 1695, 020056. [Google Scholar] [CrossRef]
- Ferrara, G.; Coppola, B.; Di Maio, L.; Incarnato, L.; Martinelli, E. Tensile strength of flax fabrics to be used as reinforcement in cement-based composites: Experimental tests under different environmental exposures. Compos. Part B Eng. 2019, 168, 511–523. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- LaRosa Thompson, J.; Silsbee, M.; Gill, P.; Scheetz, B. Characterization of silicate sealers on concrete. Cem. Concr. Res. 1997, 27, 1561–1567. [Google Scholar] [CrossRef]
- Ouarabi, M.A.; Antonaci, P.; Boubenider, F.; Gliozzi, A.; Scalerandi, M. Ultrasonic Monitoring of the Interaction between Cement Matrix and Alkaline Silicate Solution in Self-Healing Systems. Materials 2017, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, A.; Scalerandi, M.; Anglani, G.; Antonaci, P.; Salini, L. Correlation of elastic and mechanical properties of consolidated granular media during microstructure evolution induced by damage and repair. Phys. Rev. Mater. 2018, 2, 13601. [Google Scholar] [CrossRef]
- Formia, A.; Terranova, S.; Antonaci, P.; Pugno, N.M.; Tulliani, J.-M. Setup of Extruded Cementitious Hollow Tubes as Containing/Releasing Devices in Self-Healing Systems. Materials 2015, 8, 1897–1923. [Google Scholar] [CrossRef]
- Phair, J.; Van Deventer, J. Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers. Int. J. Miner. Process. 2002, 66, 121–143. [Google Scholar] [CrossRef]
- Sakulich, A.; Anderson, E.; Schauer, C.L.; Barsoum, M.W. Mechanical and microstructural characterization of an alkali-activated slag/limestone fine aggregate concrete. Constr. Build. Mater. 2009, 23, 2951–2957. [Google Scholar] [CrossRef]
- Wright, K.; Cygan, R.T.; Slater, B. Structure of the (1014) surfaces of calcite, dolomite and magnesite under wet and dry conditions. Phys. Chem. Chem. Phys. 2001, 3, 839–844. [Google Scholar] [CrossRef]
