Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties
2.4. Corrosion Properties
3. Results and Discussion
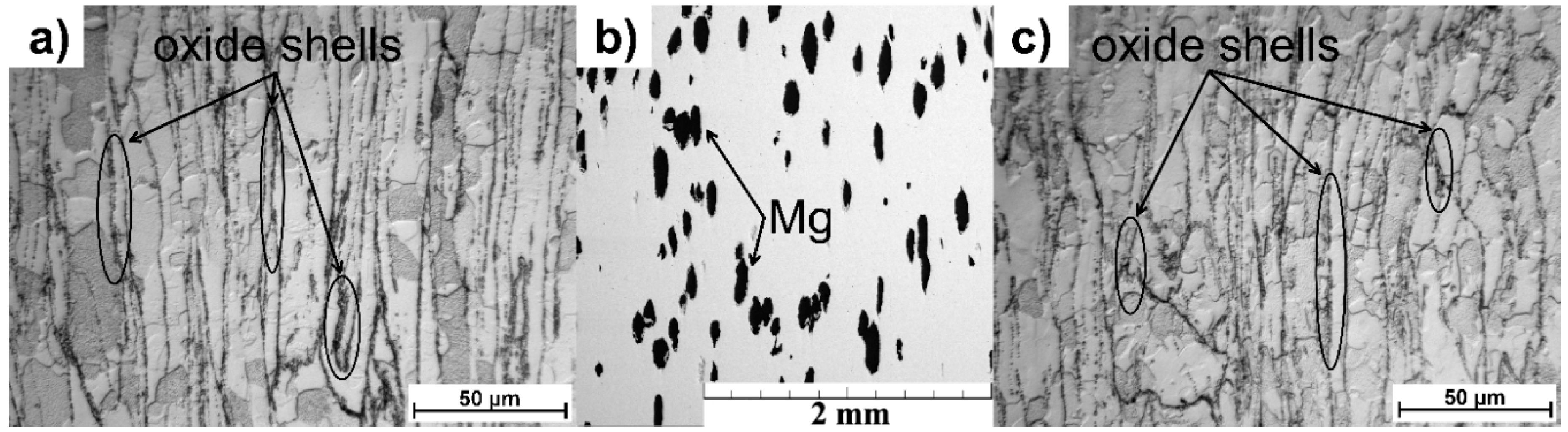
3.1. Microstructure Characterization
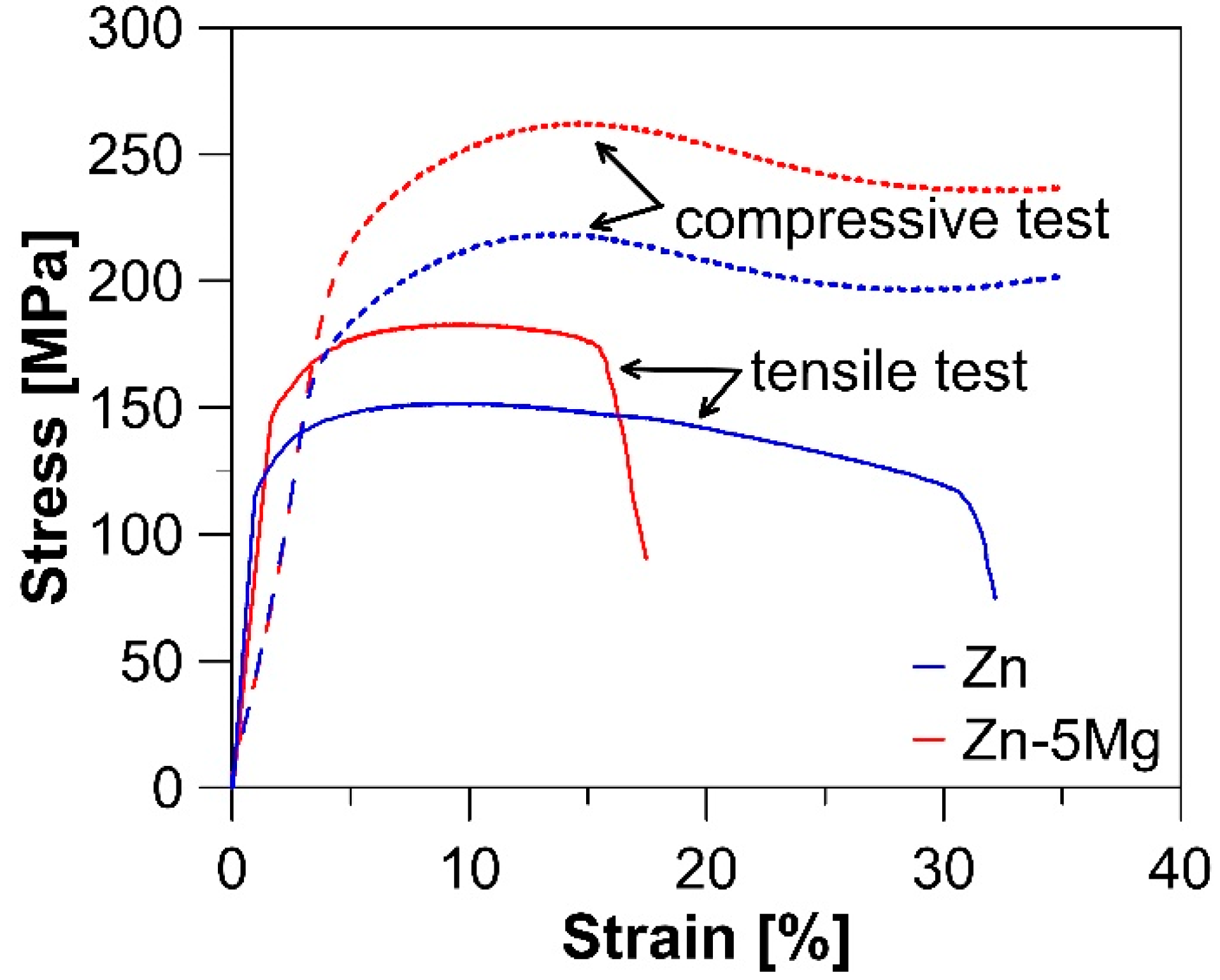
3.2. Mechanical Properties
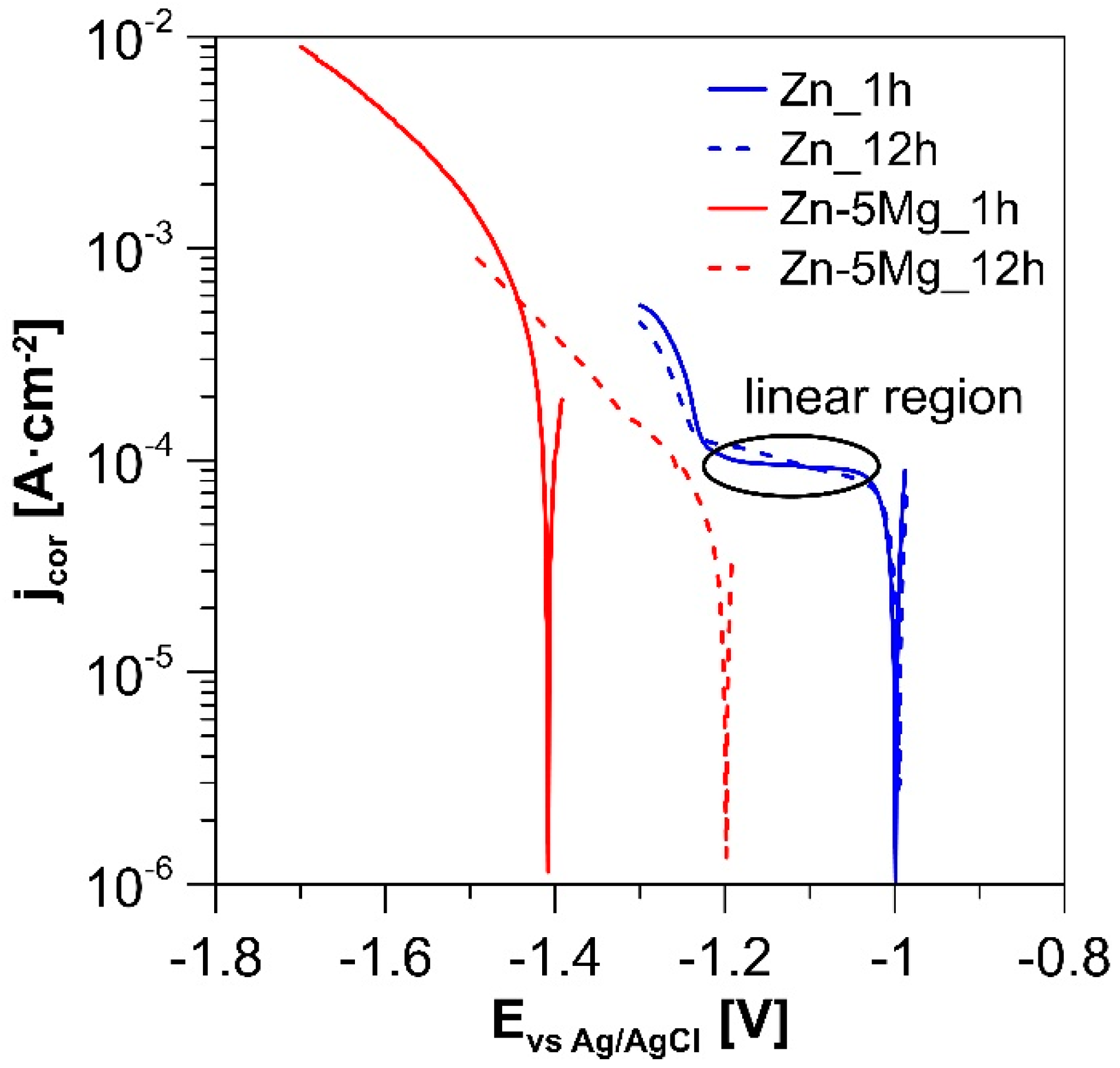
3.3. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vimalanandan, A.; Bashir, A.; Rohwerder, M. Zn-Mg and Zn-Mg-Al alloys for improved corrosion protection of steel: Some new aspects. Mater. Corros. 2014, 65, 392–400. [Google Scholar] [CrossRef]
- Hausbrand, R.; Stratmann, M.; Rohwerder, M. Corrosion of zinc–magnesium coatings: Mechanism of paint delamination. Corros. Sci. 2009, 51, 2107–2114. [Google Scholar] [CrossRef]
- Prosek, T.; Nazarov, A.; Bexell, U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corros. Sci. 2008, 50, 2216–2231. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (<0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C 2018, 84, 67–79. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Vojtech, D.; Kubasek, J.; Serak, J.; Novak, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Cockerill, I.; Wang, Y.D.; Qin, Y.X.; Chang, L.Q.; Zheng, Y.F.; Zhu, D.H. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef] [PubMed]
