Colossal Permittivity and Low Dielectric Loss of Thermal Oxidation Single-Crystalline Si Wafers
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
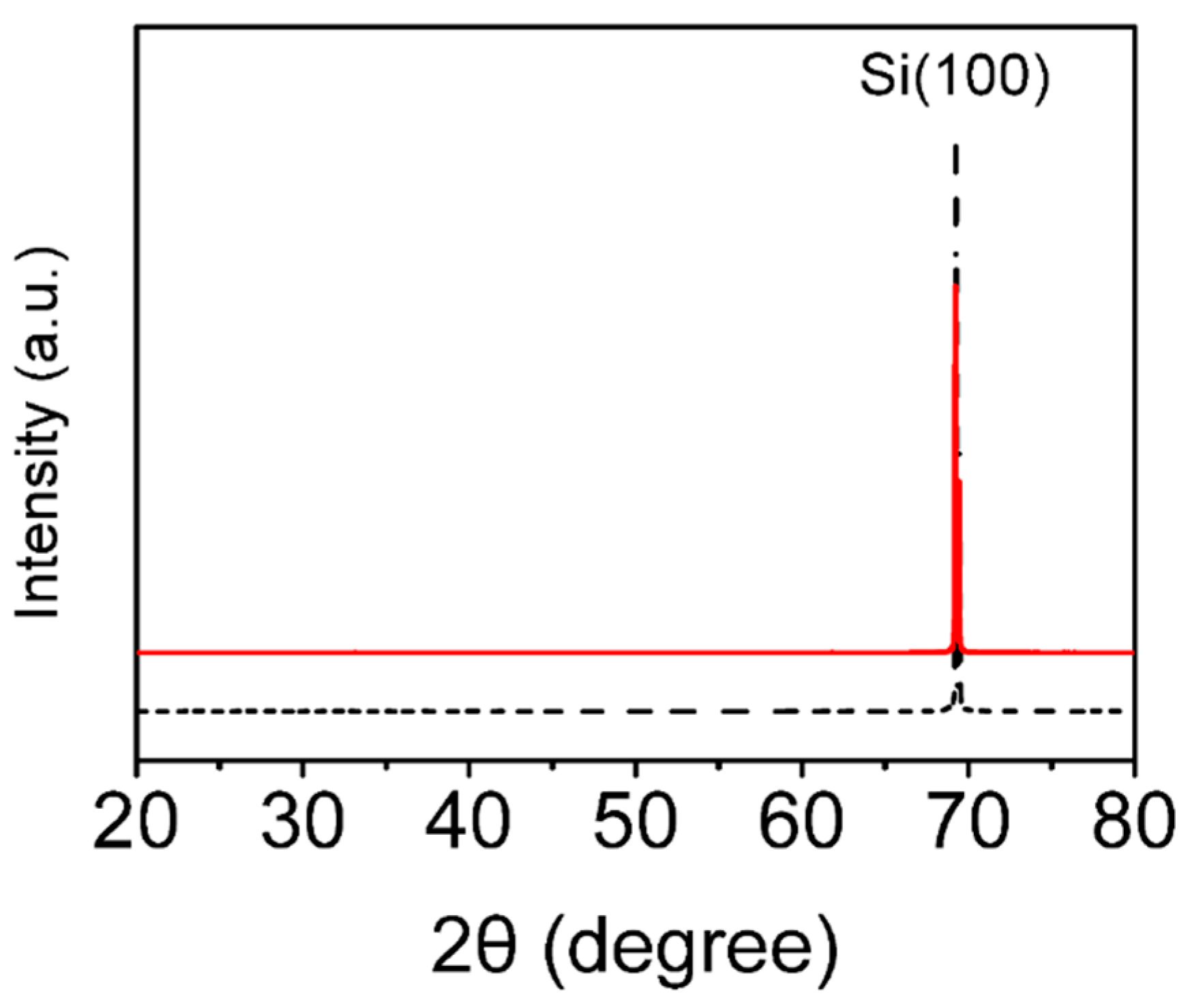
3.1. Microstructure Analysis of the Single-Crystal Semiconductor Si Plates after Thermal Oxidation
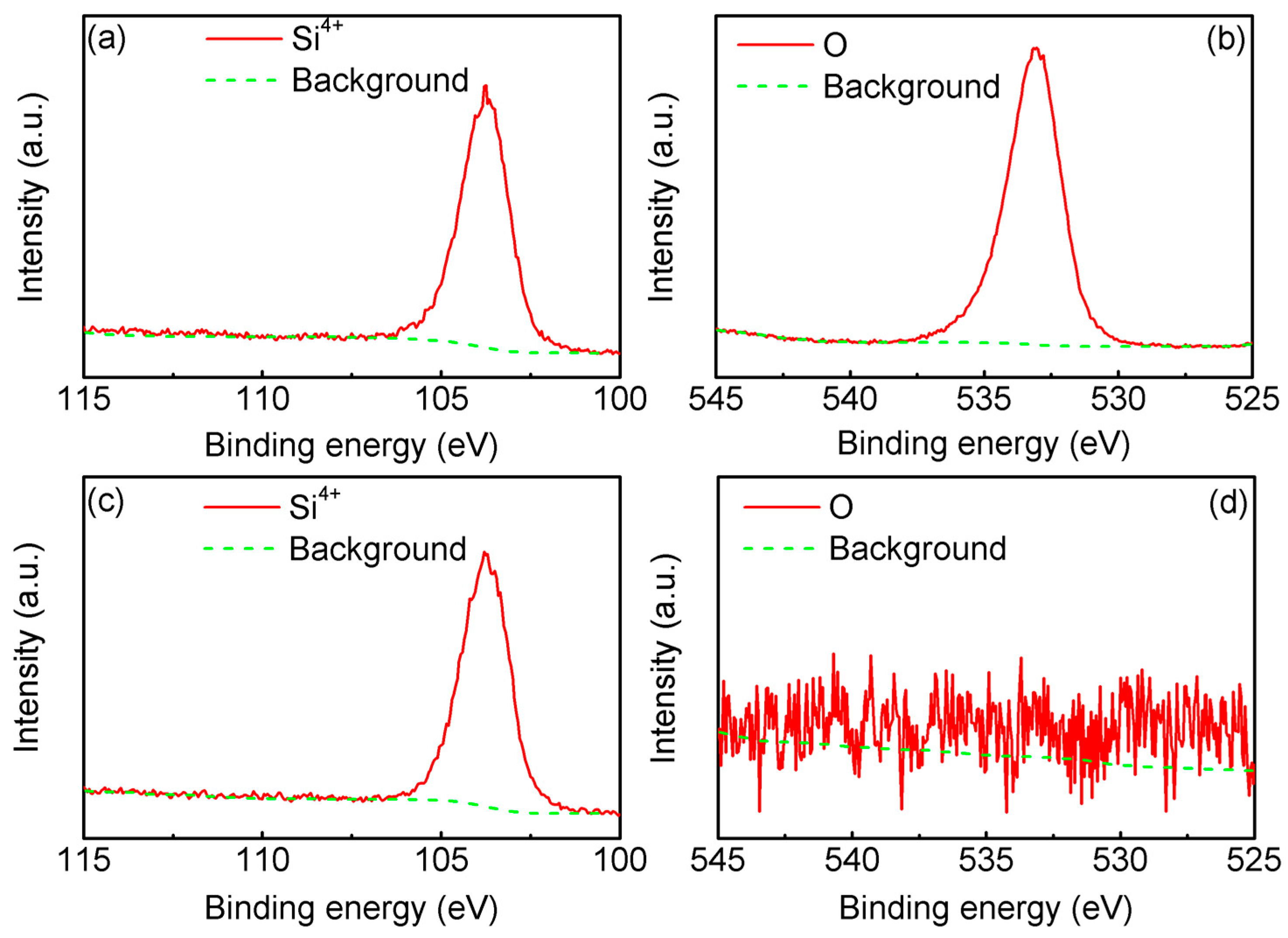
3.2. XPS Analysis and SiO2 Film Thickness Measurement of the Single-Crystalline Silicon Plates after Thermal Oxidation
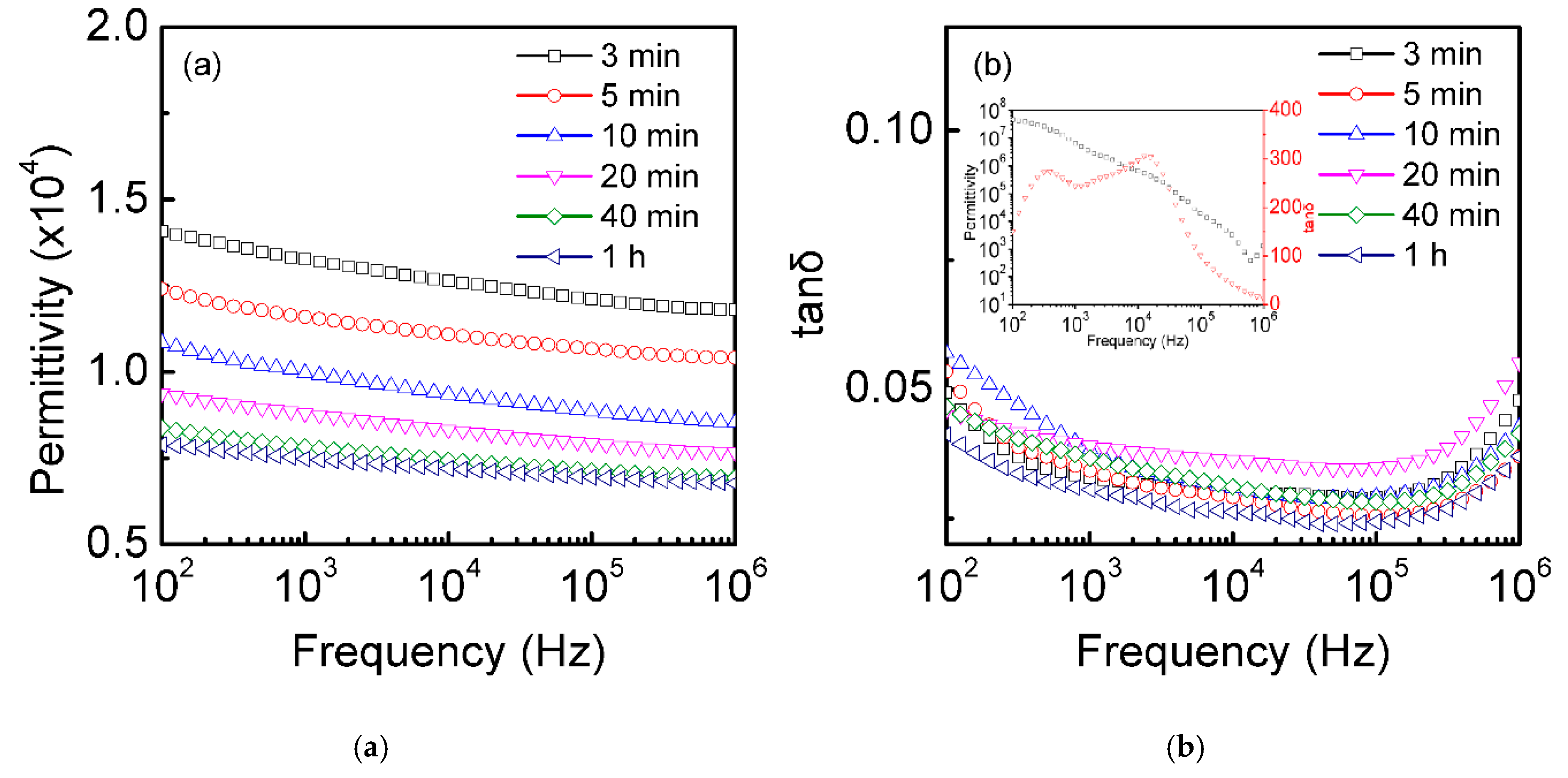
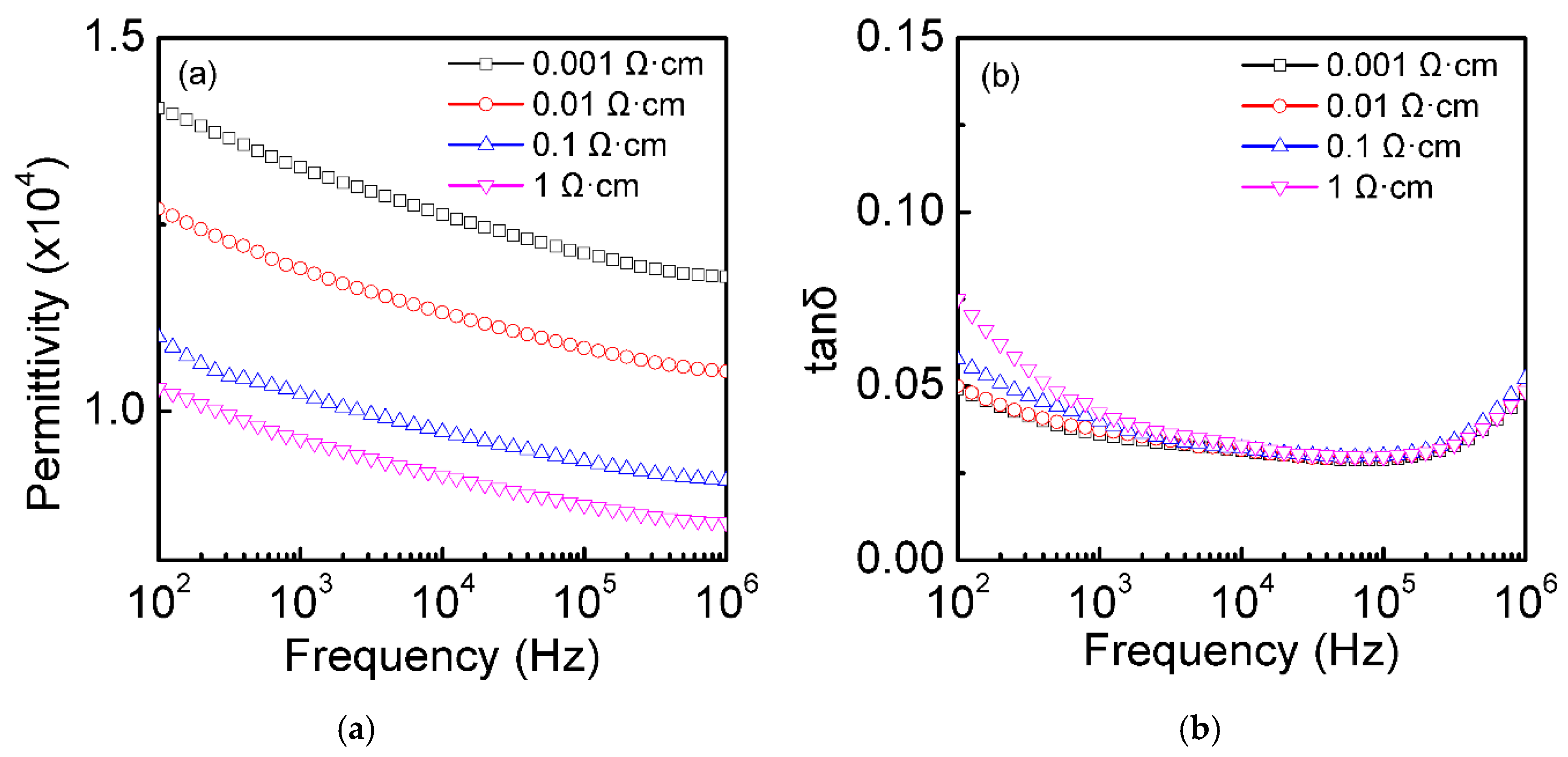
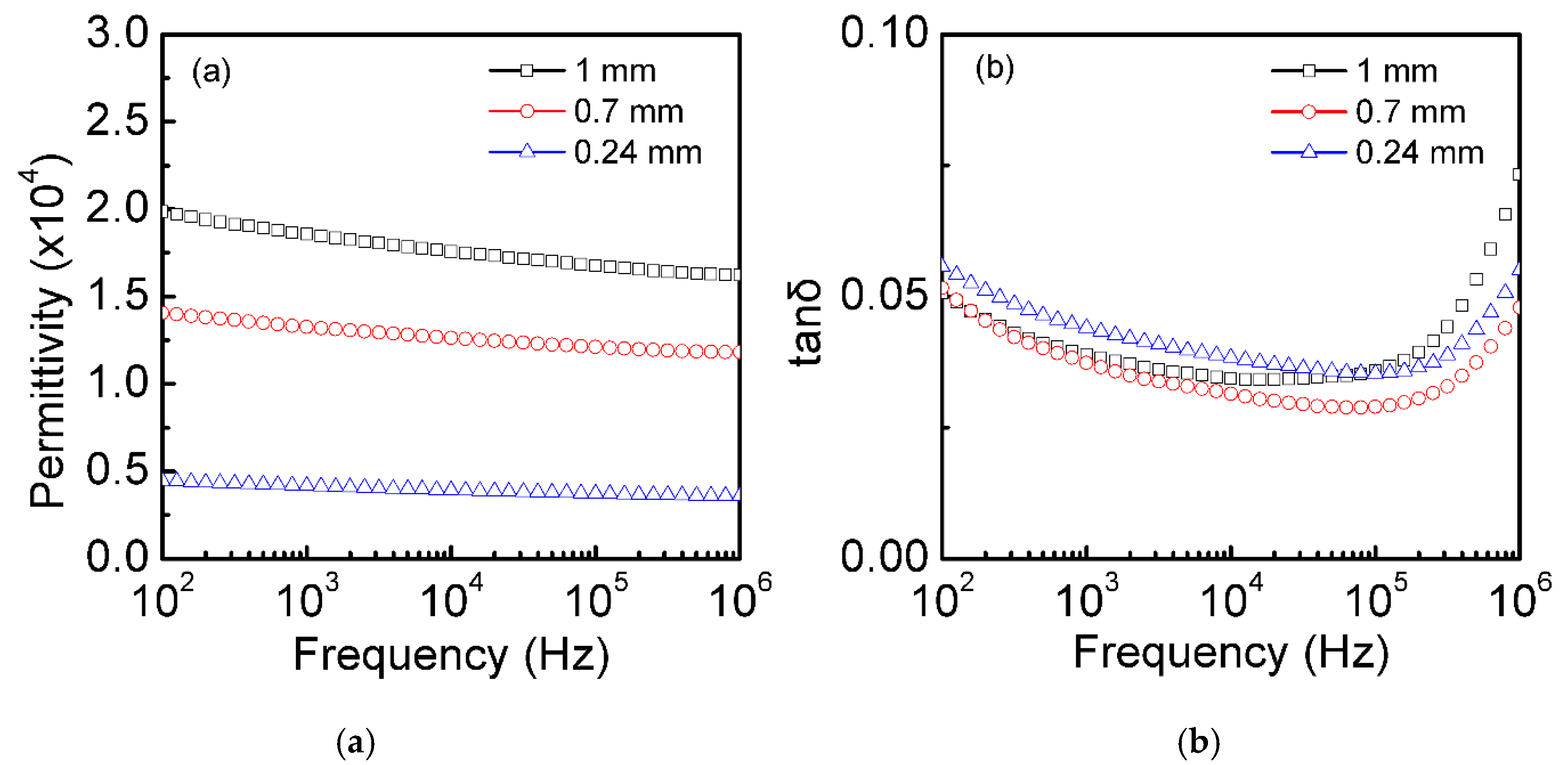
3.3. Dielectric Properties at Room Temperature of the Single-Crystal Semiconductor Si Plates after Thermal Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buscaglia, M.T.; Viviani, M.; Buscaglia, V.; Mitoseriu, L.; Galassi, C. High dielectric constant and frozen macroscopic polarization in dense nanocrystalline BatiO3 ceramics. Phys. Rev. B 2006, 73, 064114. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Dong, L.; Duan, N.; Reisner, B.A.; Sleight, A.W. High Dielectric Constant in CaCu3Ti4O12 and CaCu3Ti3FeO12 Phases. J. Solid State Chem. 2000, 151, 323. [Google Scholar] [CrossRef]