- Fenter, P.; Zhang, Z.; Park, C.; Sturchio, N.C.; Hu, X.; Higgins, S.R. Structure and reactivity of the dolomite (104)–water interface: New insights into the dolomite problem. Geochim. Cosmochim. Acta 2007, 71, 566–579. [Google Scholar] [CrossRef]
- Konno, H.; Nanri, Y.; Kitamura, M. Crystallization of aragonite in the causticizing reaction. Powder Technol. 2002, 123, 33–39. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2004, 34, 857–868. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Kalousek, G.L. Crystal Chemistry of Hydrous Calcium Silicates: I, Substitution of Aluminum in Lattice of Tobermorite. J. Am. Ceram. Soc. 1957, 40, 74–80. [Google Scholar] [CrossRef]
- Haas, J.; Nonat, A. From C–S–H to C–A–S–H: Experimental study and thermodynamic modelling. Cem. Concr. Res. 2015, 68, 124–138. [Google Scholar] [CrossRef]
- Skibsted, J.; Andersen, M.D. The Effect of Alkali Ions on the Incorporation of Aluminum in the Calcium Silicate Hydrate (C-S-H) Phase Resulting from Portland Cement Hydration Studied by 29 Si MAS NMR. J. Am. Ceram. Soc. 2012, 96, 651–656. [Google Scholar] [CrossRef]
- Siddique, R.; Khan, M.I. Supplementary Cementing Materials; Springer Science and Business Media LLC: New York, NY, USA, 2011; Volume 37. [Google Scholar]
- Dolgaleva, I.V.; Gorichev, I.G.; Izotov, A.D.; Stepanov, V.M. Modeling of the Effect of pH on the Calcite Dissolution Kinetics. Theor. Found. Chem. Eng. 2005, 39, 614–621. [Google Scholar] [CrossRef]
- Choquette, M.; Berube, M.A.; Locat, J. Behavior of common rock-forming minerals in a strongly basic NaOH solution. Can. Mineral. 1991, 29, 163–173. [Google Scholar]
- Humad, A.; Habermehl-Cwirzen, K.; Cwirzen, A. Effects of Fineness and Chemical Composition of Blast Furnace Slag on Properties of Alkali-Activated Binder. Materials 2019, 12, 3447. [Google Scholar] [CrossRef]
- Risdanareni, P.; Ekaputri, J.; Triwulan, M. The Influence of Alkali Activator Concentration to Mechanical Properties of Geopolymer Concrete with Trass as a Filler. Mater. Sci. Forum 2014, 803, 125–134. [Google Scholar] [CrossRef]