- Kubasek, J.; Vojtech, D.; Jablonska, E.; Pospisilova, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular scent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants-A Review Paper. Metals 2017, 7, 18. [Google Scholar] [CrossRef]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular Zn(2+) and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Young, M.L.; Ma, J.; Zheng, Y.; Tang, L. Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef]
- Jablonska, E.; Vojtech, D.; Fousova, M.; Kubasek, J.; Lipov, J.; Fojt, J.; Ruml, T. Influence of surface pre-treatment on the cytocompatibility of a novel biodegradable ZnMg alloy. Mater. Sci. Eng. C 2016, 68, 198–204. [Google Scholar] [CrossRef]
- Krystýnová, M.; Doležal, P.; Fintová, S.; Březina, M.; Zapletal, J.; Wasserbauer, J. Preparation and Characterization of Zinc Materials Prepared by Powder Metallurgy. Metals 2017, 7, 396. [Google Scholar] [CrossRef]
- Sadighikia, S.; Abdolhosseinzadeh, S.; Asgharzadeh, H. Production of high porosity Zn foams by powder metallurgy method. Powder Metall. 2015, 58, 61–66. [Google Scholar] [CrossRef]
- Samson, S. Die kristallstruktur von Mg2Zn11—Isomorphie zwischen Mg2Zn11 und Mg2Cu6Al5. Acta Chem. Scand. 1949, 3, 835–843. [Google Scholar] [CrossRef]
- Smithells, C.J.; Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands; Boston, MA, USA, 2004. [Google Scholar]
