XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
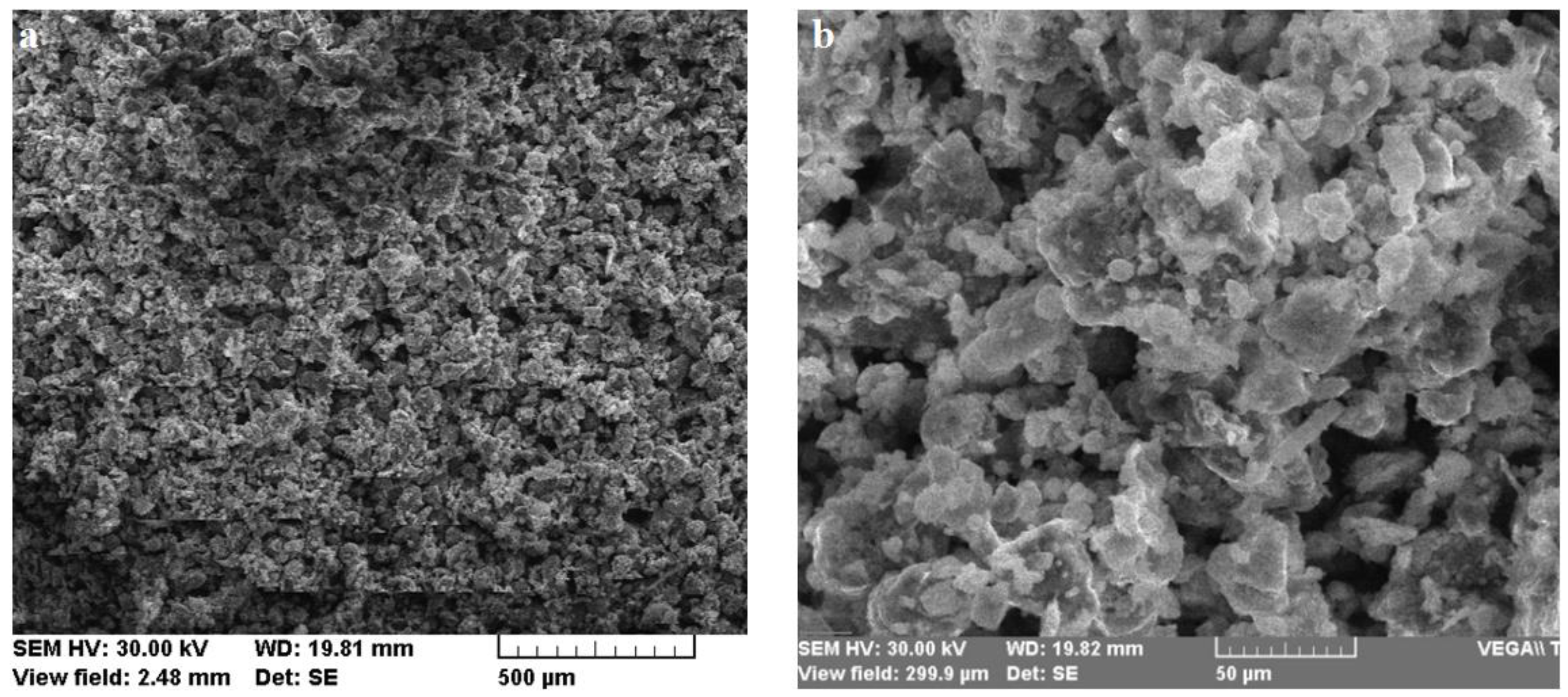
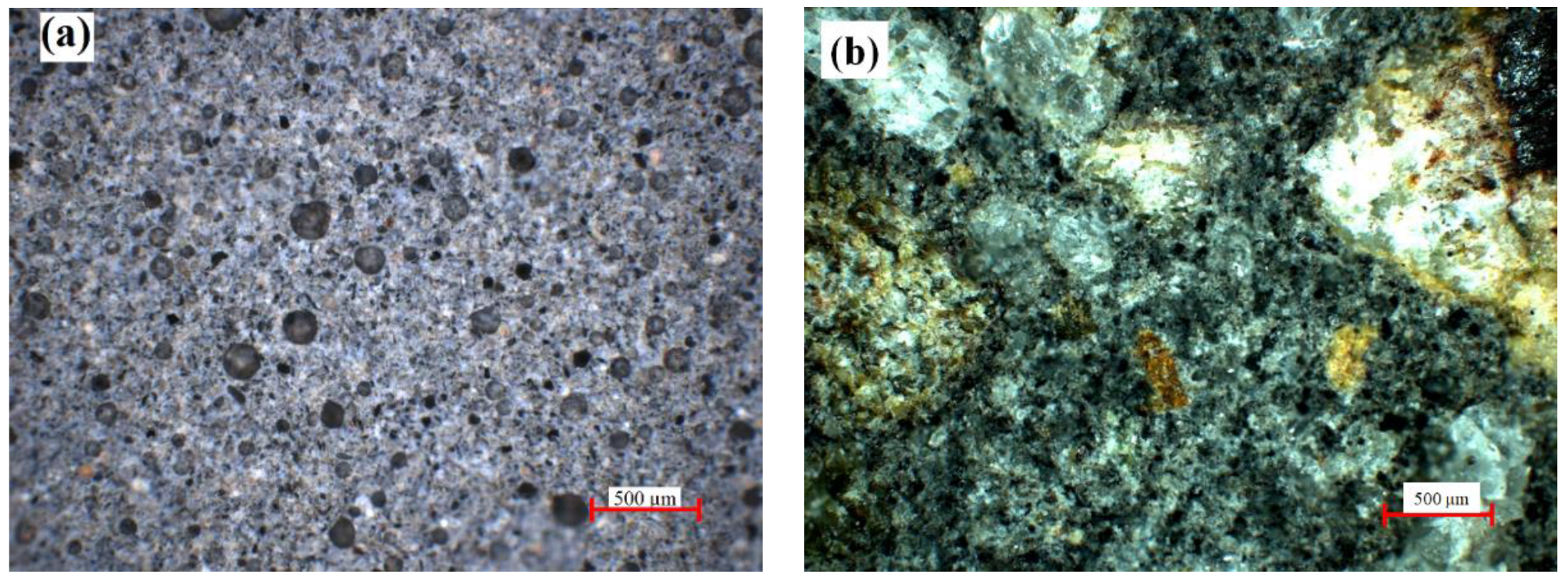
2.1.1. Indigenous Fly Ash
2.1.2. Glass Powder
2.1.3. Natural Aggregates
2.1.4. Sodium Silicate
2.1.5. Sodium Hydroxide
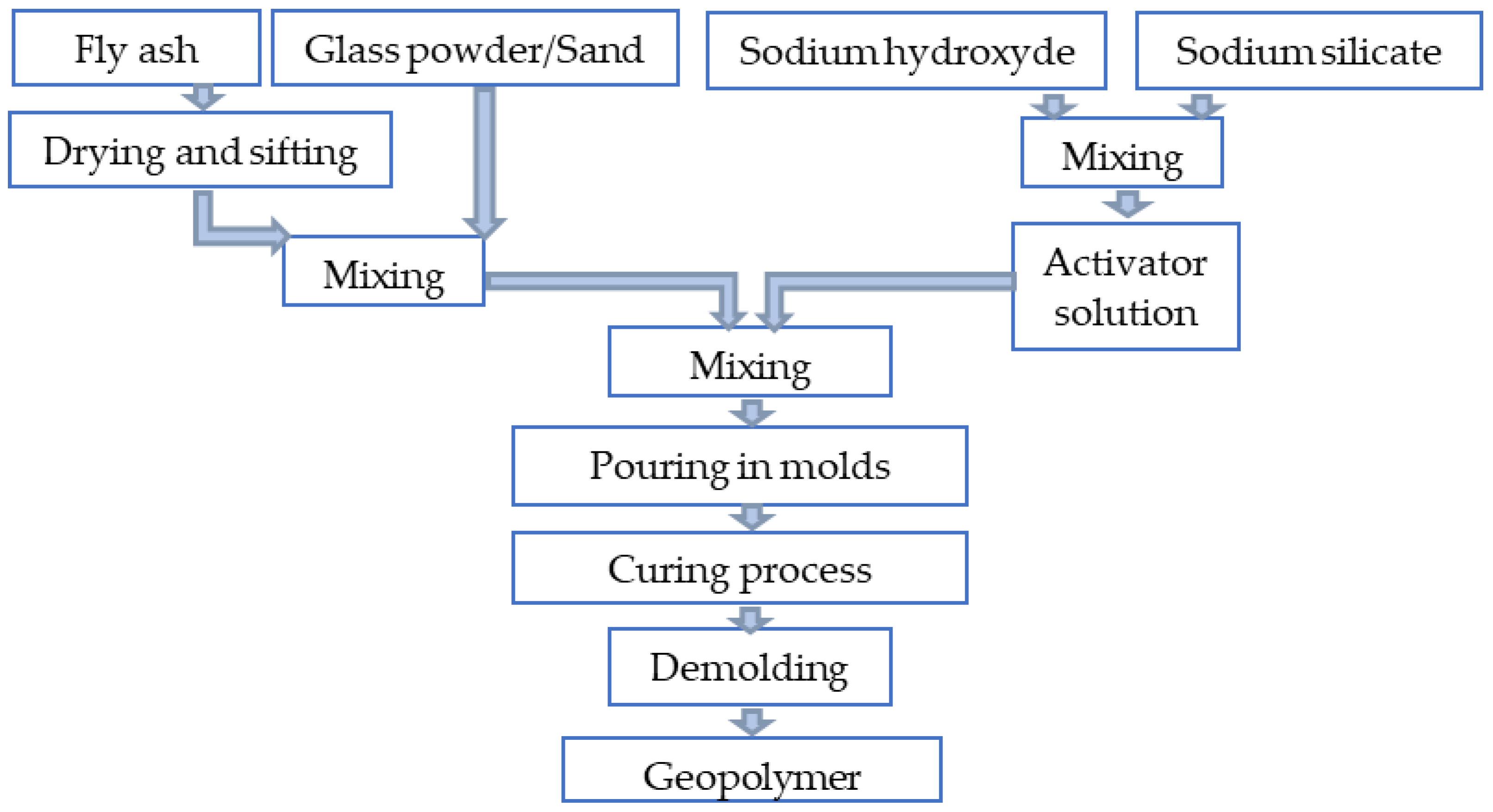
2.1.6. Sample Preparation
- -
- a raw materials relative humidity of 0;
- -
- fly ash particles lower than 80 µm;
- -
- glass powder particles lower than 100 µm;
- -
- sand particles lower than 4 mm;
- -
- curing temperature of 70 °C;
- -
- curing time of 8 h.