- Gado, R.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of the Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2011, 47, 2782–2797. [Google Scholar] [CrossRef]
- Bădănoiu, A.I.; Al-Saadi, T.H.A.; Voicu, G. Synthesis and properties of new materials produced by alkaline activation of glass cullet and red mud. Int. J. Miner. Process. 2015, 135, 1–10. [Google Scholar] [CrossRef]
- Silva, I.; Castro-Gomes, J.; Albuquerque, A. Effect of immersion in water partially alkali-activated materials obtained of tungsten mine waste mud. Constr. Build. Mater. 2012, 35, 117–124. [Google Scholar] [CrossRef]
- Celerier, H.; Jouin, J.; Tessier-Doyen, N.; Rossignol, S. Influence of various metakaolin raw materials on the water and fire resistance of geopolymers prepared in phosphoric acid. J. Non-Cryst. Solids 2018, 500, 493–501. [Google Scholar] [CrossRef]
- Slaty, F.; Khoury, H.; Rahier, H.; Wastiels, J. Durability of alkali activated cement produced from kaolinitic clay. Appl. Clay Sci. 2015, 104, 229–237. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-Ngernkham, T.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Bignozzi, M.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on metakaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]

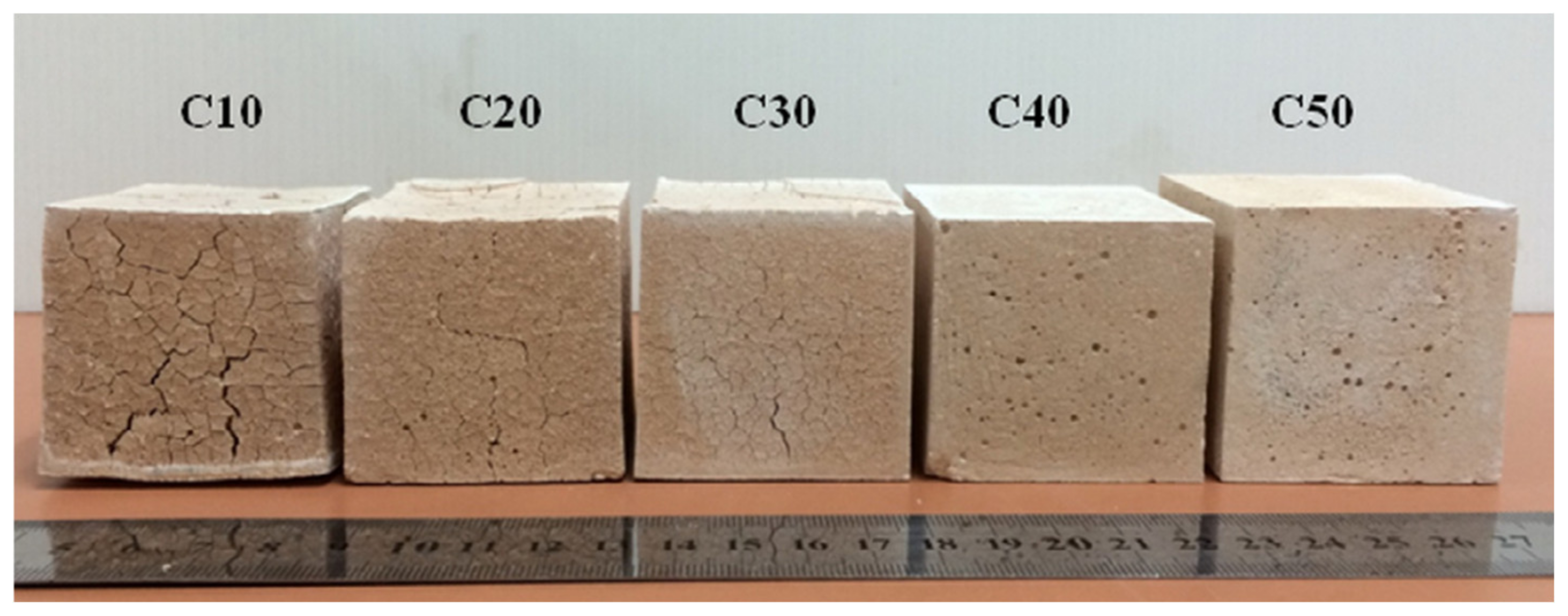

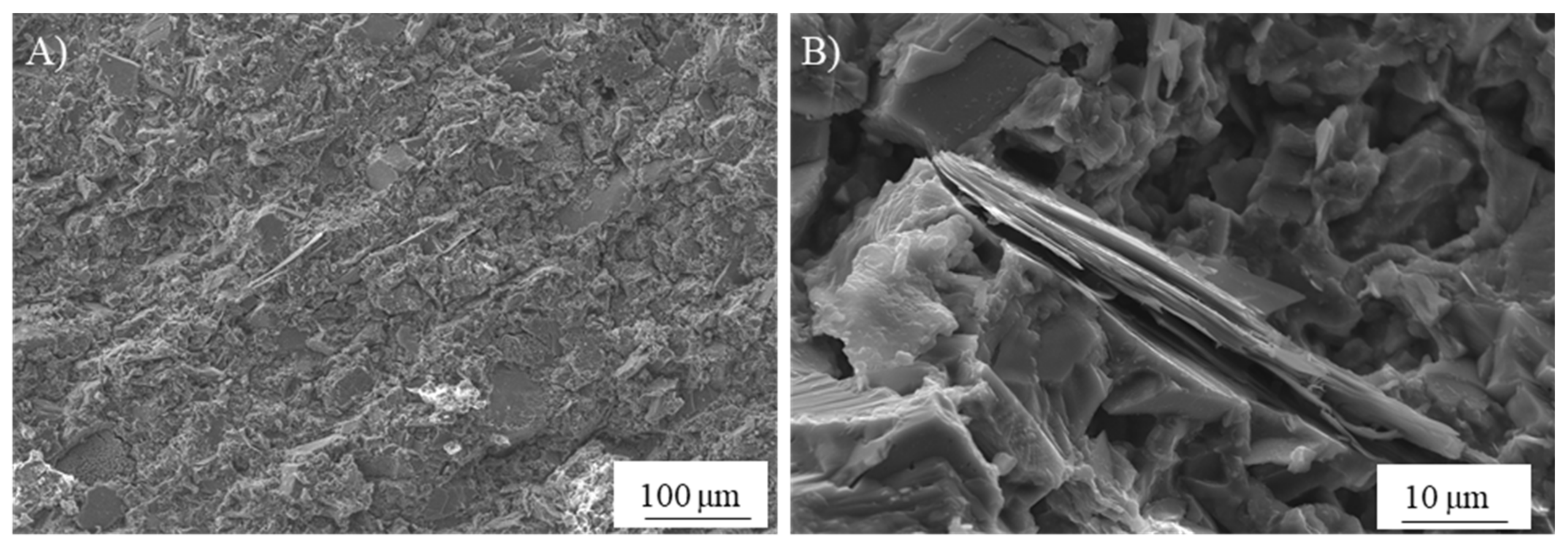
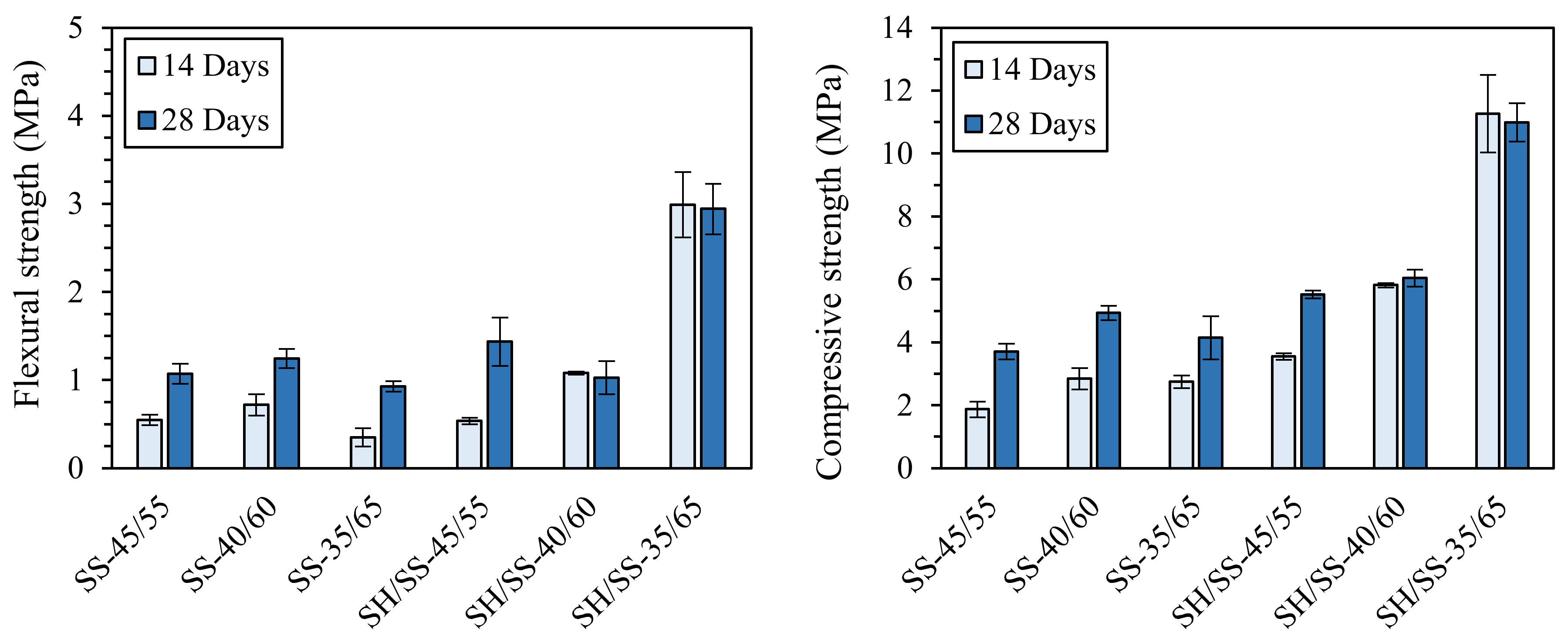
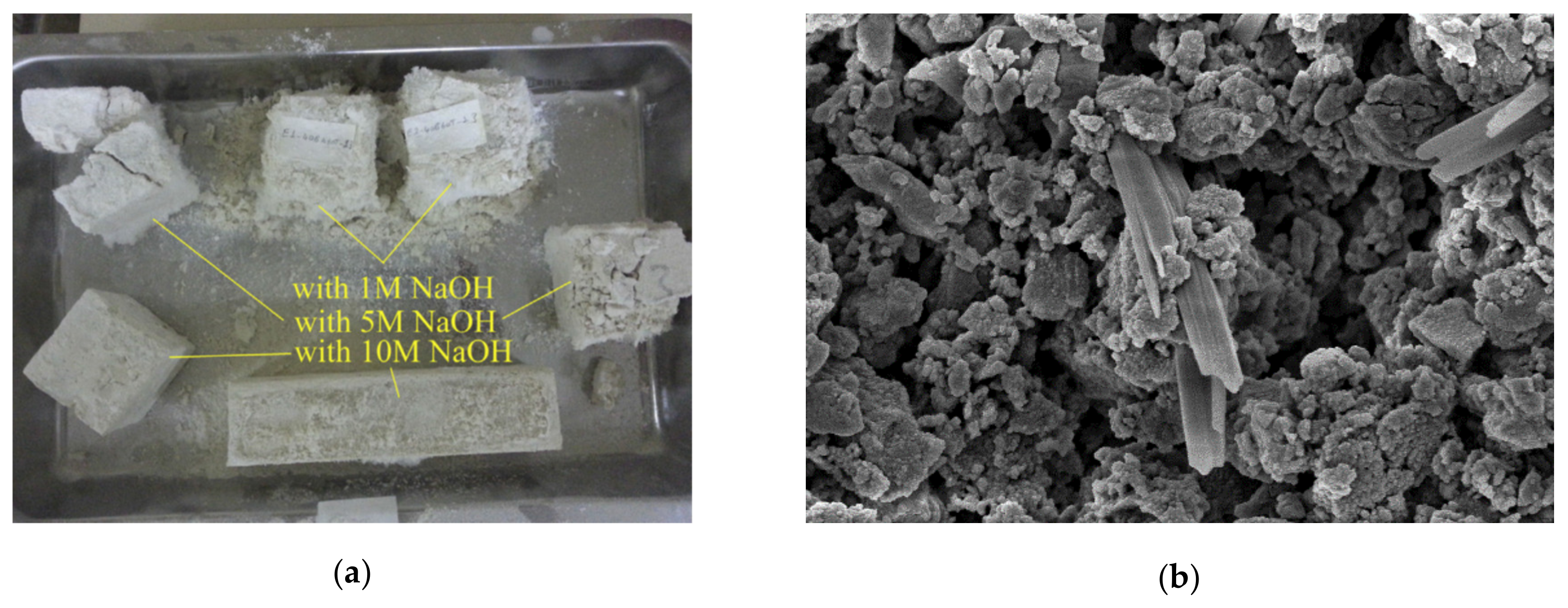
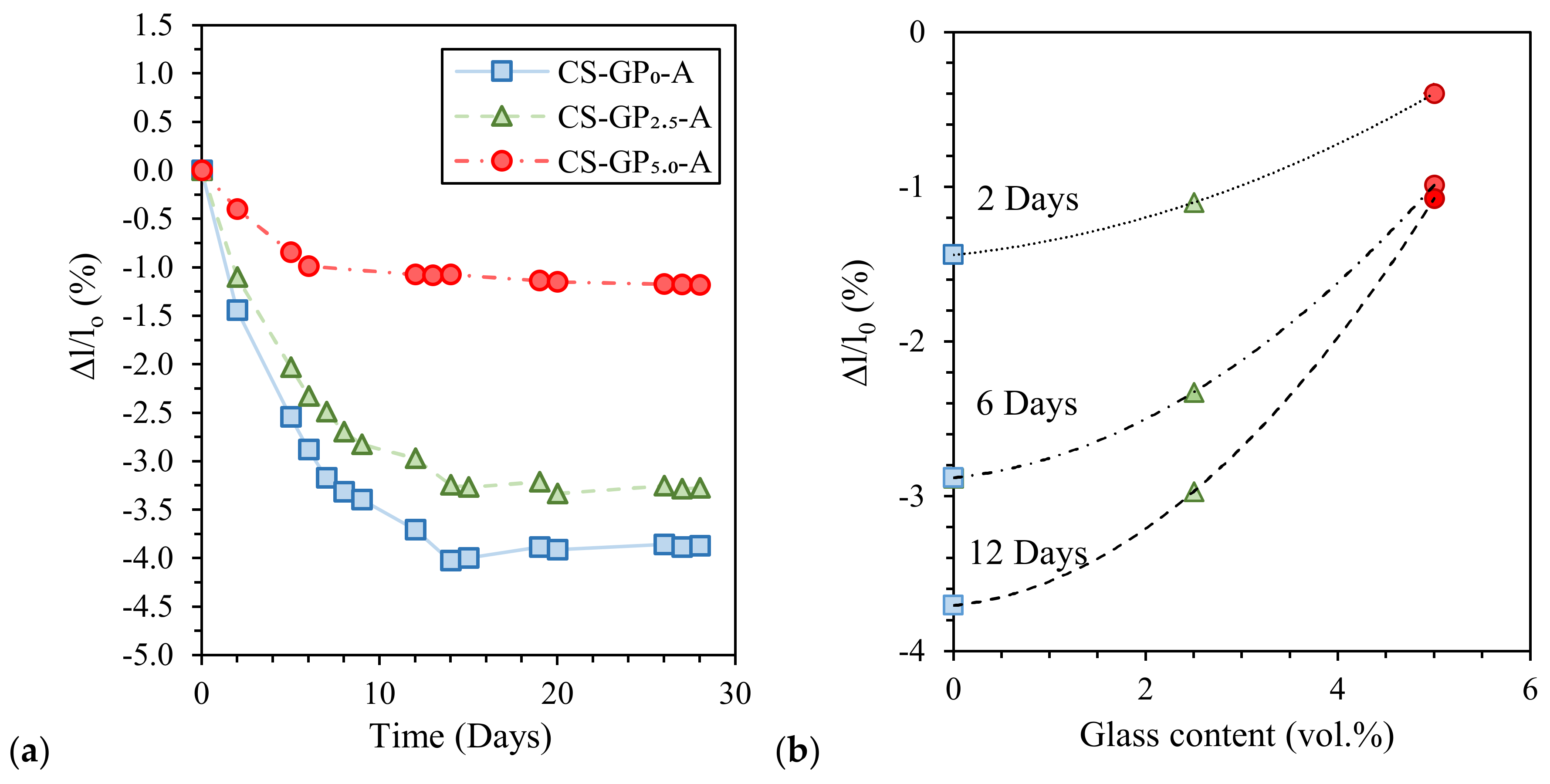
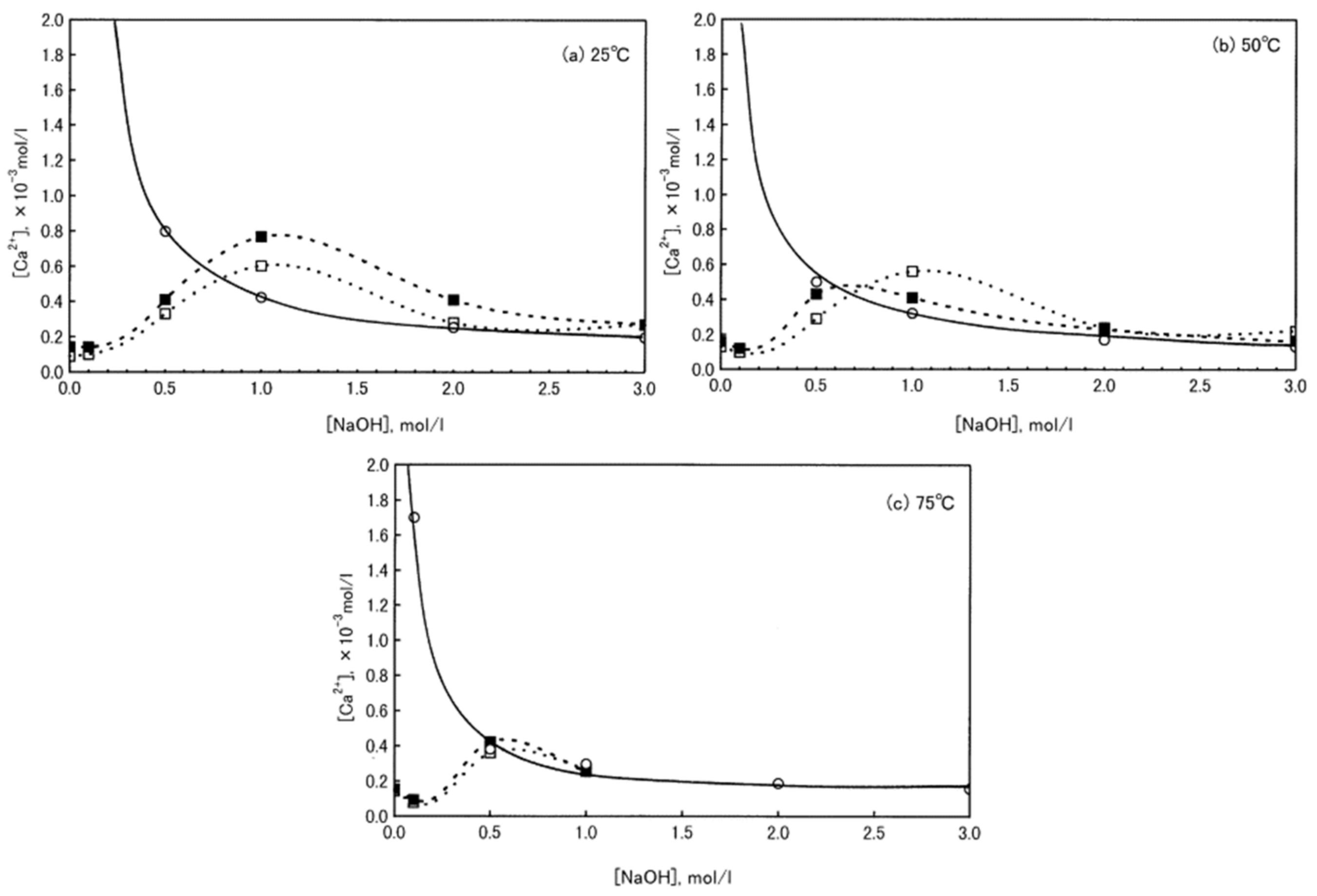
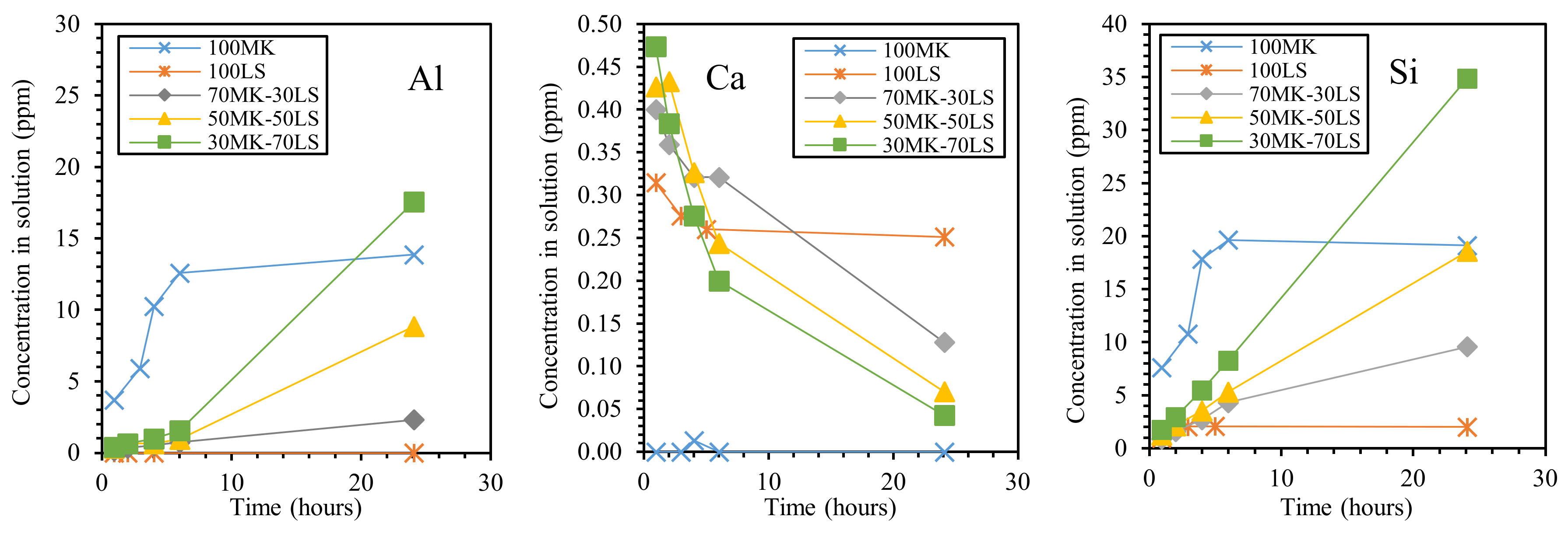

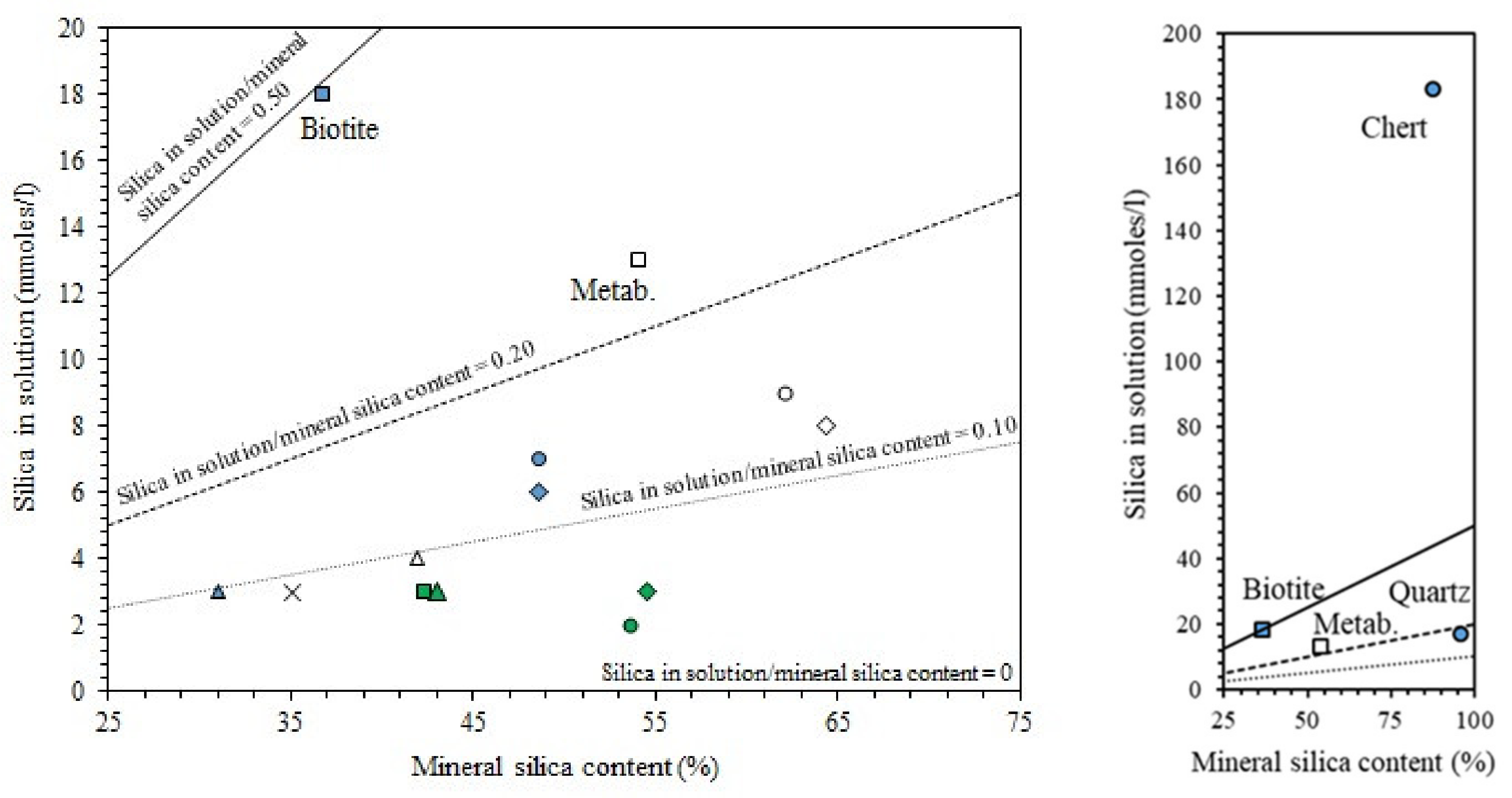
| Classification | Mineral Additive | Alkali-Activation Source | Activator | Reference |
|---|---|---|---|---|
| Alumino-silicates | Granite | Fly Ash/Granulated Blast Furnace Slag | NaOH + Na2SiO3 + H2O | [47] |
| Granite | Metakaolin | NaOH + Na2SiO3 + H2O | [48] | |
| Granite | - | NaOH + Na2SiO3 + H2O | [46] | |
| Albite + | - | Na2CO3/NaOH | [49] | |
| Pietra Serena | Metakaolin | NaOH + Na2SiO3 + H2O | [50] | |
| Pisha sandstone | -/Fly Ash | NaOH + Na2SiO3 + H2O | [51] | |