- Yang, H.T.; Qu, X.H.; Lin, W.J.; Chen, D.F.; Zhu, D.H.; Dai, K.R.; Zheng, Y.F. Enhanced Osseointegration of Zn-Mg Composites by Tuning the Release of Zn Ions with Sacrificial Mg-Rich Anode Design. ACS Biomater. Sci. Eng. 2019, 5, 453–467. [Google Scholar] [CrossRef]

| HV1 | Tensile Test | Compression Test | ||||
|---|---|---|---|---|---|---|
| TYS (0.2 Proof Stress) (MPa) | UTS (MPa) | Elongation (%) | CYS (0.2 Proof Stress) (MPa) | UCS (MPa) | ||
| Zn | 35 | 114 ± 5 | 156 ± 5 | 35 ± 4 | 170 ± 6 | 215 ± 4 |
| Zn-5Mg | 36 (Zn) 51 (Mg) | 148 ± 6 | 183 ± 4 | 16 ± 2 | 209 ± 6 | 256 ± 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubásek, J.; Dvorský, D.; Čapek, J.; Pinc, J.; Vojtěch, D. Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties. Materials 2019, 12, 3930. https://doi.org/10.3390/ma12233930
Kubásek J, Dvorský D, Čapek J, Pinc J, Vojtěch D. Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties. Materials. 2019; 12(23):3930. https://doi.org/10.3390/ma12233930
Chicago/Turabian StyleKubásek, Jiří, Drahomír Dvorský, Jaroslav Čapek, Jan Pinc, and Dalibor Vojtěch. 2019. "Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties" Materials 12, no. 23: 3930. https://doi.org/10.3390/ma12233930
APA StyleKubásek, J., Dvorský, D., Čapek, J., Pinc, J., & Vojtěch, D. (2019). Zn-Mg Biodegradable Composite: Novel Material with Tailored Mechanical and Corrosion Properties. Materials, 12(23), 3930. https://doi.org/10.3390/ma12233930