- Junbo, W.; Ce-Wen, N.; Yuanhua, L.; Yuan, D. Giant Dielectric Permittivity Observed in Li and Ti Doped NiO. Phys. Rev. Lett. 2002, 89, 217601. [Google Scholar]
- Krohns, S.; Lunkenheimer, P.; Kant, C.; Pronin, A.V.; Brom, H.B.; Nugroho, A.A.; Diantoro, M.; Loidl, A. Colossal dielectric constant up to gigahertz at room temperature. Appl. Phys. Lett. 2009, 94, 122903. [Google Scholar] [CrossRef]
- Wanbiao, H.; Yun, L.; Ray, L.W.; Terry, J.F.; Lasse, N.; Amanda, S.; Melanie, K.; Paul, S.; Bill, G.; Hua, C.; et al. Electron-pinned defect-dipoles for high-performance colossal permittivity materials. Nat. Mater. 2013, 12, 821. [Google Scholar]
- Tkach, A.; Okhay, O.; Almeida, A. Giant dielectric permittivity and high tunability in Y-doped SrTiO3 ceramics tailored by sintering atmosphere. Acta Mater. 2017, 130, 249–260. [Google Scholar] [CrossRef]
- Adams, T.B.; Sinclair, D.C.; West, A.R. Giant Barrier Layer Capacitance Effects in CaCu3Ti4O12 Ceramics. Adv. Mater. 2002, 14, 1321–1323. [Google Scholar] [CrossRef]
- Guillemet-Fritsch, S.; Valdez-Nava, Z.; Tenailleau, C. Colossal Permittivity in Ultrafine Grain Size BaTiO3−x and Ba0.95La0.05TiO3−x Materials. Adv. Mater. 2008, 20, 551. [Google Scholar] [CrossRef]
- Ramesh, S.; Shutzberg, B.A.; Huang, C.; Gao, J.; Giannelis, E.P. Dielectric nanocomposites for integral thin film capacitors: Materials design, fabrication and integration issues. IEEE Trans. Adv. Packag. 2003, 26, 17–24. [Google Scholar] [CrossRef]
- Phansamdaeng, P.; Khemprasit, J. Study on magnetic and dielectric properties of BaTiO3/MnCr0.2Fe1.8O4 composite material. J. Alloys Compd. 2019, 776, 105–110. [Google Scholar] [CrossRef]
- Fan, J.T.; Leng, S.L.; Cao, Z.Z.; He, W.Y.; Gao, Y.F.; Liu, J.R.; Li, G.R. Colossal permittivity of Sb and Ga co-doped rutile TiO2 ceramics. Ceram. Int. 2019, 45, 1001–1010. [Google Scholar] [CrossRef]