| Pisha sandstone | - | NaOH + NaCO3 + Na2SO4 + Na2SiO3 + H2O | [52] | |
| Cordierite | Metakaolin | NaOH + Na2SiO3 + H2O | [53] | |
| Diatomite | - | NaOH + H2O + Wood Biomass Ash ^ | [54] | |
| Carbonates | Calcite | Metakaolin | NaOH + Na2SiO3 + H2O | [55] |
| Calcite | Granulated Blast Furnace Slag | NaOH + H2O | [56] | |
| Calcite | Metakaolin | NaOH/KOH + Na2SiO3 + H2O | [57] | |
| Calcite | Fly Ash | NaOH + Na2SiO3 + H2O | [58] | |
| Dolomite | Fly Ash/Granulated Blast Furnace Slag | Na2CO3/NaOH | [59] | |
| Dolomite | Metakaolin | NaOH + Na2SiO3 + H2O | [55] | |
| Dolomite + | Bentonite + Na2CO3 | H2O | [60] | |
| Dolomite + | Granulated Blast Furnace Slag + Na2CO3 | H2O | [61] | |
| Dolomite | Fly ash/cement | NaOH + Na2SiO3 + H2O | [62] | |
| Marble ° | - | NaOH + H2O | [63] | |
| Marble | - | NaOH + Na2SiO3 + H2O | [45] | |
| Marble | Smectite clay | NaOH + Na2SiO3/Sodium citrate + H2O | [64] | |
| Marble | Cement/Fly ash/GBFS/Gypsum/Clay | NaOH + Na2SiO3 + H2O | [65] | |
| Marble | Fly ash | NaOH + Na2SiO3 + H2O | [66] | |
| Pietra di Angera | Metakaolin | NaOH + Na2SiO3 + H2O | [50] | |
| Travertine ° | - | NaOH + H2O | [63] | |
| Limestone | Metakaolin | NaOH + H2O | [67] | |
| Limestone | Fly Ash/Granulated Blast Furnace Slag | NaOH + Na2SiO3 + H2O | [68] | |
| Limestone | Granulated Blast Furnace Slag | Na2CO3 + H2O | [69,70] | |
| Limestone | - | NaOH + Na2SiO3 + H2O | [40] | |
| Limestone | Halloysite clay | NaOH + Na2SiO3+H2O | [71] | |
| Limestone * | Granulated Blast Furnace Slag | NaOH + Na2CO3 + H2O | [72] | |
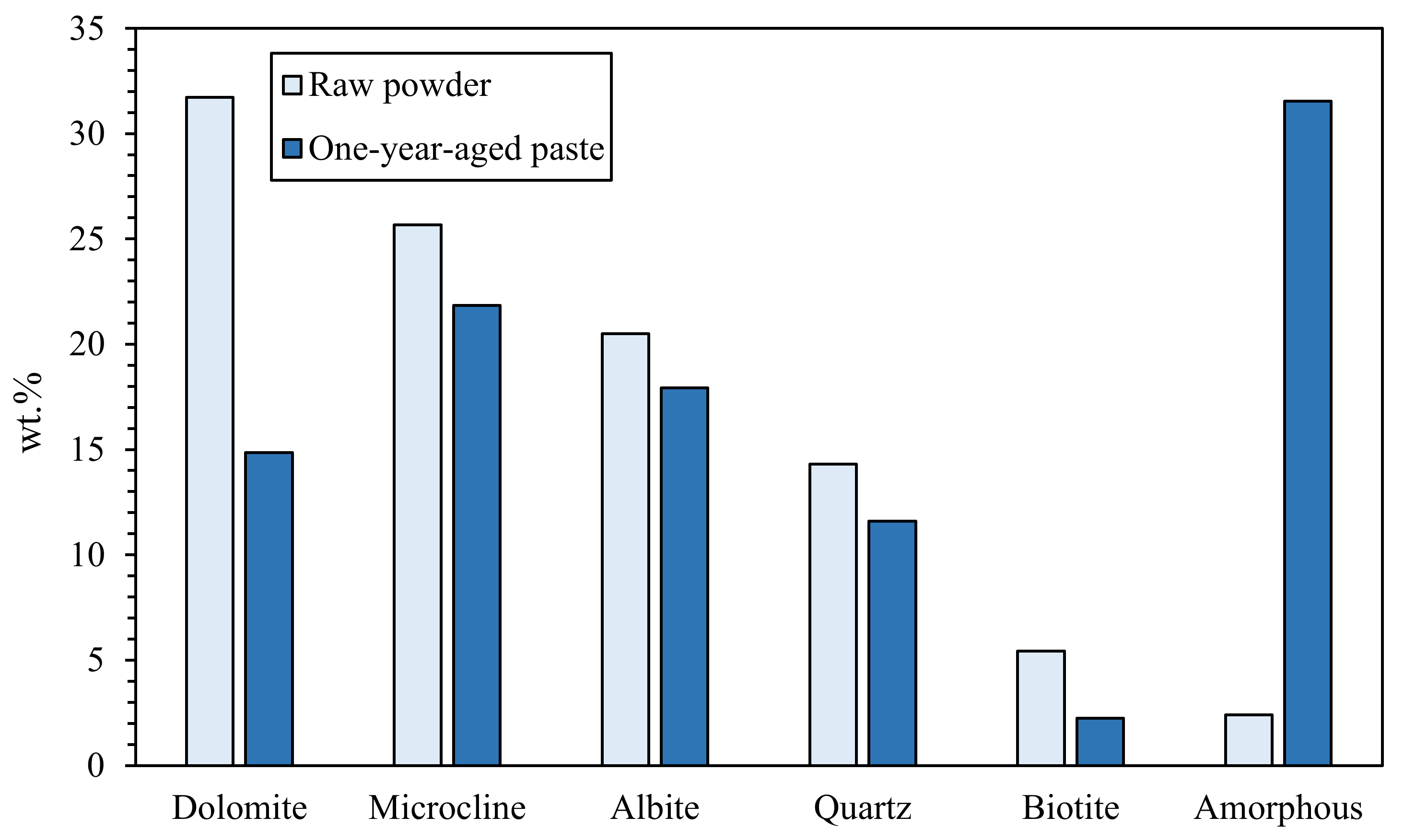
| Mixed | Dolomite + Microcline + Albite + Quartz | - | NaOH + Na2SiO3 + H2O | [46] |
| Marl + | - | NaOH + Na2SiO3 + H2O | [73] | |
| Marl ++Limestone | - | Na2SiO3 + H2O | [74] | |
| Marl/Marl + | Granulated Blast Furnace Slag | Na2SiO3 + H2O | [75] | |
| Pietra serena sludge | Fly ash/metakaolin | NaOH + H2O | [76] | |
| Garnet tailings | Metakaolin | Na2SiO3 + NaOH + H2O | [77] |
