Abstract
The paper presents the results of ample investigations performed on industrial and traditional ceramics of fired clay used in processes of water potabilization in the last stage of filtration, after that of active charcoal. Using the data obtained through the scanning electron microscope coupled with energy dispersive X-ray analysis (SEM-EDX) and pH analyses, on the basis of the atomic composition and free concentration of hydronium ions, the normal caustic (Si/Al) and summative [(Si+Ti+FeIII+Cl)/(Al+Ca+Mg+Na+K)] modules were assessed, which were correlated with the free acidity and, respectively, the capacity of absorption and ionic exchange of the Fe3+ and Al3+ ions. The study allowed the selection, on the basis of the caustic module, of the ceramics with high capacity for ionic exchange.
1. Introduction
The capacity for ionic exchange is due to the acidic marginal structures of the type Si(IV)–O–H+ and of the hydroxide ones Al(III)–OH. The base-acidic activity of these groups from the structure of the fired-clay ceramics is due to the Si:Al stoichiometric ratio (known in practice as the caustic module, which varies from 1:1 to 4:1), but also to the position of the two coordination centers of the basic elementary cell. It is known that the basic units of fired-clay ceramics are tetrahedrons of silicate anions and octahedrons of aluminate anions, connected by piro-links in various stratified or coplanar two-dimensional and, respectively, three-dimensional structural forms, which at the surface, present hydroxide (HO–) groups, amphoteric, with double-linked oxygen and acidic structures (–O–H+). Generally, because of the different mineralogical nature of the base clay (composed of kaolinite, montmorillonite, chlorite, halloysite, illite, vermiculite, smectite, pyrophyllite, etc.) and of the firing technology, but also the foundations, plasticizers and other additives employed in the manufacturing, the acid-basic character, given by the marginal groups, varies widely, from weakly acidic (pH ≤ 4,5) to weakly alkaline (pH ≤ 9,5) [1,2,3,4,5,6]. An important role in the capacity for ionic exchange is that of the porosity and, respectively, the active surface area of the granulite’s. The capacity for ionic exchange is, thus, directly proportional to the capacity of absorption and the active surface.
The number and distribution of groups capable of ionic exchange is given both by the mineralogical composition of the raw materials, the composition before the plastic and thermal processing, as well as the conditions of calcination, vitrification, and glazing, which have variable parameters: heating speed, firing temperature, firing duration, firing system (closed, open or semi-open), with a direct or hidden source, oxidative, or reducing, etc. [6,7,8,9].
The capacity for ionic exchange is due only to the acidic structures Si(IV)–O–H+, Ti(IV)–O–H+, and Fe(III)–O–H+. The ceramics with high concentrations of Al(III), Ca(II), and Mg(II) have a character that varies from amphoteric to weakly basic, while those with Si(IV), Ti(IV), and Fe(III) vary from amphoteric to acidic [10,11,12,13,14,15,16].
The main advantage of ceramic products used in treating and purifying water is due to the mechanic resistance, the acid-basic and redox stability, but also to the thermal and photochemical stability, as well as to an extensive series of very important characteristics, such as the apparent density or specific weight, under 1500 kg/m3, and compression resistance, which varies between 50 and 200 daN/cm2; the maximum quantity of absorbed water, which varies between 8% and 20%; the gelifraction or the phenomenon of mechanical deterioration (breakup) of the products saturated with water due to freezing and thawing, then their biodegradation and biodeterioration through biochemical and chemical processes, due to the specific loads of the treated waters. Likewise, another advantage of the industrial ceramics is given by the minimum concentration of soluble components in aqueous systems [17,18,19,20,21,22,23,24,25,26,27,28,29].
For this reason, the granules used in water treatment are beforehand subjected to cleaning processes by dispersion in containers with deionized—weakly acidic water in which they are lightly agitated for several tens of minutes, after which the water is decanted and the ceramics are dried with warm air [6].