2.2. Methods
2.2.1. Simultaneous Thermal Analysis
2.2.2. X-ray Diffraction
3. Results and Discussion
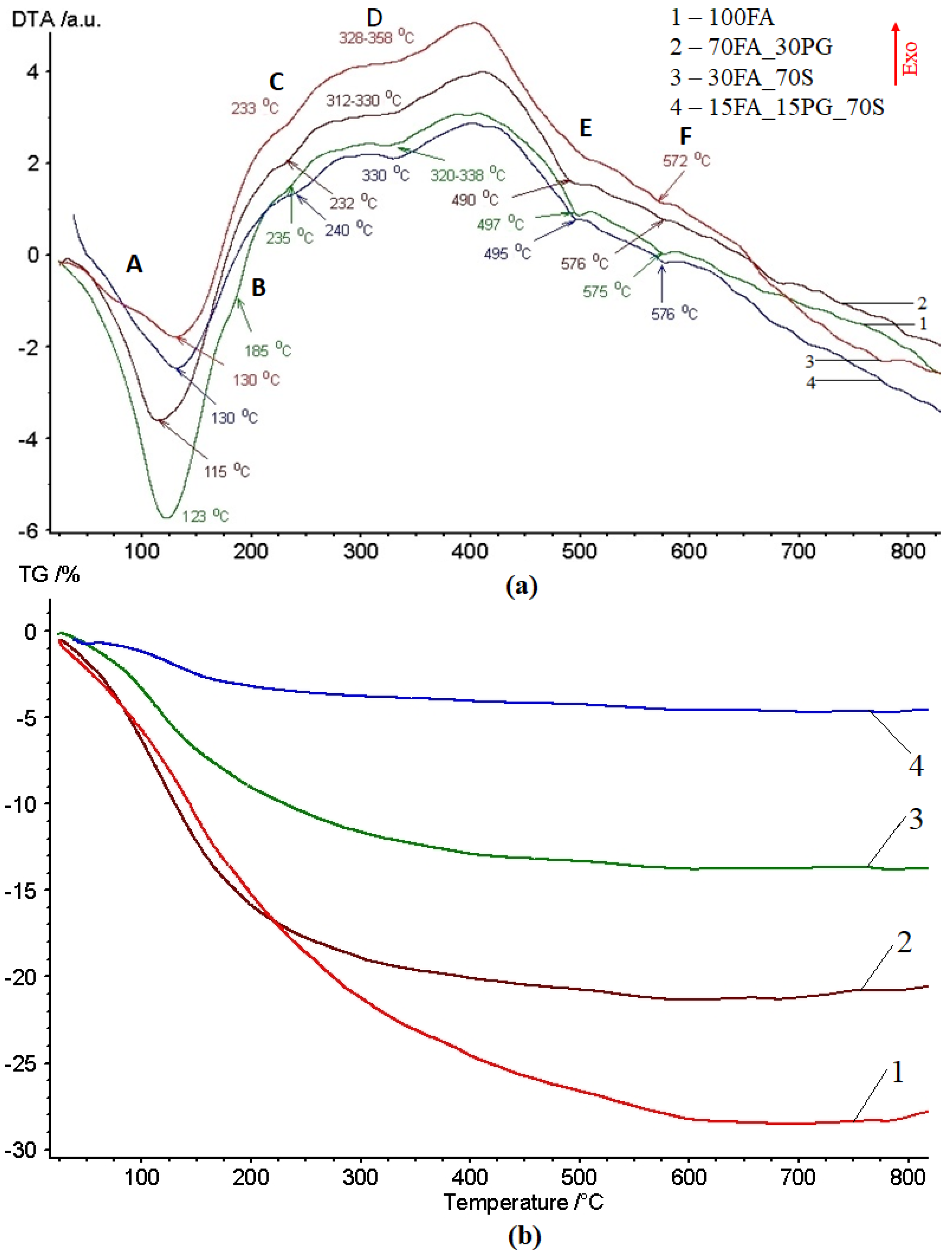
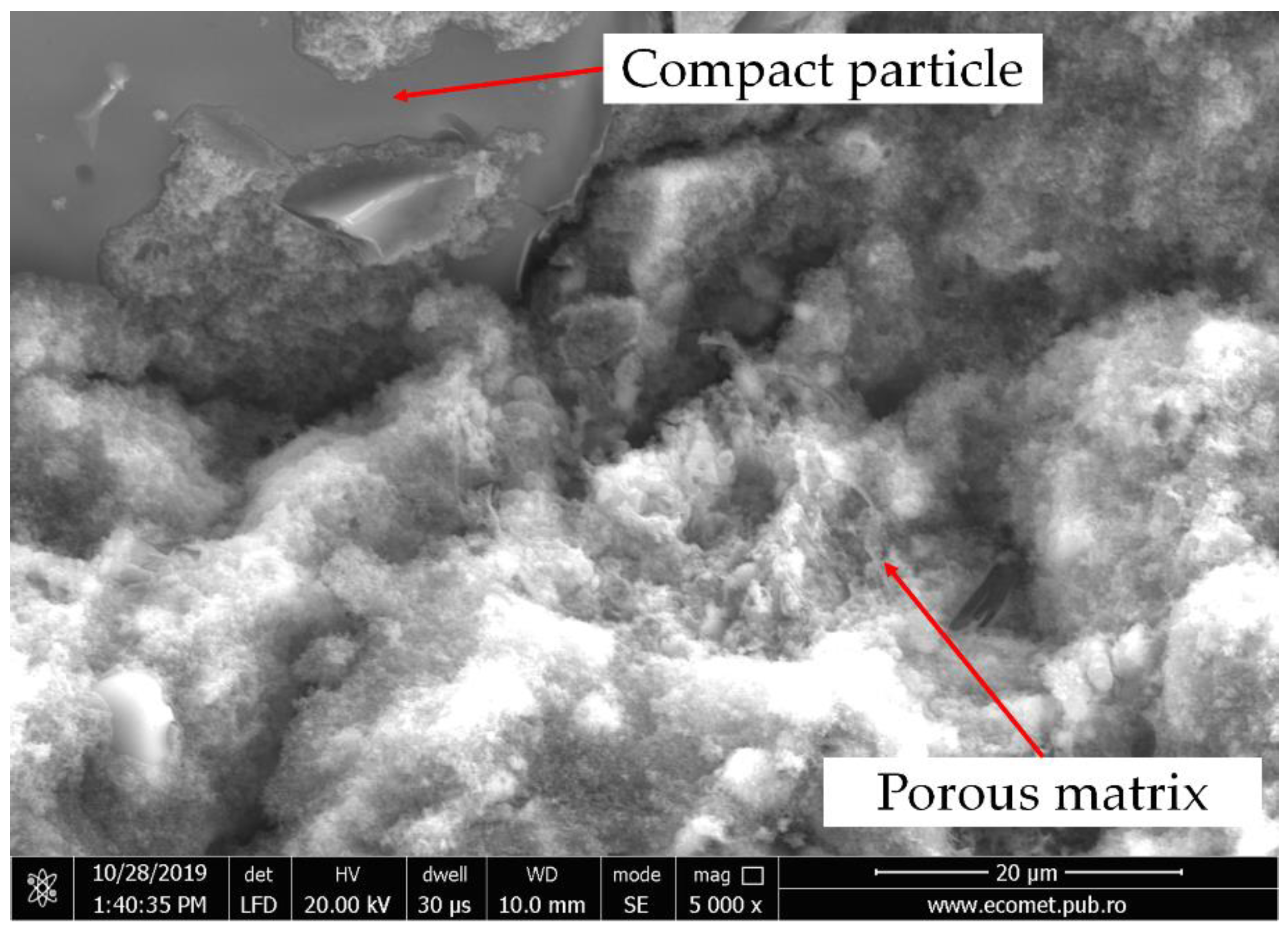
3.1. Thermal Behavior Evaluation
- -
- crystallization water (anionic and cationic or coordinative) which is removed from the structure in the 120–200 °C temperature range. This sub-type of water molecules are bonded in the structure during the formation of crystals from aqueous solution [38].