| Mineral Additive (Chemical Composition) | Alkali Activation and Curing Regimen | Max. Compressive Strength + | Reference |
|---|---|---|---|
| Granite (62.11% SiO2; 15.72% Al2O3; 4.98% K2O) | 10 M NaOH | 30.5 MPa (10% of granite in FA-based geopolymers); 72.6 MPa (10% of granite in GBFS-based geopolymers) | [47] |
| 80 °C for 24 h | |||
| Granite (68.10% SiO2; 15.80% Al2O3; 5.32% K2O) | Ms * = 1.64 (H2O/Na2O molar ratio of 13) | 35 MPa | [46] |
| 80 °C for 24 h | |||
| Pietra Serena (59% SiO2; 16% Al2O3; 6.3% MgO) | 10, 14, 16 and 20 H2O/Na2O molar ratio (mixing H2O + Na2SiO3 + NaOH) | 21 MPa (H2O/Na2O molar ratio = 20; metakaolin:pietra Serena = 1:1) | [50] |
| 20 °C and 90% RH | |||
| Granite (60.51% SiO2; 17.49% Al2O3; 8.71% Fe2O3) | Na2SiO3 was used to activate fused granite (with several Ms *) and MK (added to balance Na2/Al2O3 ratio) | 40.5 MPa (for mortars containing fused granite wastes with SiO2/Na2O = 0.47 and Al2O3/Na2O = 0.08) | [48] |
| 24 h at room temperature closed in plastic bags, then 25 °C and 90% RH | |||
| Pisha sandstone (65.64% SiO2; 14.35% Al2O3; 8.02% CaO) | 0.7, 1.2, and 1.6 wt.% of NaOH with respect to 100 g of Pisha sandstone | 6 MPa (ambient cured and 1.2 wt.% NaOH) | [51] |
| 80 °C for 24 h then ambient and water immersed | |||
| Pisha sandstone (62.46% SiO2; 20.08% Al2O3; 5.10% CaO) | Na2SiO3 (Ms * = 1.5, 2.0 and 3.0); Na2CO3, Na2SO4; NaOH | 14.4 MPa (Na2SiO3 with Ms = 3, cured at 80 °C and milled Pisha stone) | [52] |
| (i) 80 °C for 24 h and (ii) ambient temperature | |||
| Cordierite (52.85% SiO2; 34.62% Al2O3; 11.66% MgO) | H2O/Na2O = 13, 15, and 20 | 57.5 MPa (30% of Cordierite; H2O/Na2O = 13) | [53] |
| sealed and room temperature | |||
| Diatomite (80.3% SiO2; 6.1% Al2O3; 6.79% Fe2O3) | 3 wt.% of NaOH; w/p = 0.27 | 48 MPa | [54] |
| 23 °C and 99% RH | |||
| Albite (70.9% SiO2; 17% Al2O3; 9.75% Na2O) | Albite calcined with NaOH or Na2CO3; w/s = 0.3 | 44.2 MPa (Albite calcined with 50% NaOH at 1000 °C) | [49] |
| sealed and room temperature |
