The Effect of Exposure on the Autogenous Self-Healing of Ordinary Portland Cement Mortars
Abstract
1. Introduction
2. Materials and Methods
3. Results
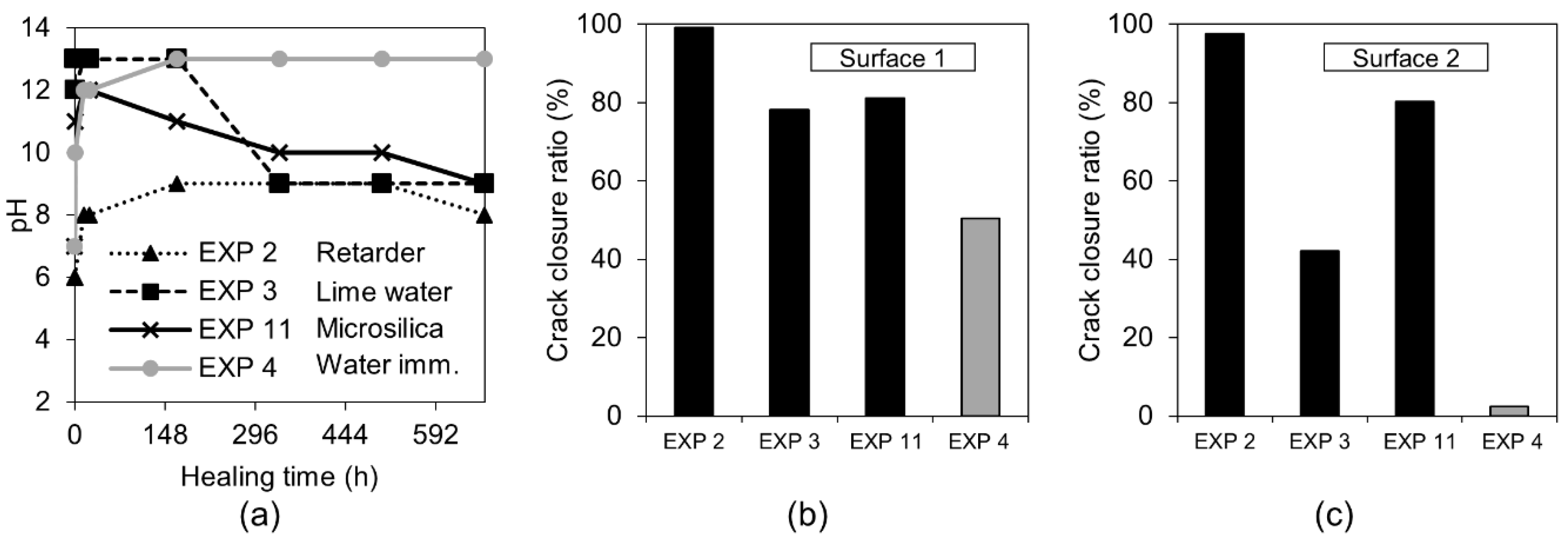
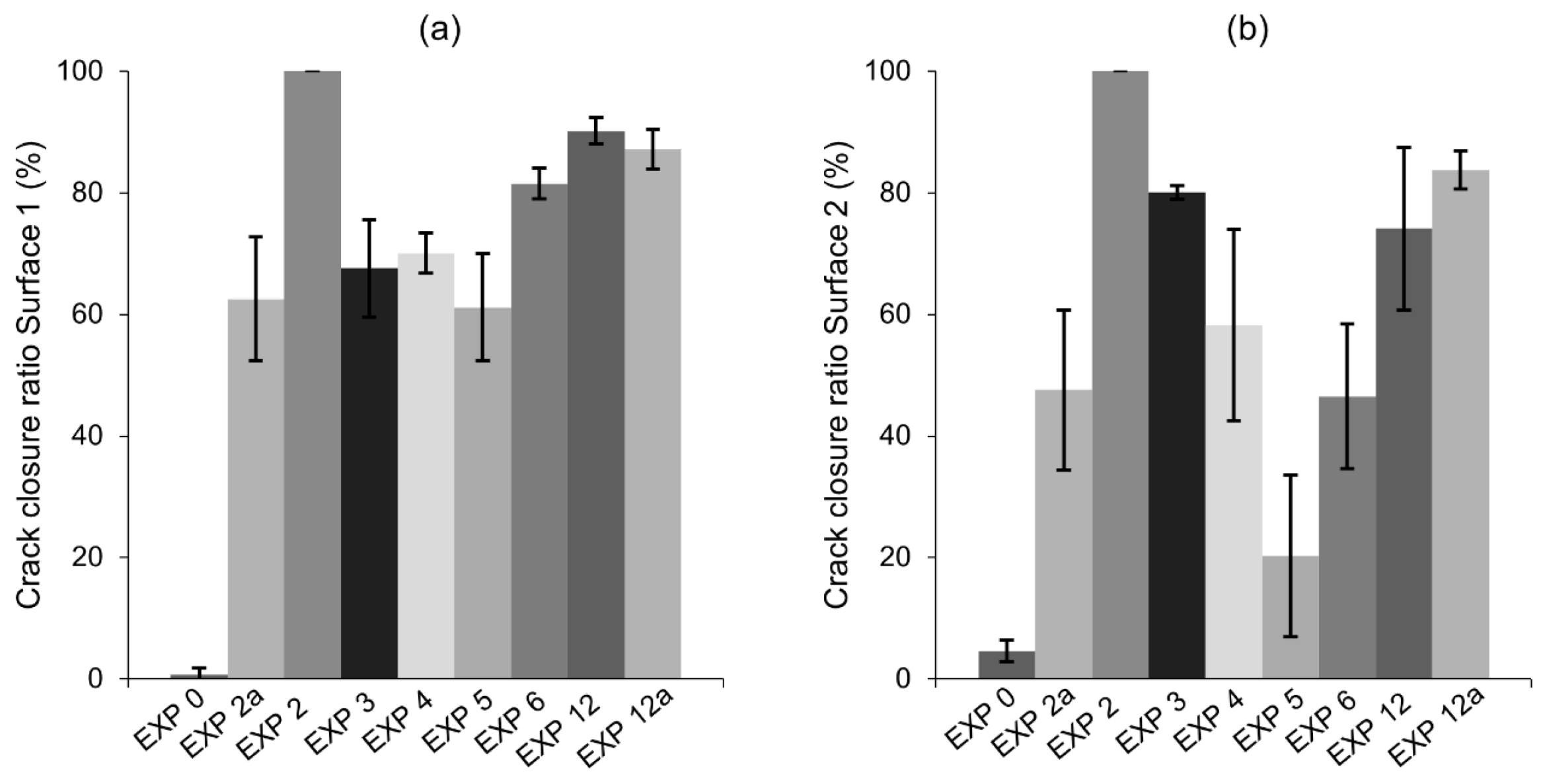
3.1. Crack Closure Ratio, pH Changes and Self-Healing Products
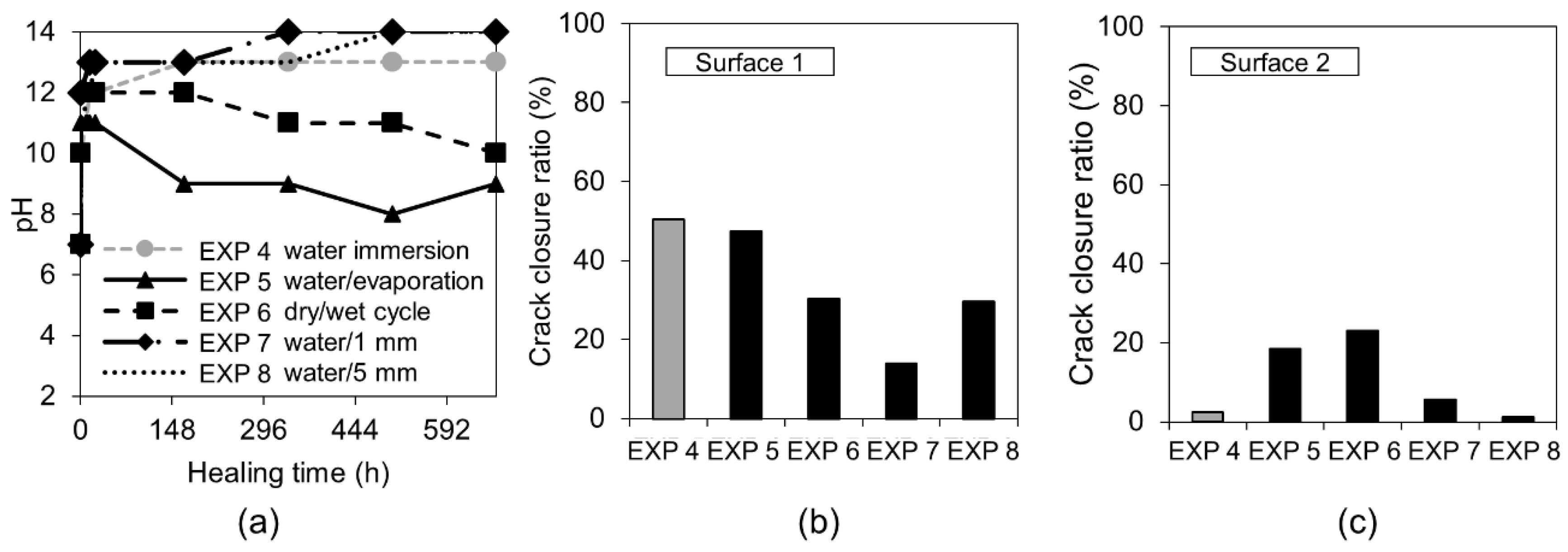
3.1.1. Water Immersion Regime
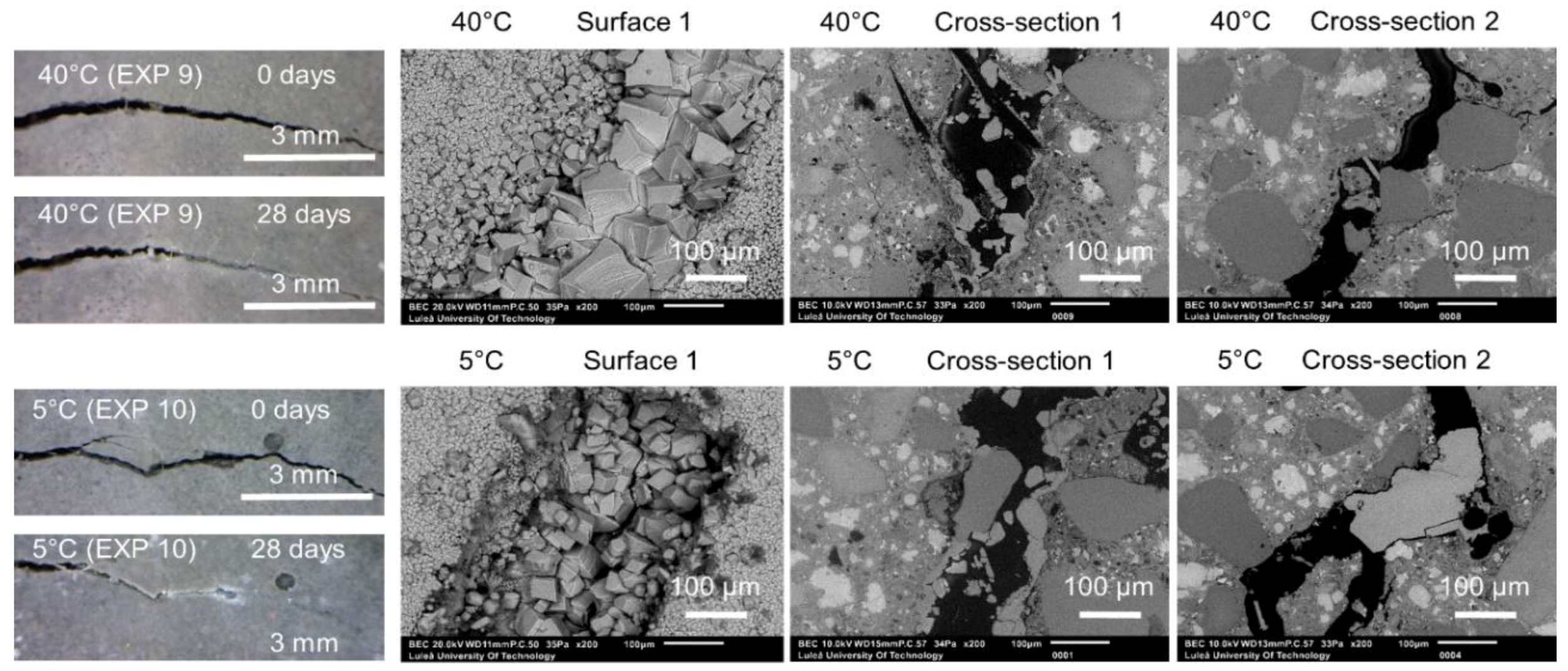
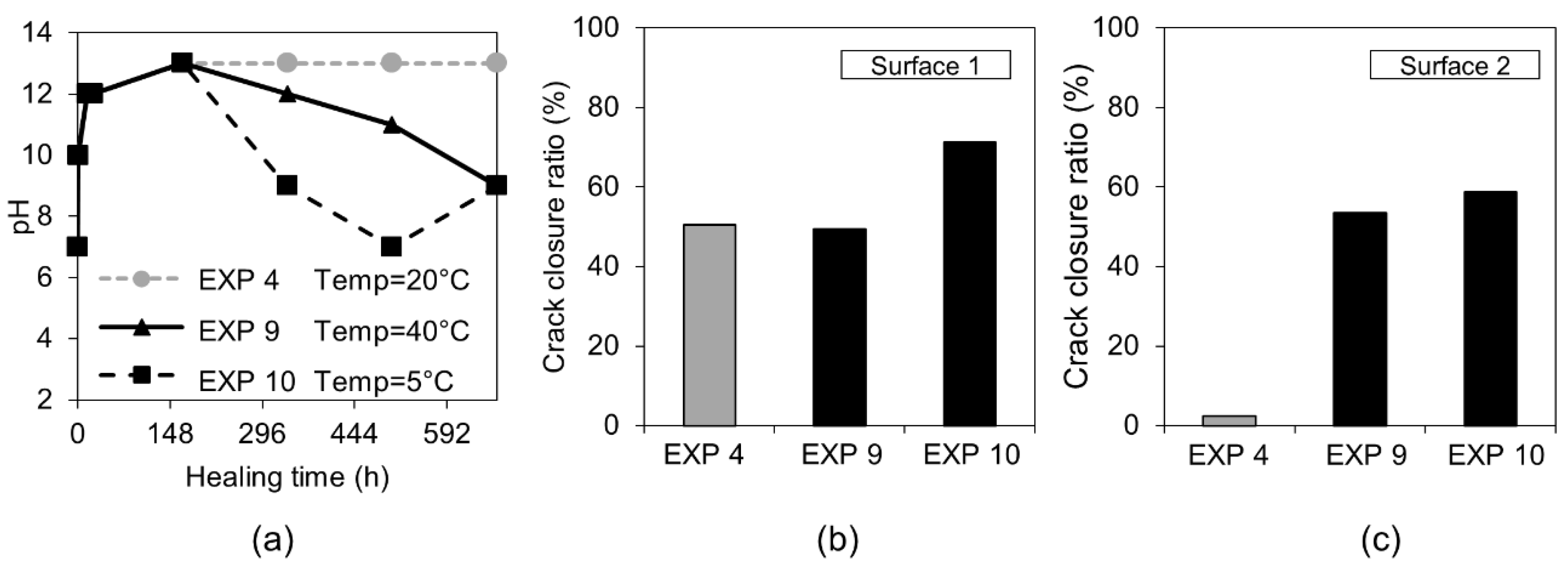
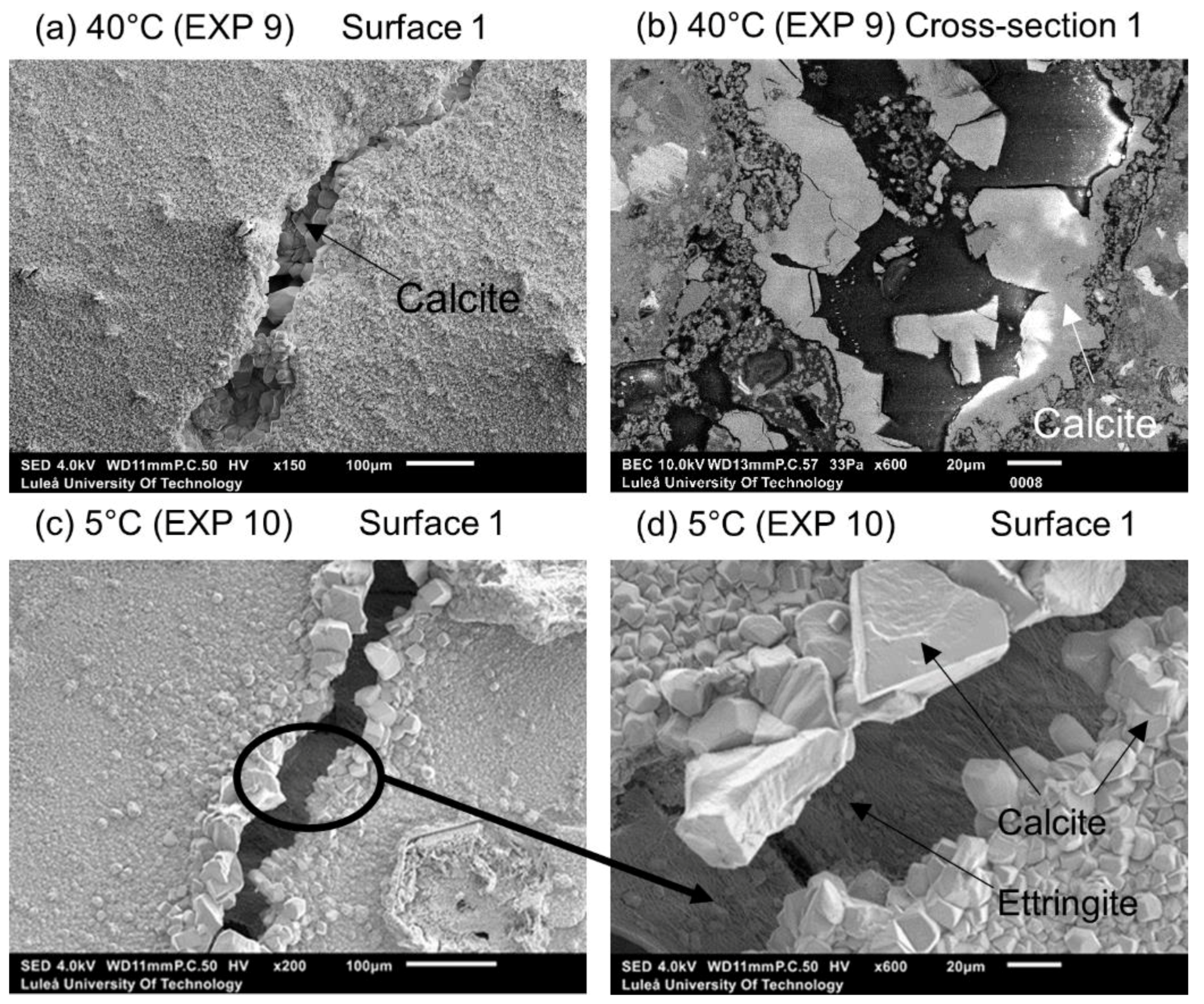
3.1.2. Temperature
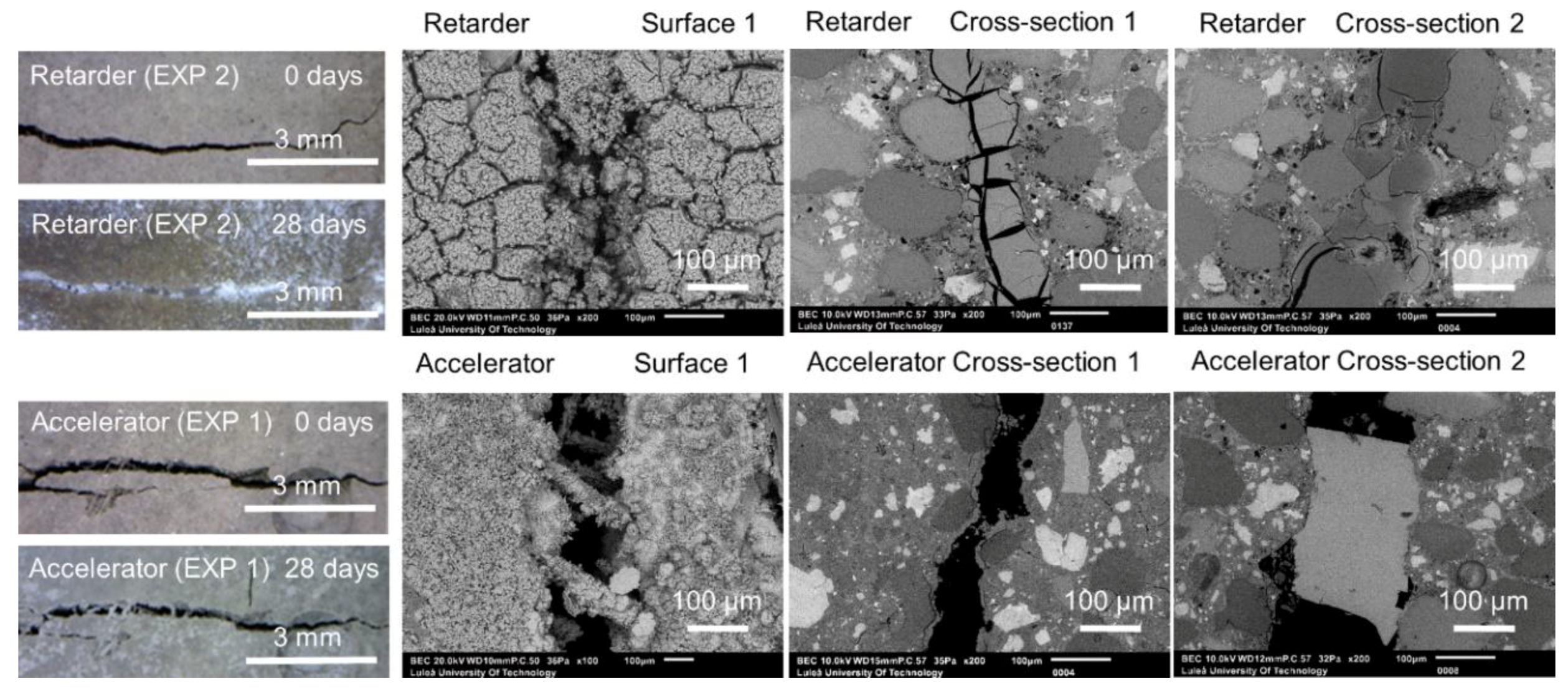
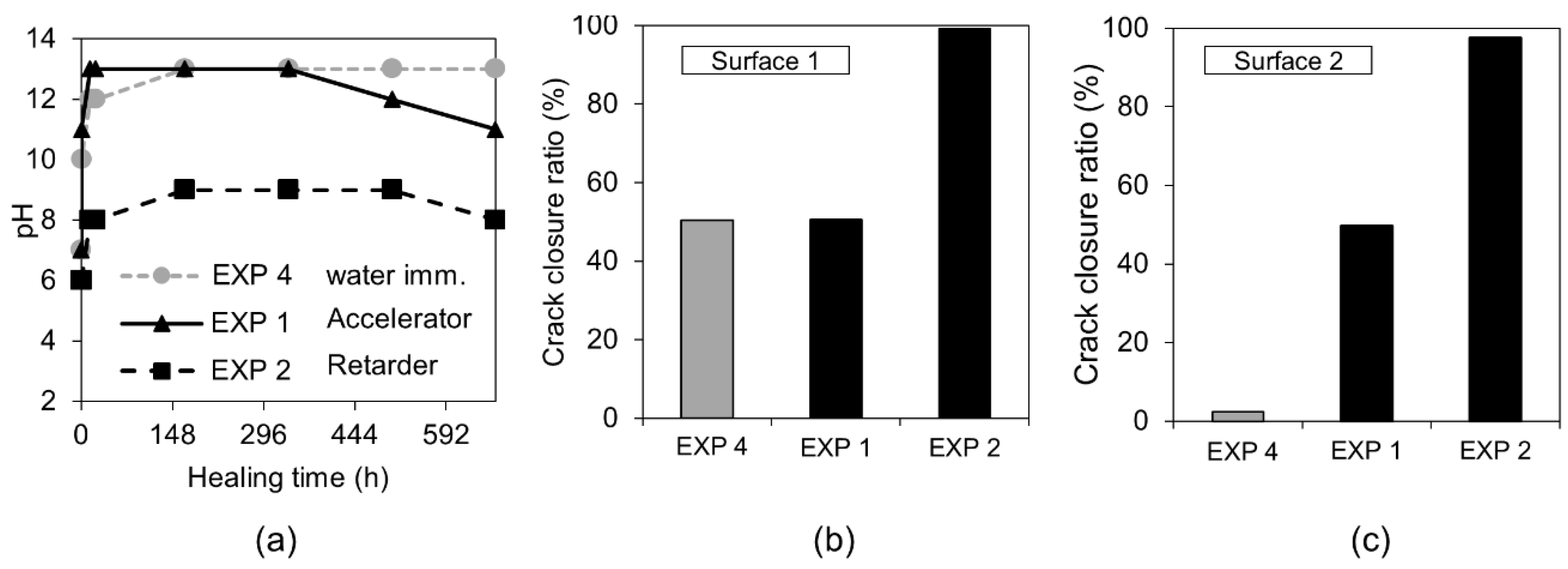
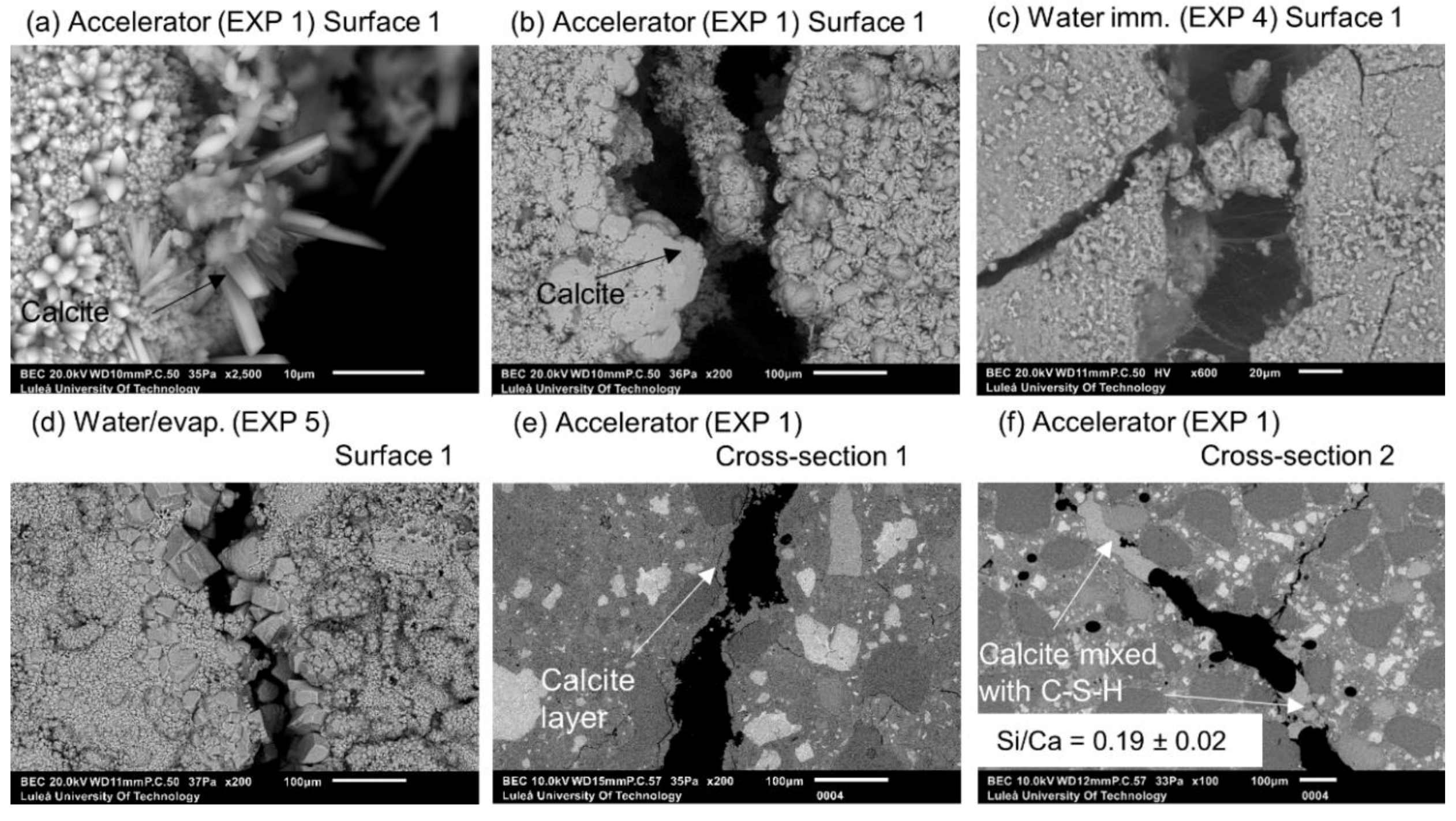
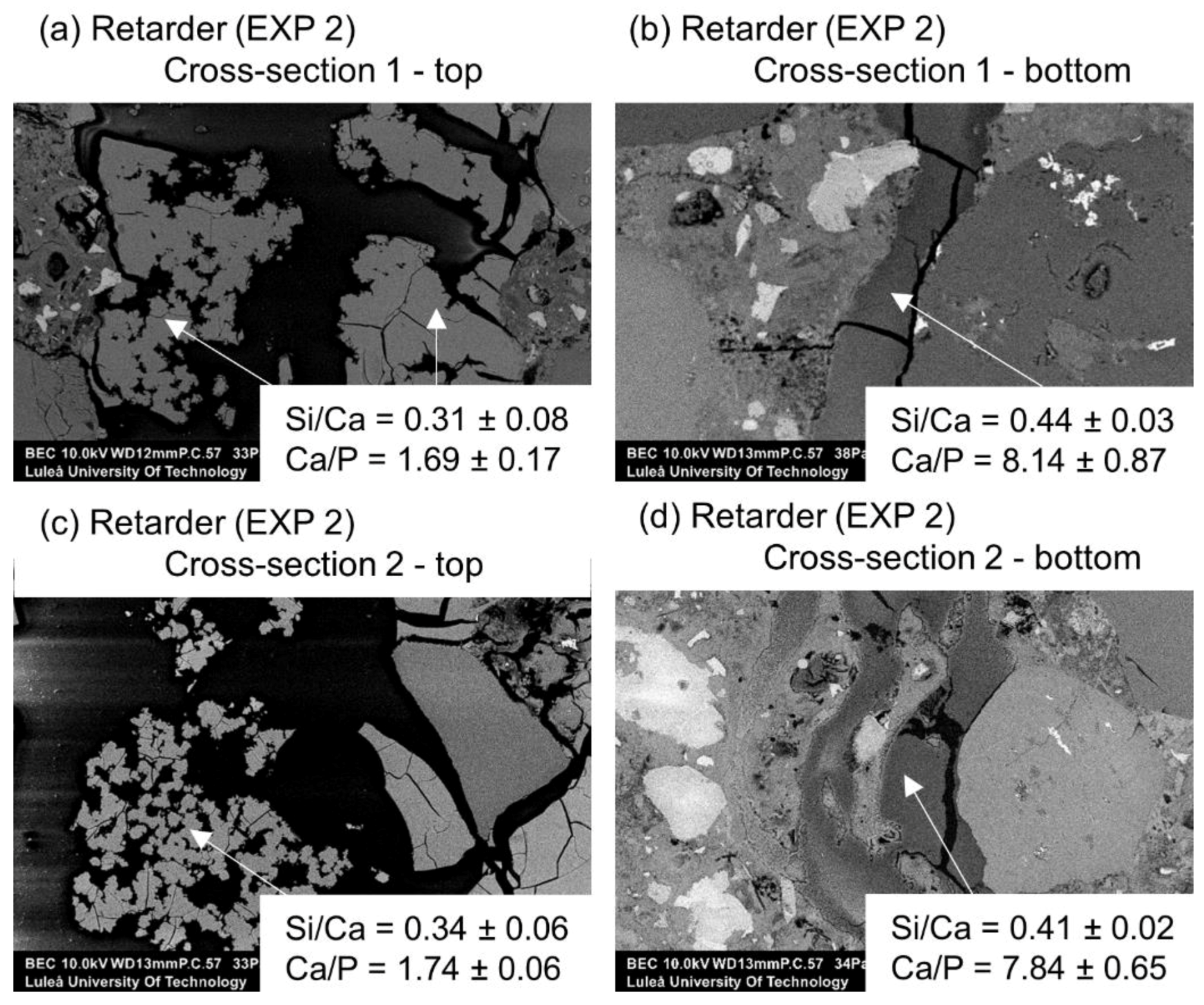