- -
- water from hydrogels that can be intercrystalline and network types that interact with the crystallization water. This sub-type of water is removed during heating in the 180–300 °C temperature range [39].
- -
- -
- Acids: M-O−H+ (Si(IV), Ti(IV), Fe(III))
- -
- Basics: M+HO− hydroxide (Na, Ca (II), K, Mg (II))
- -
- Neutral: M-OH hydroxyl (Al (III), Mn (III)).
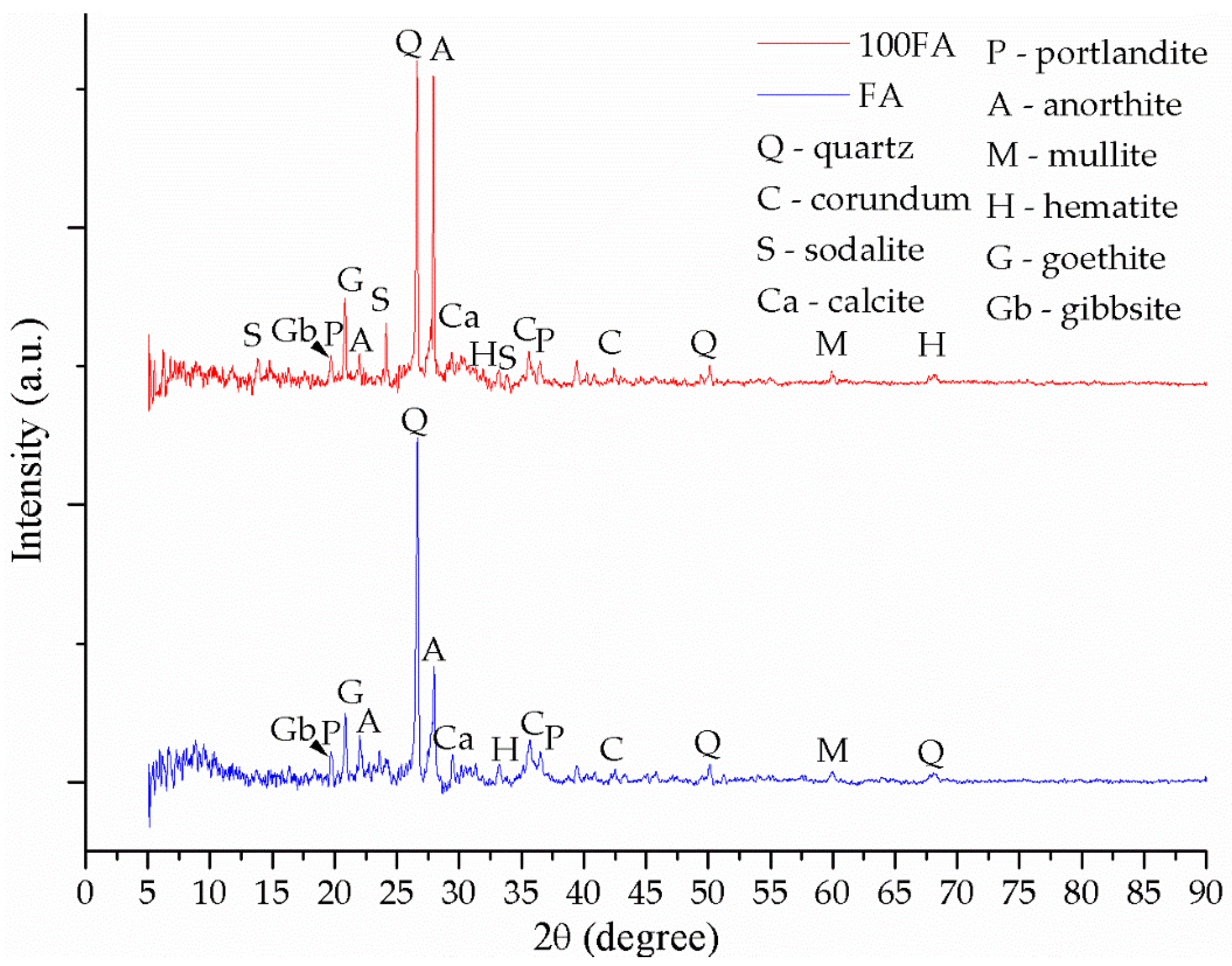
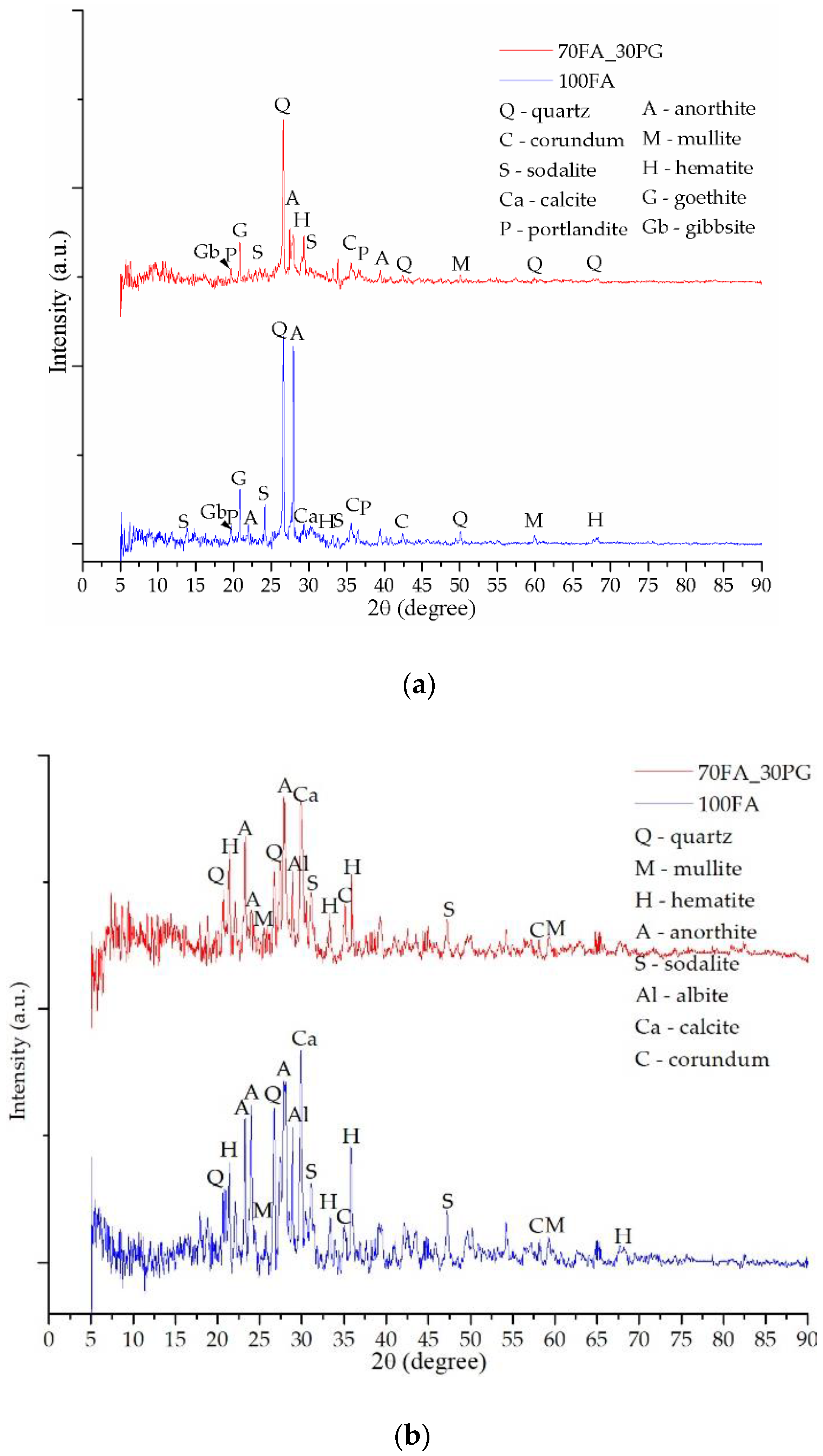
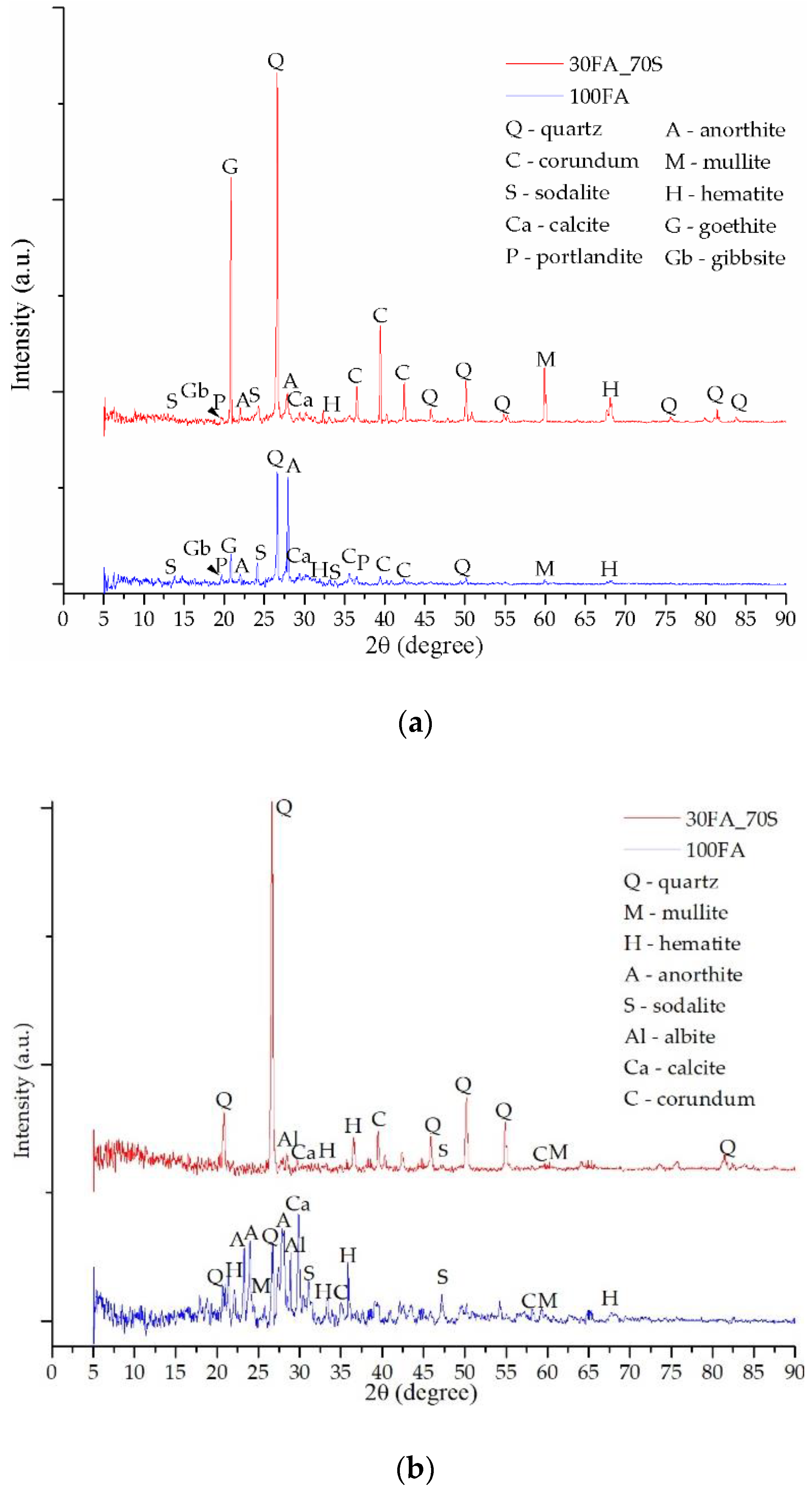
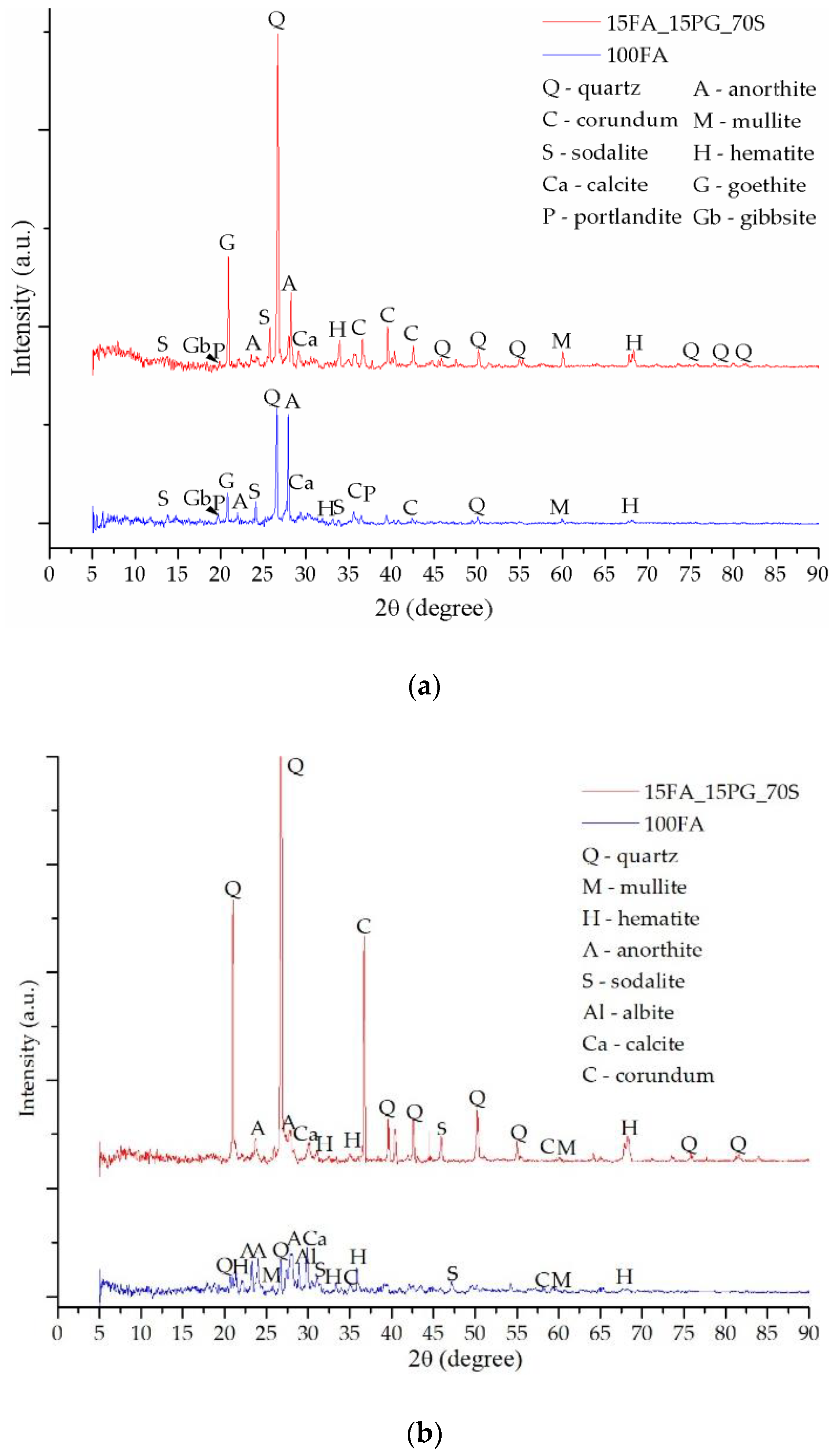
3.2. Mineralogical Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nergis, D.D.B.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 12019. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef] [PubMed]
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-geopolymer composite materials: Production and characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- North, M.R.; Swaddle, T.W. Kinetics of Silicate Exchange in Alkaline Aluminosilicate Solutions. Inorg. Chem. 2000, 39, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Farhana, Z.F.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M.M. A Study on Relationship between Porosity and Compressive Strength for Geopolymer Paste. Key Eng. Mater. 