- Li, M.; Feteira, A.; Sinclair, D.C.; West, A.R. Influence of Mn doping on the semiconducting properties of CaCu3Ti4O12 ceramics. Appl. Phys. Lett. 2006, 88, 232903. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.C.; Wu, L.; Frenkel, A.I.; Hanson, J.; Northrup, P.; Ku, W. Nanoscale Disorder in CaCu3Ti4O12: A New Route to the Enhanced Dielectric Response. Phys. Rev. Lett. 2007, 99, 037602. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.T.; Wang, Y.H.; Kuo, J.C. Role of strained nano-regions in the formation of subgrains in CaCu3Ti4O12. J. Appl. Phys. 2011, 110, 024103. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.-J. Polaron relaxation and variable-range-hopping conductivity in the giant-dielectric-constant material CaCu3Ti4O12. Phys. Rev. B 2004, 70, 174306. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P. Dieletric and electric relaxations induced by the complex defect clusters in (Yb + Nb) co-doped rutile TiO2 ceramics. J. Am. Ceram. Soc. 2017, 100, 3505–3513. [Google Scholar] [CrossRef]
- Yang, C.; Sun, E.W.; Yang, B.; Cao, W.W. Theoretical study on local domain pinning effect due to defect dipole alignment. J. Phys. D Appl. Phys. 2018, 51, 415303. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Yubero, F.; Tougaard, S.; Elizalde, E.; Sanz, J.M. Dielectric loss function of Si and SiO2 from quantitative analysis of REELS spectra. Surf. Interface Anal. 1993, 20, 719–726. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Zheng, F.; Tse, M.-Y.; Sun, Q.; Liu, Y.; Hao, J. A General Strategy to Achieve Colossal Permittivity and Low Dielectric Loss through Constructing Insulator/Semiconductor/Insulator Multilayer Structures. J. Low Temp. Phys. 2018, 192, 346–358. [Google Scholar] [CrossRef]
- De Almeida, R.N.C.; Goncalves, S.; Baumvol, I.J.R. Dynamics of thermal growth of silicon oxide films on Si. Phys. Rev. B 2000, 61, 12992. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef]
- Deepthi, G.; Christos, G.T. Diffusion-reaction modeling of silicon oxide interlayer growth during thermal annealing of high dielectric constant materials on silicon. Phys. Rev. B 2008, 77, 205304. [Google Scholar]