The aim of this study is to assess the acid-basic character in correlation with the scanning electron microscopy coupled with energy dispersive X-Ray Spectroscopy (SEM-EDX), the free acidity, and, respectively, to assess the capacity for absorption and ionic exchange of the cations Fe3+ and Al3+, of the fired-clay ceramics originating from industrial bricks and traditional pots. The study allowed the selection of the ceramics optimal for eliminating the traces of Fe3+ and Al3+, of the taste and smell of the treated waters. The ceramics will be employed in the final filtration stage of the treatment of underground and surface waters in order to make them drinkable, producing quality water with impressive organoleptic characteristics.
2. Experimental
2.1. Ceramics Under Consideration
The research focused on two types of ceramics:
- -
- Industrial ceramics, labelled CI, in the form of granules from freshly-fired bricks that were crushed;
- -
- Traditional ceramics, labelled CT, in the form of granules obtained by crushing the neck of glazed pottery, freshly fired.
Three groups of samples were collected from the two sample groups, separated using a granulometric sieve with openings of 1.5–2.5 mm, 2.5–3.5 mm, and, respectively, 3.5–6.5 mm. The resulting samples were stored in plastic containers with screwed caps.
2.2. Processing and Analyzing the Samples
The composition of each lot was determined by collecting small chips weighing ca. 10–20g, with two parallel surfaces and indexed as following:
- -
- CIa, acidic industrial ceramics;
- -
- CIsa, weakly-acidic industrial ceramics;
- -
- CIam, amphoteric industrial ceramics;
- -
- CIsaHCl, weakly-acidic industrial ceramics treated with HCl 3M;
- -
- CIamHCl, amphoteric industrial ceramics treated with HCl 3M;
- -
- CTa, acidic traditional ceramics;
- -
- CTsa, weakly-acidic traditional ceramics;
- -
- CTamsa, amphoteric to weakly-acidic traditional ceramics;
- -
- CTam, amphoteric traditional ceramics;
- -
- CTamsb, weakly-basic amphoteric traditional ceramics;
- -
- CTamHCl, amphoteric traditional ceramics treated with HCl 3M;
- -
- CTamsbHCl, weakly acidic amphoteric traditional ceramics treated with HCl 3M.
These samples were analyaed from the point of view of elemental chemical composition and internal structure by means of scanning electron microscopy coupled with X-ray spectrometry (SEM-EDX). The equipment used for this stage consisted of an SEM VEGA II LSH scanning electron microscope produced by Tescan (Brno, Czech Republic) and a Quantax QX2 EDX detector produced by Bruker/Roentec (Berlin, Germany).
For each sample, we determined the free acidity and, respectively, the pH of the solution resulting from dispersing 90 g of finely grounded (1.5–2.5 mm fraction separated using the granulometric sieve) material from each sample into 100 mL of twice-distilled water. The resulting data were correlated with the elemental composition provided by the EDX.
The free acidity was determined from the value of the pH when the granules with a diameter of 1.5–2.5 mm were dispersed in the twice-distilled water, namely:
[H+] = 10−pH, moles/L.
2.3. Evaluation of retention capacity
To evaluate the capacity of retention of ions of Fe3+ and Al3+ for those two groups of ceramics three sets of samples (1.5–2.5mm; 2.5–3.5mm and 3.5–6.5mm granulometry) were involved. The granules were indexed as follow:
- -
- CIm, control industrial ceramic (untreated with aqueous solution of chloride acid for solubilization of labile components) having granulometry: CIm(1.5–2.5); CIm(2.5–3.5); CIm(3.5–6.5);
- -
- CIHCl, industrial ceramic treated with aqueous solution of chloride acid 5M, having granulometry: CIHCl(1.5–2.5); CIHCl(2.5–3.5); CIHCl(3.5–6.5);
- -
- CTm, control traditional ceramic (untreated), having granulometry: CTm(1.5–2.5); CTm(2.5–3.5); CTm(3.5–6.5);
- -
- CTHCl, treated traditional ceramic (aqueous solution of HCl 5M), having granulometry CTHCl(1.5–2.5); CTHCl(2.5–3.5); CTHCl(3.5–6.5).