2013, 594–595, 1112–1116. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.-G.; Shi, C.-J. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Muthu, M.; Vogt, O.; Koenders, E. Geopolymer, Calcium Aluminate, and Portland Cement-Based Mortars: Comparing Degradation Using Acetic Acid. Materials 2019, 12, 3115. [Google Scholar] [CrossRef]
- Rangan, B.V. Design, Properties, and Applications of Low-Calcium Fly Ash-Based Geopolymer Concrete. In Developments in Porous, Biological and Geopolymer Ceramics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 347–362. [Google Scholar]
- Ismail, N.; El-Hassan, H. Development and Characterization of Fly AshSlag Blended Geopolymer Mortar and Lightweight Concrete. J. Mater. Civ. Eng. 2018, 30, 4018029. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Capmas, A. Calcium Aluminate Cements. In Leas Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 1998; pp. 713–782. [Google Scholar]
- Vafaei, M.; Allahverdi, A. Influence of calcium aluminate cement on geopolymerization of natural pozzolan. Constr. Build. Mater. 2016, 114, 290–296. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Azmi, A.A.; Abdullah, M.M.A.B.; Ghazali, C.M.R.; Sandu, A.V.; Hussin, K.; Sumarto, D.A. A Review on Fly Ash Based Geopolymer Rubberized Concrete. Key Eng. Mater. 2016, 700, 183–196. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and characteristics of local fly ash based geopolymers mixed with natural aggregates. Rev. Chim. 2019, 70, 1262–1267. [Google Scholar]
- Ma, H.; Qian, S.; Li, V.C. Influence of Fly Ash Type on Mechanical Properties and Self-Healing Behavior of Engineered Cementitious Composite (ECC). In Proceedings of the 9th International Conference on Fracture Mechanics of Concrete and Concrete Structures, Berkeley, CA, USA, 28 May–1 June 2016. [Google Scholar]
- Ibrahim, W.M.W.; Hussin, K.; Abdullah, M.M.A.B.; Kadir, A.A.; Binhussain, M. A Review of Fly Ash-Based Geopolymer Lightweight Bricks. Appl. Mech. Mater. 2015, 754–755, 452–456. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 22–27 January 2017; pp. 201–214. [Google Scholar]
- Available online: http://www.anpm.ro/documents/839616/33839940/Raport_Amplasament_CET2+Holboca+Rev.+Sep2017.pdf/7931f8ce-1e21-4af7-9abf-a16c602acc5b (accessed on 12 December 2019).