- Brugemann, L.; Bloch, R.; Press, W.; Gerlach, P. Surface and interface topography of amorphous SiO2/crystalline Si (100) studied by X-ray diffraction. J. Phys. Condens. Matter 1990, 2, 8869. [Google Scholar] [CrossRef]
- He, G.; Liu, M.; Zhu, L.Q.; Chang, M.; Fang, Q.; Zhang, L.D. Effect of postdeposition on the thermal stability and structural characteristics of sputtered HfO2 films on Si(100). Sur. Sci. 2005, 576, 67–75. [Google Scholar] [CrossRef]
- Mitchell, D.F.; Clark, K.B.; Bardwell, J.A.; Lennard, W.N.; Massoumi, G.R.; Mitchell, I.V. Film thickness measurements of SiO2 by XPS. Surf. Interface Anal. 1994, 21, 44–50. [Google Scholar] [CrossRef]
- Seah, M.P.; Spencer, S.J. Ultrathin SiO2 on Si IV. Intensity measurement in XPS and deduced thickness linearity. Surf. Interface Anal. 2003, 35, 515–524. [Google Scholar] [CrossRef]
- Seah, M.P. Ultrathin SiO2 on Si II. Issues in quantification of the oxide thickness. Surf. Interface Anal. 2002, 33, 640–652. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chen, T.P.; Ding, L.; Liu, Y.; Tse, M.S.; Fung, S.; Dong, Z.L. Static dielectric constant of isolated silicon nanocrystals embedded in a SiO2 thin film. Appl. Phys. Lett. 2006, 88, 63103. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, D.; Liu, K.; Zheng, F. Colossal Permittivity and Low Dielectric Loss of Thermal Oxidation Single-Crystalline Si Wafers. Materials 2019, 12, 1102. https://doi.org/10.3390/ma12071102
Sun Y, Wu D, Liu K, Zheng F. Colossal Permittivity and Low Dielectric Loss of Thermal Oxidation Single-Crystalline Si Wafers. Materials. 2019; 12(7):1102. https://doi.org/10.3390/ma12071102
Chicago/Turabian StyleSun, Yalong, Di Wu, Kai Liu, and Fengang Zheng. 2019. "Colossal Permittivity and Low Dielectric Loss of Thermal Oxidation Single-Crystalline Si Wafers" Materials 12, no. 7: 1102. https://doi.org/10.3390/ma12071102
APA StyleSun, Y., Wu, D., Liu, K., & Zheng, F. (2019). Colossal Permittivity and Low Dielectric Loss of Thermal Oxidation Single-Crystalline Si Wafers. Materials, 12(7), 1102. https://doi.org/10.3390/ma12071102