The capacity of retention of ions of Fe3+ and Al3+ from aqueous solutions of 0.5 M was performed by dispersion in 100 mL water of 90 g ceramic granules, under weak agitation at room temperature for 20 min. Before dispersion, ceramic granules were washed in bi-distilled water, dried for 4 h at 105 °C in an oven. After 20 min of dynamic dispersion, residual iron quantity was determined by atomic absorption spectroscopy. The retaining capacity was calculated by the formula: CR = (Cr/Ci)×100%, where CR is the retaining capacity (%), Cr—is the final concentration (mol/L), and Ci—initial concentration (mol/L).
3. Results and Discussions
3.1. Determining the Chemical Nature and the Physical Microstructures of the Ceramics
Figure 1 and Figure 2 presents the SEM microphotographs of the 12 samples analyzed (five from industrial bricks and seven from traditional pots).
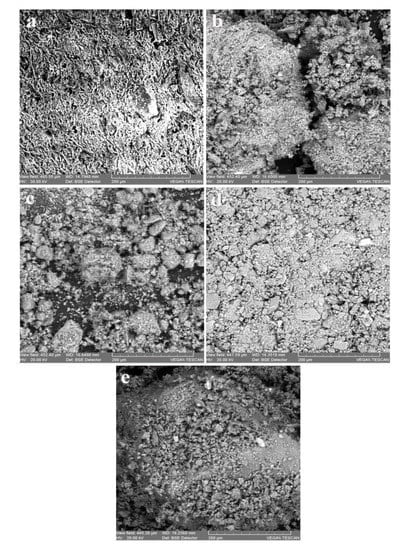
Figure 1.
Scanning electron microscope images of the industrial ceramic samples: (a) CIa acidic ceramic; (b) CIsa weakly-acidic ceramic; (c) CIam amphoteric ceramic; (d) CIsaHCl weakly-acidic ceramic treated with HCl 3M; (e) CIamHCl amphoteric ceramic treated with HCl 3M
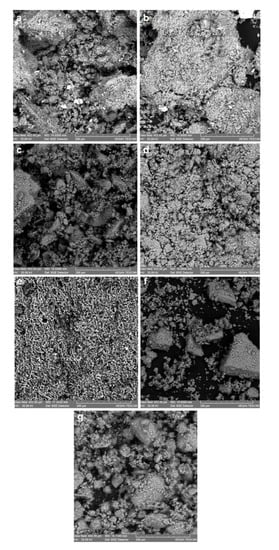
Figure 2.
Scanning electron microscope images of the traditional ceramic samples (CT): (a) CTa acidic ceramic; (b) CTsa weakly-acidic ceramic; (c) CTamsa amphoteric low-acidic ceramic; (d) CTamsb amphoteric low-alkaline ceramic, (e) CTam amphoteric ceramic, f) CTamHCl amphoteric ceramic treated with HCl 3M, (g) CTamsbHCl amphoteric low-alkaline ceramic treated with HCl 3M.
The SEM analyzes allow, on the basis of the microphotographs from Figure 1 and Figure 2, assessing the structure and morphology of the sintering granules of the ceramic material.
The industrial ceramics display in the volume phase a finer granulation and the somewhat uniform distribution of the acidic alumino-silicate granulite’s, whereas the traditional ceramics have a coarse and nonhomogeneous granulation, in which besides the acidic base structures there are often fragments of glazing and basic glasses.
Table 1 presents the elemental composition of the 12 ceramics under analysis.

Table 1.
Chemical elemental composition of the studied ceramics.
The data from the energy dispersive X-ray (EDX) elemental analysis from Table 1 confirms the high compositional similarity of the industrial ceramics, explainable by the rigorously controlled dosing of the bisque or paste ingredients, whereas in the case of the traditional ceramics, the compositions vary widely for the same and between potters, who moreover used different clays and compositions for producing the bisque.