| Mineral Additive (Chemical Composition) | Alkali-Activation and Curing Regimen | Max. Compressive Strength + | Reference |
|---|---|---|---|
| Calcite (53.5% CaO; 1.7% MgO; 1.5% SiO2) | Ms * = 2.0, 1.5, and 1.2 | ~60 MPa (Ms = 1.5, MK with 20% of calcite) | [55] |
| 40 °C for 24 h | |||
| Calcite (55.91% CaO; 0.18% K2O; 0.09% SiO2) | 2%, 4%, and 6% of NaOH | ~70 MPa (4% of NaOH, 5% of calcite, and 91% of GBFS) | [56] |
| 37 °C 100% RH | |||
| Calcite (purity 98.5%) | 13% KOH, 10% NaOH, 27% Na2SiO3, 50% H2O | ~28 MPa (MK with 6% of calcite) | [57] |
| 40 °C for 12 h | |||
| Calcite (50.1% CaO; 3.9% SiO2; 1.7% Al2O3) | 3, 6, and 12 M NaOH | 19.2 MPa (12 M, 67% mineral addition, 5 h curing) | [58] |
| 80 °C for 1, 3, and 5 h | |||
| Marble (53.68% CaO; 1.32% Fe2O3; 0.26% SiO2) | 1, 5, and 10 M NaOH | 37.48 MPa (10 M NaOH, curing 20 °C, 20% Marble) | [63] |
| (1) 22 °C and 40% RH; (2) 45 °C for 24 h; (3) 75 °C for 24 h. Afterwards, wet condition (min. 95% RH) or 22 °C and 35% RH | |||
| Marble (55.9% CaO; 0.6% MgO; 0.1% Fe2O3) | (1) 0.1 mol of Na2O; (2) 0.1 mol of Na2O and 0.1 mol SiO2; (3) substitution of sodium citrate (Na3C6H5O7) | 60.7 MPa (25% Marble/75% calcined smectite clay; containing Na-citrate) | [64] |
| 24 h at 95% RH then dry-cured at room temperature | |||
| Marble (44.20% CaO; 1.41% MgO; 0.07% Fe2O3) | Ms * = 1.65 and 3.50 | 38.30 MPa (Ms = 1.65, dry curing) | [45] |
| 24 h at 80 °C then: (1) 20 °C and 95% RH; (2) 20 °C and 18% RH; (3) immersed in water | |||
| Marble (45.60% CaO; 6.82% MgO; 0.70% SiO2) | 8 M NaOH or Na2SiO3·nH2O:NaOH (w:w = 5) | 52 MPa (Na2SiO3·nH2O + NaOH; cement, GBFS, marble, FA) | [65] |
| Room temperature | |||
| Marble (38.02% CaO; 34.66% SiO2; 13.12% Fe2O3; 7.21% MgO) | 2 and 4 M NaOH + Na2SiO3 | 6.52 MPa (7 days, 4 M, 60% marble + 40% FA) | [66] |
| 70 °C for 24 h and 7 days in plastic bags | |||
| Travertine (55.10% CaO; 0.70% SiO2; 0.20% Fe2O3) | 1, 5, and 10 M NaOH | 42.24 MPa (10 M NaOH, dry curing at 20 °C, 20% travertine) | [63] |