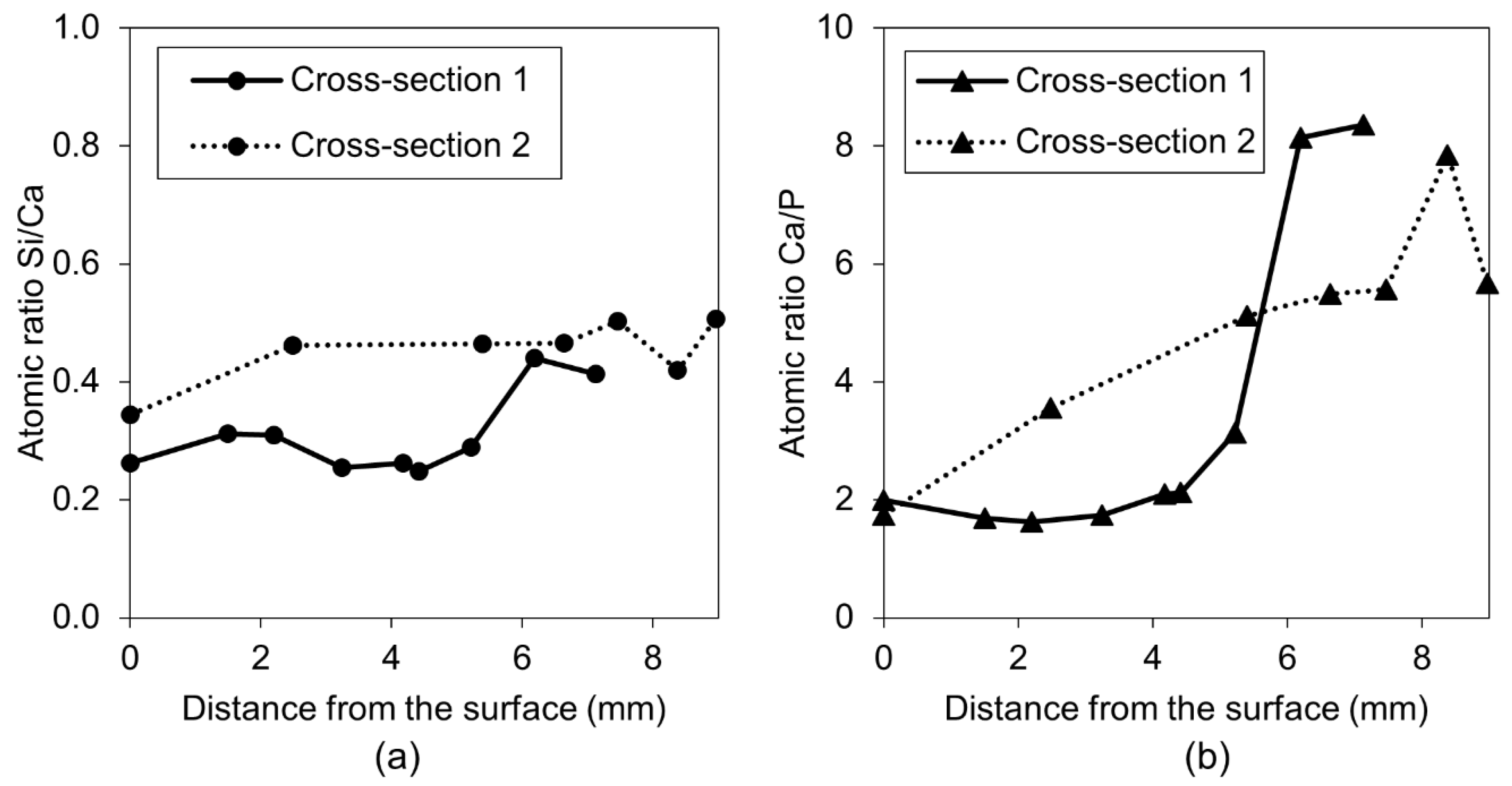
3.1.3. Accelerating and Retarding Admixtures
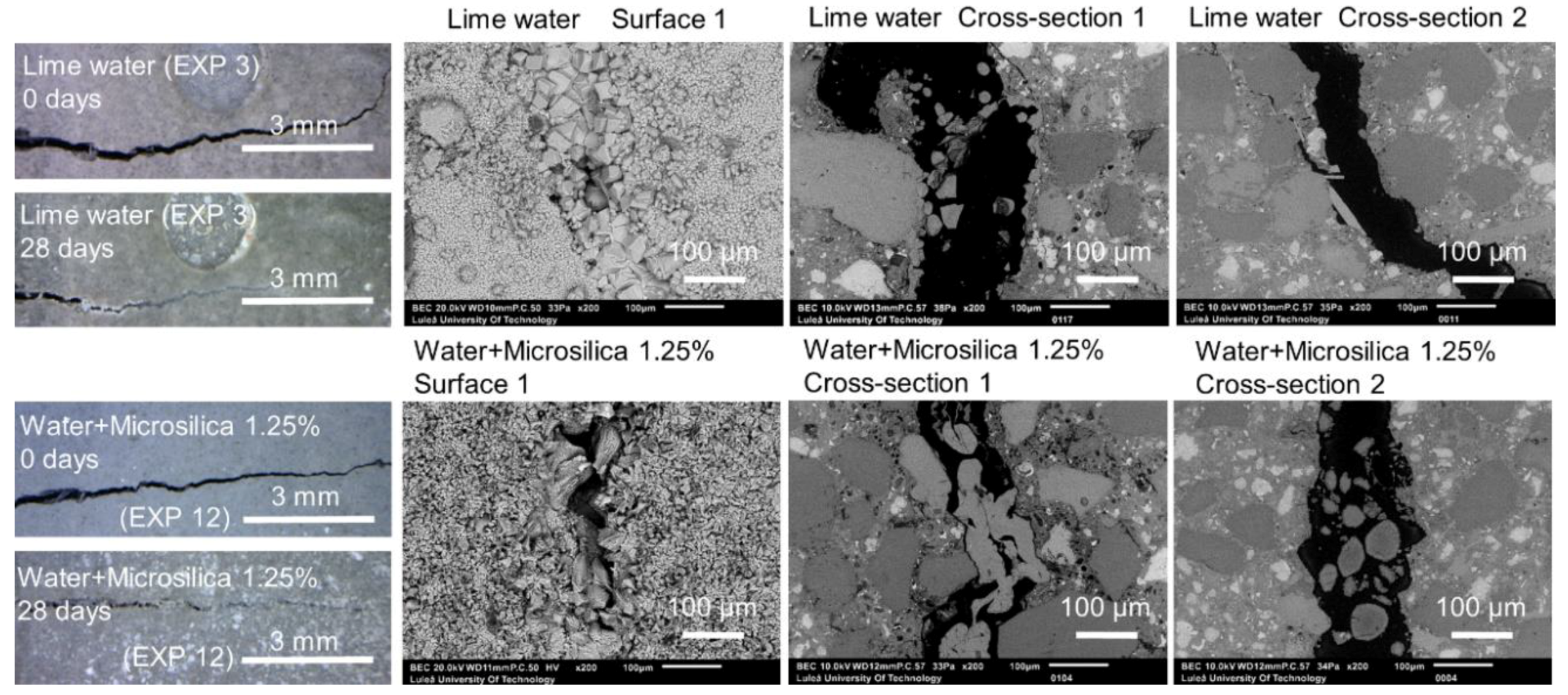
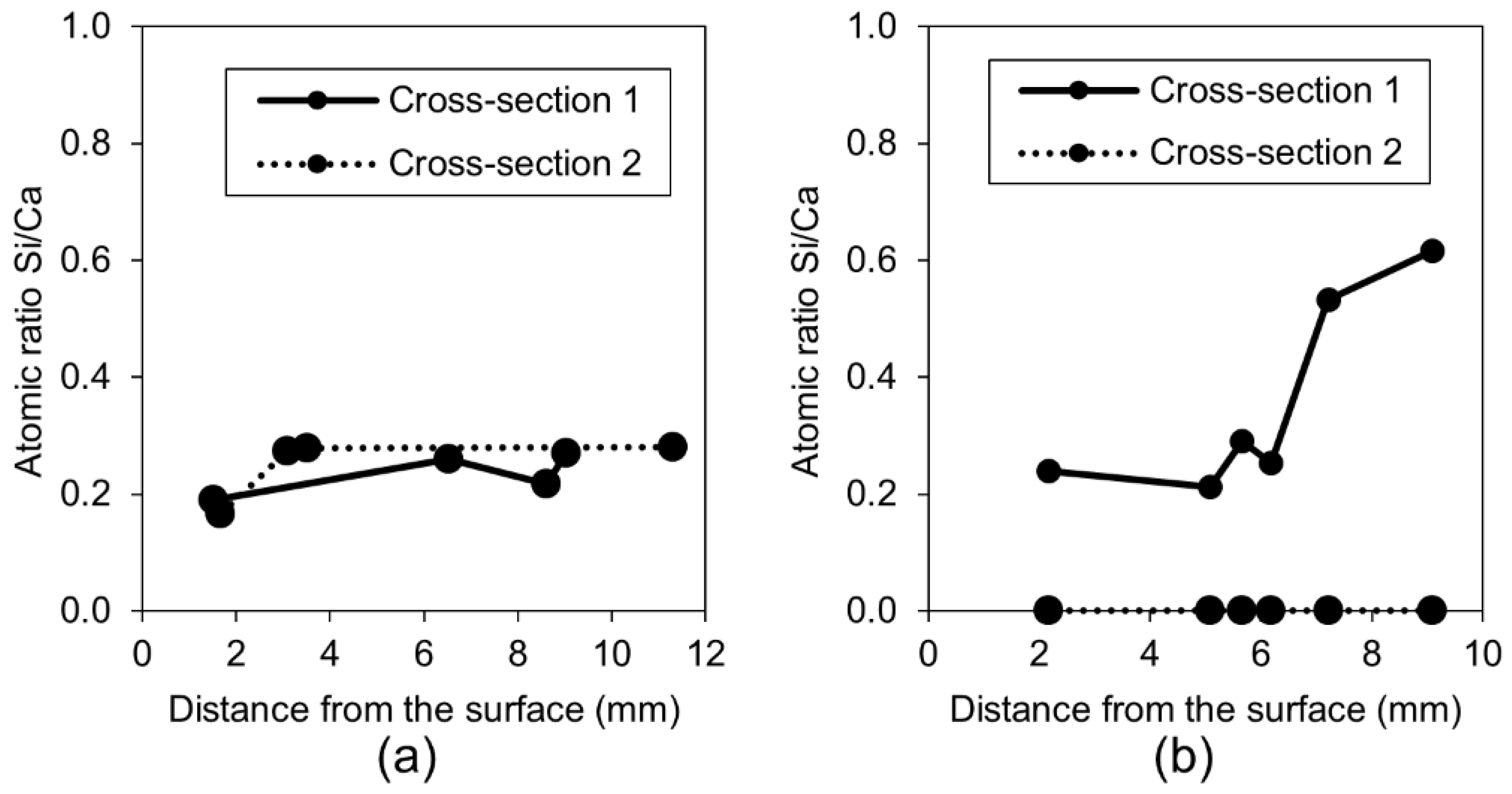
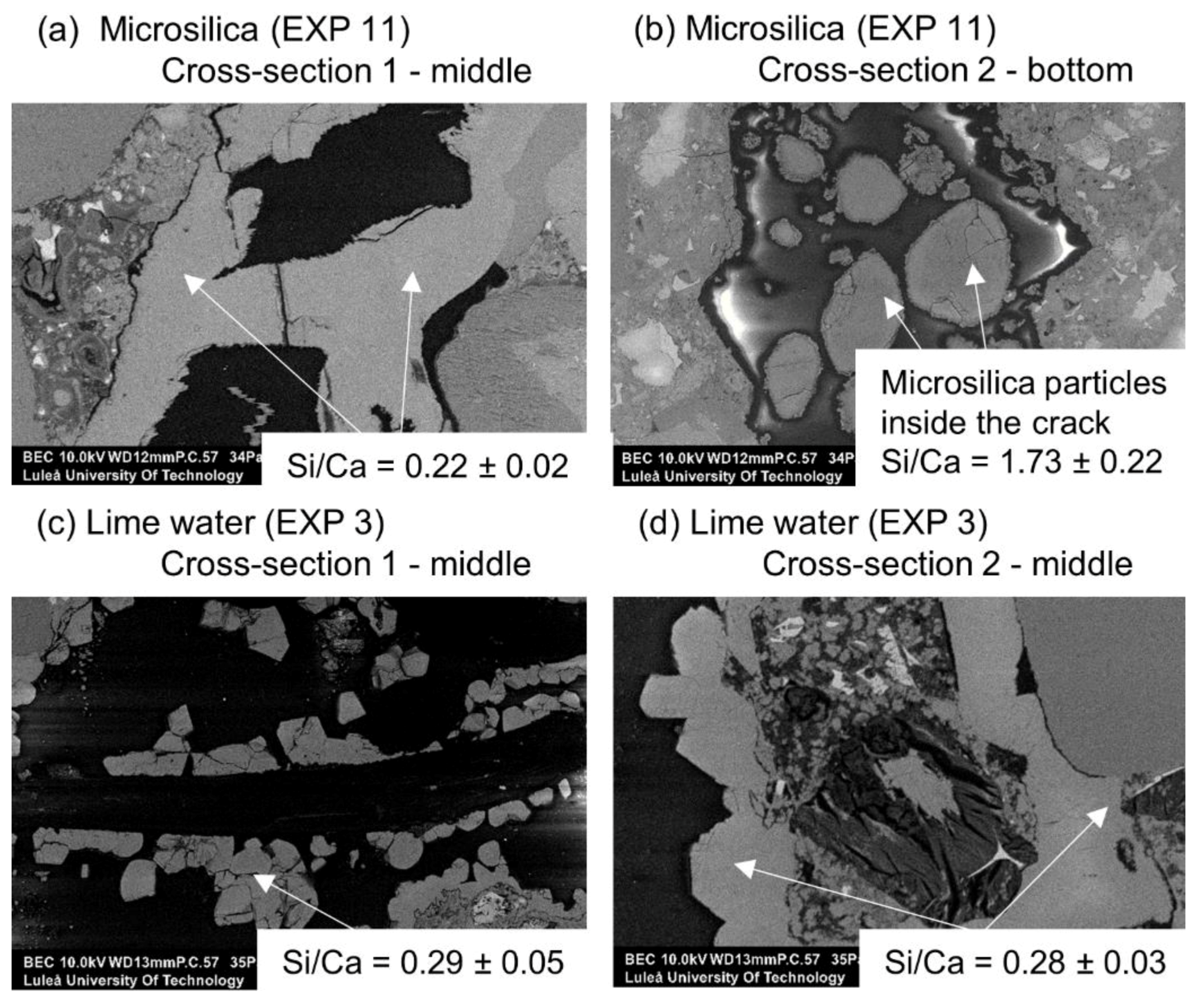
3.1.4. Calcium Ions and Silica Microparticles
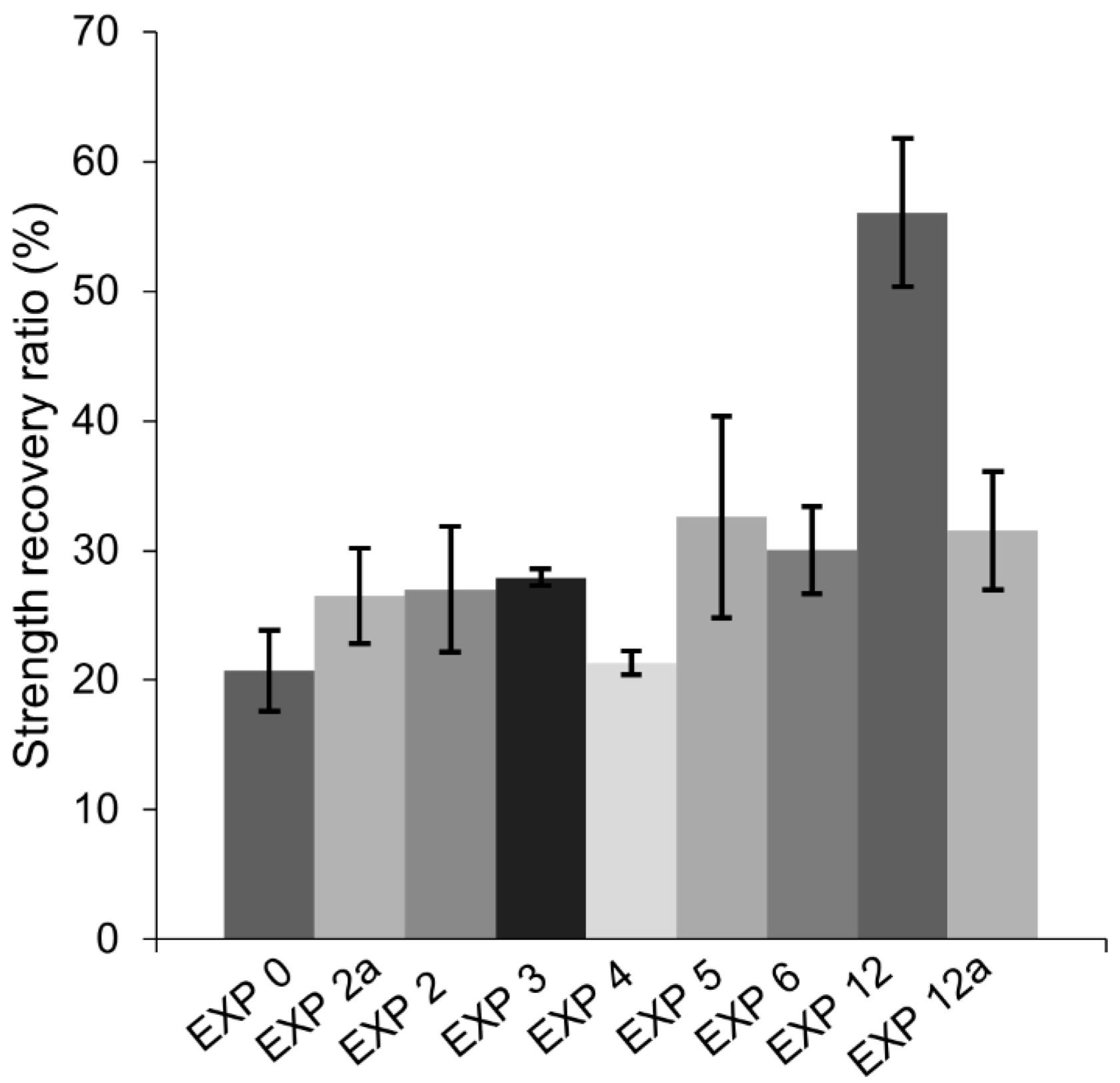
3.2. Strength Recovery
4. Discussion
5. Conclusions
- The water-related exposures did not give satisfying self-healing results despite the application of different cycles or water volumes. A higher ion concentration, expected in the case of a smaller amount of water present inside of the crack, did not support the healing process;
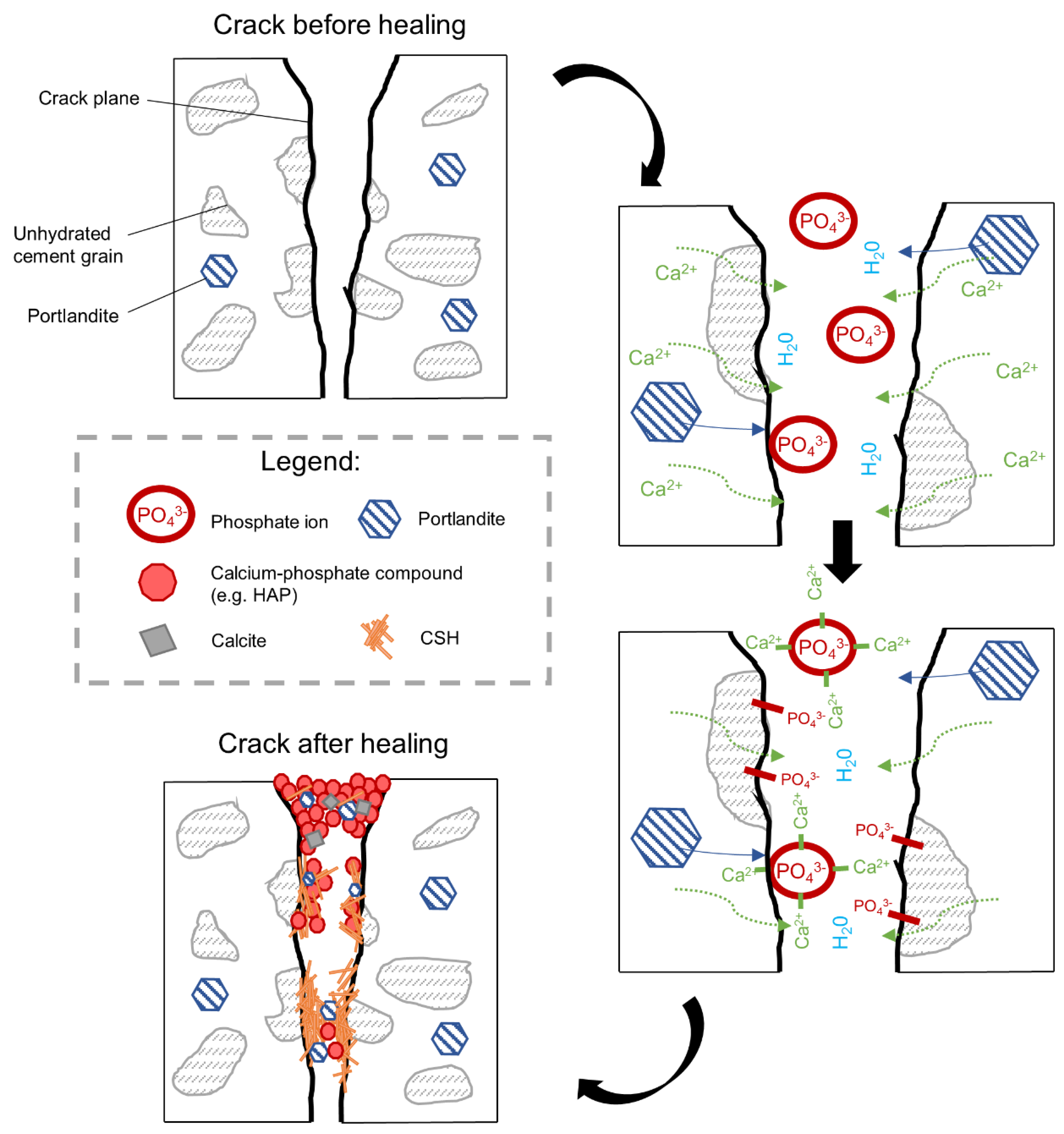