It must be recalled that initially, a much higher number of samples were advanced for these analyses, both from industrial ceramics (20) and traditional wares (30), from which only the most representative, in terms of chemical composition as ascertained from the SEM-EDX analyses, were selected.
The data from the EDX elementary analysis (Table 1) allow correlations between the value of the normal caustic module and that of the summative module and the free acidity. It must be stressed that in the case of the free acidity, an important role is played by the Cl− ion, native, or induced from the HCl treatment.
The basic module of a ceramic structure that determines its acid-base behavior is given by the Si(IV)/Al(III) ratio. With respect to the three-dimensional structure of a ceramic, we speak of structures with acidic functions (titanium [TiO4] octahedrons or tetrahedrons), interspersing those of silicate [SiO4] and aluminate [AlO3], which are joined by the groups with basic functions (Ca–OH, Mg–OH, etc.) that co-act in the acid-base behavior. The exception is the marginal ions Cl− and S2−, which have dominant acidic function.
Table 2 presents the values of the normal caustic Si/Al modules, and the summative Si(Ti/FeIII+Cl)/Al(Ca/Mg+Na/K) of the 12 types of ceramics, which are correlated with their free acidity.

Table 2.
Correlation between the normal caustic and summative modules, and the free acidity.
3.2. The Free Acidity of the Ceramics
The free acidity of the samples listed above and, respectively, the pH of the solution resulting from dispersing 90 g of finely grounded (1.5–2.5 mm fraction separated using the granulometric sieve) material from each sample into 100 mL of twice-distilled water. The resulting data were correlated with the elemental composition provided by the EDX.
Table 2 presents the correlation between the normal caustic and summative modules, and the free acidity, expressed through the value of the pH obtained by dispersing the 1.5–2.5 mm granules into the twice-distilled water and, respectively, the [H+] concentration calculated by the formula:
[H+] = 10−pH, moles/L.
The resulting data show a high correlation between the normal caustic module and the summative one, obtained on the basis of the EDX atomic composition of the 12 types of ceramics. The data allowed sorting the 12 types of untreated and treated ceramics according to the decrease in the free acidity.
The present study allowed selecting the optimal ceramics for use in processes of chemo-absorption from the final stage of filtering ground and surface waters in order to make them drinkable, respectively, to obtain pure water with high organoleptic characteristics. In this regard, of interest are the ceramics with the caustic module between 1.1 and 2.8.
These ceramics have been featured in a new procedure for water potabilization using current city wastewater plants [6].
3.3. Retention Capacity of Fe3+ and Al3+ Ions
To evaluate the capacity of retention of ions Fe3+ and Al3+ of two types of considered granules, using only acid ceramics, CIa and CTa, after fine crushing the samples were separated in sieves having stitches of: 1.5–2.5mm; 2.5–3.5mm and 3.5–6.5mm, and indexed as follow:
- -
- Control industrial ceramic, granulometry: CIm(1.5–2.5); CIm(2.5–3.5); CIm(3.5–6.5);
- -
- Industrial ceramic treated with solution of HCl 5M, granulometry CIHCl(1.5–2.5); CIHCl(2.5–3.5); CIHCl(3.5–6.5);
- -
- Control traditional ceramic, granulometry: CTm(1.5–2.5); CTm(2.5–3.5); CTm(3.5–6.5);
- -
- Traditional ceramic treated with solution of HCl 5M, granulometry CTHCl(1.5–2.5); CTHCl(2.5–3.5); CTHCl(3.5–6.5).
Figure 3 and Figure 4 present the retaining capacity of ions Fe3+ and Al3+ by percent of iron and aluminum changed by chemo-sorption for analyzed ceramics, function of three granulometric groups (for industrial ceramics CI and traditional ceramics CT), as such or treated with an aqueous solution of HCl 3 M.