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cement Concrete Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef]
- Embong, R.; Kusbiantoro, A.; Shafiq, N.; Nuruddin, M.F. Strength and microstructural properties of fly ash based geopolymer concrete containing high-calcium and water-absorptive aggregate. J. Clean. Prod. 2016, 112, 816–822. [Google Scholar] [CrossRef]
- Joseph, B.; Mathew, G. Influence of aggregate content on the behavior of fly ash based geopolymer concrete. Sci. Iran. 2012, 19, 1188–1194. [Google Scholar] [CrossRef]
- Dave, S.; Bhogayata, A.; Arora, D.N. Impact Resistance of Geopolymer Concrete Containing Recycled Plastic Aggregates. EasyChair 2017, 1, 137–143. [Google Scholar]
- Provis, J.L. Alkali-activated Binders and Concretes: The Path to Standardization. In Geopolymer Binder Systems; ASTM International: West Conshohocken, PA, USA, 2013; pp. 185–195. [Google Scholar]
- Nordin, N.; Abdullah, M.M.A.B.; Fakri, W.M.N.R.W.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K.; Zailani, W.W.A. Exploration on fly ash waste as global construction materials for dynamics marketability. In Proceedings of the Applied Physics of Condensed Matter (APCOM 2019), Pleso, Slovak, 19–21 June 2019; AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Xu, H.; van Deventer, J.S.J. Effect of source materials on geopolymerization. Ind. Eng. Chem. Res. 2003, 42, 1698–1706. [Google Scholar] [CrossRef]
- Mo, B.H.; Zhu, H.; Cui, X.M.; He, Y.; Gong, S.Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Yahya, Z.; Tahir, M.F.M.; Hussin, K.; Binhussain, M.; Sandhu, A.V. Fly Ash Based Lightweight Geopolymer Concrete Using Foaming Agent Technology. Appl. Mech. Mater. 2014, 679, 20–24. [Google Scholar] [CrossRef]
- Harja, M.; Barbuta, M.; Rusu, L. Obtaining and Characterization of the Polymer Concrete with Fly Ash. J. Appl. Sci. 2009, 9, 88–96. [Google Scholar] [CrossRef]
- Corbu, O.; Ioani, A.M.; Al Bakri Abdullah, M.M.; Meita, V.; Szilagyi, H.; Sandu, A.V. The pozzoolanic activity level of powder waste glass in comparisons with other powders. Key Eng. Mater. 2015, 660, 237–243. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Chemical interactions between siliceous aggregates and low-Ca alkali-activated cements. Cem. Concr. Res. 2007, 37, 844–855. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2012, 46, 361–373. [Google Scholar] [CrossRef]
- Wuddivira, M.N.; Robinson, D.A.; Lebron, I.; Bréchet, L.; Atwell, M.; de Caires, S.; Oatham, M.; Jones, S.B.; Abdu, H.; Verma, A.K.; et al. Estimation of soil clay content from hygroscopic water content measurements. Soil Sci. Soc. Am. J. 2012, 76, 1529–1535. [Google Scholar] [CrossRef]
- Rico, P.; Adriano, A.; Soriano, G.; Duque, J.V. Characterization of Water Absorption and Desorption properties of Natural Zeolites in Ecuador. In International Symposium on Energy; Puerto Rico Energy Center-Laccei: Puerto Rico, 2013. [Google Scholar]
- Longhi, M.A.; Zhang, Z.; Rodríguez, E.D.; Kirchheim, A.P.; Wang, H. Efflorescence of alkali-activated cements (geopolymers) and the impacts on material structures: A critical analysis. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Smart Nanoconcretes and Cement-Based Materials: Properties, Modelling and Applications. Available online: https://books.google.ro/books?id=oaq-DwAAQBAJ&pg=PA173&lpg=PA173&dq=strong+physically+bound+water&source=bl&ots=F2VLsZWYHy&sig=ACfU3U11W_kRxqwL2yooFg6auXynm-80ww&hl=ro&sa=X&ved=2ahUKEwjjptPehrLmAhV_wAIHHVmYCG8Q6AEwDnoECAoQAQ#v=onepage&q=strong%20physically%20bound%20water&f=false (accessed on 13 December 2019).