- The addition of the phosphate-based retarding admixture demonstrated the highest crack closure both internally and externally. Phosphate ions were found to contribute to the filling of the crack, most likely by preventing the formation of a dense shell composed of the hydration phases on the exposed crack by unhydrated cement grains. Phosphate ions also caused the formation of calcium–phosphate based compounds;
- The highest strength recovery and a very good crack closure ratio was achieved by immersion in water mixed with microsilica particles. The micro sized silica particles presumably served as nucleation sites for the formation of the CSH, calcium carbonate, and Portlandite.
- The applied chemicals were of an industrial/technical grade, which generated a number of different factors that should be considered. Even though this is the case in real-life concrete applications, in order to fully understand the mechanisms of the autogenous self-healing, higher purity chemical substances should be used to separate the variables;
- The SEM evaluation should be performed on a larger number of specimens in order to enable quantitative evaluation of the internal self-healing products. It is especially crucial due to the strong effects of the crack width and shape;
- In order to fully confirm the chemical composition of the precipitated self-healing phases, the performed elemental analysis should be complemented by an evaluation of the mineralogical composition using, for example an X-ray powder diffraction (XRD). Larger specimens should be tested, thus increasing the amount of the analyzed phases;
- As the used retarding admixture significantly enhanced the self-healing efficiency, different kinds of retarders could be compared;
- Mineralogical composition, solubility, durability, and other physical properties of the calcium phosphate phase should be verified with respect to its full scale applicability;
- The combination of different exposures, which showed the highest self-healing efficiency, could be tested, e.g., a retarding admixture together with microsilica particles.