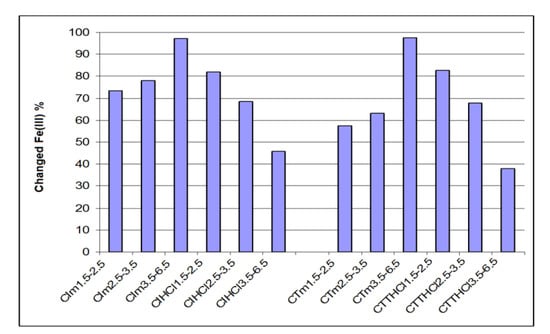
Figure 3.
Iron percent changed through chemo-sorption in industrial and traditional ceramics treated with HCl and untreated.
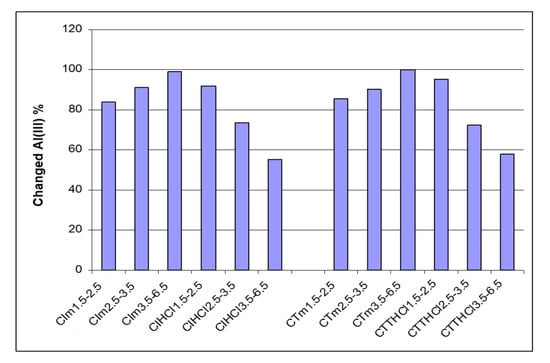
Figure 4.
Aluminum percent changed through chemo-sorption in industrial and traditional ceramics treated with HCl and untreated.
Conforming of data from Figure 3, the change in iron percent of traditional ceramics treated with a solution of HCl 3 M decreased with increasing granulometry, so for granulometry of 1.5–2.5 mm, compared to 3.5–6.5 mm, the change in iron percent decreased from 81.9% to 45.6%. Tendency is inverted for the untreated traditional ceramics, where increases in iron percent were directly proportional with granulometry. So, for granulometry of 1.5–2.5 mm, comparing to 3.5–6.5mm, percent of the changed iron increase was proportional from 73.5% to 97.2%.
The same tendency was observed for industrial ceramic. Treated industrial ceramic presented for granulometry of 1.5–3.6 mm comparing to 3.5–6.5 mm a percent of changed iron in decreasing from 82.7% to 37.9%, while for the untreated one, the effect was inverted, the values of the change in iron percent increased from 57.5% to 97.5%.
Similar, the data from Figure 4 prove that for traditional ceramics treated with HCl 3 M, the percent of changed aluminum decreased when granulometry increased, so for granulometry of 1.5–2.5 mm, compared with 3.5–6.5 mm, the percent of changed aluminum decreased from 92.1% to 55.3%. The tendency is inverted for untreated traditional ceramic because the percent of changed aluminum increase was directly proportional with granulometry. So, for granulometry of 1.5–2.5 mm, compared to 3.5–6.5 mm, the percent of changed aluminum increased proportionally from 83.8% to 99.2%.
The same tendency was noted for industrial ceramic. So, treated industrial ceramic presented a granulometry of 1.5–3.6 mm compared to 3.5–6.5 mm, a decreasing percent of changed iron from 95.2% to 57.8%, while for those untreated, the effect was inverted, the percentage change in aluminum increased from 85.7% to 99.8%.
Initially, the capacity of ionic change for five industrial and seve traditional ceramics was studied, and it was observed that only those acids (CIa and CTa) presented a good reproducibility of the results. These ceramics were used for ionic change of other cations, considered as dangerous to surpass the limit levels of toxicity, like Hg2+, Pb4+, Pb3+, Ni2+, As3+, etc., but of which results did not present reproducibility similar to Fe3+ and Al3+. This fact is caused by the acid-base and redox action of ceramics in the caustic module, with polyhedron structures Si(IV), Ti(IV), and Fe(III) which confer a redoxite behavior with detoxification of some molecular structures and retention in potabilization process, as molecular chlorine reduced to chloride ion and a serial of cations (Hg2+, Pb3+, Pb4+, As5+, etc.), which are reduced to hardly soluble structures (Hg(I), Pb(II), As(III), etc.) retained in the ceramic. A cumulative effect was not be observed.