| (1) 22 °C and 40% RH; (2) 45 °C for 24 h; (3) 75 °C for 24 h. Afterwards, wet condition (min. 95% RH) or 22 °C and 35% RH | |||
| Dolomite (33.4% CaO; 17.1% MgO; 2.5% SiO2) | Ms * = 2.0, 1.5, and 1.2 | ~45 MPa (Ms = 1.5, MK with 20% of dolomite) | [55] |
| 40 °C for 24 h | |||
| Dolomite (42.48% CaO, 19.15% MgO) | Ms * = 2.5 (Na2CO3 and bentonite) | 38.3 MPa (bentonite, dolomite and Na2CO3 calcined at 1110 °C and (CaO + MgO)/SiO2 = 2.1) | [60] |
| 80 °C for 3 days | |||
| Dolomite (74.8% CaO; 18.3% MgO; 3.7% SiO2) | Ms *= 0.93 (10 M NaOH + Na2SiO3) | ~60 MPa (Cement with 40% of dolomite); ~40 MPa (FA with 40% of dolomite); | [62] |
| Cement-based samples 24 h at 100% RH then immersed in lime water; FA-based samples 24 h at 40 °C and 100 % RH then sealed and at room temperature | |||
| Dolomite (31.4% CaO, 21.3% MgO, 1.1% SiO2) | Na2CO3 + calcined dolomite (Na2O = 4.9%–7.6% in the dry mixture) | 41.6 MPa (GBFS with 10% of calcined dolomite and 10% of Na2CO3) | [61] |
| 20 °C and RH > 95% ± 2% | |||
| Dolomite (27.13% CaO; 24.53% MgO; 0.13% SiO2) | 4 M NaOH or 2 M Na2CO3 | ~60 MPa (NaOH, GBFS and 20% of dolomite); ~80 MPa (Na2CO3, GBFS and 20% of dolomite); | [59] |
| 20 °C 100% RH | |||
| Pietra di Angera (64% CaO; 33% MgO; 2.2% Fe2O3) | 10, 14, 16, and 20 H2O/Na2O molar ratio (obtained mixing H2O + Na2SiO3 + NaOH) | 18 MPa (H2O/Na2O molar ratio = 20; MK:pietra di Angera = 1:1) | [50] |
| 20 °C and 90% RH | |||
| Limestone (53.5% CaO; 1.7% MgO; 1.5% SiO2) | 3 and 5 M NaOH | 7 MPa (50% LM/50% MK; 5 M NaOH; 20 °C; dry curing) | [67] |
| 24 h at 20 or 80 °C then wet curing (water immersed) or dry curing (laboratory conditions) | |||
| Limestone (53.96% CaO; 1.01% MgO; 0.84% SiO2) | Ms * = 1.4 | 83.5 MPa (30% LM/10% FA/60% GBFS) | [68] |
| 20 °C and 95% RH | |||
| Limestone (53.96% CaO; 1.01% MgO; 0.84% SiO2) | 4 wt.% of Na2O (starting from Na2CO3) | ~55 MPa (GBFS with 10% of LM) | [69,70] |
| 20 °C and 95% RH | |||
| Limestone (57.43% CaO; 1.06% SiO2) | Ms * = 0 (only NaOH), 1, and 1.5 | ~15 MPa (Ms = 1, 10% Na2O) | [40] |