Author Contributions
Funding
Conflicts of Interest
References
- Luo, M.; Qian, C.; Li, R. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. Crack repair by concrete-immobilized bacteria. In Proceedings of the First International Conference on Self-Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007; pp. 18–20. [Google Scholar]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.M.; Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016, 122, 118–125. [Google Scholar] [CrossRef]
- Wang, J.; Dewanckele, J.; Cnudde, V.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. X-ray computed tomography proof of bacterial-based self-healing in concrete. Cem. Concr. Compos. 2014, 53, 289–304. [Google Scholar] [CrossRef]
- Wang, J.Y.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Snoeck, D.; Wiktor, V.; Van Vliergerghe, S.; Boon, N.; De Belie, N. Application of modified-alginate encapsulated carbonate producing bacteria in concrete: A promising strategy for crack self-healing. Front. Microbiol. 2015, 6, 1088. [Google Scholar] [CrossRef] [PubMed]
- Ducasse-Lapeyrusse, J.; Gagné, R.; Lors, C.; Damidot, D. Effect of calcium gluconate, calcium lactate, and urea on the kinetics of self-healing in mortars. Constr. Build. Mater. 2017, 157, 489–497. [Google Scholar] [CrossRef]
- Qiu, J.; Tan, H.S.; Yang, E. Coupled effects of crack width, slag content, and conditioning alkalinity on autogenous healing of engineered cementitious composites. Cem. Concr. Compos. 2016, 73, 203–212. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G.; Damidot, D. Effect of blast furnace slag on self-healing of microcracks in cementitious materials. Cem. Concr. Res. 2014, 60, 68–82. [Google Scholar] [CrossRef]
- Danner, T.; Hjorth Jakobsen, U.; Geiker, M.R. Mineralogical Sequence of Self-Healing Products in Cracked Marine Concrete. Minerals 2019, 9, 284. [Google Scholar] [CrossRef]
- Qian, S.; Zhou, J.; De Rooij, M.R.; Schlangen, E.; Ye, G.; Van Breugel, K. Self-healing behavior of strain hardening cementitious composites incorporating local waste materials. Cem. Concr. Compos. 2009, 31, 613–621. [Google Scholar] [CrossRef]
- Qian, S.Z.; Zhou, J.; Schlangen, E. Influence of curing condition and precracking time on the self-healing behavior of engineered cementitious composites. Cem. Concr. Compos. 2010, 32, 686–693. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, E.; Li, V.C. Autogenous healing of engineered cementitious composites at early age. Cem. Concr. Res. 2011, 41, 176–183. [Google Scholar] [CrossRef]
- Sahmaran, M.; Yildirim, G.; Erdem, T.K. Self-healing capability of cementitious composites incorporating different supplementary cementitious materials. Cem. Concr. Compos. 2013, 35, 89–101. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Yuan, Z. Influence of mineral additives and environmental conditions on the self-healing capabilities of cementitious materials. Cem. Concr. Compos. 2015, 57, 116–127. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Yuan, Z.; Yang, Z. Self-healing of cracks in concrete with various crystalline mineral additives in underground environment. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 938–944. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Innovative carboxylic acid waterproofing admixture for self-sealing watertight concretes. Constr. Build. Mater. 2018, 171, 817–824. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Nehdi, M.L. Effect of environmental exposure on autogenous self-healing of cracked cement-based materials. Cem. Concr. Res. 2018, 111, 197–208. [Google Scholar] [CrossRef]
- Reinhardt, H.; Jooss, M. Permeability and self-healing of cracked concrete as a function of temperature and crack width. Cem. Concr. Res. 2003, 33, 981–985. [Google Scholar] [CrossRef]
- Yang, Y.; Lepech, M.D.; Yang, E.; Li, V.C. Autogenous healing of engineered cementitious composites under wet–dry cycles. Cem. Concr. Res. 2009, 39, 382–390. [Google Scholar] [CrossRef]
- Kan, L.; Shi, H. Investigation of self-healing behavior of Engineered Cementitious Composites (ECC) materials. Constr. Build. Mater. 2012, 29, 348–356. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Yao, Y. Autogenous self-healing of engineered cementitious composites under freeze–thaw cycles. Constr. Build. Mater. 2012, 34, 522–530. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H.M. Autogenous healing of marine exposed concrete: Characterization and quantification through visual crack closure. Cem. Concr. Res. 2015, 73, 17–24. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G. Self-healing of cracks in cement paste affected by additional Ca2 ions in the healing agent. J. Intell. Mater. Syst. Struct. 2015, 26, 309–320. [Google Scholar] [CrossRef]
- Rajczakowska, M.; Nilsson, L.; Habermehl-Cwirzen, K.; Hedlund, H.; Cwirzen, A. Does a High Amount of Unhydrated Portland Cement Ensure an Effective Autogenous Self-Healing of Mortar? Materials 2019, 12, 3298. [Google Scholar] [CrossRef]