- Van Reeuwijk, L.P. The Thermal Dehydration of Natural Zeolites (with a Summary in Dutch); H. Veenman & Zonen: Wageningen, The Netherlands, 1974. [Google Scholar]
- Valdiviés-Cruz, K.; Lam, A.; Zicovich-Wilson, C.M. Chemical interaction of water molecules with framework Al in acid zeolites: A periodic ab initio study on H-clinoptilolite. Phys. Chem. Chem. Phys. 2015, 17, 23657–23666. [Google Scholar] [CrossRef] [PubMed]
- Calero, S.; Gómez-Álvarez, P. Hydrogen bonding of water confined in zeolites and their zeolitic imidazolate framework counterparts. RSC Adv. 2014, 4, 29571–29580. [Google Scholar] [CrossRef]
- Glad, B.E.; Kriven, W.M. Geopolymer with Hydrogel Characteristics via Silane Coupling Agent Additives. J. Am. Ceram. Soc. 2014, 97, 295–302. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, 22. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated Colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Musicá, S.M.; Krehula, S.; Popovićb, S.; Popovićb, P. Thermal decomposition of h-FeOOH. Mater. Lett. 2004, 58, 444–448. [Google Scholar]
- Derie, R.; Ghodsi, M.; Calvo-Roche, C. DTA study of the dehydration of synthetic goethite αFeOOH. J. Therm. Anal. 1976, 9, 435–440. [Google Scholar] [CrossRef]
- Rendon, J.L.; Cornejo, J.; de Arambarri, P.; Serna, C.J. Pore structure of thermally treated goethite (α-FeOOH). J. Colloid Interface Sci. 1983, 92, 508–516. [Google Scholar] [CrossRef][Green Version]
- Giovanoli, R.; Brütsch, R. Kinetics and mechanism of the dehydration of γ-FeOOH. Thermochim. Acta 1975, 13, 15–36. [Google Scholar] [CrossRef]
- Walter, D.; Buxbaum, G.; Laqua, W. The mechanism of the thermal transformation from goethite to hematite*. J. Therm. Anal. Calorim. 2001, 63, 733–748. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, 45355. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.H.; Togra, B.; Baykara, H.; Soriano, G.; Paredes, C.; Elsen, J. Effect of Calcium Hydroxide and Water to Solid Ratio on Compressive Strength of Mordenite-Based Geopolymer and the Evaluation of Its Thermal Transmission Property. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition (IMECE), Pittsburgh, PA, USA, 9–15 November 2018; American Society of Mechanical Engineers (ASME): New York, NY, USA, 2018; Volume 12. [Google Scholar]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Bajare, D.; Vitola, L.; Dembovska, L.; Bumanis, G. Waste stream porous alkali activated materials for high temperature application. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Dehydration Reactions and Kinetic Parameters of Gibbsite—Science Direct. Available online: https://www.sciencedirect.com/science/article/pii/S0272884210002592 (accessed on 13 December 2019).
- MacKenzie, K.J.D.; Temuujin, J.; Okada, K. Thermal decomposition of mechanically activated gibbsite. Thermochim. Acta 1999, 327, 103–108. [Google Scholar] [CrossRef]
- Zhu, B.; Fang, B.; Li, X. Dehydration reactions and kinetic parameters of gibbsite. Ceram. Int. 2010, 36, 2493–2498. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Raghdi, A. Mechanism and Kinetic Parameters of the Thermal Decomposition of Gibbsite Al(OH)3 by Thermogravimetric Analysis. Acta Phys. Pol. Ser. A 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Hao, H.; Lin, K.-L.; Wang, D.; Chao, S.-J.; Shiu, H.-S.; Cheng, T.-W.; Hwang, C.-L. Elucidating Characteristics of Geopolymer with Solar Panel Waste Glass. Environ. Eng. Manag. J. 2015, 14, 79–87. [Google Scholar]
- Grawe, S.; Augustin-Bauditz, S.; Clemen, H.C.; Ebert, M.; Eriksen Hammer, S.; Lubitz, J.; Reicher, N.; Rudich, Y.; Schneider, J.; Staacke, R.; et al. Coal fly ash: Linking immersion freezing behavior and physicochemical particle properties. Atmos. Chem. Phys. 2018, 18, 13903–13923. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Paunović, P.; Petrovski, A.; Načevski, G.; Grozdanov, A.; Marinkovski, M.; Andonović, B.; Makreski, P.; Popovski, O.; Dimitrov, A. Pathways for the production of non-stoichiometric titanium oxides. In Nanoscience Advances in CBRN Agents Detection, Information and Energy Security; Springer: Dordrecht, The Netherlands, 2015; pp. 239–253. ISBN 9789401796972. [Google Scholar]
- Anderson, P.J.; Horlock, R.F. Thermal decomposition of magnesium hydroxide. Trans. Faraday Soc. 1962, 58, 1993–2004. [Google Scholar] [CrossRef]
- Zulkifly, K.; Yong, H.C.; Abdullah, M.M.A.B.; Ming, L.Y.; Panias, D.; Sakkas, K. Review of Geopolymer Behaviour in Thermal Environment. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 26–27 July 2016; Institute of Physics Publishing: Bristol, UK, 2017; Volume 209. [Google Scholar]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]

| Oxide | SiO2 | Al2O3 | FexOy | CaO | K2O | MgO | TiO2 | Na2O | P2O5 | Oth 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| %, weight | 47.80 | 28.60 | 10.20 | 6.40 | 2.40 | 2.00 | 1.30 | 0.60 | 0.40 | 0.30 |
| Stat. error, % | 0.32 | 0.27 | 0.95 | 0.77 | 0.71 | 1.09 | 1.81 | 0.63 | 0.24 | - |
| Oxide | SiO2 | Al2O3 | FexOy | CaO | MgO | Na2O | Oth 1 |
|---|---|---|---|---|---|---|---|
| %, weight | 70–71 | 1.5–2 | 0.8–1 | 9–11 | 2–3 | 12–14 | <0.1 |
| Sample | Liquid Component, % Weight | Solid Component, % Weight | |||
|---|---|---|---|---|---|
| Na2SiO3 | NaOH | Fly Ash | Glass Powder | Sand | |
| 100FA | 60 | 40 | 100 | 0 | 0 |
| 70FA_30PG | 60 | 40 | 70 | 30 | 0 |
| 30FA_70S | 60 | 40 | 30 | 0 | 70 |
| 15FA_15PG_70S | 60 | 40 | 15 | 15 | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials 2020, 13, 343. https://doi.org/10.3390/ma13020343
Burduhos Nergis DD, Abdullah MMAB, Sandu AV, Vizureanu P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials. 2020; 13(2):343. https://doi.org/10.3390/ma13020343
Chicago/Turabian StyleBurduhos Nergis, Dumitru Doru, Mohd Mustafa Al Bakri Abdullah, Andrei Victor Sandu, and Petrică Vizureanu. 2020. "XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder" Materials 13, no. 2: 343. https://doi.org/10.3390/ma13020343
APA StyleBurduhos Nergis, D. D., Abdullah, M. M. A. B., Sandu, A. V., & Vizureanu, P. (2020). XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials, 13(2), 343. https://doi.org/10.3390/ma13020343