| 24 h at 60 °C then dry stored in plastic bags at 20 °C and 80%–90% RH | |||
| Limestone (47.85% CaO; 9.07% SiO2; 1.51% Al2O3) | 5, 10, and 8 M NaOH + Na2SiO3 | 47.77 MPa (8 M, 45% LM/55% Clay) | [71] |
| 24 °C in open air | |||
| Limestone 1 (90% calcite, 9% quartz) (43.31% CaO; 14.26% SiO2; 2.44% Al2O3) | NaOH + Na2CO3 + H2O | 39 MPa (30% LM_3/70% GBFS; Ssp 600 m2/kg) | [72] |
| Limestone 2 (33% calcite, 66% dolomite) (39.79% CaO; 1.26% SiO2; 12.94% MgO) | |||
| Limestone 3 (100% calcite) (55.06% CaO; 0.47% SiO2; 0.49% MgO) | Room temperature and 95%–100% RH |
| Pure Alumino-Silicate | Mixed Alumino-Silicate/Carbonate | Mixed Carbonate/Silica | Pure Carbonate | |
|---|---|---|---|---|
| Composition | 100% granite | ~70% granite | 95.4% marble | 100% marble |
| ~30% dolomite | 4.6% waste glass | |||
| 28 day compressive strength | ~30 MPa | ~30 MPa | ~45 MPa | >35 MPa |
| 28 day flexural strength | ~12 MPa | ~14 MPa | ~17 MPa | ~11 MPa |
| Water resistance | Rapid dissolution | Crack formation | Optimal behavior | Crack formation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, B.; Tulliani, J.-M.; Antonaci, P.; Palmero, P. Role of Natural Stone Wastes and Minerals in the Alkali Activation Process: A Review. Materials 2020, 13, 2284. https://doi.org/10.3390/ma13102284
Coppola B, Tulliani J-M, Antonaci P, Palmero P. Role of Natural Stone Wastes and Minerals in the Alkali Activation Process: A Review. Materials. 2020; 13(10):2284. https://doi.org/10.3390/ma13102284
Chicago/Turabian StyleCoppola, Bartolomeo, Jean-Marc Tulliani, Paola Antonaci, and Paola Palmero. 2020. "Role of Natural Stone Wastes and Minerals in the Alkali Activation Process: A Review" Materials 13, no. 10: 2284. https://doi.org/10.3390/ma13102284
APA StyleCoppola, B., Tulliani, J.-M., Antonaci, P., & Palmero, P. (2020). Role of Natural Stone Wastes and Minerals in the Alkali Activation Process: A Review. Materials, 13(10), 2284. https://doi.org/10.3390/ma13102284