- Matschei, T.; Glasser, F.P. Temperature dependence, 0 to 40 C, of the mineralogy of Portland cement paste in the presence of calcium carbonate. Cem. Concr. Res. 2010, 40, 763–777. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.; Sharp, J.H. Effect of temperature on the hydration of the main clinker phases in Portland cements: Part I, neat cements. Cem. Concr. Res. 1998, 28, 1245–1257. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Palacios, M.; Puertas, F. Effect of curing temperature on cement hydration. Ceram. Silik. 2009, 53, 65–75. [Google Scholar]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Development of microstructures in plain cement pastes hydrated at different temperatures. Cem. Concr. Res. 1991, 21, 179–189. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, W.; Sha, A.; Gao, J.; Han, Z.; Xu, W. Portland Cement Hydration Behavior at Low Temperatures: Views from Calculation and Experimental Study. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Zhang, G. Formation of ettringite in Portland cement/calcium aluminate cement/calcium sulfate ternary system hydrates at lower temperatures. Constr. Build. Mater. 2012, 31, 347–352. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.; Bollmann, K. Delayed ettringite formation in concrete. Nord. Concr. Res. Publ. 2000, 23, 4–28. [Google Scholar]
- Collepardi, M. A state-of-the-art review on delayed ettringite attack on concrete. Cem. Concr. Compos. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Taylor, H.; Famy, C.; Scrivener, K.L. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
- Divet, L.; Randriambololona, R. Delayed ettringite formation: The effect of temperature and basicity on the interaction of sulphate and CSH phase. Cem. Concr. Res. 1998, 28, 357–363. [Google Scholar] [CrossRef]
- Kirov, G.K.; Vesselinov, I.; Cherneva, Z. Conditions of formation of calcite crystals of tabular and acute rhombohedral habits. Krist. Tech. 1972, 7, 497–509. [Google Scholar] [CrossRef]
- Choi, H.; Choi, H.; Inoue, M.; Sengoku, R. Control of the polymorphism of calcium carbonate produced by self-healing in the cracked part of cementitious materials. Appl. Sci. 2017, 7, 546. [Google Scholar] [CrossRef]
- Kalina, L.; Bílek, V.; Novotný, R.; Mončeková, M.; Másilko, J.; Koplík, J. Effect of Na3PO4 on the hydration process of alkali-activated blast furnace slag. Materials 2016, 9, 395. [Google Scholar] [CrossRef]
- Bénard, P.; Garrault, S.; Nonat, A.; Cau-Dit-Coumes, C. Hydration process and rheological properties of cement pastes modified by orthophosphate addition. J. Eur. Ceram. Soc. 2005, 25, 1877–1883. [Google Scholar] [CrossRef]
- Maeda, H.; Yokota, S.; Kasuga, T. Structural changes in calcium silicate hydrate gel and resulting improvement in phosphate species removal properties after mechanochemical treatment. R. Soc. Open Sci. 2018, 5, 181403. [Google Scholar] [CrossRef]
- Karageorgiou, K.; Paschalis, M.; Anastassakis, G.N. Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J. Hazard Mater. 2007, 139, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Naus, D.J.; Mattus, C.H.; Dole, L.R. Final Report–Assessment of Potential Phosphate Ion-Cementitious Materials Interactions; No. ORNL/TM--2007/005; Oak Ridge National Laboratory (United States) Funding organisation (ORNL Work for Others): Oak Ridge, TN, USA, 2007. [Google Scholar]
- Guan, W.; Ji, F.; Chen, Q.; Yan, P.; Zhang, Q. Preparation and phosphorus recovery performance of porous calcium–silicate–hydrate. Ceram. Int. 2013, 39, 1385–1391. [Google Scholar] [CrossRef]
- Bénard, P.; Coumes, C.C.D.; Garrault, S.; Nonat, A.; Courtois, S. Dimensional stability under wet curing of mortars containing high amounts of nitrates and phosphates. Cem. Concr. Res. 2008, 38, 1181–1189. [Google Scholar] [CrossRef]
- Van Kemenade, M.; De Bruyn, P.L. A kinetic study of precipitation from supersaturated calcium phosphate solutions. J. Colloid Interface Sci. 1987, 118, 564–585. [Google Scholar] [CrossRef]
- Meyer, J.L.; Weatherall, C.C. Amorphous to crystalline calcium phosphate phase transformation at elevated pH. J. Colloid Interface Sci. 1982, 89, 257–267. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Liu, M.; Ma, B.; Guo, Y.; Jian, S. Effect of the adsorbing behavior of phosphate retarders on hydration of cement paste. J. Mater. Civ. Eng. 2017, 29, 04017088. [Google Scholar] [CrossRef]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Gallucci, E.; Mathur, P.; Scrivener, K. Microstructural development of early age hydration shells around cement grains. Cem. Concr. Res. 2010, 40, 4–13. [Google Scholar] [CrossRef]
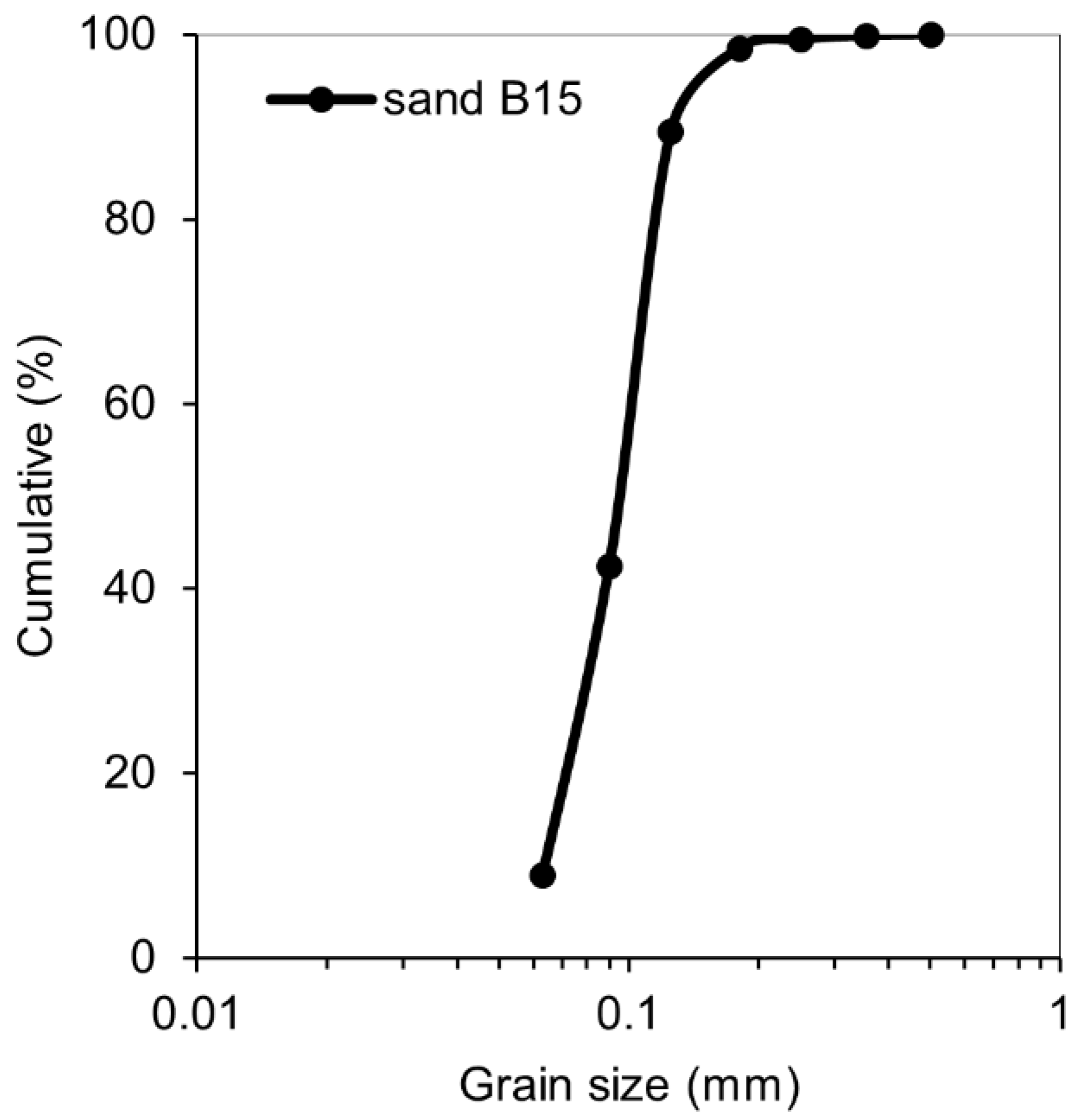
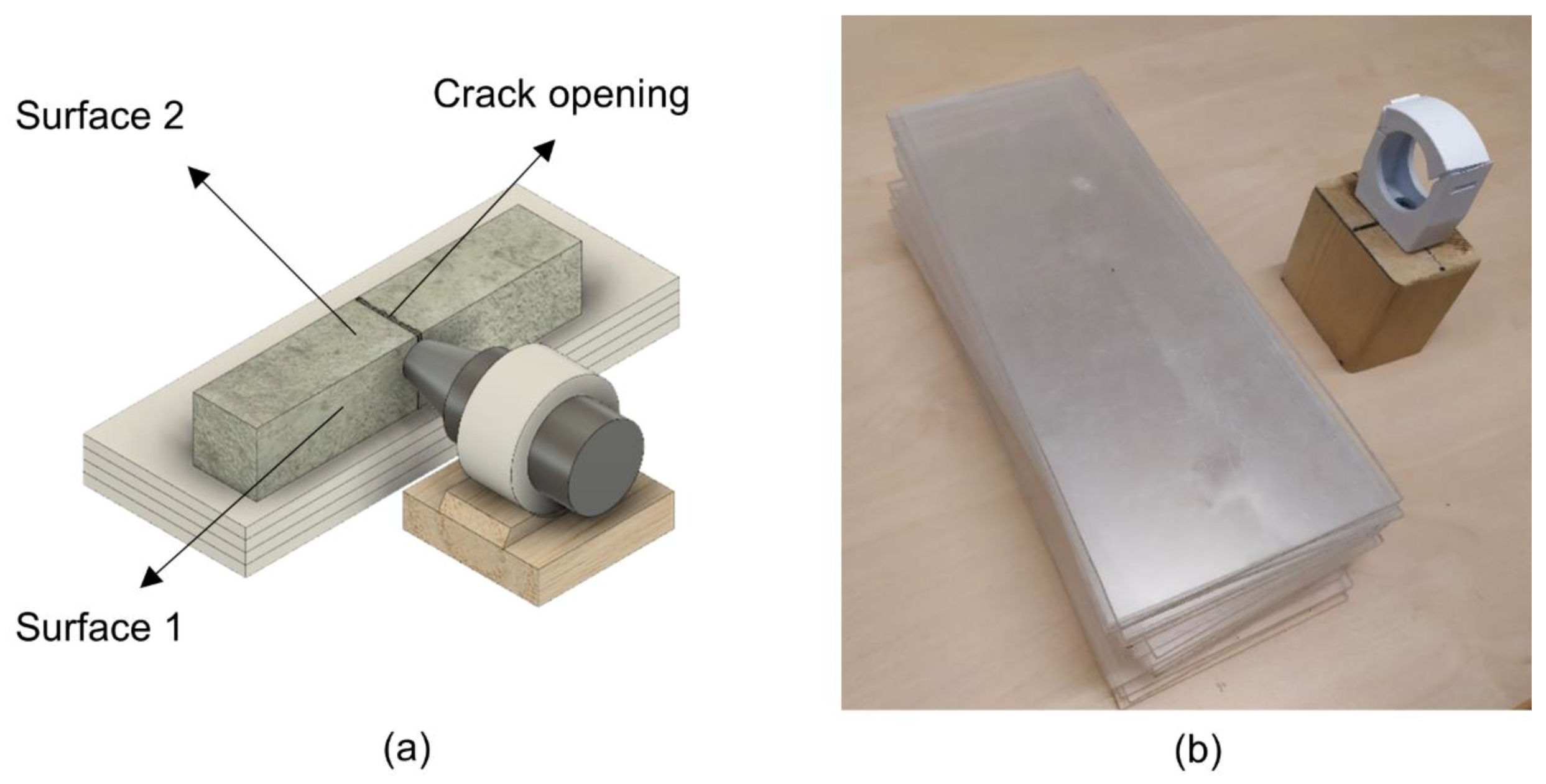
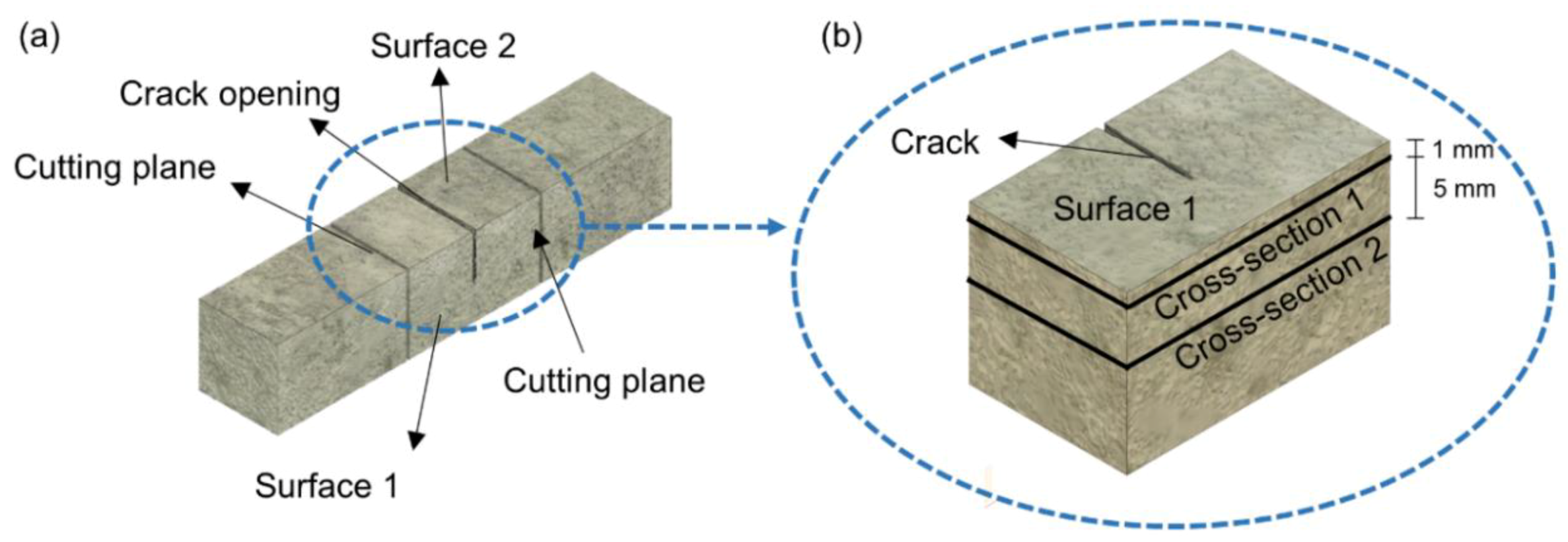
| Chemical Analysis | Mean Value (%) |
|---|---|
| CaO | 63.30 |
| SiO2 | 21.20 |
| Al2O3 | 3.40 |
| Fe2O3 | 4.10 |
| MgO | 2.20 |
| Na2O | 0.18 |
| K2O | 0.56 |
| SO3 | 2.70 |
| Cl | <0.01 |
| Loss of ignition | 2.50 |
| Water soluble Cr6+ | <2 mg/kg |
| Na2O-eq. | 0.55 |
| Exposure | Abbreviation | Justification |
|---|---|---|
| Air | EXP 0 | Non-healed samples |
| Deionized water mixed with Accelerator in proportions 3:1 (immersion) | EXP 1 | Increasing the rate of hydration process inside the crack; possibly faster healing; different composition of hydrates [26,27,28] |
| Deionized water mixed with Retarder in proportions 3:1 (immersion) | EXP 2 | Slowing down the hydration—more hydrates can precipitate on the surface of unhydrated cement grains [27,29] |