This domain is very interesting for science and technology; therefore, deeper research must be conducted. This study allowed the selection of optimum ceramics which could be used for chemiorption processes in the final stage of filtering and treating of ground and surface waters. These ceramics were the base to elaborate a new method of potabilization of waters [6].
The resulted data were re-validated using statistical, probabilistic, and numerical methods for analysis, keeping in mind future research involving IoT-based methods for the analysis.
4. Conclusions
As stated by the specialized literature, the capacity for ionic exchange of the normal fired-clay ceramics is due to the acidic structures of the type Si(Ti)–O–H+. The capacity for ionic exchange is influenced by the caustic module (Si/Al ratio), the porosity, and active surface of the ceramic granules. The number and distribution of grouping capable of ionic exchange is given, on the one hand, by the mineralogical composition of the raw materials, and the composition of the mixture before the plastic processing and the firing, and, on the other hand, by the conditions of calcination and vitrification, with their variable parameters—heating speed, firing temperature, firing duration, firing system (closed, open, semi-open), source type (direct or hidden), firing type (oxidative or reducing), etc.
The present study was carried out on two lots of freshly-produced ceramics, namely industrial bricks and traditional pots, in order to select the optimal type, from the acid-base point of view, for use in chemo-absorption processes during the final filtration stage of ground and surface water potabilization treatment, allowing the following conclusions.
The normal ceramic materials used in treating and purifying waters have, compared to other filtration materials, a series of advantages, including good mechanical resistance, acid-basic, and redox chemical stability, thermal and photochemical stability, but also an extensive series of very important characteristics for the purpose at hand, such as the apparent density or specific weight under 1500 Kg/m3 and resistance to compression, which varies between 50 and 200 daN/cm2; the maximum quantity of water absorbed (between 8% and 20%); gelifraction, or mechanical deterioration (breaking), and degradation of products saturated with water under the action of freezing and thawing and the biochemical and chemical processes, under the influence of the specific composition of the waters undergoing treatment, and last but not least, the low price.
Conforming with data from ionic change for ion Fe3+, it was noted that for traditional ceramics treated with HCl 3 M, the percent of change in iron decreased when granulometry increased; This tendency is inverted for untreated traditional ceramic where the percentage of changed iron increased when granulometry increased.
The same tendency was noted for industrial ceramic. Treated industrial ceramic exhibit an decreasing of percent of changed iron when granulometry increase, while for the untreated ceramic, an increasing of percent of changed iron when granulometry increase;
Regarding the percent of changed aluminum, for traditional ceramics treated with HCl 3M, this decrease when granulometry increase, and the behavior inverted for untreated ceramics, when the percent of changed aluminum increase when granulometry increase; The same tendency was deduced for treated industrial ceramic, when the percent of changed aluminum decreased with increased granulometry, while for untreated industrial ceramics the percentage of changed aluminum increased when granulometry increased.
The study allowed selecting ceramics with optimum properties regarding the ionic change, able to be used in the final stage of filtering and treating the process of ground and surface waters to obtain fresh potable water. The better results exhibit acid granules notes as CIa and CTa;
The ceramics with the caustic module between 1.1 and 2.8 can be used for the ionic exchange of other cations that risk exceeding the threshold for toxicity.
As a general conclusion, these materials are very good to be used in water treatment due to their low cost, being waste materials or scraps from the technological flow of construction bricks/tiles or traditional ceramics. The resulting water has good organoleptic properties.
Author Contributions
writing and editing, formal analysis, A.V.S.; investigation, writing original draft, V.V.; investigation, reviewing I.G.S.; validation, J.M.S. and I.K.K.; data curation and visualization, P.D.M.; Conceptualization and supervision, I.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, H.H. Applied Clay Mineralogy; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Niculica, B.P.; Vasilache, V.; Boghian, D.; Sandu, I. An archaeometric Study of Several Ceramic Fragments from the Komariv (Komarow) Settlement of Adancata—Sub Padure, Suceva Conty, Fragments and Mineral Pigments from the Cucutenian Site of Tacuta—Dealul Miclea/Paic, Vaslui Conty. In Third Arheoinvest Congress Programme and Abstracts, Proceedings of Third Arheoinvest Congress, Interdisciplinary Research in Archaeology, Iasi, Romania, 6–8 June 2013; Cotiuga, V., Ed.; Alexandru Ioan Cuza University: Iasi, Romania, 2013; pp. 67–68. [Google Scholar]
- Vasilache, V.; Filote, C.; Cretu, M.A.; Sandu, I.; Coisin, V.; Vasilache, T. Monitoring of groundwater quality in some vulnerable areas in Botosani County—Nitrates and nitrites based pollutants. In Proceedings of the 6th International Conference on Environmental Engineering and Management ICEEM 06, Balatonalmádi, Hungary, 1–4 September 2011. [Google Scholar]
- Vasilache, V.; Sandu, I.; Vasilache, T. Studies Regarding the Quality of Waters for Some Rivers from Suceava County, Annals of “Dunarea de Jos” University of Galati, fasc. Ix Met. Mater. Sci. 2011, 29, 169–174. [Google Scholar]
- Sandu, I.; ENitescu, E.; Calu, N.; Berdan, I.; Smocot, R.; Bialus, A.; Popa, G.; Vascu, V.; Stanila, A. Procedeu Şi Instalaţie Pentru Obţinerea Apei Potabile. Patent RO114318(B1), 30 March 1999. [Google Scholar]
- Sandu, I.; Cretu, M.A.; Lupascu, T.; Vasilache, V.; Sandu, A.V.; Sandu, I.G.; Sieliechi, J.-M.; Kouame, I.K.; Kayem, J.G.; Sandu, A.V.; et al. Process for Water Treatment of Ground and Surface Waters. Patent MD 4298(B1), 31 October 2014. [Google Scholar]
- Sentana, I.; Puche, R.D.S.; Sentana, E.; Prats, D. Reduction of chlorination byproducts in surface water using ceramic nanofiltration membranes. Desalination 2011, 277, 147–155. [Google Scholar] [CrossRef]
- Koster van Gross, A.F.; Guggenheim, S. Dehydroxylation of Ca- and Mg-exchanged montmorillonite. Am. Miner. 1989, 74, 627–636. [Google Scholar]
- Grim, R.E. Clay Mineralogy; McGraw-Hill Book Comp. Inc.: New York, NY, USA; Toronto, ON, Canada; London, UK; Sydney, Australia, 1968. [Google Scholar]
- Martin, D.; Aparicio, P.; Galan, E. Time evolution of the mineral carbonation of ceramic bricks in a simulated pilot plant using a common clay as sealing material at superficial conditions. Appl. Clay Sci. 2019, 180, 105191. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Design and optimization of a new reactor based on biofilm-ceramic for industrial wastewater treatment. Environ. Pollut. 2019, 15, 113298. [Google Scholar] [CrossRef]
- Yasui, K.; Goto, S.; Kinoshita, H.; Kamiunten, S.; Yuji, T.; Okamura, Y.; Mungkung, N.; Sezaki, M. Ceramic waste glass fiber-reinforced plastic-containing filtering materials for turbid water treatment. Environ. Earth Sci. 2016, 75, 1135. [Google Scholar] [CrossRef]
- Moga, I.C.; Ardelean, I.; Donu, O.G.; Moisescu, C.; Bǎran, N.; Petrescu, G.; Voicea, I. Materials and Technologies Used in Wastewater Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012079. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, Q.; Yan, Z.; Liu, J.; Liang, J.; He, L.; He, J.; Yan, M.; He, L. Effect of waste ceramic adsorbent on wastewater treatment. MATEC Web Conf. 2018, 175, 01010. [Google Scholar] [CrossRef][Green Version]