| Saturated lime water immersion | EXP 3 | More Ca2+ ions in the solution, higher pH |
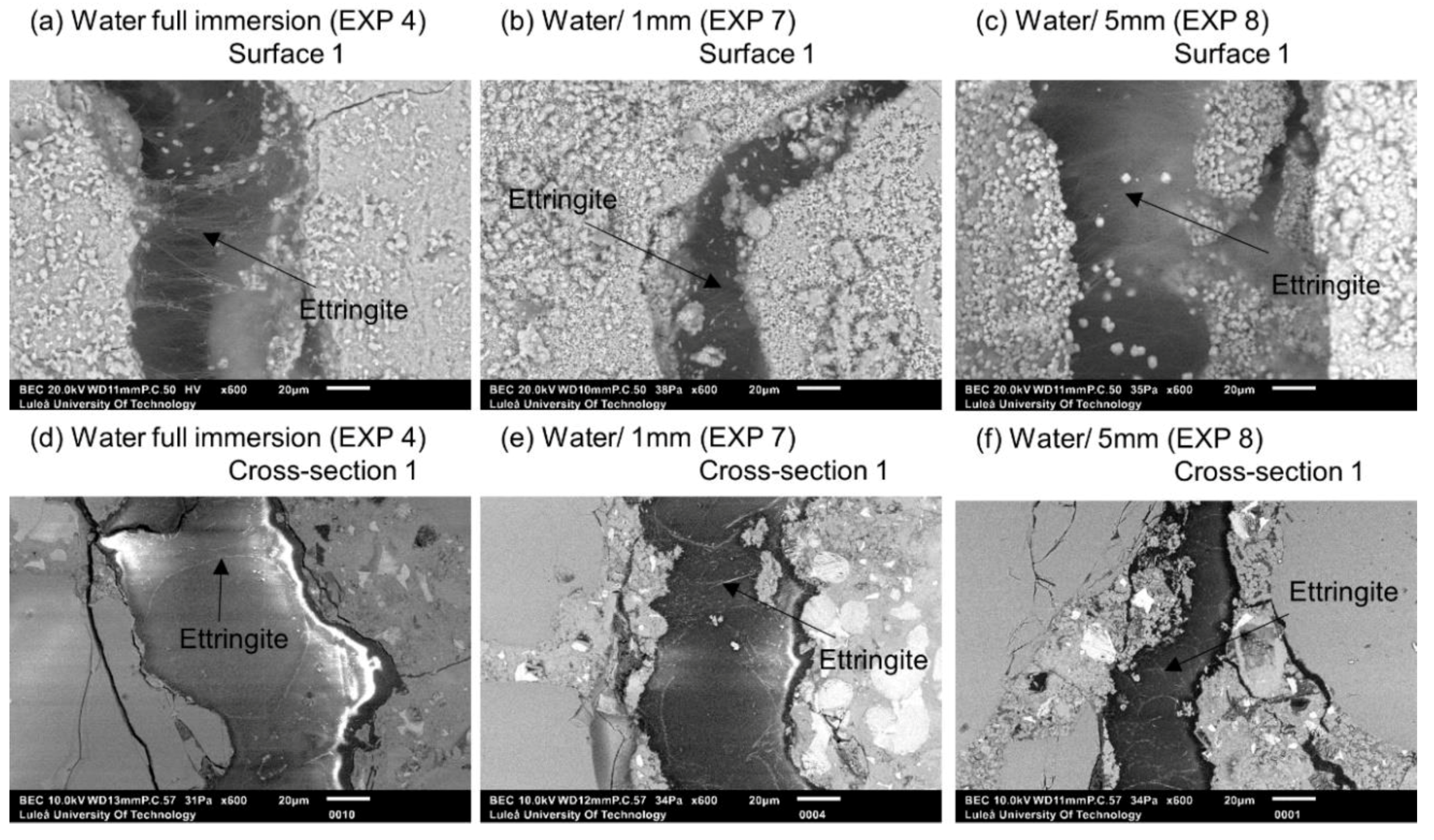
| Deionized water immersion | EXP 4 | Reference exposure |
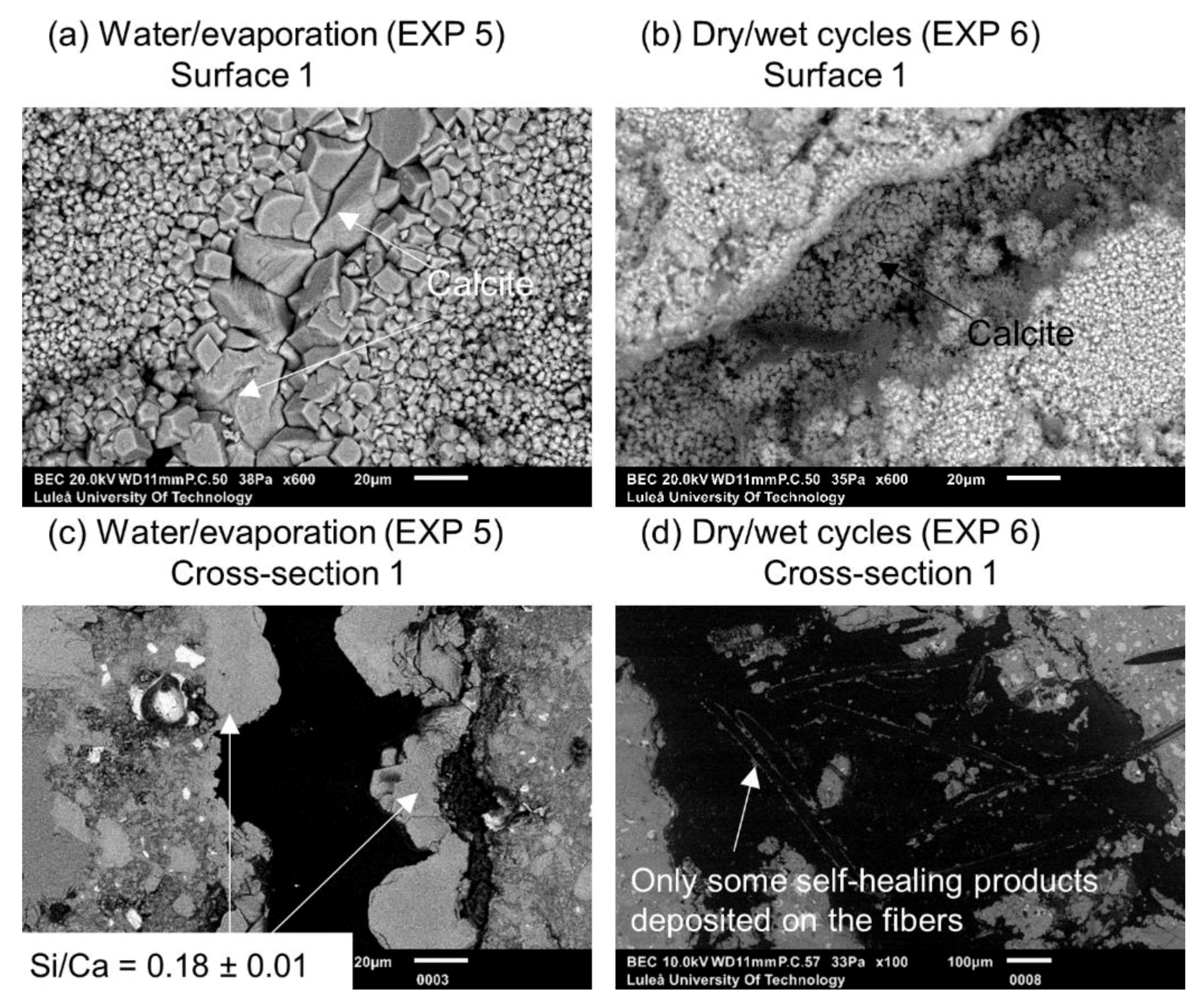
| Deionized water immersion with cyclic evaporation (72 h cycle) | EXP 5 | Changing of the water regime by introducing the cycles of evaporation as well as different volume of water in order to modify the concentration of ions inside the crack |
| Dry/wet (deionized water) cycles; 24 h dry and 24 h immersion in water | EXP 6 | |
| Deionized water immersion up to 1 mm height of the sample | EXP 7 | |
| Deionized water immersion up to 5 mm height of the sample | EXP 8 | |
| Water immersion temperature cycle 24 h/20 °C and 24 h/40 °C | EXP 9 | Increasing/decreasing the rate of the hydration process as well changing the hydration products composition. Possible ettringite formation leading to a higher strength regain in case of lower temperature [30,31] |
| Water immersion temperature cycle 24 h/20 °C and 24 h/5 °C | EXP 10 | |
| Deionized immersion with microsilica particles 1.25 %w | EXP 11 | Providing the nucleation sites inside the crack for the self-healing products |
| Phase | Composition | Ca/P |
|---|---|---|
| Brushite (DCPD) | CaHPO4 2H2O | 1.00 |
| Monetite (DCPA) | CaHPO4 | 1.00 |
| Octacalcium phosphate (OCP) | Ca4H(PO4)3 2.5H2O | 1.33 |
| Whitlockite/tricalcium phosphate (TCP) | Ca3H(PO4)2 | 1.50 |
| Hydroxyapatite (HAP) | Ca5(PO4)3OH | 1.67 |
| Amorphous calcium phosphate (ACP) | - | - |
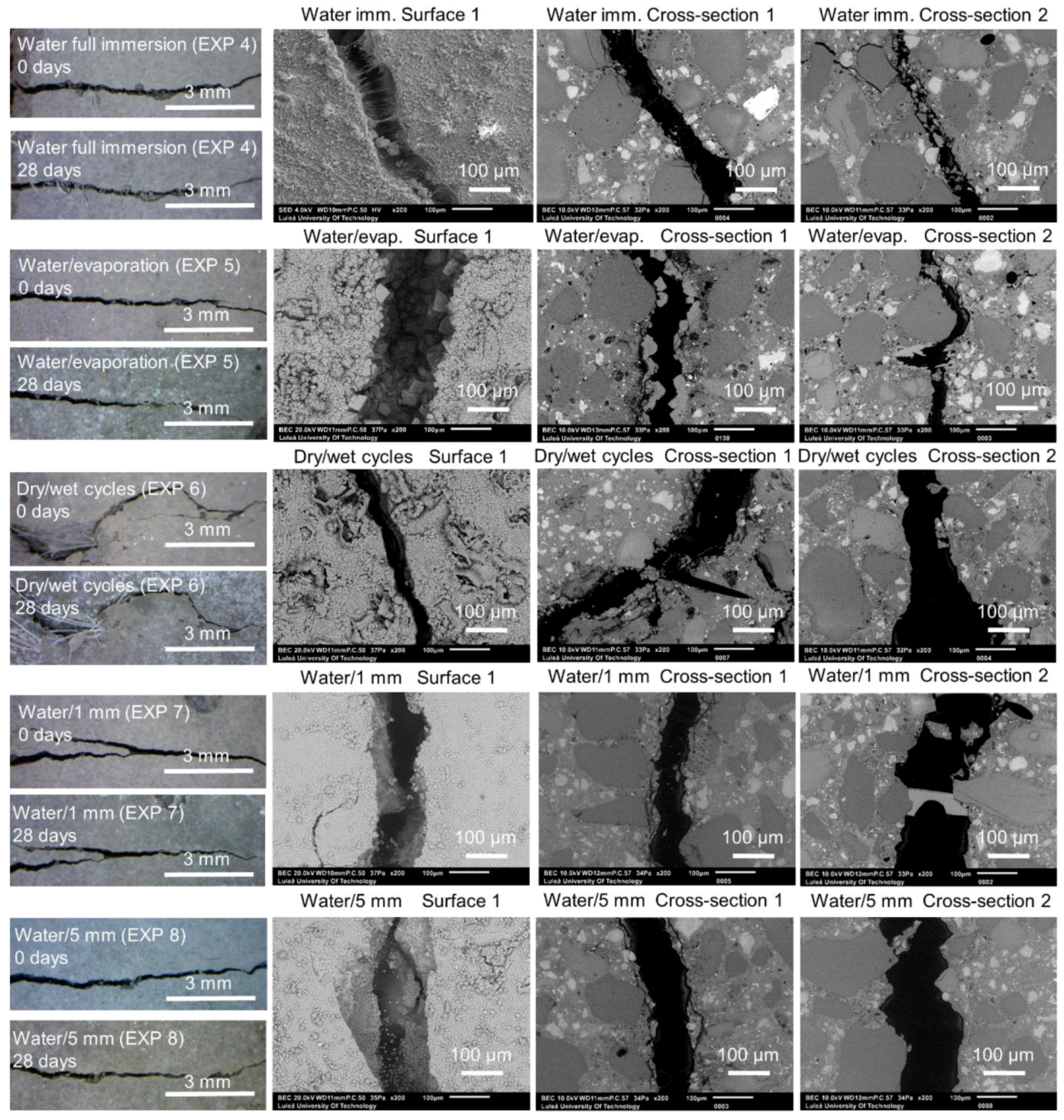
| Exposure | Abbreviation | External Self-Healing | Internal Self-Healing |
|---|---|---|---|
| Deionized water mixed with Accelerator in proportions 3:1 (immersion) | EXP 1 | Very limited crack closure; Several calcite crystals of various shapes | Almost no healing; Several microns thick calcite layer under the surface, inside—few thicker deposits of calcite mixed with CSH (Si/Ca = 0.19) |
| Deionized water mixed with Retarder in proportions 3:1 (immersion) | EXP 2 | The crack almost completely healed; calcium phosphate compounds on the surface with some amount of sodium originating from the self-healing mixture | Very high internal crack closure; Calcium phosphate compounds as well as CSH; Si/Ca and Ca/P increasing with crack depth |
| Saturated lime water immersion | EXP 3 | Very good external self-healing; dense layer of calcite crystals present at the surface | Almost no internal self-healing; few self-healing products with average Si/Ca of 0.3 |
| Deionized water immersion | EXP 4 | Very limited external healing, ettringite and calcite crystals filling the crack | Ettringite visible close to the surface; no internal self-healing |
| Deionized water immersion with cyclic evaporation (72 h cycle) | EXP 5 | Some crack closure; bigger calcite crystals covering the crack | Layer of calcite inside of the sample closure to the surface, few self-healing products in deeper parts of the crack |
| Dry/wet (deionized water) cycles 24 h/24 h | EXP 6 | Almost no external self-healing with small calcite crystal layer at the surface | No internal self-healing except for few healing products deposited on the PVA fibers surface |
| Deionized water immersion up to 1 mm height of the sample | EXP 7 | Minimal external healing, ettringite present; no noticeable differences between exposure 8 and 9 | Ettringite visible close to the surface; no internal self-healing |
| Deionized water immersion up to 5 mm height of the sample | EXP 8 | ||
| Water immersion temperature cycle 24 h/20 °C and 24 h/40 °C | EXP 9 | Very limited external healing, calcite crystals filling the crack | Hardly any internal healing with only single calcite crystals |
| Water immersion temperature cycle 24 h/20 °C and 24 h/5 °C | EXP 10 | Efficient external crack closing; calcite crystals inside the crack as well as thick layer of ettringite | |
| Deionized immersion with microsilica particles 1.25%w | EXP 11 | Very high external self-healing with densified calcite structure filling the crack | Agglomerates of microsilica particles inside the crack without self-healing products. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajczakowska, M.; Habermehl-Cwirzen, K.; Hedlund, H.; Cwirzen, A. The Effect of Exposure on the Autogenous Self-Healing of Ordinary Portland Cement Mortars. Materials 2019, 12, 3926. https://doi.org/10.3390/ma12233926
Rajczakowska M, Habermehl-Cwirzen K, Hedlund H, Cwirzen A. The Effect of Exposure on the Autogenous Self-Healing of Ordinary Portland Cement Mortars. Materials. 2019; 12(23):3926. https://doi.org/10.3390/ma12233926
Chicago/Turabian StyleRajczakowska, Magdalena, Karin Habermehl-Cwirzen, Hans Hedlund, and Andrzej Cwirzen. 2019. "The Effect of Exposure on the Autogenous Self-Healing of Ordinary Portland Cement Mortars" Materials 12, no. 23: 3926. https://doi.org/10.3390/ma12233926
APA StyleRajczakowska, M., Habermehl-Cwirzen, K., Hedlund, H., & Cwirzen, A. (2019). The Effect of Exposure on the Autogenous Self-Healing of Ordinary Portland Cement Mortars. Materials, 12(23), 3926. https://doi.org/10.3390/ma12233926