- Tociu, C.; Deák, G.; Maria, C.; Ivanov, A.A.; Ciobotaru, I.E.; Marcu, E.; Marinescu, F.; Cimpoeru, C.; Savin, I.; Constandache, A.C. Advanced treatment solutions intended for the reuse of livestock wastewater in agricultural applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012109. [Google Scholar] [CrossRef]
- Olteanu, M.; Baraitaru, A.; Panait, A.-M.; Dumitru, D.; Boboc, M.; Deák, G. Advanced SiO2 Composite Materials for Heavy Metal Removal from Wastewater. Water Air Soil Pollut. 2019, 230, 179. [Google Scholar] [CrossRef]
- Peterson, S. The Craft and Art of Clay; Harry N. Abrams: London, UK, 2003. [Google Scholar]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J. Clean. Prod. 2012, 29–30, 208–213. [Google Scholar] [CrossRef]
- Bociort, D.; Gherasimescu, C.; Berariu, R.; Butnaru, R.; Branzila, M.; Sandu, I. Comparative Studies on Making the Underground Raw Water Drinkable, by Coagulation-Flocculation and Adsorption on Granular Ferric Hydroxide Processes. Rev. Chim. 2012, 63, 1243–1248. [Google Scholar]
- Bociort, D.; Gherasimescu, C.; Berariu, R.; Butnaru, R.; Branzila, M.; Sandu, I. Research on the Degree of Contamination of Surface and Groundwater used as Sources for Drinking Water. Rev. Chim. 2012, 63, 1152–1157. [Google Scholar]
- Goldan, E.; Nedeff, V.; Barsan, N.; Mosnegutu, E.; Sandu, A.V.; Panainte, M. The effect of biochar mixed with compost on heavy metal concentrations in a greenhouse experiment and on Folsomia candida and Eisenia andrei in laboratory conditions. Rev. Chim. 2019, 70, 809–813. [Google Scholar]
- Besnea, D.; Gheorghe, G.I.; Dontu, O.; Moraru, E.; Constantin, V.; Moga, I.C. Experimental researches regarding realization of wastewater treatment elements by means of modern technologies. Int. J. Mechatron. Appl. Mech. 2018, 4, 61–65. [Google Scholar]
- Tataru, L.; Nedeff, V.; Barsan, N.; Sandu, A.V.; Mosnegutu, E.; Panainte-Lehadus, M.; Sandu, I. Applications of polymeric membranes ultrafiltration process on the retention of bentonite suspension. Mater. Plast. 2019, 56, 97–102. [Google Scholar]
- Vu, D.H.; Wang, K.S.; Bac, B.H. Humidity control porous ceramics prepared from waste and porous materials. Mater. Lett. 2011, 65, 940–943. [Google Scholar] [CrossRef]
- Chen, R.Z.; Lei, Z.F.; Yang, S.J.; Zhang, Z.Y.; Yang, Y.N.; Sugiura, N. Characterization and modification of porous ceramic sorbent for arsenate removal. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 393–399. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, V.K.; Mbuya, O.S. Removal of arsenite by Fe(VI), Fe(VI)/Fe(III), and Fe(VI)/Al(III) salts: Effect of pH and anions. J. Hazard. Mater. 2009, 169, 339–344. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Slingh, S. Evaluation of iron oxide and aluminium oxide as potential arsenic(V) adsorbents. Chem. Eng. Process. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Keely, J.; Smith, A.D.; Judd, S.J.; Jarvis, P. Reuse of recovered coagulants in water treatments: An investigation on the effect coagulants purity has on treatment performance. Sep. Purif. Technol. 2014, 131, 69–78. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.H. Differential natural organic matter fouling of ceramic versus polymeric ultrafiltration membranes. Water Res. 2014, 48, 43–51. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).